A Carbon Nanofiber Electrochemical Sensor Made of FeMn@C for the Rapid Detection of Tert-Butyl Hydroquinone in Edible Oil
Abstract
1. Introduction
2. Experimental Section
2.1. Instruments and Reagents
2.2. Synthesis of FeMn-PBAs
2.3. Preparation of Fe/Mn@C/CNFs
2.4. Preparation of FeMn@C/CNFs/GCE
2.5. Electrochemical Testing
3. Results and Discussion
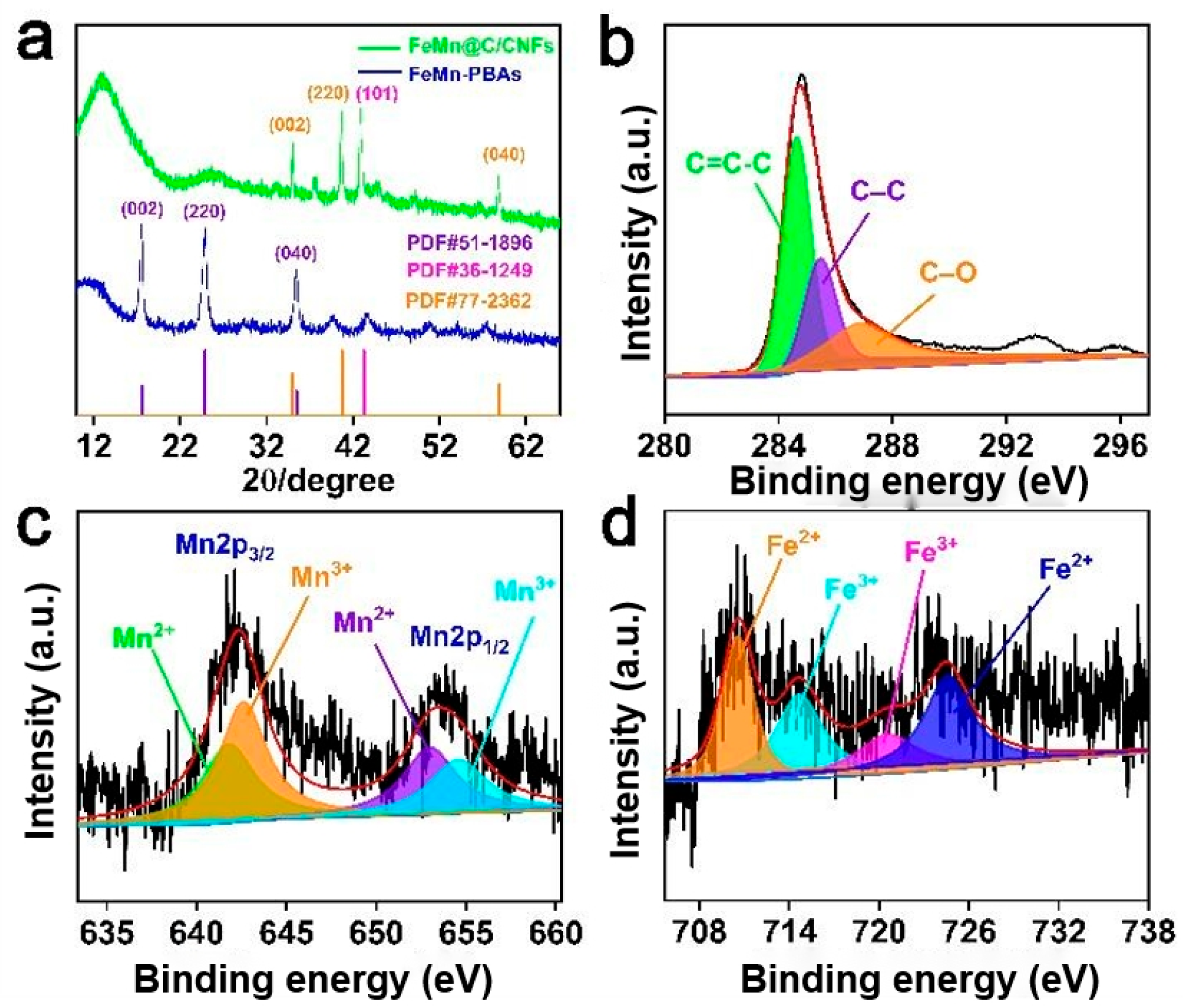
3.1. Material Characterization
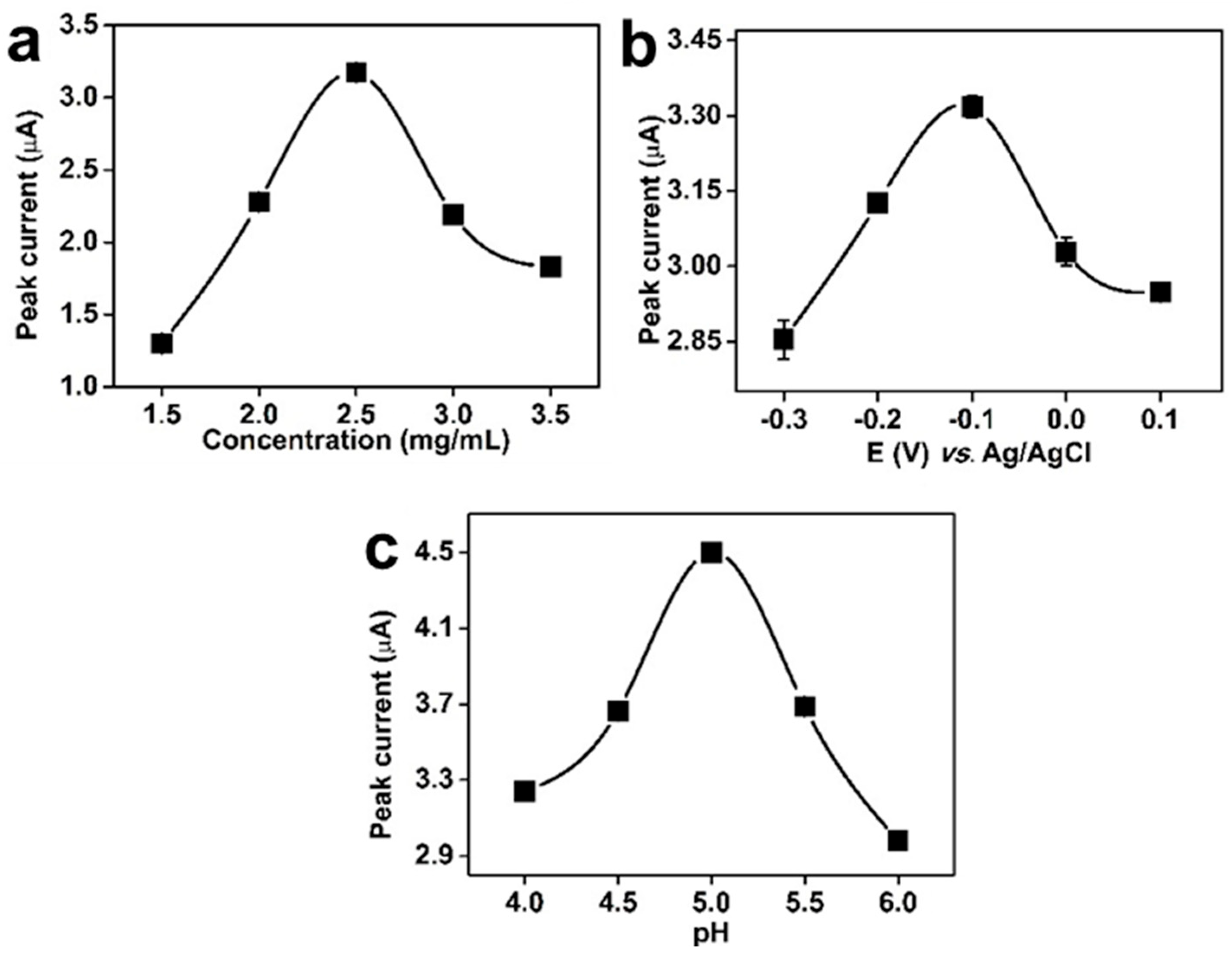
3.2. Optimization of Electrochemical Conditions
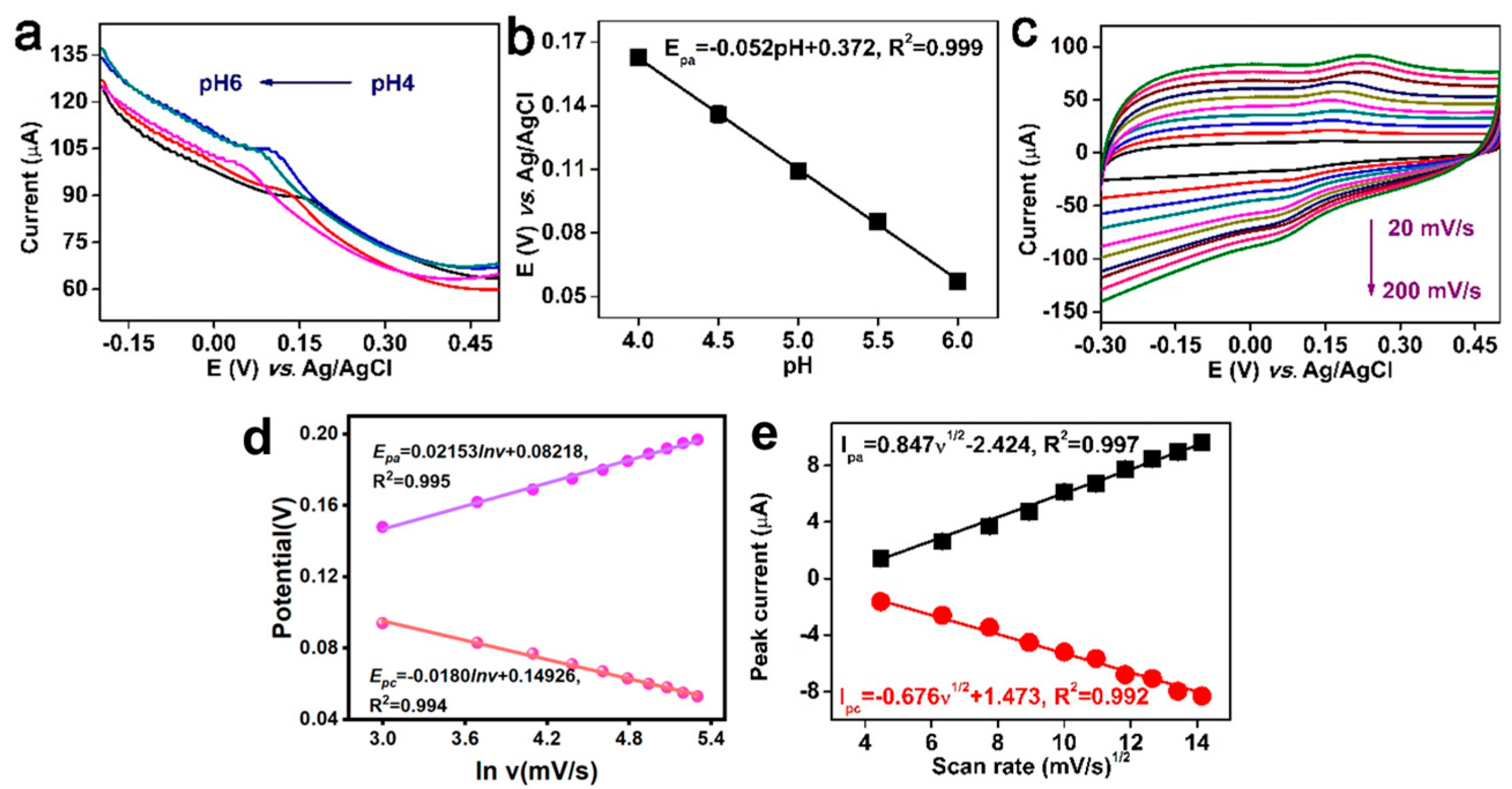
3.3. Influence of pH and Scan Rate
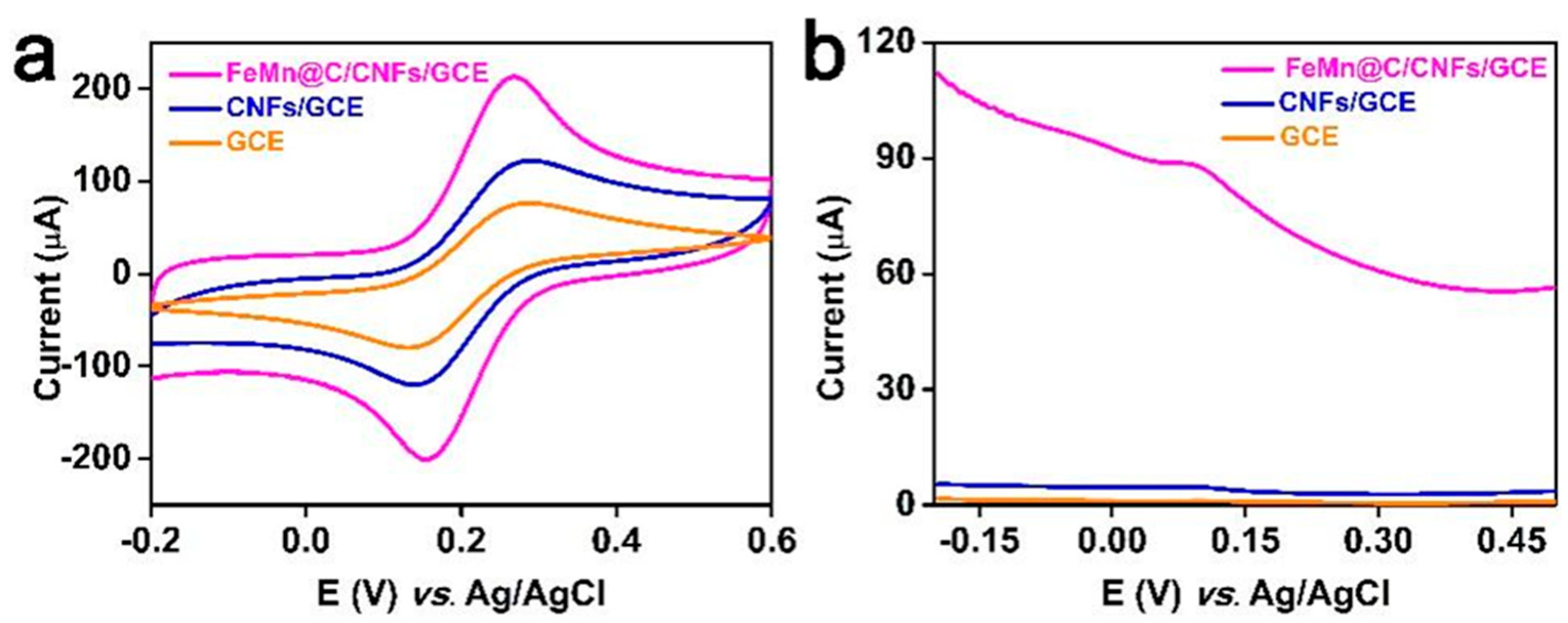
3.4. Electrochemical Behaviors of Different Electrodes
3.5. Establishment of Standard Curves
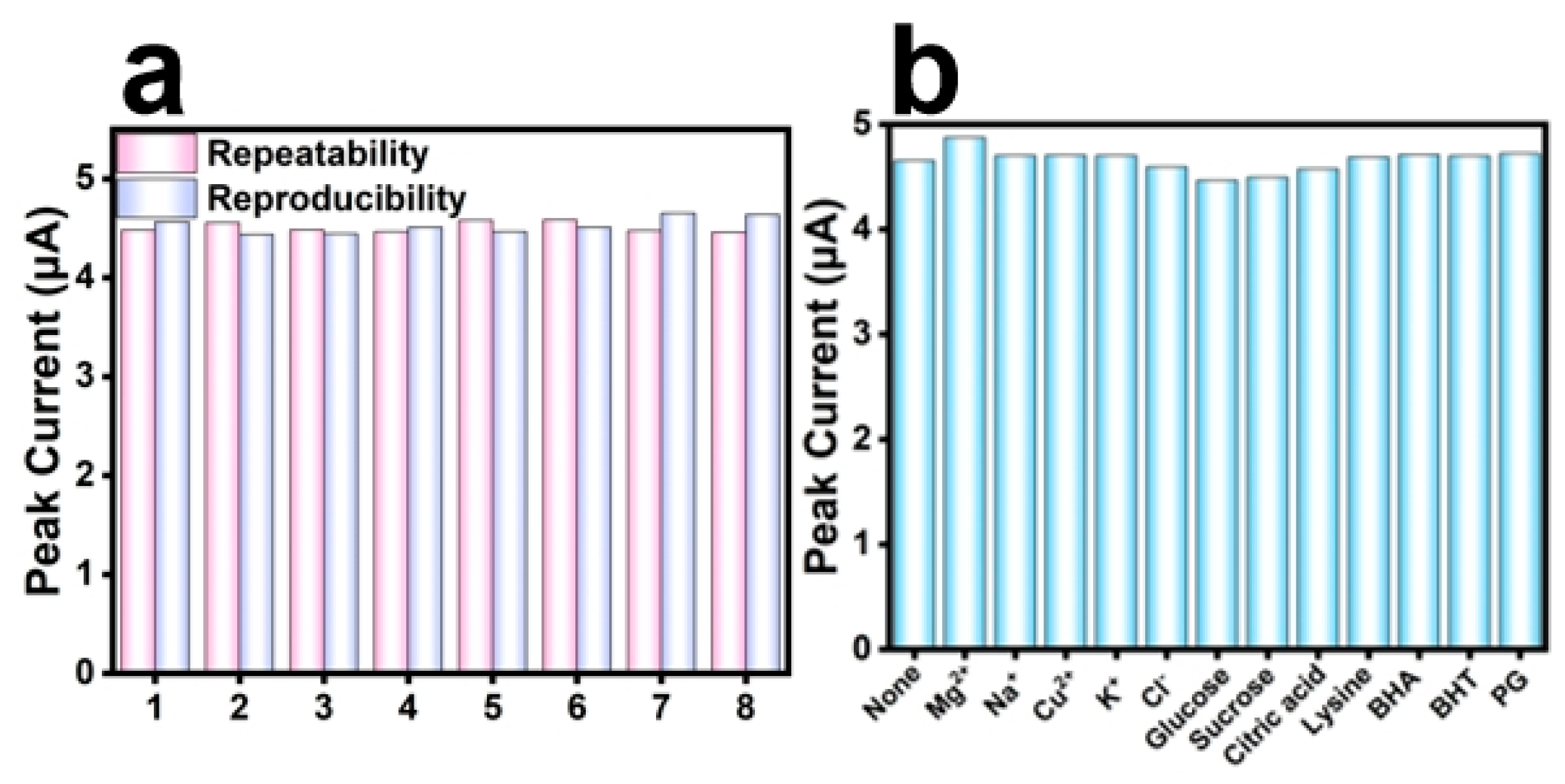
3.6. Assessment of Repeatability and Reproducibility
3.7. Testing Actual Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TBHQ | Tert-butylhydroquinone |
| PBAs | Prussian blue analogues |
| PB | Prussian blue |
| NPs | Nanoparticles |
| CNFs | Carbon nanofibers |
| SEM | Scanning electron microscope |
| TEM | Transmission electron microscope |
| XRD | X-ray diffractometer |
| XPS | X-ray photoelectron spectrometer |
| GCE | Glassy carbon electrode |
| PAN | Polyacrylonitrile |
| DMF | N, N-dimethylformamide |
| PBS | Phosphate-buffered saline |
| DPV | Differential pulse voltammetry |
| CV | Cyclic voltammetry |
| CC | Chronocoulometry |
| Powder diffraction file |
References
- Balram, D.; Lian, K.-Y.; Sebastian, N.; Rasana, N. Ultrasensitive detection of cytotoxic food preservative tert-butylhydroquinone using 3D cupric oxide nanoflowers embedded functionalized carbon nanotubes. J. Hazard. Mater. 2021, 406, 124792. [Google Scholar] [CrossRef]
- Khezerlou, A.; Akhlaghi, A.p.; Alizadeh, A.M.; Dehghan, P.; Maleki, P. Alarming impact of the excessive use of tert-butylhydroquinone in food products: A narrative review. Toxicol. Rep. 2022, 9, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Leonard, E.R.; Marques, E.S.; Roy, M.A.; Conlin, S.M.; Ranjan, R.; Timme-Laragy, A.R. Dietary exposure to the food preservative tert-Butylhydroquinone (tBHQ) impairs zebrafish (Danio rerio) survival, growth, organ development, and gene expression in Nrf2a-dependent and independent ways. Food Chem. Toxicol. 2023, 176, 113788. [Google Scholar] [CrossRef]
- Tang, J.; Wang, W.; Zheng, S.; Zhang, Y.; Wei, J.; Wang, J. Electrochemical Determination of Tert-Butyl Hydroquinone in Edible Oil Samples at Poly (Crystal Violet) Modified Glassy Carbon Electrode. Food Anal. Methods 2016, 9, 3044–3052. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Statement on the refined exposure assessment of tertiary-butyl hydroquinone (E 319). EFSA J. 2016, 14, 4363. [Google Scholar] [CrossRef][Green Version]
- Esazadeh, K.; Ezzati Nazhad Dolatabadi, J.; Andishmand, H.; Mohammadzadeh-Aghdash, H.; Mahmoudpour, M.; Naemi Kermanshahi, M.; Roosta, Y. Cytotoxic and genotoxic effects of tert-butylhydroquinone, butylated hydroxyanisole and propyl gallate as synthetic food antioxidants. Food Sci. Nutr. 2024, 12, 7004–7016. [Google Scholar] [CrossRef]
- Sun, J.; Hu, H.; Ren, X.; Simpkins, J.W. Tert-butylhydroquinone compromises survival in murine experimental stroke. Neurotoxicol. Teratol. 2016, 54, 15–21. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kumagai, T.; Yoshida, C.; Naito, Y.; Miyamoto, M.; Ohigashi, H.; Osawa, T.; Uchida, K. Pivotal role of electrophilicity in glutathione S-transferase induction by tert-butylhydroquinone. Biochemistry 2003, 42, 4300–4309. [Google Scholar] [CrossRef] [PubMed]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.-Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Capitán-Vallvey, L.F.; Valencia, M.C.; Nicolás, E.A. Monoparameter sensors for the determination of the antioxidants butylated hydroxyanisole and n-propyl gallate in foods and cosmetics by flow injection spectrophotometry. Analyst 2001, 126, 897–902. [Google Scholar] [CrossRef]
- Ma, Y.; Li, J.; Wang, L. Porous carbon derived from ZIF-8 modified molecularly imprinted electrochemical sensor for the detection of tert-butyl hydroquinone (TBHQ) in edible oil. Food Chem. 2021, 365, 130462. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, T.; Wang, X.; Zhang, T.; Zhang, R.; Xu, Z.; Ma, M.; Ma, Y.; Shi, F. Nanoparticles based on Prussian Blue for biosensor applications: A review. ACS Appl. Nano Mater. 2023, 6, 22568–22593. [Google Scholar] [CrossRef]
- Ma, Q.; Dong, R.; Liu, H.; Zhu, A.; Qiao, L.; Ma, Y.; Wang, J.; Xie, J.; Pan, J. Prussian blue analogue-derived Mn–Fe oxide nanocubes with controllable crystal structure and crystallinity as highly efficient OER electrocatalysts. J. Alloys Compd. 2020, 820, 153438. [Google Scholar] [CrossRef]
- Wang, W.; Yu, X.; He, H.; Wang, Y.; Li, Y.; Deng, L.; Liu, Y.-N. Electrochemical reconstitution of Prussian blue analogue for coupling furfural electro-oxidation with photo-assisted hydrogen evolution reaction. Chem. Eng. J. 2023, 465, 142865. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, J.; Wang, L.; Jiao, Z.; Xiao, M.; Huang, Q.-a.; Liu, M.; Shao, Q.; Sun, X.; Zhang, J. Prussian blue and its analogues as cathode materials for Na-, K-, Mg-, Ca-, Zn- and Al-ion batteries. Nano Energy 2022, 99, 107424. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, S.; Tang, H.; Guo, X.; Xue, H.; Pang, H. Prussian blue and its derivatives as electrode materials for electrochemical energy storage. Energy Storage Mater. 2017, 9, 11–30. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Chen, M.; Wu, W.; Qiu, S.; Wu, H.; Zheng, M.; Zhang, X.; Wu, X. Suppressing vacancies and crystal water of sodium manganese iron-based Prussian blue analogue by potassium doping for advanced sodium-ion batteries. Chem. Eng. Sci. 2025, 302, 120848. [Google Scholar] [CrossRef]
- Tang, Z.; Hu, B.; Nie, P.; Shang, X.; Yang, J.; Liu, J. Bimetallic Fe, Ni-PBA on hollow graphite tube for capacitive deionization with exceptional stability. Chem. Eng. J. 2023, 466, 143216. [Google Scholar] [CrossRef]
- Janjani, P.; Bhardwaj, U.; Gupta, R.; Singh Kushwaha, H. Bimetallic Mn/Fe MOF modified screen-printed electrodes for non-enzymatic electrochemical sensing of organophosphate. Anal. Chim. Acta 2022, 1202, 339676. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, D.; Zhang, Y.; Li, J.; Meng, W.; Tong, B.; Zhang, J.; Han, C.; Dai, L. In-situ integrated electrodes of FeM-MIL-88/CP for simultaneous ultra-sensitive detection of dopamine and acetaminophen based on crystal engineering strategy. Anal. Chim. Acta 2023, 1283, 341936. [Google Scholar] [CrossRef]
- Chen, C.; Xiong, D.; Gu, M.; Lu, C.; Yi, F.-Y.; Ma, X. MOF-Derived Bimetallic CoFe-PBA Composites as Highly Selective and Sensitive Electrochemical Sensors for Hydrogen Peroxide and Nonenzymatic Glucose in Human Serum. ACS Appl. Mater. Interfaces 2020, 12, 35365–35374. [Google Scholar] [CrossRef] [PubMed]
- Hurlbutt, K.; Wheeler, S.; Capone, I.; Pasta, M. Prussian Blue Analogs as Battery Materials. Joule 2018, 2, 1950–1960. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.; Cai, Y.; Zhang, H.; Li, Y.; Liu, D. Ni-doping Cu-Prussian blue analogue/carbon nanotubes composite (Ni-CuPBA/CNTs) with 3D electronic channel-rich network structure for capacitive deionization. Desalination 2022, 528, 115622. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, S.; Wang, J.; Yu, A.; Wei, G. Carbon Nanofiber-Based Functional Nanomaterials for Sensor Applications. Nanomaterials 2019, 9, 1045. [Google Scholar] [CrossRef]
- Medeiros, G.B.; Lima, F.d.A.; de Almeida, D.S.; Guerra, V.G.; Aguiar, M.L. Modification and Functionalization of Fibers Formed by Electrospinning: A Review. Membranes 2022, 12, 861. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhahebi, A.M.; Ling, J.; Krishnan, S.G.; Yousefzadeh, M.; Elumalai, N.K.; Saheed, M.S.M.; Ramakrishna, S.; Jose, R. Electrospinning research and products: The road and the way forward. Appl. Phys. Rev. 2022, 9, 031307. [Google Scholar] [CrossRef]
- Yang, X.; Feng, C.; Peng, A.; Wang, Q.; Liu, Z.-Y.; Pei, F.; Mu, J.; Yang, E.-C. Bimetallic FeMn@C derived from Prussian blue analogue as efficient nanozyme for glucose detection. Anal. Bioanal. Chem. 2022, 414, 7773–7782. [Google Scholar] [CrossRef]
- Yimtrakarn, T.; Liao, Y.-C.; Mv, A.S.; Chen, J.-L.; Chuang, Y.-C.; Lerkkasemsan, N.; Kaveevivitchai, W. Mn-Fe Prussian blue analogue as low-cost robust cathode for non-aqueous Zn-ion batteries. Mater. Today Commun. 2023, 34, 105231. [Google Scholar] [CrossRef]
- Li, L.; Huang, Z.; Liu, Y.; Zhu, Z.; Xu, M.; Wei, W.; Zhang, Q.; Hong, J. Novel porous Mn-Fe nanocubes toward peroxymonosulfate activation via non-radical/radical pathways for emerging contaminants degradation. Appl. Surf. Sci. 2022, 581, 152390. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Liu, Y.; Wang, W.; Sun, Q.; Wang, X.; Zhao, X.; Hu, H.; Wu, M. Sulfur bridges between Co9S8 nanoparticles and carbon nanotubes enabling robust oxygen electrocatalysis. Carbon 2019, 144, 259–268. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Yang, S.; Shan, J. A novel electrochemical sensor based on TiO2–Ti3C2TX/CTAB/chitosan composite for the detection of nitrite. Electrochim. Acta 2020, 359, 136938. [Google Scholar] [CrossRef]
- Zheng, Y.; Du, X.; Song, G.; Gu, J.; Guo, J.; Zhou, M. Degradation of carbamazepine over MOFs derived FeMn@C bimetallic heterogeneous electro-Fenton catalyst. Chemosphere 2023, 312, 137353. [Google Scholar] [CrossRef]
- Varshini, K.S.; Kishore, K.R.; Jeyaprakash, B.G.; Balamurugan, D. Investigation of Ammonia-Sensing Characteristics of Electrospun Fe2O3 Nanograins. J. Electron. Mater. 2023, 52, 4853–4864. [Google Scholar] [CrossRef]
- Li, L.; Fu, L.; Wang, R.; Sun, J.; Li, X.; Fu, C.; Fang, L.; Zhang, W. Cobalt, manganese zeolitic-imidazolate-framework-derived Co3O4/Mn3O4/CNx embedded in carbon nanofibers as an efficient bifunctional electrocatalyst for flexible Zn-air batteries. Electrochim. Acta 2020, 344, 136145. [Google Scholar] [CrossRef]
- Ezhil Vilian, A.T.; Umapathi, R.; Hwang, S.-K.; Lee, M.J.; Huh, Y.S.; Han, Y.-K. Simple synthesis of a clew-like tungsten carbide nanocomposite decorated with gold nanoparticles for the ultrasensitive detection of tert-butylhydroquinone. Food Chem. 2021, 348, 128936. [Google Scholar] [CrossRef]
- Ahmad, M.W.; Dey, B.; Sarkhel, G.; Yang, D.-J.; Choudhury, A. Sea-urchin-like cobalt-MOF on electrospun carbon nanofiber mat as a self-supporting electrode for sensing of xanthine and uric acid. J. Electroanal. Chem. 2022, 920, 116646. [Google Scholar] [CrossRef]
- Dey, B.; Sarkhel, G.; Choudhury, A. Facile synthesis of copper MOF/carbon nanofiber nanocomposite paper for electrochemical detection of toxic 4-nitrophenol. J. Macromol. Sci. Part A 2023, 60, 150–160. [Google Scholar] [CrossRef]
- Tang, J.; Zheng, S.-B.; Jiang, S.-X.; Li, J.; Guo, T.; Guo, J.-H. Metal organic framework (ZIF-67)-derived Co nanoparticles/N-doped carbon nanotubes composites for electrochemical detecting of tert-butyl hydroquinone. Rare Met. 2020, 40, 478–488. [Google Scholar] [CrossRef]
- Dey, B.; Ahmad, M.W.; Sarkhel, G.; Ho Lee, G.; Choudhury, A. Fabrication of niobium metal organic frameworks anchored carbon nanofiber hybrid film for simultaneous detection of xanthine, hypoxanthine and uric acid. Microchem. J. 2023, 186, 108295. [Google Scholar] [CrossRef]
- Lin, X.; Ni, Y.; Kokot, S. Glassy carbon electrodes modified with gold nanoparticles for the simultaneous determination of three food antioxidants. Anal. Chim. Acta 2013, 765, 54–62. [Google Scholar] [CrossRef]
- Cacho, J.I.; Campillo, N.; Viñas, P.; Hernández-Córdoba, M. Determination of synthetic phenolic antioxidants in edible oils using microvial insert large volume injection gas-chromatography. Food Chem. 2016, 200, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Song, W.; Zhu, W.; Wang, J.; Wang, Y. In situ surface electrochemical co-reduction route towards controllable construction of AuNPs/ERGO electrochemical sensing platform for simultaneous determination of BHA and TBHQ. Electrochim. Acta 2015, 182, 847–855. [Google Scholar] [CrossRef]
- Wang, P.; Han, C.; Zhou, F.; Lu, J.; Han, X.; Wang, Z. Electrochemical determination of tert-butylhydroquinone and butylated hydroxyanisole at choline functionalized film supported graphene interface. Sens. Actuators B Chem. 2016, 224, 885–891. [Google Scholar] [CrossRef]
- de Oliveira, T.R.; Grawe, G.F.; Moccelini, S.K.; Terezo, A.J.; Castilho, M. Enzymatic biosensors based on inga-cipo peroxidase immobilised on sepiolite for TBHQ quantification. Analyst 2014, 139, 2214–2220. [Google Scholar] [CrossRef]
- Qin, R.; Wang, Q.; Ren, C.; Dai, X.; Han, H. Development of a Molecularly Imprinted Electrochemical Sensor for Tert-Butylhydroquinone Recognition. Int. J. Electrochem. Sci. 2017, 12, 8953–8962. [Google Scholar] [CrossRef]
- Sebastian, N.; Yu, W.-C.; Balram, D.; Al-Mubaddel, F.S.; Tayyab Noman, M. Nanomolar detection of food additive tert-butylhydroquinone in edible oils based on novel ternary metal oxide embedded β-cyclodextrin functionalized carbon black. Food Chem. 2022, 377, 131867. [Google Scholar] [CrossRef]




| Modified Electrode | LOD (μM) | Linear Range (μM) | References |
|---|---|---|---|
| AuNPs/GCE | 0.48 | 1.2–16.8 | [40] |
| SPE-MWCNT | 0.34 | 0.5–10 | [41] |
| Peroxidase/Naf/SEP/CNT/CPE | 2.47 | 9.9–59.1 | [42] |
| ZnO TPHS@GO/GCE | 0.02 | 0.13–10.83 | [43] |
| MWCNT/CPE | 0.487 | 1–10 | [44] |
| MIP/AgNP/POM/rGO/GCE | 0.0000148 | 0.0005–0.0015 | [45] |
| ZnCuMg TMO/β-CD-CB/SPCE | 0.001 | 0.031–12.56 | [46] |
| FeMn@C/CNFs | 0.041 | 0.5–50 | This work |
| Sample | Added/(μmol/L) | Found ± SD/(μmol/L) | Recovery/% |
|---|---|---|---|
| Soybean oil | 0.000 | – | – |
| 3.000 | 3.073 ± 0.081 | 102.433 | |
| 20.000 | 19.886 ± 0.436 | 99.330 | |
| 40.000 | 41.049 ± 0.383 | 102.623 | |
| Peanut oil | 0.000 | – | – |
| 1.000 | 0.954 ± 0.030 | 95.540 | |
| 15.000 | 14.985 ± 0.368 | 99.900 | |
| 30.000 | 29.583 ± 1.039 | 98.610 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Zhang, Y.; He, Z.; Zhang, L.; Wang, T.; Tang, T.; Chen, J.; Cheng, H. A Carbon Nanofiber Electrochemical Sensor Made of FeMn@C for the Rapid Detection of Tert-Butyl Hydroquinone in Edible Oil. Molecules 2025, 30, 2725. https://doi.org/10.3390/molecules30132725
Xiao Y, Zhang Y, He Z, Zhang L, Wang T, Tang T, Chen J, Cheng H. A Carbon Nanofiber Electrochemical Sensor Made of FeMn@C for the Rapid Detection of Tert-Butyl Hydroquinone in Edible Oil. Molecules. 2025; 30(13):2725. https://doi.org/10.3390/molecules30132725
Chicago/Turabian StyleXiao, Yan, Yi Zhang, Zhigui He, Liwen Zhang, Tongfei Wang, Tingfan Tang, Jiaxing Chen, and Hao Cheng. 2025. "A Carbon Nanofiber Electrochemical Sensor Made of FeMn@C for the Rapid Detection of Tert-Butyl Hydroquinone in Edible Oil" Molecules 30, no. 13: 2725. https://doi.org/10.3390/molecules30132725
APA StyleXiao, Y., Zhang, Y., He, Z., Zhang, L., Wang, T., Tang, T., Chen, J., & Cheng, H. (2025). A Carbon Nanofiber Electrochemical Sensor Made of FeMn@C for the Rapid Detection of Tert-Butyl Hydroquinone in Edible Oil. Molecules, 30(13), 2725. https://doi.org/10.3390/molecules30132725






