Hybrid Uracil Derivatives with Caffeine and Gramine Obtained via Click Chemistry as Potential Antioxidants and Inhibitors of Plant Pathogens
Abstract
1. Introduction
2. Results and Discussion
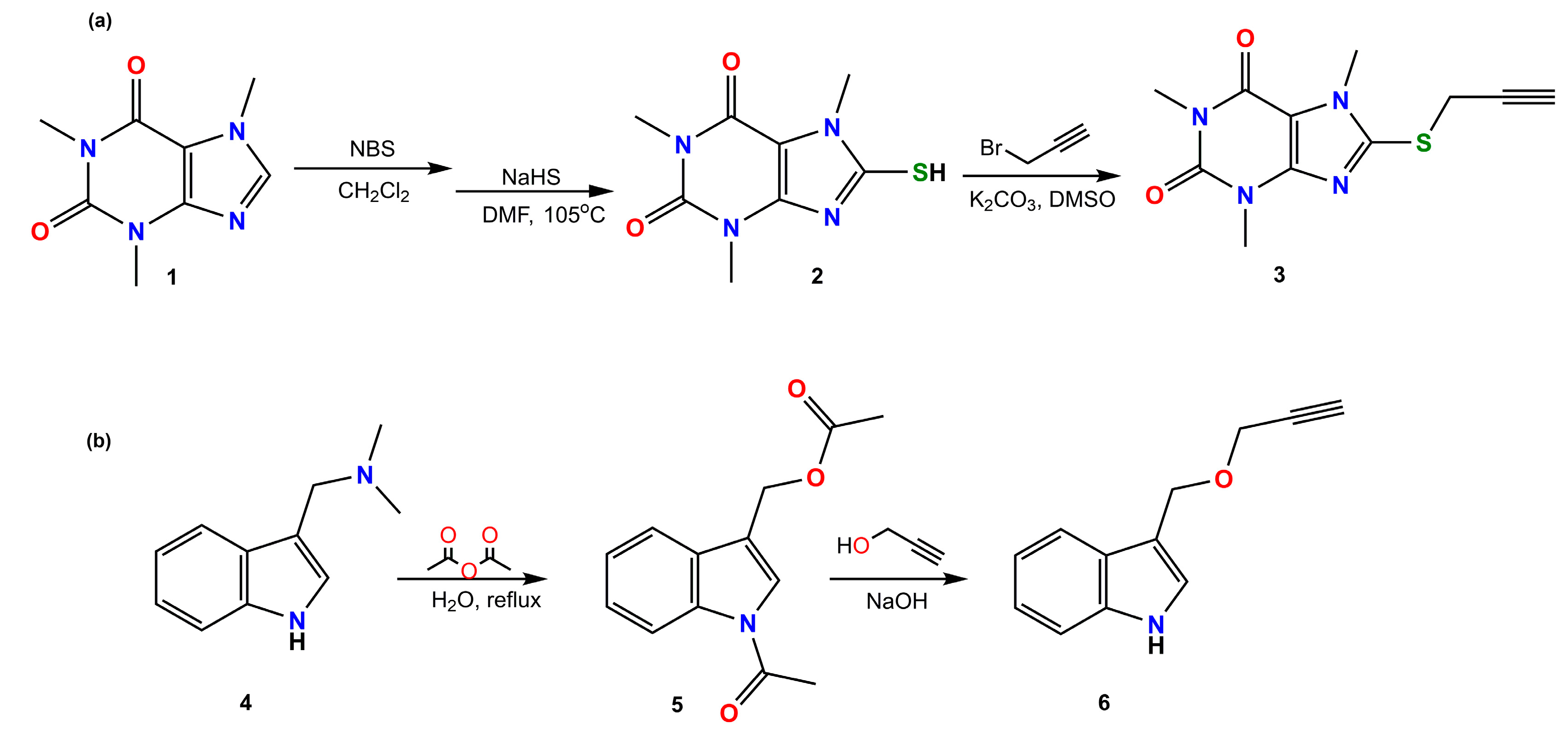
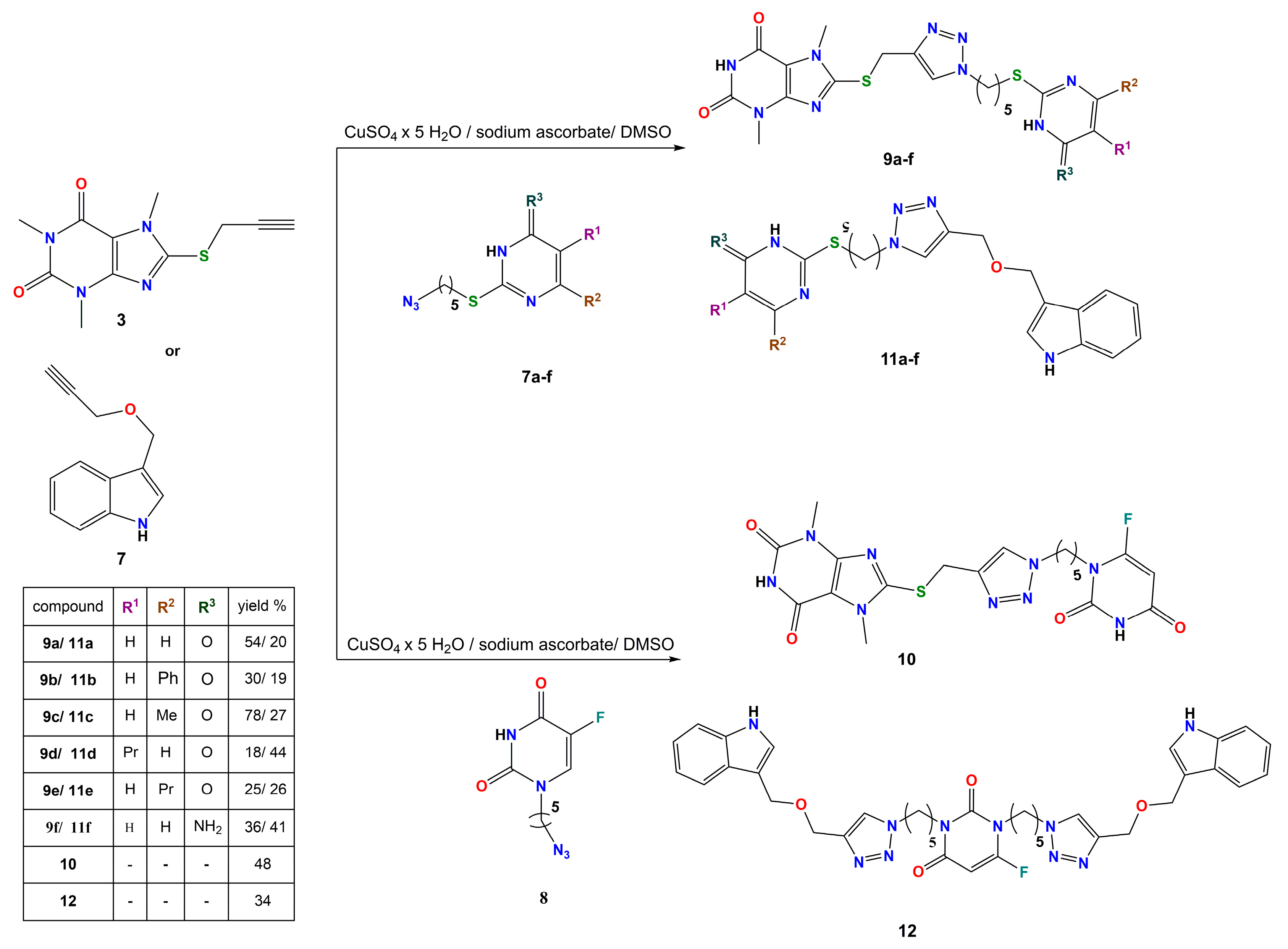
2.1. Synthesis and Spectroscopy
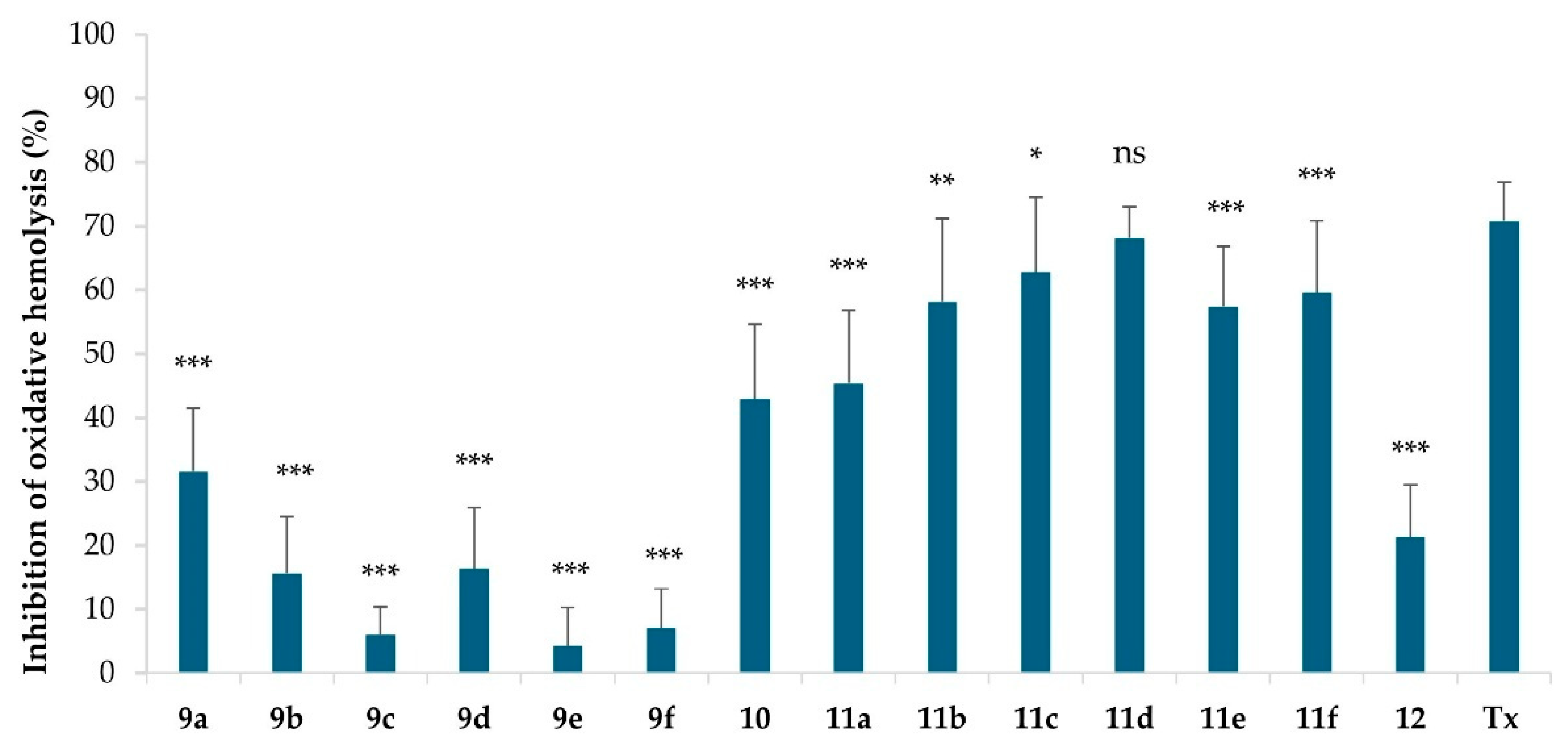
2.2. Hemolytic Activity
2.3. Physicochemical Data—ADME
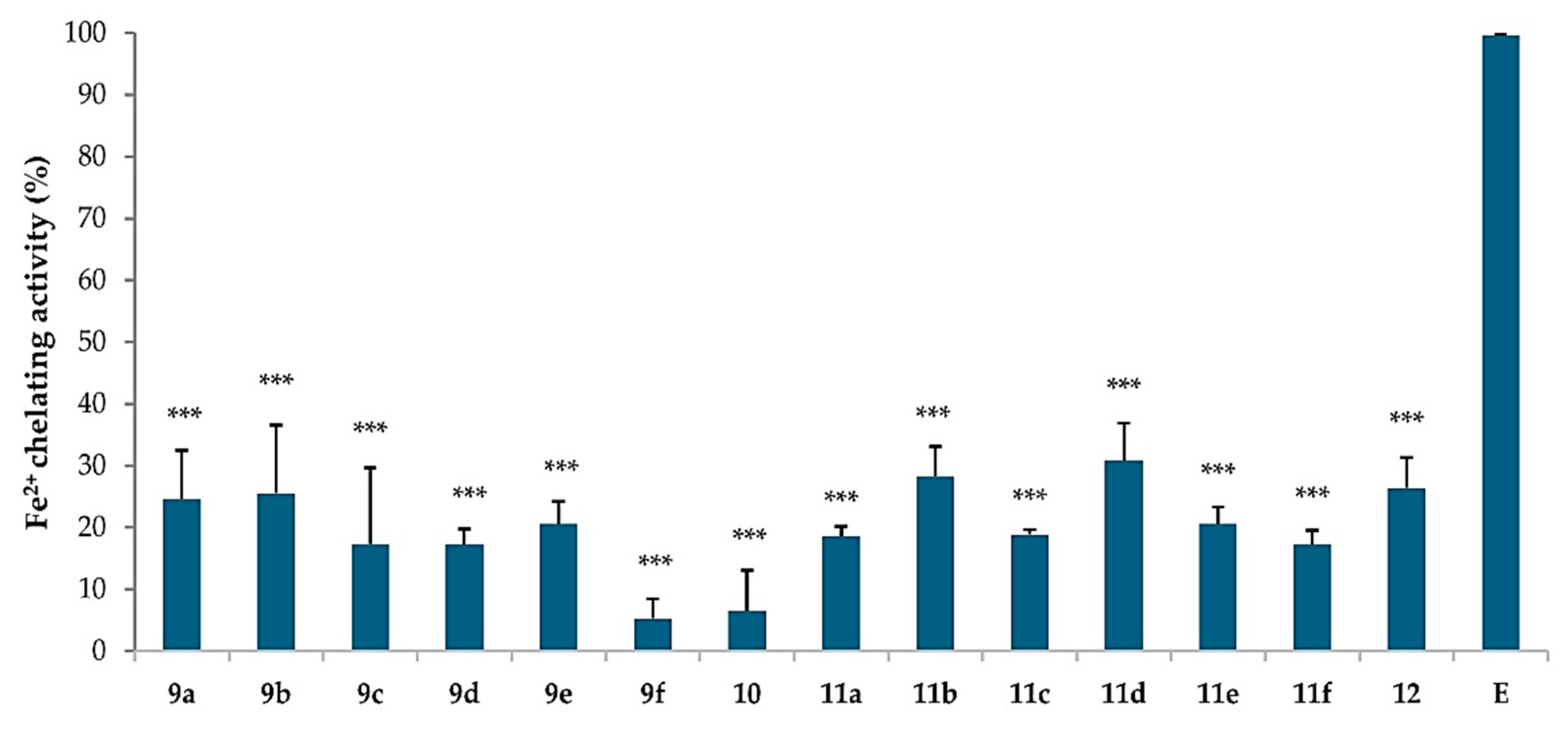
2.4. Chelating Activity of Ferrous Ions (Fe2+)
2.5. Cytoprotective Activity
2.6. Spectral Scans of Hemoglobin
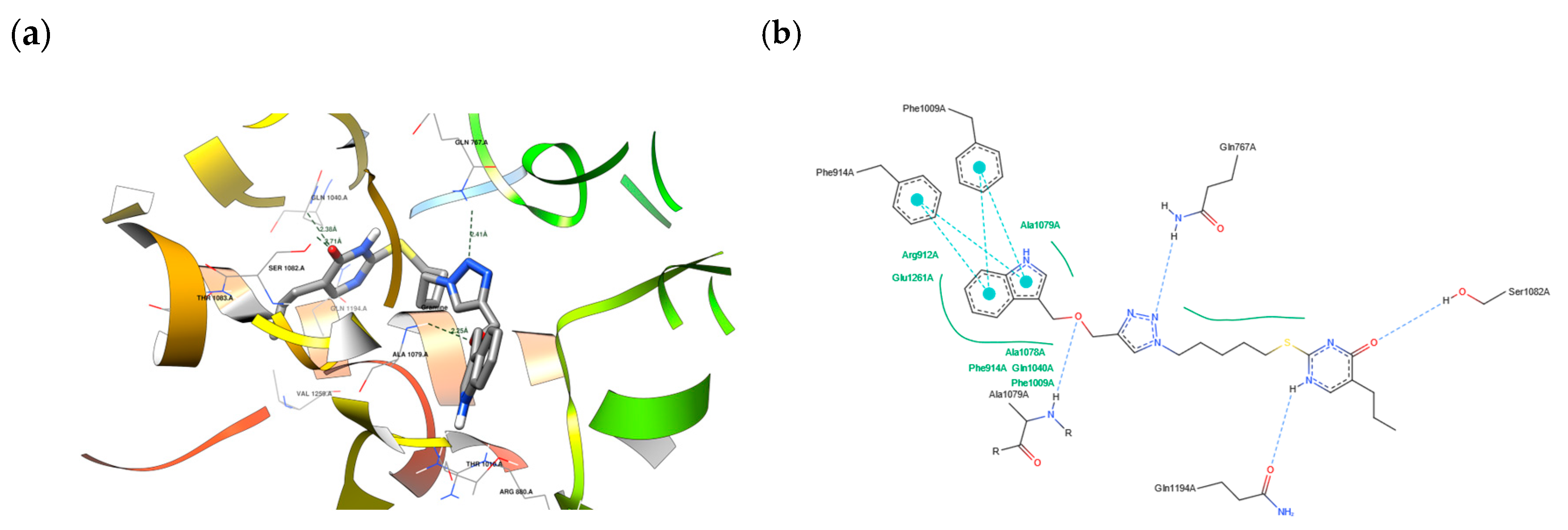
2.7. Molecular Docking
2.8. Fungicidal and Antibacterial Activity
3. Materials and Methods
3.1. Chemistry
3.2. Biological Activity
3.2.1. Human Erythrocyte
3.2.2. Hemolytic Activity
3.2.3. Chelating Activity on Ferrous Ions (Fe2+)
3.2.4. Cytoprotective Activity
3.2.5. Fungicidal and Antibacterial Activity
3.3. Physicochemical Data
3.4. Molecular Docking
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rusu, A.; Moga, I.M.; Uncu, L.; Hancu, G. The Role of Five-Membered Heterocycles in the Molecular Structure of Antibacterial Drugs Used in Therapy. Pharmaceutics 2023, 15, 2554. [Google Scholar] [CrossRef]
- Pałasz, A.; Ciez, D. In Search of Uracil Derivatives as Bioactive Agents. Uracils and Fused Uracils: Synthesis, Biological Activity and Applications. Eur. J. Med. Chem. 2015, 97, 582–611. [Google Scholar] [CrossRef]
- Kwak, S.; Ku, S.-K.; Kang, H.; Baek, M.-C.; Bae, J.-S. Methylthiouracil, a New Treatment Option for Sepsis. Vasc. Pharmacol. 2017, 88, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bahn, R.S.; Burch, H.S.; Cooper, D.S.; Garber, J.R.; Greenlee, C.M.; Klein, I.L.; Laurberg, P.; Ross Mcdougall, I.; Rivkees, S.A.; Ross, D.; et al. The Role of Propylthiouracil in the Management of Graves’ Disease in Adults: Report of a Meeting Jointly Sponsored by the American Thyroid Association and the Food and Drug Administration. Thyroid 2009, 19, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.M.; Lotfallah, A.H.; Gaballah, M.S.; Awad, S.M.; Soltan, M.K. Novel 2-Thiouracil-5-Sulfonamide Derivatives: Design, Synthesis, Molecular Docking, and Biological Evaluation as Antioxidants with 15-LOX Inhibition. Molecules 2023, 28, 1925. [Google Scholar] [CrossRef] [PubMed]
- Kadi, A.A.; El-Tahir, K.E.H.; Jahng, Y.; Motiur Rahman, A.F.M.S. Synthesis, Biological Evaluation and Structure Activity Relationships (SARs) Study of 8-(Substituted)Aryloxycaffeine. Arab. J. Chem. 2015, 12, 2356–2364. [Google Scholar] [CrossRef]
- Turati, F.; Galeone, C.; Edefonti, V.; Ferraroni, M.; Lagiou, P.; La Vecchia, C.; Tavani, A. A Meta-Analysis of Coffee Consumption and Pancreatic Cancer. Ann. Oncol. 2012, 23, 311–318. [Google Scholar] [CrossRef]
- Rosales, P.F.; Bordin, G.S.; Gower, A.E.; Moura, S. Indole Alkaloids: 2012 Until Now, Highlighting the New Chemical Structures and Biological Activities. Fitoterapia 2020, 143, 104558. [Google Scholar] [CrossRef]
- Sierakowska, A.; Jasiewicz, B.; Piosik, Ł.; Mrówczyńska, L. New C8-Substituted Caffeine Derivatives as Promising Antioxidants and Cytoprotective Agents in Human Erythrocytes. Sci. Rep. 2023, 13, 1785. [Google Scholar] [CrossRef]
- Jasiewicz, B.; Kozanecka-Okupnik, W.; Przygodzki, M.; Warżajtis, B.; Rychlewska, U.; Pospieszny, T.; Mrówczyńska, L. Synthesis, Antioxidant and Cytoprotective Activity Evaluation of C-3 Substituted Indole Derivatives. Sci. Rep. 2021, 11, 15425. [Google Scholar] [CrossRef]
- Kozanecka-Okupnik, W.; Jasiewicz, B.; Pospieszny, T.; Jastrząb, R.; Skrobańska, M.; Mrówczyńska, L. Spectroscopy, Molecular Modeling and Anti-Oxidant Activity Studies on Novel Conjugates Containing Indole and Uracil Moiety. J. Mol. Struct. 2018, 1169, 130–137. [Google Scholar] [CrossRef]
- Kozanecka-Okupnik, W.; Sierakowska, A.; Berdzik, N.; Kowalczyk, I.; Mrówczyńska, L.; Jasiewicz, B. New Triazole-Bearing Gramine Derivatives—Synthesis, Structural Analysis and Protective Effect against Oxidative Haemolysis. Nat. Prod. Res. 2022, 36, 3413–3419. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Babijczuk, K.; Berdzik, N.; Nowak, D.; Warżajtis, B.; Rychlewska, U.; Starzyk, J.; Mrówczyńska, L.; Jasiewicz, B. Novel C3-Methylene-Bridged Indole Derivatives with and without Substituents at N1: The Influence of Substituents on Their Hemolytic, Cytoprotective, and Antimicrobial Activity. Int. J. Mol. Sci. 2024, 25, 5364. [Google Scholar] [CrossRef]
- Waeterschoot, J.; Gosselé, W.; Lemež, Š.; i Solvas, X.C. Artificial Cells for In Vivo Biomedical Applications through Red Blood Cell Biomimicry. Nat. Commun. 2024, 15, 2504. [Google Scholar] [CrossRef] [PubMed]
- Umbreit, J. Methemoglobin—It’s Not Just Blue: A Concise Review. Am. J. Hematol. 2007, 82, 134–144. [Google Scholar] [CrossRef]
- Gulcin, I.; Alwasel, S.H. Metal Ions, Metal Chelators and Metal Chelating Assay as Antioxidant Method. Processes 2022, 10, 132. [Google Scholar] [CrossRef]
- Meng, X.Y.; Zhang, H.X.; Mezei, M.; Cui, M. Molecular docking: A powerful approach for structure-based drug discovery. Curr. Comput. Aided Drug Des. 2011, 7, 146–157. [Google Scholar] [CrossRef]
- Sitalu, K.; Babu, B.H.; Latha, J.N.L.; Rao, A.L. Synthesis, characterization and antimicrobial activities of copper derivatives of NHC-II Complexes. Pak. J. Biol. Sci. 2017, 20, 82–91. [Google Scholar] [CrossRef]
- Arsenyan, P.; Vasiljeva, J.; Domracheva, I.; Kanepe-Lapsa, I. 8-Ethynylxanthines as promising antiproliferative agents, angiogenesis inhibitors, and calcium channel activity modulators. Chem. Heterocycl. Comp. 2020, 56, 776–785. [Google Scholar] [CrossRef]
- Yan, B.; Liu, L.; Huang, S.; Ren, Y.; Wang, H.; Yao, Z.; Li, L.; Chen, S.; Wang, X.; Zhang, Z. Discovery of a new class of highly potent necroptosis inhibitors targeting the mixed lineage kinase domain-like protein. Chem. Commun. 2017, 53, 3637–3640. [Google Scholar] [CrossRef] [PubMed]
- Sowemimo-Coker, S.O. Red blood cell hemolysis during processing. Transfus. Med. Rev. 2002, 16, 46–60. [Google Scholar] [CrossRef]
- Nowak, D.; Babijczuk, K.; Jaya, L.O.I.; Bachorz, R.A.; Mrówczyńska, L.; Jasiewicz, B.; Hoffmann, M. Artificial Intelligence in Decrypting Cytoprotective Activity under Oxidative Stress from Molecular Structure. Int. J. Mol. Sci. 2023, 24, 11349. [Google Scholar] [CrossRef] [PubMed]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef] [PubMed]
- Jasiewicz, B.; Sierakowska, A.; Jankowski, W.; Hoffmann, M.; Piorońska, W.; Górnicka, A.; Bielawska, A.; Bielawski, K.; Mrówczyńska, L. Antioxidant and cytotoxic activity of new di- and polyamine caffeine analogues. Free Radic. Res. 2018, 52, 724–736. [Google Scholar] [CrossRef]
- Teixeira, J.P.; de Castro, A.A.; Soares, F.V.; da Cunha, E.F.F.; Ramalho, T.C. Future therapeutic perspectives into the Alzheimer’s disease targeting the oxidative stress hypothesis. Molecules 2019, 24, 4410. [Google Scholar] [CrossRef]
- Michelini, E.; Cevenini, L.; Mezzanotte, L.; Coppa, A.; Roda, A. Cell-based assays: Fuelling drug discovery. Anal. Bioanal. Chem. 2010, 398, 227–238. [Google Scholar] [CrossRef]
- Werber, J.; Wang, Y.J.; Milligan, M.; Li, X.; Ji, J.A. Analysis of 2,2′-Azobis (2-amidinopropane) dihydrochloride degradation and hydrolysis in aqueous solutions. J. Pharm. Sci. 2011, 100, 3307–3315. [Google Scholar] [CrossRef]
- Jasiewicz, B.; Babijczuk, K.; Warżajtis, B.; Rychlewska, U.; Starzyk, J.; Cofta, G.; Mrówczyńska, L. Indole Derivatives Bearing Imidazole, Benzothiazole-2-Thione or Benzoxazole-2-Thione Moieties-Synthesis, Structure and Evaluation of Their Cytoprotective, Antioxidant, Antibacterial and Fungicidal Activities. Molecules 2023, 28, 708. [Google Scholar] [CrossRef]
- León-Carmona, J.R.; Galano, A. Is caffeine a good scavenger of oxygenated free radicals? J. Phys. Chem. B 2011, 115, 4538–4546. [Google Scholar] [CrossRef]
- Santos, J.L.F.; Kauffmann, A.C.; da Silva, S.C.; Silva, V.C.P.; de Souza, G.L.C. Probing structural properties and antioxidant activity mechanisms for eleocarpanthraquinone. J. Mol. Model. 2020, 26, 244. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Alvarez-Idaboy, J.R. Computational strategies for predicting free radical scavengers’ protection against oxidative stress: Where are we and what might follow? Int. J. Quantum Chem. 2019, 119, e25665. [Google Scholar] [CrossRef]
- Silveira, C.C.; Mendes, S.R.; Soares, J.R.; Victoria, F.N.; Martinez, D.M.; Savegnago, L. Synthesis and antioxidant activity of new C-3 sulfenyl indoles. Tetrahedron Lett. 2013, 54, 4926–4929. [Google Scholar] [CrossRef]
- Kostova, I.; Atanasov, P.Y. Antioxidant Properties of Pyrimidine and Uracil Derivatives. Curr. Org. Chem. 2017, 21, 2020–2032. [Google Scholar] [CrossRef]
- Fan, J.; Fu, A.; Zhang, L. Progress in molecular docking. Quant. Biol. 2019, 7, 83–89. [Google Scholar] [CrossRef]
- Ramírez, D.; Caballero, J. Is It Reliable to Take the Molecular Docking Top Scoring Position as the Best Solution without Considering Available Structural Data? Molecules 2018, 23, 1038. [Google Scholar] [CrossRef]
- Al-Mulla, A. A Review: Biological Importance of Heterocyclic Compounds. Der Pharma Chem. 2017, 9, 141–147. [Google Scholar]
- Schreiber, K.J.; Nasmith, C.G.; Allard, G.; Singh, J.; Subramaniam, R.; Desveaux, D. Found in Translation: High-Throughput Chemical Screening in Arabidopsis thaliana Identifies Small Molecules That Reduce Fusarium Head Blight Disease in Wheat. Mol. Plant Microbe Interact. 2011, 24, 640–648. [Google Scholar] [CrossRef]
- Barmaki, M.; Valiyeva, G.; Maharramovm, A.A.; Allaverdiyev, M.M. Synthesis of 2,3-Dihydro-6-Methyl-2-Thiopyrimidin-4(1H)-One (6-Methylthiouracil) Derivatives and Their Reactions. J. Chem. 2013, 2013, 176213. [Google Scholar] [CrossRef]
- Whitehead, C.W.; Traverso, J.J.; Whitehead, B.W.C. Synthesis of 2-Thiocytosines and 2-Thiouracils. J. Am. Chem. Soc. 1952, 74, 5294–5299. [Google Scholar] [CrossRef]
- Spengler, J.-P.; Schunack, W. H2-Antihistaminika, 18. Mitt.’’ 5,6-Alkylsubstituierte 4-Pyrimidinone mit H2-Antihistaminischer Wirkung. Arch. Pharm. 1984, 317, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L. Efficient Drug Lead Discovery and Optimization. Acc. Chem. Res. 2009, 42, 724–733. [Google Scholar] [CrossRef]
- Mrówczyńska, L.; Hagerstrand, H. Platelet-Activating Factor Interaction with the Human Erythrocyte Membrane. J. Biochem. Mol. Toxicol. 2009, 23, 345–348. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open Chemical Toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Raschka, S. Biopandas: Working with Molecular Structures in Pandas Dataframes. J. Open Source Softw. 2017, 2, 14. [Google Scholar] [CrossRef]
- Berman, H.M.; Battistuz, T.; Bhat, T.N.; Bluhm, W.F.; Bourne, P.E.; Burkhardt, K.; Feng, Z.; Gilliland, G.L.; Iype, L.; Jain, S.; et al. The Protein Data Bank. Acta Crystallogr. D 2002, 58, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Blair-Johnson, M.; Fiedler, T.; Fenna, R. Human Myeloperoxidase: Structure of a Cyanide Complex and Its Interaction with Bromide and Thiocyanate Substrates at 1.9 Å Resolution. Biochemistry 2001, 40, 13990–13997. [Google Scholar] [CrossRef]
- Okamoto, K.; Eger, B.T.; Nishino, T.; Kondo, S.; Pai, E.F.; Nishino, T. An Extremely Potent Inhibitor of Xanthine Oxidoreductase: Crystal Structure of the Enzyme-Inhibitor Complex and Mechanism of Inhibition. J. Biol. Chem. 2003, 278, 1848–1855. [Google Scholar] [CrossRef]
- Kurumbail, R.G.; Stevens, A.M.; Gierse, J.K.; McDonald, J.J.; Stegeman, R.A.; Pak, J.Y.; Gildehaus, D.; Iyashiro, J.M.; Penning, T.D.; Seibert, K.; et al. Structural Basis for Selective Inhibition of Cyclooxygenase-2 by Anti-Inflammatory Agents. Nature 1996, 384, 644–648. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Kargapolova, Y.; Geißen, S.; Zheng, R.; Baldus, S.; Winkels, H.; Adam, M. The Enzymatic and Non-Enzymatic Function of Myeloperoxidase (MPO) in Inflammatory Communication. Antioxidants 2021, 10, 562. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.; Winterbourn, C.C. Reactive Oxidants and Myeloperoxidase and Their Involvement in Neutrophil Extracellular Traps. Front. Immunol. 2012, 3, 424. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Baek, B.S.; Song, S.H.; Kim, M.S.; Huh, J.I.; Shim, K.H.; Kim, K.W.; Lee, K.H. Xanthine Dehydrogenase/Xanthine Oxidase and Oxidative Stress. Age 1997, 20, 127–140. [Google Scholar] [CrossRef]
- Berry, C.E.; Hare, J.M. Xanthine Oxidoreductase and Cardiovascular Disease: Molecular Mechanisms and Pathophysiological Implications. J. Physiol. 2004, 555, 589–606. [Google Scholar] [CrossRef]
- Laube, M.; Kniess, T.; Pietzsch, J. Development of Antioxidant COX-2 Inhibitors as Radioprotective Agents for Radiation Therapy—A Hypothesis-Driven Review. Antioxidants 2016, 5, 14. [Google Scholar] [CrossRef]
- Desai, S.J.; Prickril, B.; Rasooly, A. Mechanisms of Phytonutrient Modulation of Cyclooxygenase-2 (COX-2) and Inflammation Related to Cancer. Nutr. Cancer 2018, 70, 350–375. [Google Scholar] [CrossRef]


| Compound | MW [g/mL] | logP | HBD | HBA | TPSA [Å2] | GI ABS | BBB Perm | Lipinski’s Rule |
|---|---|---|---|---|---|---|---|---|
| 9a | 503.6 | 1.47 | 1 | 7 | 188.88 | low | no | No |
| 9b | 579.7 | 2.67 | 1 | 7 | 188.88 | low | no | No |
| 9c | 517.6 | 1.81 | 1 | 7 | 188.88 | low | no | No |
| 9d | 545.7 | 2.45 | 1 | 7 | 188.88 | low | no | no |
| 9e | 545.7 | 2.51 | 1 | 7 | 188.88 | low | no | no |
| 9f | 502.7 | 0.9 | 2 | 7 | 193.54 | low | no | no |
| 10 | 505.5 | 1.06 | 1 | 8 | 172.69 | low | no | no |
| 11a | 424.52 | 2.82 | 2 | 5 | 126.78 | high | no | yes |
| 11b | 500.62 | 4.17 | 2 | 5 | 126.78 | low | no | yes |
| 11c | 438.55 | 3.07 | 2 | 5 | 126.78 | high | no | yes |
| 11d | 466.6 | 3.77 | 2 | 5 | 126.78 | low | no | yes |
| 11e | 466.6 | 3.82 | 2 | 5 | 126.78 | low | no | yes |
| 11f | 425.55 | 2.51 | 3 | 5 | 131.44 | high | no | yes |
| 12 | 722.81 | 4.92 | 2 | 9 | 155.46 | low | no | no |
| PDB ID | Compound | Binding Energy [kcal/mol] | Standard Deviation of Binding Energy [kcal/mol] |
|---|---|---|---|
| 1DNU | Melatonin | −5.6 | 0.19 |
| 11d | −6.3 | 0.17 | |
| 1N5X | Febuxostat | −7.4 | 0.50 |
| 11d | −10.6 | 0.54 | |
| 4COX | Indomethacin | −8.6 | 0.45 |
| 11d | −9.4 | 0.29 |
| Compound | Fusarium culmorum | Fusarium graminearum | Trichoderma atroviridae | Trichoderma harzianum | Alternaria alternata | Botrytis cinerea |
|---|---|---|---|---|---|---|
| 9a | 16 | 0 | 8 | 7 | 6.5 | 5.5 |
| 9b | 1 | 2.5 | 0 | 0 | 7 | 12 |
| 9c | 0 | 0 | 9 | 6 | 10 | 0 |
| 9d | 1 | 4 | 0 | 0 | 7 | 13.4 |
| 9e | 1.2 | 6.4 | 4.5 | 0 | 8 | 17 |
| 9f | 16 | 0 | 8.5 | 6.5 | 4 | 5.5 |
| 10 | 10.5 | 0 | 4 | 8 | 8 | 0 |
| 11a | 1.5 | 10 | 4.5 | 8.4 | 11.2 | 11.8 |
| 11b | 0 | 0 | 8 | 10 | 7 | 0 |
| 11c | 0 | 0 | 7.5 | 7 | 0 | 6.5 |
| 11d | 1 | 3 | 9 | 0 | 9 | 16 |
| 11e | 0 | 0 | 12 | 8.5 | 8.5 | 6 |
| 11f | 3 | 0 | 7 | 6.5 | 8.5 | 6 |
| 12 | 15 | 12 | 6 | 8 | 6.5 | 5 |
| PDB ID | Search Space Center (x, y, z) | Size of the Search Space (x, y, z) |
|---|---|---|
| 1DNU | 40, −38, −5 | 22, 19, 20 |
| 1N5X | 97, 55, 39 | 35, 37, 43 |
| 4COX | 25, 22, 15 | 38, 37, 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szlaużys, M.; Ostrowski, K.; Nowak, D.; Prukała, W.; Starzyk, J.; Jasiewicz, B.; Mrówczyńska, L. Hybrid Uracil Derivatives with Caffeine and Gramine Obtained via Click Chemistry as Potential Antioxidants and Inhibitors of Plant Pathogens. Molecules 2025, 30, 2714. https://doi.org/10.3390/molecules30132714
Szlaużys M, Ostrowski K, Nowak D, Prukała W, Starzyk J, Jasiewicz B, Mrówczyńska L. Hybrid Uracil Derivatives with Caffeine and Gramine Obtained via Click Chemistry as Potential Antioxidants and Inhibitors of Plant Pathogens. Molecules. 2025; 30(13):2714. https://doi.org/10.3390/molecules30132714
Chicago/Turabian StyleSzlaużys, Milda, Kamil Ostrowski, Damian Nowak, Wiesław Prukała, Justyna Starzyk, Beata Jasiewicz, and Lucyna Mrówczyńska. 2025. "Hybrid Uracil Derivatives with Caffeine and Gramine Obtained via Click Chemistry as Potential Antioxidants and Inhibitors of Plant Pathogens" Molecules 30, no. 13: 2714. https://doi.org/10.3390/molecules30132714
APA StyleSzlaużys, M., Ostrowski, K., Nowak, D., Prukała, W., Starzyk, J., Jasiewicz, B., & Mrówczyńska, L. (2025). Hybrid Uracil Derivatives with Caffeine and Gramine Obtained via Click Chemistry as Potential Antioxidants and Inhibitors of Plant Pathogens. Molecules, 30(13), 2714. https://doi.org/10.3390/molecules30132714







