1. Introduction
Nucleosides, the fundamental components of nucleic acids, play a key role in many crucial biochemical processes in all organisms. Chemically modified nucleoside analogs mimic their endogenous counterparts, exploiting cellular metabolism and becoming incorporated into both DNA and RNA. This property, which is related to their ability to inhibit enzymes essential for the replication of pathogens and the proliferation of cancer cells, makes nucleoside analogs effective drugs in the treatment of various conditions, mainly viral, bacterial, and cancer diseases [
1,
2,
3].
In recent years, there has been increasing interest in nucleosides as potential agents for the treatment of fungal infections, especially in light of the rising resistance to conventional antifungal drugs [
4,
5]. Over the last decade, the incidence of fungal infections has increased, particularly among immunocompromised individuals or those hospitalized with severe illnesses. These infections often arise as secondary complications in patients with such conditions as cancer, AIDS, asthma, diabetes, organ transplants, chemotherapy, and corticosteroid treatment. Among fungal infections,
Candida,
Aspergillus,
Cryptococcus, and
Pneumocystis are major risk factors worldwide due to the severity and higher incidence of the disease [
6].
Candida species represent an important source of yeast infections. In 2022, the World Health Organization (WHO) released a list of fungal priority pathogens categorized into three priority groups. In this classification,
C. auris (currently named
Candidozyma auris) and
C. albicans are designated as critical pathogens.
C. glabrata (currently named
Nakaseomyces glabratus),
C. parapsilosis, and
C. tropicalis are classified into the high-priority group, and
C. krusei (currently named
Pichia kudriavzevii) is placed in the medium-priority group [
7]. These opportunistic microorganisms are not only the most common cause of superficial vaginal or mucosal oral infections but can also enter the bloodstream leading to hard-to-treat deep-tissue infections [
8].
Currently, antifungal treatments for invasive mycoses are limited to five major classes of compounds: echinocandins, imidazoles, triazoles, polyenes, and nucleoside analogs [
9]. Among antifungal nucleosides, flucytosine, whose active compound is 5-fluorocytosine (5-FC), a fluorinated pyrimidine derivative, is widely used. Flucytosine belongs to the group of antifungal antimetabolites. It penetrates fungal cells via cytosine deaminase, converting to fluorouracil, which is then incorporated into RNA, thereby inhibiting protein synthesis. Mammalian cells lack this enzyme, rendering flucytosine non-toxic to humans. Additionally, flucytosine is metabolized into fluorodeoxyuridine, which disrupts DNA synthesis and impairs fungal cell division. Flucytosine is effective against species from the genera
Candida,
Cryptococcus,
Cladosporium,
Phialophora,
Cyberlindnera,
Debaryomyces,
Diutina,
Rhodotorula,
Saccharomyces, and
Aspergillus. In combination with the polyene agent amphotericin B, flucytosine is a reliable treatment for resistant
Candida infections and cryptococcal meningitis [
10]. However, its clinical utility is limited by the frequent emergence of both primary and acquired resistance, particularly when used as monotherapy. Primary resistance to 5-FC is found in less than 5% of all
Candida species, except for
C. krusei, where resistance is observed in up to 35% of isolates. Resistance mechanisms are complex and can involve mutations or loss of function in any of the three key enzymes: FCY2 (cytosine permease), FCY1 (cytosine deaminase), or FUR1 (uracil phosphoribosyltransferase). FCY2 is responsible for the active transport of flucytosine into fungal cells, while FCY1 and FUR1 convert it into its toxic metabolite, 5-fluoro-uridylate. Additionally, increased endogenous pyrimidine production can help fungi circumvent the toxic effects of antifungal agents [
11].
Given the growing burden of fungal diseases and antimicrobial resistance, new therapeutic options are urgently needed. Among the most innovative approaches is de novo drug discovery, which involves the identification of both synthetic compounds and natural products derived from various sources, such as microbial strains, plants, algae, endophytic fungi, and marine fungi. Importantly, antifungal therapies in current clinical development are being designed based on a broad range of strategies, including the targeting of known molecular pathways (e.g., cell wall synthesis inhibitors like fosmanogepix or nikkomycin Z), novel or previously unexploited targets such as calcineurin, Hsp90, lipid biosynthesis, and kinases, as well as the development of entirely new molecular scaffolds (e.g., tetrazoles like oteseconazole and VT-1598, BSG005, encochleated amphotericin B, rezafungin, or AM-2-19). In addition, another promising strategy is also drug repurposing and re-evaluating existing non-antifungal compounds for potential antifungal activity, thus bypassing the high costs and lengthy timelines of traditional drug development [
12,
13].
Since nucleoside analogs are a class of drugs which are important from a clinical perspective and show promise in the treatment of mycoses, in this study, we used 5-fluorouridine (riboside of 5-fluoro-2,4(1H, 3H)-pyrimidinedione, 5-FUrd), i.e., a fluoropyrimidine nucleoside analog, to evaluate its efficacy against
Candida species. 5-FUrd is a cell-permeable modified RNA precursor and a prodrug of 5-fluorouracil (5-FU) [
14]. Previous studies have demonstrated its antifungal activity against
C. albicans attributed to inhibition of thymidylate kinase (CaTMPK), a key enzyme in the dTTP biosynthesis pathway. CaTMPK catalyzes the phosphorylation of dTMP to dTDP, which is subsequently converted to dTTP by nucleoside diphosphate kinase (NDPK). Several factors support the potential of 5-FUrd as a selective antifungal agent: (i) Structural differences exist between CaTMPK and its human counterpart (hTMPK), particularly in the Ca-loop, a unique surface-exposed catalytic element that drives hyperactivity in CaTMPK. The deletion of this loop significantly impairs
C. albicans growth, identifying it as a promising target for fungal-specific inhibitors. (ii) The catalytic efficiency of CaTMPK is 15-fold higher than that of hTMPK. (iii) CaTMPK exhibits strong activity in converting dUMP to dUDP and 5-FdUMP to 5-FdUDP, enhancing the cytotoxicity of 5-FUrd. (iv) 5-FUrd is effective against
C. albicans strains resistant to both 5-FC and azoles due to differences in their metabolic activation pathways. 5-FUrd enters
C. albicans via uridine permease (Fui1) and is phosphorylated by uridine kinase (Urk1) to 5-FUMP, bypassing the resistance mechanisms that impair 5-FC activation [
15].
Initial studies on the antifungal activity of 5-FUrd focused exclusively on
C. albicans, a major opportunistic fungal pathogen and a widely used model organism in antifungal research [
15]. Encouraged by the promising results obtained in this species, we substantially expanded the scope of our investigation to include additional
Candida species and a broad collection of clinical isolates of
C. albicans. This comprehensive approach aimed to assess the wider antifungal spectrum of 5-FUrd and to determine whether the observed effects could be replicated in other clinically relevant
Candida species, particularly those with distinct susceptibility profiles and virulence traits. In parallel, the study was extended beyond conventional susceptibility testing to include key virulence-associated features, such as adhesion, biofilm formation, dimorphism, hydrolase secretion, hemolytic activity, and cell surface hydrophobicity, in two species found to be particularly sensitive to 5-FUrd:
C. albicans and
C. parapsilosis. These pathogenicity-related factors are known to play a critical role in infection persistence and resistance to treatment. Collectively, this expanded investigation provides an in-depth evaluation of 5-FUrd as a promising antifungal candidate, with dual potential for both growth inhibition and attenuation of fungal virulence. Additionally, we assessed the frequency of spontaneous mutations in
C. albicans and
C. parapsilosis under drug pressure and evaluated 5-FUrd cytotoxicity using human erythrocytes and a zebrafish model.
3. Discussion
This study provides new insights into the antifungal potential of 5-FUrd, demonstrating its broad-spectrum activity against various Candida species, including clinical isolates. In addition to its growth-inhibitory effects, 5-FUrd was found to impair key virulence factors, such as biofilm formation, hyphal development, and enzymatic activity, i.e., traits that are essential for fungal pathogenicity and persistence within host environments. Notably, our evaluation of toxicity and mutagenicity offers preliminary evidence supporting a favorable safety profile and therapeutic potential.
In an era of rapidly increasing resistance to antimicrobial agents, it is extremely important to search for new antifungal substances that are effective against pathogenic fungi exhibiting resistance to conventional therapies. In this study, we investigate the effect of 5-FUrd on the growth and virulence of
Candida species. The biological activity spectrum of 5-FUrd and its derivatives primarily includes antitumor and antiviral effects [
14,
16,
17,
18]. Previous studies have also demonstrated the antifungal activity of 5-FUrd attributed to its ability to inhibit thymidylate kinase in
C. albicans cells [
15]. Thus, 5-FUrd can be classified among nucleoside derivatives with antifungal activity, alongside such compounds as tolytoxin, tubercidin, nikkomycin, polyoxin, blasticidin S, arginomycin, mildiomycin, cordycepin, sinefungin, toyocamycin, and clavines [
4,
19,
20,
21,
22,
23,
24]. To expand upon earlier research, we conducted studies on selected
Candida reference strains as well as clinical isolates of
C. albicans, aiming to further evaluate the therapeutic potential of this compound in the treatment of candidiasis. We then focused specifically on
C. albicans and
C. parapsilosis, assessing the effect of 5-FUrd on important virulence factors in both species.
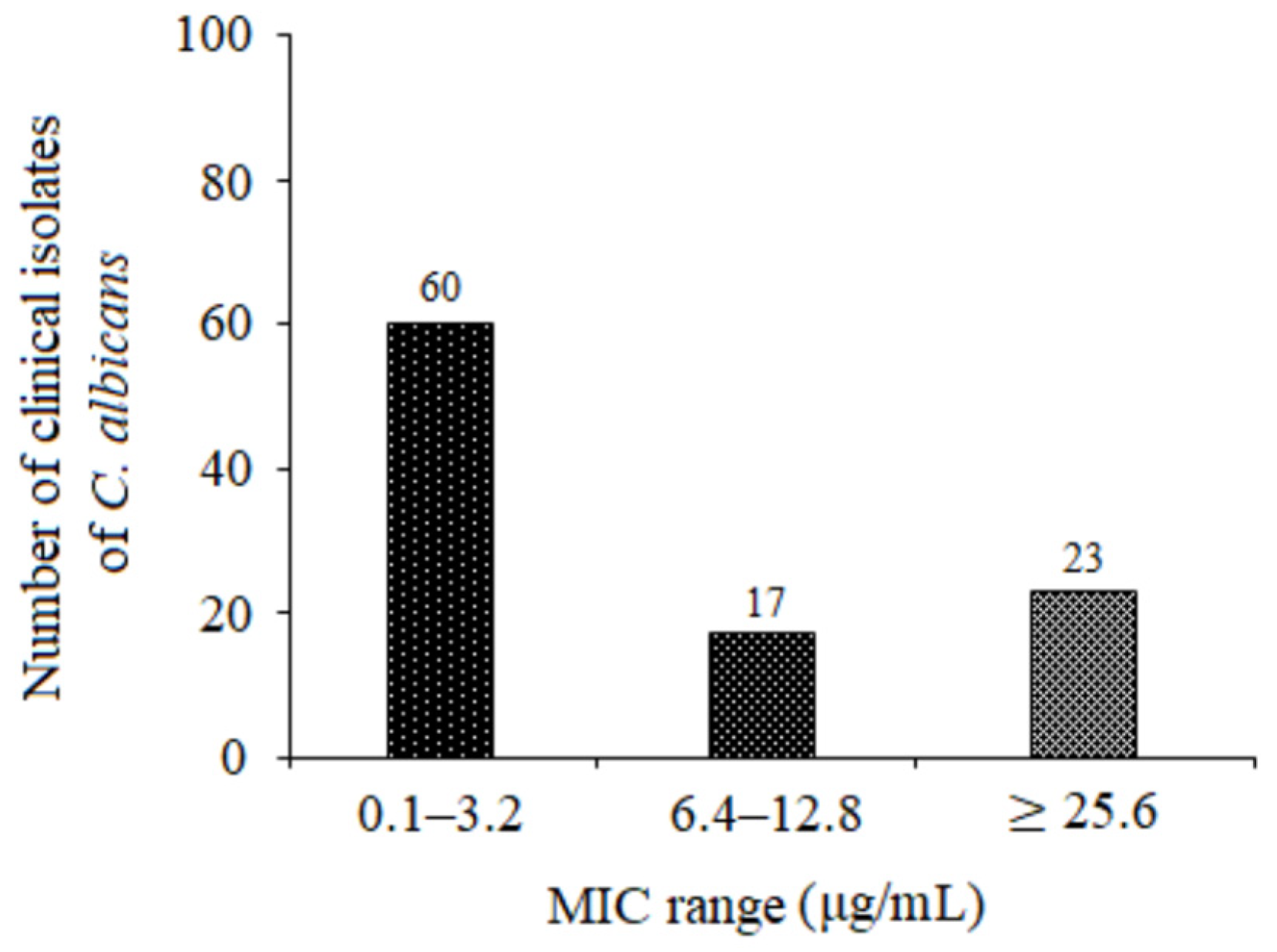
Our results demonstrate that 5-FUrd exhibits significant antifungal activity against a range of Candida species: C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, C. krusei, C. kefyr, C. lusitaniae, and C. norvegensis. An exception is multidrug-resistant C. auris, for which the MIC exceeded the highest tested concentration of 5-FUrd (>265 μg/mL). The MIC and MFC values for the other Candida species ranged from 0.2 μg/mL to 51.2 μg/mL. Among them, C. albicans and C. parapsilosis were the most susceptible, with MIC values of 0.4 μg/mL and 0.2 μg/mL, respectively. The MFC/MIC ratios observed for all the Candida species indicate that 5-FUrd is both fungistatic and fungicidal, suggesting its potential as an effective therapeutic agent. Interestingly, 5-FUrd exhibited variable activity against a panel of 100 clinical strains of C. albicans. Most strains were highly susceptible to 5-FUrd, with MIC values ranging from 0.1 to 6.4 μg/mL, although some showed relatively high MICs (≥25.6 μg/mL). This variability underscores the need for further studies on the mechanisms of resistance to 5-FUrd, which may be crucial for optimizing its clinical application.
5-FUrd is particularly effective against
C. albicans and
C. parapsilosis. While
C. albicans remains the leading cause of nosocomial invasive candidiasis worldwide, infections caused by non-albicans
Candida species (NACs), including
C. parapsilosis, have been increasingly reported.
C. parapsilosis poses a considerable risk to immunocompromised individuals, including patients with HIV and those undergoing surgical procedures, particularly involving the gastrointestinal tract. Importantly,
C. parapsilosis accounts for approximately one-third of neonatal
Candida infections and is associated with a mortality rate of about 10% [
25,
26].
Candida species have developed several specific and effective strategies to enhance their pathogenicity, including cell adhesion, biofilm, formation of hyphae, secretion of hydrolytic enzymes, and hemolytic activity. Virulence factors have also been considered as potent antifungal targets [
27,
28]. Biofilm formation by
C. albicans and
C. parapsilosis plays a crucial role in their pathogenicity. They form biofilms not only on implanted medical devices, including catheters, pacemakers, heart valves, joint prostheses, and dentures, but also on host surfaces, such as mucosal membranes, epithelial cell linings, and parenchymal organs. Biofilms are structured microbial communities embedded in the extracellular matrix (ECM). Biofilm development proceeds through three regulated stages: initial adhesion, an intermediate phase with yeast-to-hyphae transition and multilayer formation, and a maturation/dispersion phase. Biofilm architecture, morphology, and hyphae vary among
Candida species and even between strains [
29].
C. albicans generally forms larger and more complex biofilms than other
Candida species, characterized by a heterogeneous structure composed of yeast cells, hyphae, and pseudohyphae embedded in the extracellular matrix (ECM). The ECM of
C. albicans biofilms includes proteins and their glycosylated forms, carbohydrates, lipids, and extracellular DNA [
30]. In contrast,
C. parapsilosis, which does not produce true hyphae, forms biofilms consisting mainly of aggregated blastoconidia and pseudohyphae, resulting in a lower biofilm volume compared to other
Candida species. Additionally, the ECM of
C. parapsilosis biofilms is predominantly composed of carbohydrates, with relatively low protein content [
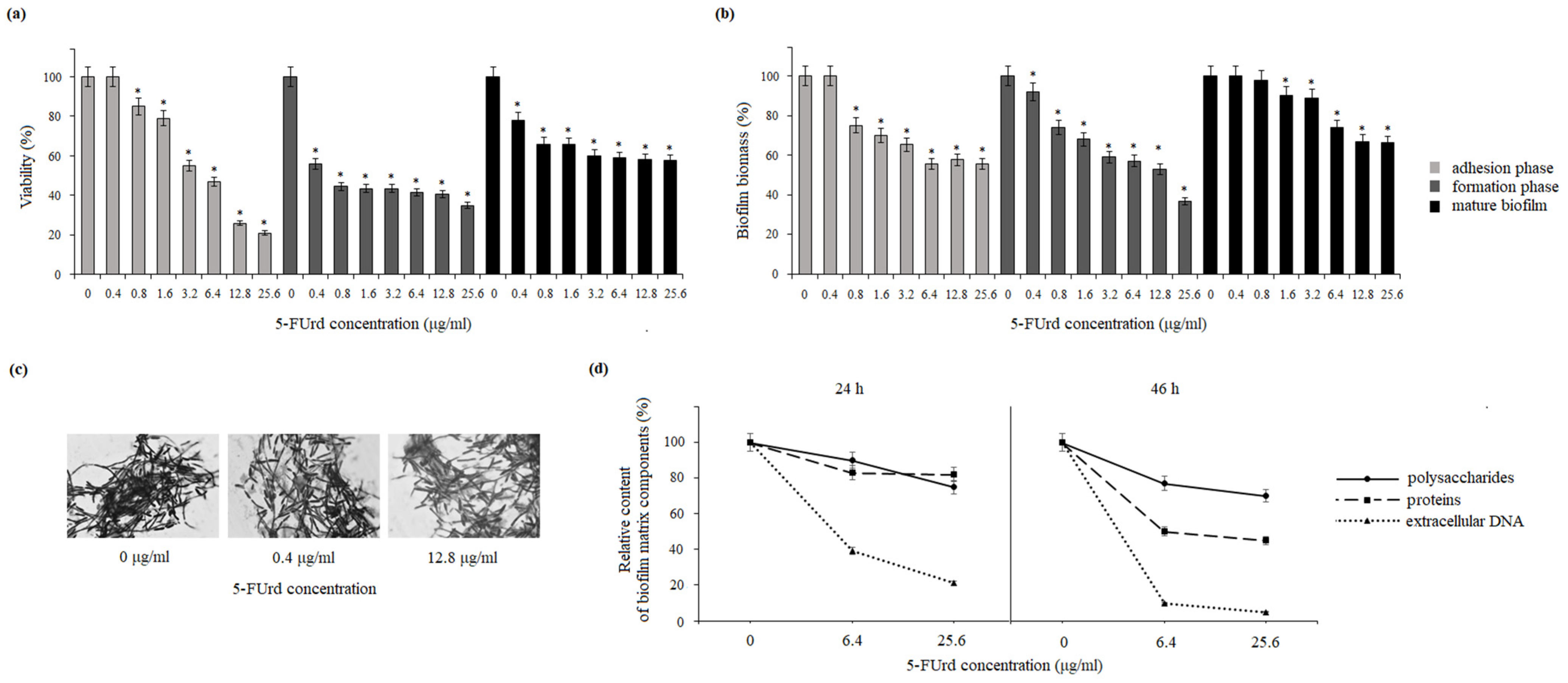
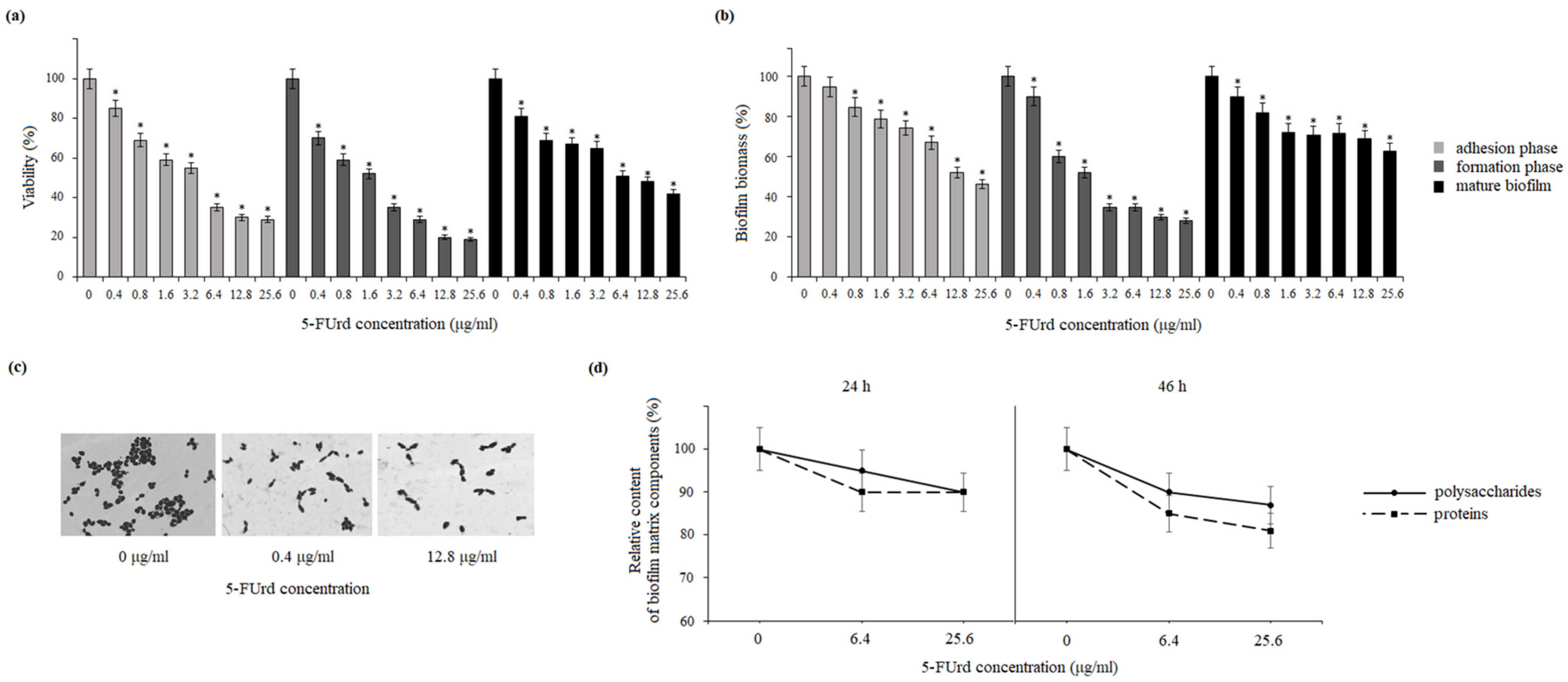
25]. Our results show that 5-FUrd has a significant impact on the biofilm structure and composition in both
Candida species. The compound reduced biofilm biomass and metabolic activity in both species during all biofilm formation phases: adhesion, biofilm formation, and even maturation. Additionally, the reduction in the extracellular matrix components, including polysaccharides, proteins, and extracellular DNA in
C. albicans or polysaccharides and proteins in
C. parapsilosis, indicates that 5-FUrd may interfere with biofilm formation through multiple pathways. Notably, the extracellular DNA in the
C. albicans matrix is significantly reduced, which is probably associated with the intracellular activity of 5-FUrd disrupting DNA synthesis. In a study conducted by Martins et al. (2012), the reduction in extracellular DNA by the addition of DNase improved the anti-biofilm activity of some antifungal drugs [
31]. This suggests that 5-FUrd may not only inhibit fungal growth but also directly target biofilm structure, which is a promising feature for combating persistent fungal infections.
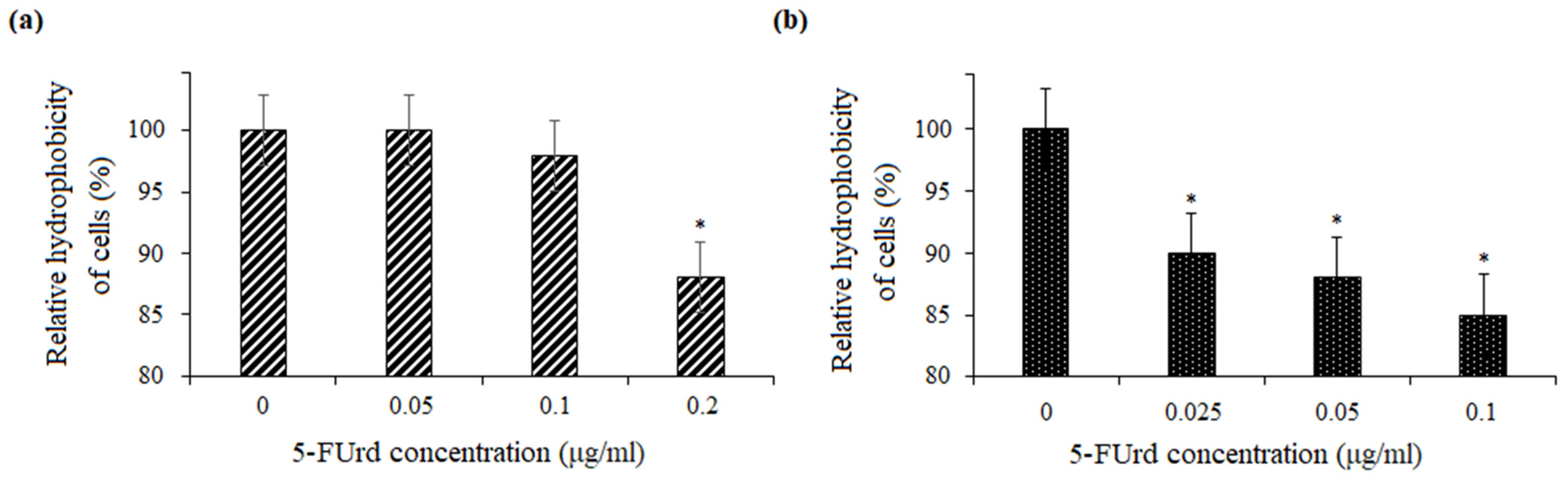
Adhesion is a critical multifactorial process mediated by both fungal and host cell characteristics, including cell surface hydrophobicity, cell wall composition, and growth conditions. Initially, yeast cell adhesion relies largely on hydrophobic interactions between the microorganism and the host surface. Cell surface hydrophobicity (CSH) is also strongly correlated with adhesion to abiotic surfaces.
Candida species generally exhibit high levels of CSH [
32]. The slight reduction in CSH observed in both
C. albicans and
C. parapsilosis following the exposure to 5-FUrd suggests that this compound disrupts key surface properties involved in the host–pathogen interaction, potentially reducing the ability of yeast cells to adhere and form mature biofilms.
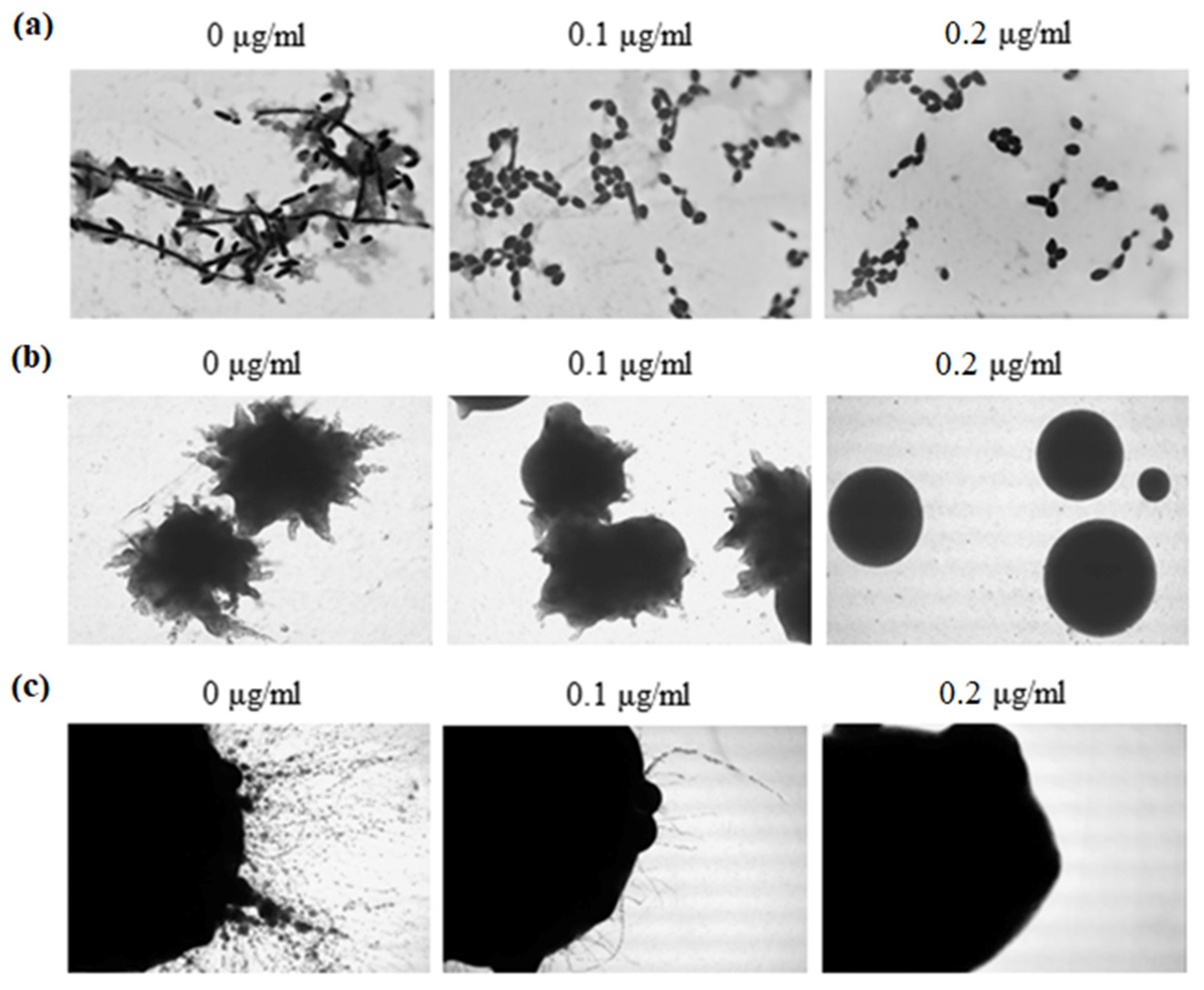
The ability to switch between yeast and hyphal growth forms (dimorphism) is one of the most discussed and best investigated virulence attributes of the human pathogenic
Candida fungi. The inhibition of hyphal formation is considered a potential therapeutic strategy [
33]. Several small molecules, including farnesol, fatty acids, rapamycin, geldanamycin, histone deacetylase inhibitors, and cell cycle inhibitors, have been shown to modulate the yeast-to-hyphae transition in
C. albicans. Additionally, some established antifungal agents, such as azoles, are also known to specifically inhibit hyphal growth [
34]. The impact of 5-FUrd on the morphology of
C. albicans and
C. parapsilosis was also evaluated in the present study. In
C. albicans, the compound inhibited hyphal formation in both liquid and solid culture conditions. In contrast,
C. parapsilosis exhibited a more limited morphological response. Fewer pseudohyphal cells were observed in liquid cultures, while no significant changes were noted in colonies grown on solid media, which did not produce hyphae even in the control conditions (without 5-FUrd). These findings suggest that 5-FUrd exerts a more pronounced morphological effect on
C. albicans than on
C. parapsilosis, indicating species-specific differences in sensitivity to the compound.
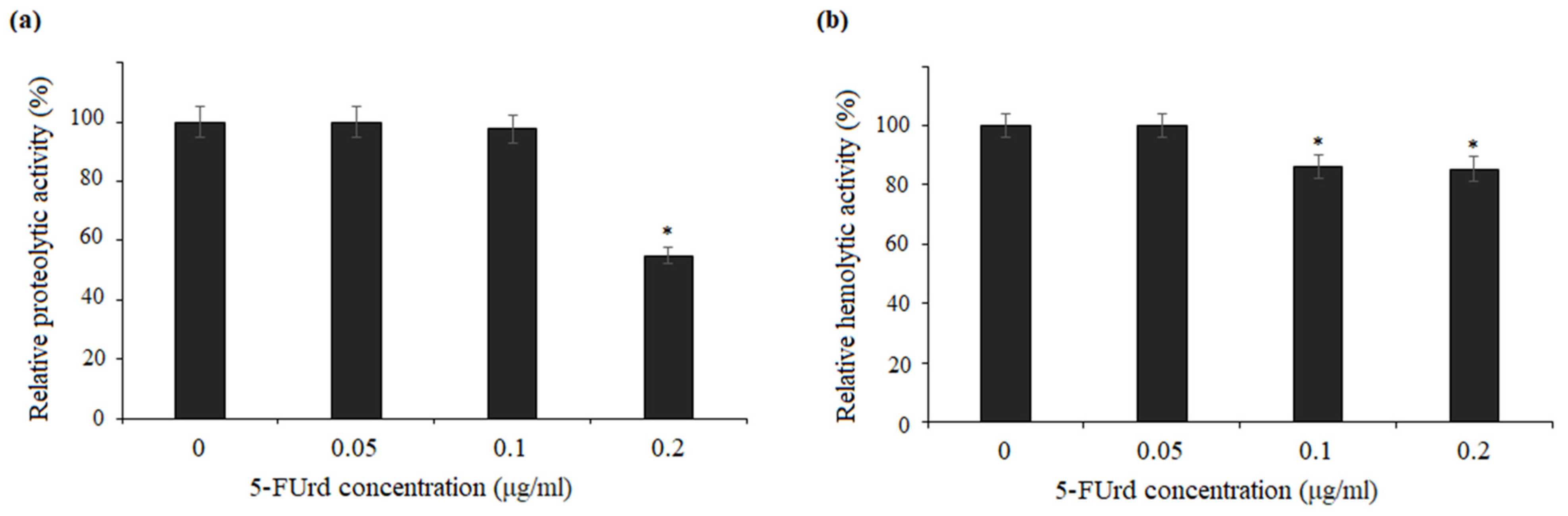
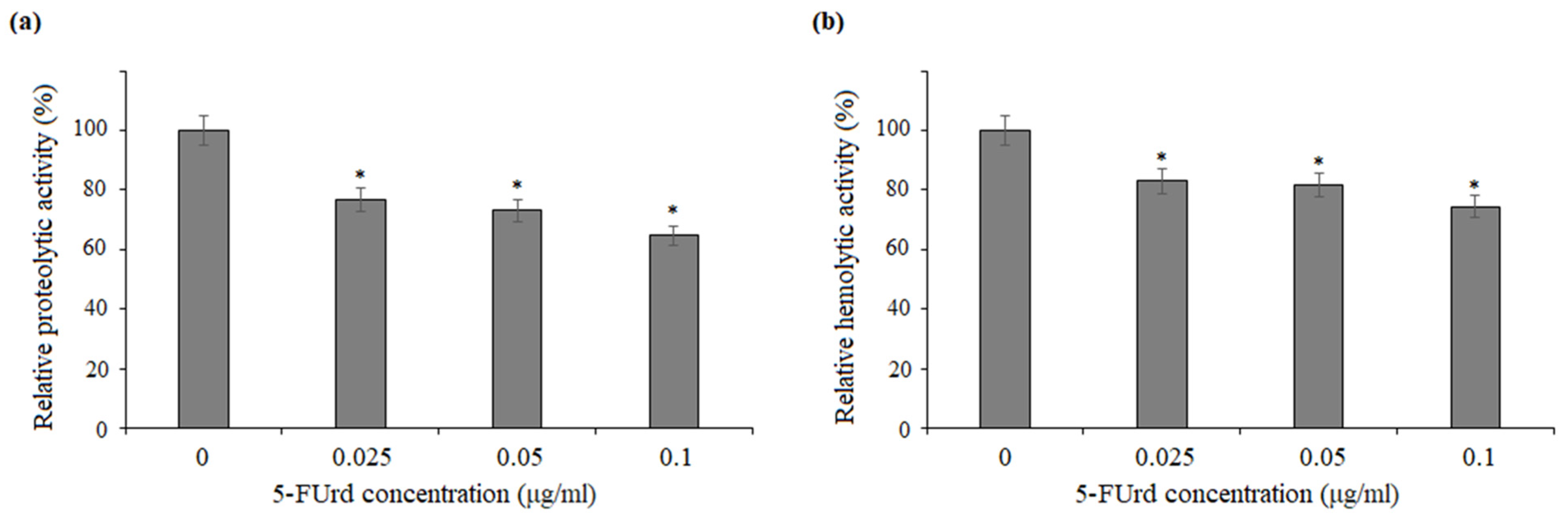
The secretion of extracellular enzymes, such as aspartyl proteases, lipases, and phospholipases, is a well-established virulence factor in
Candida species, closely associated with adhesion, host cell damage, and tissue invasion [
32]. The production of hemolytic factors by pathogenic
Candida species is also considered a key mechanism for survival within the mammalian host, enabling iron acquisition from the hemoglobin–heme complex. Free hemoglobin is an important host factor triggering the yeast cell differentiation pathway necessary for the dissemination and establishment of superficial infections. In addition, hemolytic activity may facilitate hyphal invasion in systemic candidiasis [
35,
36]. Compared to
C. albicans,
C. parapsilosis exhibits lower protease and hemolytic activity, and the role of phospholipases in
C. parapsilosis remains poorly understood [
25,
37]. Our results showed that 5-FUrd did not inhibit phospholipase activity in
C. albicans and
C. parapsilosis, indicating that its antifungal mechanism may not involve the direct inhibition of these enzymes. However, the compound slightly reduced both proteolytic and hemolytic activity of
C. albicans and
C. parapsilosis, which could contribute to its overall antifungal effects by limiting the degradation of host tissues and thus reducing the invasive potential of the pathogen.
The toxicity of 5-FUrd was evaluated using zebrafish embryos and human erythrocytes. The results showed that 5-FUrd did not cause significant mortality or morphological abnormalities in zebrafish embryos at concentrations up to 8 µg/mL, which corresponds to 20 × MIC for C. albicans and 40 × MIC for C. parapsilosis. Additionally, no changes in the heart rate or embryonic development were observed, suggesting that the compound is not toxic to zebrafish at therapeutic concentrations. Similarly, the compound caused minimal erythrocyte lysis, with less than 10% destruction of human red blood cells, indicating that 5-FUrd is safe for human erythrocytes at high concentrations. These findings support the potential of 5-FUrd as an antifungal agent with a favorable safety profile.
Spontaneous mutation frequencies were evaluated to assess the potential for resistance development. The results showed that 5-FUrd caused a moderate increase in the mutation frequency in both
C. albicans and
C. parapsilosis, which is typical of antifungal agents that interfere with nucleic acid synthesis, such as ribavirin [
38]. However, this increase was relatively higher than that observed with classical antifungals, such as rezafungin [
39]. These mutations led to a ≥4-fold shift in MIC values, indicating a potential for resistance development, albeit at relatively low frequencies. These findings suggest that 5-FUrd should preferably be used for a short time or in combination with other antifungal agents. Therefore, beyond the discovery of novel antifungals, a pragmatic strategy would be to enhance the efficacy of existing drugs through combination therapy. In this context, testing 5-FUrd in combination with conventional antifungals in vitro could be highly beneficial for broadening the antimicrobial spectrum, minimizing the emergence of resistance, and reducing side effects by allowing the use of lower effective drug concentrations.
4. Materials and Methods
4.1. Fungal Strains, Compounds, and Growth Conditions
The reference strains of Candida (C. albicans ATCC 10231, C. auris ATCC MYA-5001, C. parapsilosis ATCC 22099, C. glabrata ATCC 15126, C. krusei ATCC 14243, C. tropicalis ATCC 13803 C. lusitaniae ATCC 34449, C. kefyr ATCC 204093, and C. norvegensis ATCC 22977) were obtained from the American Type Culture Collection (ATCC, Gaithersburg, MD, USA). A total of 100 clinical isolates of C. albicans were obtained as stock cultures from the Jan Boży Independent Public Provincial Hospital in Lublin, Poland. The strains were identified using VITEK 2 YST IC CARDS (bioMérieux, Warsaw, Poland). The yeast were routinely grown in YPD (1% yeast extract, 2% peptone, 2% glucose) liquid medium at 30 °C with agitation (200 rpm). Sabouraud Dextrose (Biocorp, Warsaw, Poland) and RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO, USA) buffered with 0.165 M morpholinepropanesulfonic acid (Sigma-Aldrich, St. Louis, MO, USA) to a pH of 7.0 were used as well. 5-fluorouridine (5-FUrd), dimethyl sulfoxide (DMSO), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), menadione, crystal violet (CV), fetal bovine serum (FBS), phosphate-buffered saline (PBS), octane, and other chemicals were purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA). Human blood for hemolytic tests was purchased from Biomaxima (Lublin, Poland).
4.2. Antifungal Susceptibility Testing
The antifungal activity of 5-fluorouridine (5-FUrd) against yeast strains was evaluated using the broth microdilution method according to the Clinical and Laboratory Standards Institute (CLSI) guidelines [
40], as described by Khabnadideh et al. (2012) [
41]. Serial dilutions of 5-FUrd (0.1–256 µg/mL) were prepared in 96-well microtiter plates using RPMI-1640 medium buffered with MOPS. Stock inocula were prepared by suspending three colonies of each tested yeast strain in sterile 0.85% NaCl, followed by adjusting the turbidity to the 0.5 McFarland standard. A working suspension was then prepared by performing a 1:1000 dilution of the stock suspension in RPMI-1640 medium. After dispensing 0.1 mL of the inoculated suspension into each well, the microtiter plates were incubated at 37 °C for 24 h. Uninoculated medium was included as a sterility control (blank). The minimum inhibitory concentration (MIC) was defined as the lowest concentration of 5-FUrd that inhibited visible growth of the microorganism. Minimal fungicidal concentrations (MFCs) were determined by subculturing aliquots from the wells corresponding to the MIC, 2 × MIC, 4 × MIC, and 8 × MIC onto Sabouraud Dextrose Agar plates (without 5-FUrd). Specifically, 10 μL from each relevant well was plated and incubated at 37 °C for 48 h. The MFC was defined as the lowest concentration of the compound that prevented visible fungal growth on solid medium.
4.3. Cell Adhesion
In a 96-well flat-bottom microtiter plate, C. albicans or C. parapsilosis cell suspensions (2.5 × 105 CFU/mL) in the RPMI-1640 medium were added to the wells containing 5-FUrd at concentrations in the range of 0–51.2 μg/mL (100 μL per well). The plates were incubated at 37 °C for 2 h to allow initial yeast adherence. Subsequently, the wells were washed with phosphate-buffered saline (PBS) to remove loosely bound cells. Fresh medium was then added, and the plates were incubated for 24 h at 37 °C. After triple washing with PBS, the resulting biofilm was treated with 40 µL of MTT (1 mg/mL), 2 µL of 0.4 mM menadione, and 158 µL of PBS in order to determine the viability of the biofilm. After incubation at 37 °C for 3 h, absorbance at 490 nm was measured using a microplate reader (Biotek Synergy HT, Winooski, VT, USA).
Identically prepared
C. albicans and
C. parapsilosis biofilms treated with 5-FUrd were stained with crystal violet (CV) to determine total biofilm biomass. For this quantification, each well was washed three times with sterile PBS, and the remaining biofilms were dried at 65 °C for 2–4 h. Then, 100 μL of a 0.5% (
w/
v) CV solution was added to each well, and the plates were incubated at room temperature for 20 min. Excess dye was removed by thoroughly rinsing the plates with water. The plates were then air-dried at room temperature for 24 h. CV bound to the biofilm biomass was solubilized by adding 200 μL of methanol. After 20 min incubation at room temperature, absorbance was measured at 570 nm using a spectrophotometric reader. The percentage of biofilm eradication was calculated using the following formula:
4.4. Effect of 5-FUrd on Yeast Biofilm Formation and Preformed Biofilms
The biofilm formation assay was performed in 96-well microtiter plates. The cell suspension was prepared in RPMI-1640 medium at a final density of 1 × 106 cells/mL, and 100 μL was dispensed into each well of microtiter plates. Serially double-diluted concentrations of 5-FUrd in RPMI-1640 medium were added to the wells. In the control, 100 μL of RPMI-1640 medium containing 1% DMSO without 5-FUrd was added to selected wells. The plates were then incubated at 37 °C for 24 h.
To assess the effect of 5-FUrd on preformed biofilms, the initial biofilm formation was induced by dispensing 100 μL of a yeast cell suspension (1 × 106 cells/mL in RPMI-1640 medium) into each well of a microtiter plate, followed by incubation at 37 °C for 24 h. After incubation, the medium was aspirated, and non-adherent cells were removed by washing the wells three times with sterile phosphate-buffered saline (PBS). Subsequently, 100 μL of serial two-fold dilutions of 5-FUrd in RPMI-1640 were added to the pre-formed biofilms. The control wells received 100 μL of RPMI-1640 medium containing 1% DMSO without the tested compound. The plates were further incubated at 37 °C for another 24 h. The metabolic activity of biofilms was evaluated using the MTT assay, following the procedure described previously. Total biofilm biomass was quantified by crystal violet staining, also according to the previously described protocol.
4.5. Microscopic Analysis of Biofilms
C. albicans and C. parapsilosis biofilms were grown on poly-L-lysine (PLL)-coated glass microscope coverslips. Clean coverslips were immersed in a 0.1% PLL solution for 10 min, air-dried overnight, rinsed with sterile deionized water, and air-dried again. Overnight yeast cultures grown in YPD medium were diluted to a final concentration of 1 × 106 cells/mL in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS). Subsequently, 20 mL of the cell suspension was transferred into sterile Petri dishes containing either 5-FUrd at concentrations of 0.4 µg/mL and 12.8 µg/mL or 1% DMSO as a control. The samples were incubated for 24 h at 37 °C without agitation. After incubation, the coverslips were gently rinsed with sterile water to remove non-adherent (planktonic) cells. Biofilms were then stained with crystal violet and visualized using a light microscope.
4.6. Extraction and Chemical Composition Analysis of Extracellular Polymeric Substances (EPS)
The extracellular polymeric substances (EPS) of
C. albicans and
C. parapsilosis biofilms were extracted using a sonication-based method, followed by analysis of DNA, protein, and carbohydrate content [
42,
43]. Biofilms were formed in 96-well flat-bottom microtiter plates. Cell suspensions of
C. albicans or
C. parapsilosis (2.5 × 10
5 CFU/mL) in RPMI-1640 medium were dispensed into wells containing 5-FUrd at final concentrations of 6.4 μg/mL and 25.6 μg/mL (100 μL per well). The plates were incubated at 37 °C. After 24 h and 48 h incubation, the supernatants were aspirated, and the remaining adherent cells were gently washed with sterile phosphate-buffered saline (PBS). The biofilms were then suspended in 1 mL of a 0.01 M KCl solution and collected by vortexing and scraping. Yeast biofilm cells were dispersed using a sonicator (Sonic Ruptor 250, Omni International, The Homogenizer Company, Kennesaw, GA, USA) in six cycles of 5 s operation and 5 s pause at a power level of 20 kHz [
44,
45]. The sonicated suspension was pelleted by centrifugation for 10 min at 7000×
g at 4 °C. The supernatants were collected and filtered using a 0.22 mm membrane filter (Genoplast Biotech S. A., Rokocin, Poland). The DNA content was quantified using the Quant-iT™ PicoGreen
® dsDNA Assay Kit (Thermo Fisher Scientific, Warsaw, Poland). The protein concentration was determined using the Lowry method with the use of the Pierce™ Modified Lowry Protein Assay Kit (Thermo Fisher Scientific, Warsaw, Poland). The carbohydrate content was measured with the phenol-sulfuric acid method, using glucose as a standard [
46]. The quantities of these three EPS components were assessed at both 24 h and 48 h of biofilm maturation. Each experiment was performed in at least three independent replicates.
4.7. Cell Surface Hydrophobicity (CSH) Assay
The cell surface hydrophobicity of
Candida cells was determined using the water–hydrocarbon two-phase assay [
47]. Cell suspensions (2 mL, 1 × 10
6 CFU/mL) supplemented with 5-FUrd at 0.05 μg/mL, 0.1 μg/mL, and 0.2 μg/mL against
C. albicans or 0.025 μg/mL, 0.05 μg/mL, and 0.1 μg/mL against
C. parapsilosis were incubated in glass tubes for 18 h at 37 °C. Next, the suspensions were centrifuged for 10 min at 3000×
g, and the fungal cells were resuspended with sterile PBS at OD
600 of 1.0. An aliquot of the strain solution (1.2 mL) was transferred into a clean glass tube and octane was added (0.3 mL). The solutions were vigorously vortexed for 3 min and separated into two distinct phases. The OD
600 of the aqueous phase was determined. The control was set as the OD
600 of the aqueous phase without the octane overlay. The relative hydrophobicity was expressed as the percentage change in the optical density (OD
600) of the untreated cells (100%).
4.8. Effect of 5-FUrd on Cell and Colony Morphology
The effect of 5-FUrd on the morphology of cells and colonies of C. albicans and C. parapsilosis was evaluated using both liquid and solid RPMI-1640 medium containing 10% fetal bovine serum (FBS). To assess morphological changes in planktonic yeast cells, the cultures were incubated with 5-FUrd at concentrations of 0.1 μg/mL and 0.2 μg/mL for C. albicans and 0.05 μg/mL and 0.1 μg/mL for C. parapsilosis. Cells were grown at 37 °C for 10 h in a shaking incubator. After incubation, 10 mL aliquots of the cultures were centrifuged, and the resulting cell pellets were resuspended in phosphate-buffered saline (PBS). The cell suspensions were adjusted to 1 × 104 cells/mL and gently applied onto microscope slides. The cells were stained with crystal violet and observed under a light microscope to evaluate morphological alterations (Mikrolab, Warsaw, Poland).
For assessment on solid media, the Candida cell suspensions (1 × 107 cells/mL in PBS; 100 μL) were plated onto RPMI-1640 agar containing 10% FBS and supplemented with 5-FUrd at the same concentrations used for liquid cultures. Control plates lacking 5-FUrd served as a reference. The plates were incubated at 37 °C for 24 h. Representative colonies were examined and compared to those on the control plates using an inverted microscope imaged with a digital camera (Mikrolab, Warsaw, Poland).
4.9. Phospholipase Activity
The phospholipase activity of
C. albicans,
C. parapsilosis, and
C. glabrata (used as a negative control) was determined using the egg yolk agar plate method [
48]. The egg yolk agar medium was prepared by enriching Sabouraud Dextrose Agar (13 g) with NaCl (11.7 g), CaCl
2 (0.11 g), and 10% sterile egg yolk in a final volume of 184 mL of distilled water. The yeast cells were incubated in the presence of 5-FUrd at concentrations of 0.05 μg/mL, 0.1 μg/mL, and 0.2 μg/mL for
C. albicans and 0.025 μg/mL, 0.5 μg/mL, and 0.1 μg/mL for
C. parapsilosis. After 20 h incubation at 37 °C with shaking, the cells were harvested by centrifugation and diluted in sterile PBS. After centrifugation for 10 min at 3000×
g, the cell pellets were resuspended in sterile PBS. The final cell suspension was adjusted to 1 × 10
8 cells/mL. Then, 10 μL of each standardized
Candida suspension was inoculated onto the surface of the egg yolk agar and allowed to dry at room temperature. The plates were then incubated at 37 °C for 48 h. Phospholipase activity was assessed by observing the precipitation zone around the colonies, indicative of enzyme production. The phospholipase activity was quantified using the phospholipase index (Pz) calculated as Pz =
a/
b, where
a is the diameter of the colony plus the precipitation zone, and
b is the diameter of the colony alone. Phospholipase activity was categorized as follows: very high (Pz ≤ 0.69), high (Pz = 0.70–0.79), low (Pz = 0.80–0.89), very low (Pz = 0.90–0.99), and negative (Pz = 1) [
49]. All experiments were performed in triplicate.
4.10. Proteinase Secretion
The effect of 5-FUrd on proteinase secretion by
C. albicans and
C. parapsilosis was evaluated using a casein degradation assay, following the method described by Ramesh et al. (2011) [
50]. Yeast cells were cultured under 5-FUrd pressure using the same concentrations and incubation conditions as described above. Cell-free supernatants (1 mL) were mixed with 1 mL of a casein solution prepared in phosphate buffer (pH 7.5) and incubated at 37 °C for 1 h. The reaction was stopped by adding 0.2 M trichloroacetic acid (TCA). Then, the reaction mixture was centrifuged at 3000 rpm for 10 min. The supernatant was then combined with 0.55 M sodium carbonate and Folin–Ciocalteu reagent and incubated for 15 min at room temperature. Optical density (OD) was measured at 650 nm against the control. The proteolytic activity in the control sample (cultured without 5-FUrd) was defined as 100%. The relative reduction in enzyme activity in the presence of 5-FUrd was expressed as a percentage of the control. All experiments were performed in triplicate.
4.11. Hemolytic Factor Secretion
Candida cells were cultured in Sabouraud Dextrose Broth at 37 °C for 18 h in the presence of 5-FUrd at 0.05 μg/mL, 0.1 μg/mL, and 0.2 μg/mL for
C. albicans and 0.025 μg/mL, 0.5 μg/mL, and 0.1 μg/mL for
C. parapsilosis. Untreated cells cultured in the same conditions served as the control. Then, the cultures were harvested by centrifugation at 1000 ×
g for 15 min and washed with sterile phosphate-buffered saline (PBS). The yeast cells were resuspended in RPMI-1640 medium supplemented with 3% glucose at a final concentration of 1 × 10
4 cells/mL and incubated at 37 °C for 48 h with shaking at 180 rpm. After incubation, the cultures were centrifuged again at 1000×
g for 15 min, and the supernatants were concentrated tenfold using centrifugal filters (VWR International, USA). The concentrated culture supernatants were mixed at a 1:1 (
v/
v) ratio with a suspension of erythrocytes (1 × 10
8 cells/mL) prepared in RPMI 1640 medium. The mixtures were incubated at 37 °C for 18 h. A 1% Triton X-100 solution was the positive control (representing 100% hemolysis), while a mixture of erythrocytes and RPMI 1640 medium with PBS (1:1,
v/
v) served as the negative control. After incubation, the samples were centrifuged at 1000×
g for 2 min, and the absorbance of the supernatants was measured at 405 nm. The inhibition of hemolysis by 5-FUrd was calculated as a percentage in relation to the control (untreated cells) [
51].
4.12. Frequency of Spontaneous Mutations
The frequency of spontaneous mutations was determined in
C. albicans ATCC 10231 and
C. parapsilosis ATCC 22099. One hundred microliters of yeast suspensions were spread onto Sabouraud Dextrose Agar (SDA) plates containing 5-FUrd at concentrations corresponding to MIC, 2 × MIC and 4 × MIC. After 24 h of incubation at 37 °C, the frequency of spontaneous mutations was calculated by dividing the number of resistant colonies observed on each drug-containing plate by the number of colony-forming units (CFUs) in the initial inoculum (2.5 × 10
5 CFU/mL). To determine the initial viable count, serial dilutions of the starting inoculum were prepared and plated in triplicate onto SDA plates without 5-FUrd. Resistant phenotypes were confirmed by re-isolation of mutant colonies and subsequent determination of their MIC values [
38].
4.13. Hemolytic Assay
The hemolytic activity of 5-FUrd was determined using human red blood cells. Erythrocytes were harvested by centrifugation at 2000 rpm for 10 min at 20 °C and washed three times with phosphate-buffered saline (PBS). The erythrocyte pellet was resuspended in PBS to prepare a 10% (
v/
v) erythrocyte suspension. This suspension was further diluted 1:10 with PBS. A volume of 450 μL of the diluted erythrocyte suspension was mixed with 50 μL of PBS containing a predetermined 5-FUrd concentration gradient (ranging from 0.4 to 8 μg/mL) in microcentrifuge tubes. Total hemolysis was induced using 1% Triton X-100 as a positive control. The tubes were incubated at 37 °C for 1 h, followed by centrifugation at 2000 rpm for 10 min at room temperature. Then, 150 μL of the supernatant was transferred to a flat-bottom microtiter plate, and absorbance was measured spectrophotometrically at 450 nm. The percentage of hemolysis was calculated using the following equation:
4.14. Toxic Effects of 5-FUrd on Zebrafish Embryos
To determine the toxicity of 5-FUrd, a Fish Embryo Toxicity (FET) test was performed on zebrafish (
Danio rerio) according to OECD Test Guideline 236 [
52,
53]. Fertilized zebrafish embryos were exposed to 5-FUrd for 96 h at concentrations ranging from 0.4 µg/mL to 8 µg/mL. The E3 solution (5 mM NaCl, 0.33 mM MgCl
2, 0.33 mM CaCl
2, 0.17 mM KCl; pH 7.2) was used as the embryo culture medium and to prepare the solutions. The experiments were conducted in 24-well plates, with ten embryos per well, in triplicate. The plates were covered and incubated at 28 °C. At the end of the 96 h exposure period, acute toxicity was assessed based on a positive outcome in any of the four visual indicators of lethality: coagulation of fertilized eggs, lack of somite formation, failure of the tailbud to detach from the yolk sac, and absence of heartbeat. The mortality rate was expressed as the percentage of dead embryos. The heart rate was monitored using a stopwatch and direct microscopic observation. Zebrafish embryo development and viability were evaluated using bright-field microscopy (Zeiss Stereo Discovery V8, ZEISS, Germany). Image analysis was performed using Zeiss ZEN lite to determine the percentage of dead and malformed embryos over time.
4.15. Statistical Analysis
All data are expressed as a mean ± SD (standard deviation) of three independent experiments. Statistical significance between the treated and control groups was analyzed by Student’s t-test using GraphPad Software version 9.1.1. (San Diego, CA, USA). p value < 0.05 was considered statistically significant.