Physicochemical Properties and Aroma Profiles of Golden Mulberry Fruits at Different Harvesting Stages
Abstract
1. Introduction
2. Results
2.1. Impact of Harvesting Time on Fruit Drop, Mold Incidence, and Quality of Golden Mulberry Fruit
2.2. Effect of Harvesting Time on Fruit Shape Index and Single Fruit Weight of Golden Mulberry
2.3. Effect of Harvesting Time on the Hardness and Color Characteristics of Golden Mulberry Fruit
2.4. Sensory Evaluation of Golden Mulberry Fruit at Different Harvesting Stages
2.5. Impact of Harvesting Time on the Nutritional Quality of Golden Mulberry Fruit
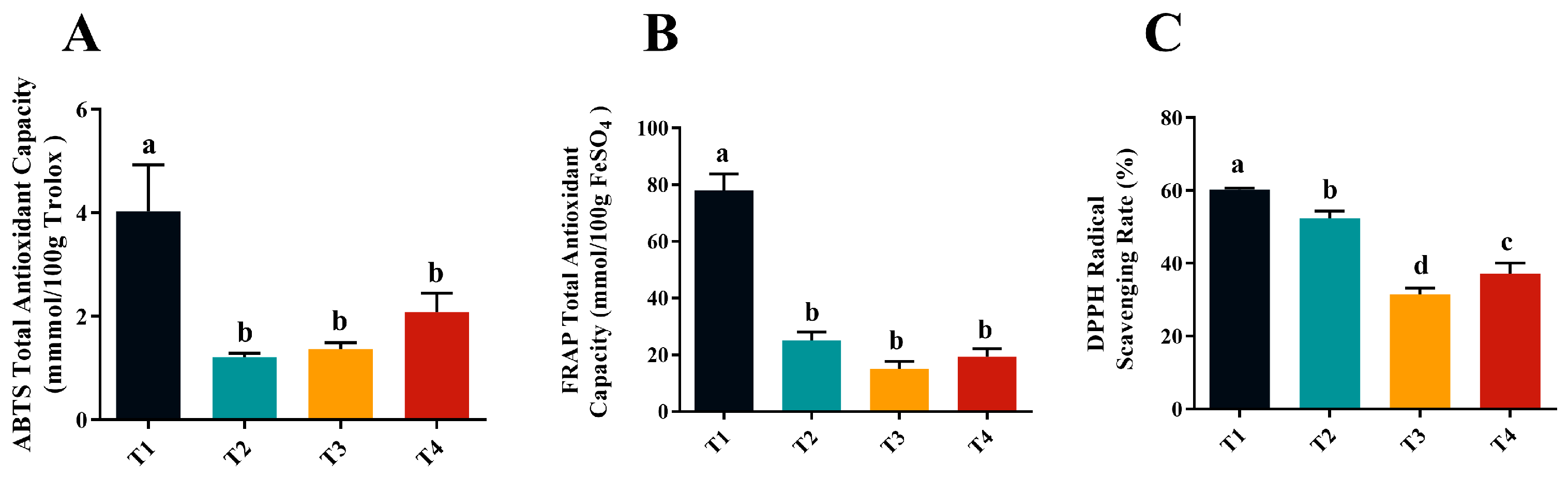
2.6. Antioxidant Capacity of Golden Mulberry Fruit at Various Harvesting Stages
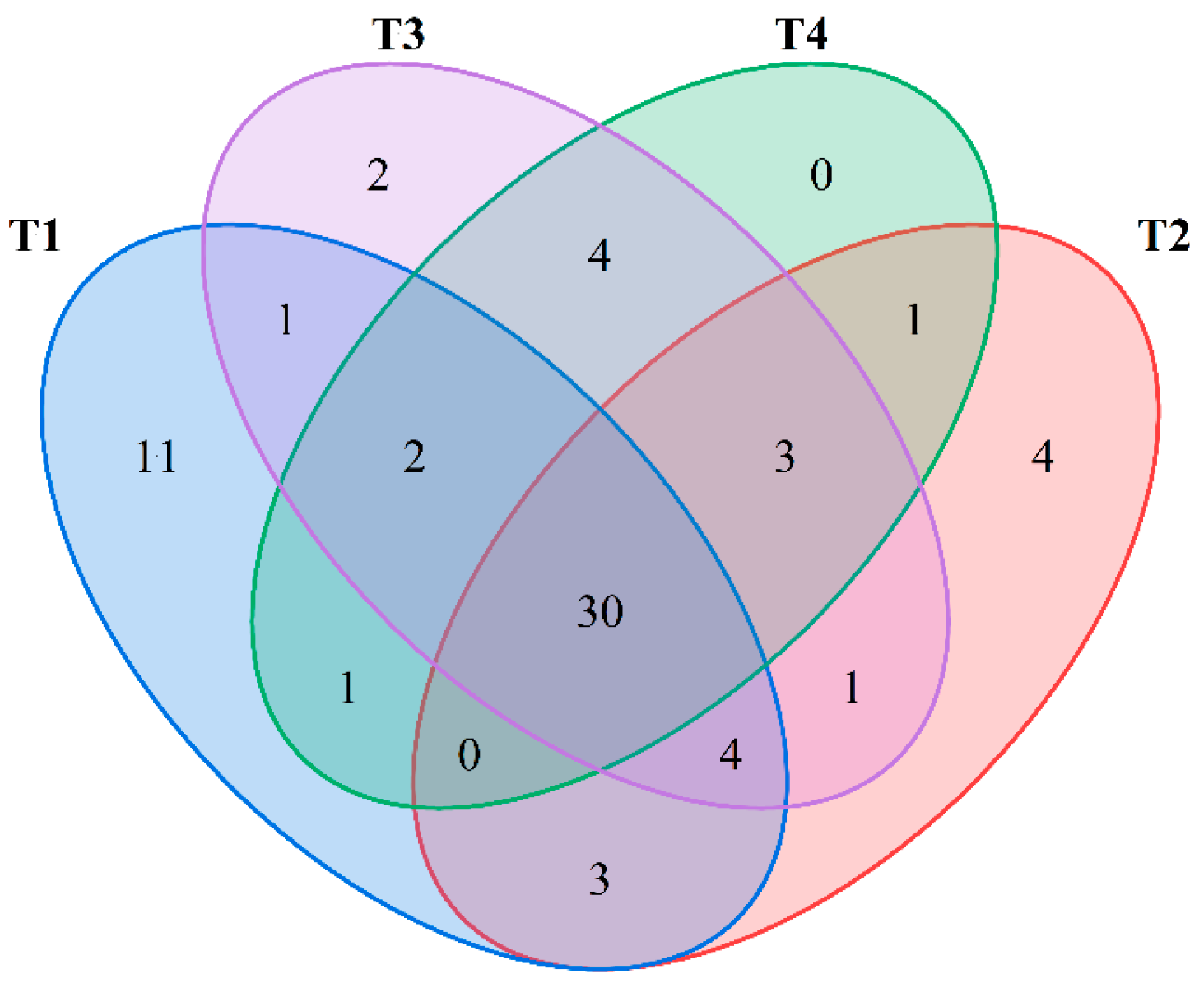
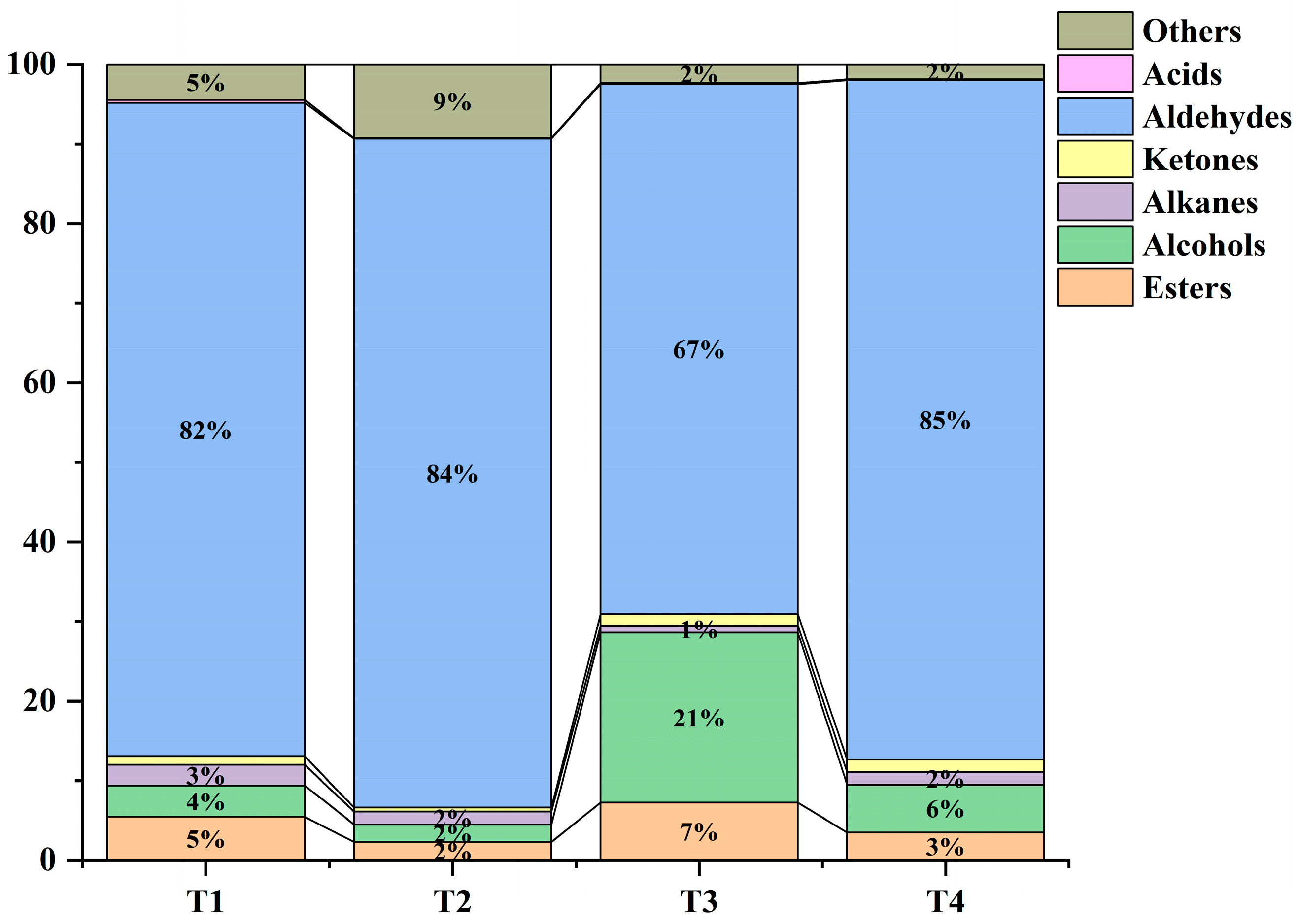
2.7. Analysis of Aroma Components in Golden Mulberry Fruit Across Different Harvesting Stages
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sample Preparation
4.3. The Percentage of Mulberry Fruit Drop, Mold, and Marketable Fruit
4.4. Measurement of Appearance Indicators
4.5. Material Composition Analysis
4.6. Evaluation of Antioxidant Activity
4.7. Determination of Aroma Composition
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| Chromaticity | C* |
| DPPH | 1-diphenyl-2-picrylhydrazyl radical |
| FRAP | ferric reducing antioxidant power |
| GAE | gallic acid equivalent |
| SD | standard deviation |
| SSs | soluble sugars |
| TSSs | total soluble solids |
| TA | titratable acidity |
References
- He, X.M.; Sun, J.; Liang, G.Q.; Qiu, C.Y.; Li, J.M.; Li, C.B.; Sheng, J.F.; Liu, G.M.; Ling, Q.N.; Tang, Y.Y. Nutritional and Medicinal Quality of Mulberry Fruit from 13 Main Varieties Grown in Guangxi. Food Sci. 2018, 39, 250–256. [Google Scholar]
- Yu, H.L.; Ma, Y.K.; Zhang, R.; Liu, L.; Li, J.F.; Li, X. Principal component analysis of aroma components in mulberry from different varieties. Sci. Technol. Food Ind. 2016, 37, 62–71. [Google Scholar]
- Chen, X.; Sououl, M.H.; Nateghi, M.; Melekoglu, E.; Fatahi, S. Impact of mulberry consumption on cardiometabolic risk factors: A systematic review and meta-analysis of randomized-controlled trials. J. Clin. Pharm. Ther. 2022, 47, 1982–1993. [Google Scholar] [CrossRef] [PubMed]
- Erden, Y. Sour black mulberry (Morus nigra L.) causes cell death by decreasing mutant p53 expression in HT-29 human colon cancer cells. Food Biosci. 2021, 42, 101113. [Google Scholar] [CrossRef]
- Cheng, K.; Wang, C.; Chang, Y. Mulberry fruits extracts induce apoptosis and autophagy of liver cancer cell and prevent hepatocarcinogenesis in vivo. J. Food Drug Anal. 2019, 28, 84–93. [Google Scholar] [CrossRef]
- Samira, Y.; Mohammad, H.; Hossein, A.M. The antidiabetic potential of Black Mulberry extract enriched Pasta through inhibition of enzymes and glycemic index. Plant Foods Hum. Nutr. 2019, 74, 149–155. [Google Scholar]
- Kim, T.E.; Jo, Y.H.; Kim, C.T. Improvement of Quality Characteristics of Mulberry (Morus alba L.) Fruit Extract Using High-Pressure Enzymatic Treatment. Food Bioprocess Technol. 2024, 17, 4106–4114. [Google Scholar] [CrossRef]
- Kakaei, S.; Saba, M.K.; Mansouri, S.; Darvishi, H. Melatonin postharvest spray influence on white mulberry browning, storage life, and biochemical changes. Postharvest Biol. Technol. 2024, 213, 8. [Google Scholar] [CrossRef]
- Li, K.F.; Ni, L.; Yang, P. Preliminary study on trellage cultivation technology of mulberry. China Seric. 2019, 40, 14–21. [Google Scholar]
- Zhang, J.Q.; Li, C.; Huang, Q.; Li, J.Y.; Chun, C.; Xiong, F.; Rui, H.L. Comparative study on the physicochemical properties and bioactivities of polysaccharide fractions extracted from Fructus Mori at different temperatures. Food Funct. 2019, 10, 410–421. [Google Scholar] [CrossRef]
- Chen, C.; You, L.J.; Huang, Q.; Xiong, F.; Zhang, B.; Liu, R.H.; Li, C. Modulation of gut microbiota by mulberry fruit polysaccharide treatment of obese diabetic db/db mice. Food Funct. 2018, 9, 3732–3742. [Google Scholar] [CrossRef] [PubMed]
- Herman, R.A.; Ayepa, E.; Fometu, S.S.; Shittu, S.; Davids, J.S.; Wang, J. Mulberry fruit post-harvest management: Techniques, composition and influence on quality traits-A review. Food Control. 2022, 140, 109126. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, L. The Mulberry (Morus alba L.) Fruit A Review of Characteristic Components and Health Benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Wang, L.X.; Wu, Y.J. Fruit quality analysis and comprehensive evaluation of 18 Huyou (Citrus changshanensis) accessions. J. Fruit Sci. 2024, 41, 1033–1043. [Google Scholar]
- Li, H.M.; Zheng, J.X.; Zeng, K.F. Effect of Different Harvest Maturity on the Nutritional Quality of Midknight Valencia Orange Fruit. Sci. Technol. Food Ind. 2023, 44, 390–400. [Google Scholar]
- Esmaeili, M.R.; Khodabakhshian, R.; Khojastehpour, M. Effect of Asafoetida Extract Solution on Red Apple Quality with a Focus on the Mechanical Properties of Peduncle, Rate Fruit Drop and Orchard Pest Control. Applied Fruit Sci. 2024, 66, 2145–2155. [Google Scholar] [CrossRef]
- Geng, Y.; Zhao, X.M.; Tan, Y.P. Effects of harvest maturity on storage quality and antioxidative capacity of Ziziphus jujuba ‘Jingcang1′. Food Ferment. Ind. 2023, 49, 269–275. [Google Scholar]
- Li, H.Y.; Ma, Q.L.; Lin, X. Effects of harvest maturity on storage quality and antioxidative capacity of Taoxi pomelo. Food Ferment. Ind. 2019, 45, 191–198. [Google Scholar]
- Lin, Y.J.; Ye, X.F.; Fang, Z.Z. Nutritional Quality and Aroma Characteristics of Fuhong Plum (Prunus salicina Lindl) during Three Different Mature Stages. Chin. J. Nucl. Agric. 2024, 38, 725–735. [Google Scholar]
- Kong, F.N.; Li, X.Y.; Zhang, J. Effect of Different Maturity on Quality and Aroma Composition of C. lancifolius Fruit. Sci. Technol. Food Ind. 2024, 45, 63–71. [Google Scholar]
- Gao, L.; Long, Y.; Li, H. Effects of harvest maturity on quality and flavor of late-maturing Daya citrus. Food Ferment. Ind. 2023, 49, 140–148. [Google Scholar]
- Li, K.F.; Wang, X.X.; Wang, Y. Preliminary Study on rain-protected root-limiting cultivation technology of mulberry tree. Seric. China 2022, 43, 54–56. [Google Scholar]
- Kattil, A.; Hamid; Dash, K.K.; Shams, R.; Sharma, S. Nutritional composition, phytochemical extraction, and pharmacological potential of mulberry: A comprehensive review. Future Foods 2024, 9, 100295. [Google Scholar] [CrossRef]
- Fan, Z.Y.; Li, X.Y.; Li, J.X. Antioxidant and Antiglycation Activities of Phenolic Compounds Extracted from Mulberry Fruits. Food Sci. 2016, 37, 19–26. [Google Scholar]
- Zhang, W.L.; Chen, T.T.; Tang, J.M. Tracing the production area of citrus fruits using aroma—active compounds and their quality evaluation models. J. Sci. Food Agric. 2020, 100, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.S.; Xu, Y.J.; Wu, J.J. Effect of ultra-high pressure homogenization processing on phenolic compounds, antioxidant capacity and anti-glucosidase of mulberry juice. Food Chem. 2014, 153, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.W.; Lu, L.Y.; Wang, N. Analysis of mulberry quality at different maturities. Res. Econ. For. 2019, 42, 282–292. [Google Scholar]
- Park, J.H.; Hong, S.I.; Jeong, M.C.; Kim, D. Quality characteristics and changes in mulberry (Morus alba L.) depending on their maturity during distribution. Food Sci. Preserv. 2013, 20, 304–316. [Google Scholar]
- Nayab, S.; Razzaq, K.; Ullah, S.; Rajwana, I.A.; Amin, M.; Faried, H.N.; Akhtar, G.; Khan, A.S.; Asghar, Z.; Hassan, H.; et al. Genotypes and harvest maturity influence the nutritional fruit quality of mulberry. Sci. Hortic. 2020, 266, 109311. [Google Scholar] [CrossRef]
- Cindrić, I.J.; Krizman, I.; Zeiner, M. ICP-AES determination of minor- and major elements in apples after microwave assisted digestion. Food Chem. 2012, 135, 2675–2680. [Google Scholar] [CrossRef]
- Suriyaprom, S.; Kaewkod, T.; Promputtha, I.; Desvaux, M.; Tragoolpua, Y. Evaluation of antioxidant and antibacterial activities of white mulberry (Morus alba L.) fruit extracts. Plants 2021, 10, 2736. [Google Scholar] [CrossRef] [PubMed]
- Makavelou, M.; Kafkaletou, M.; Tsantili, E.; Harizanis, P. Physiological and Quality Characteristics in Four Genotypes of Mulberry Fruit (Morus alba L.) During Ripening. In II Balkan Symposium on Fruit Growing; ISHS: Leuven, Belgium, 2013. [Google Scholar]
- Meng, Y.; Lian, Y.; Li, J.; Suo, H.; Song, J.; Wang, M.; Zhang, Y. Quality Characteristics and Flavor Analysis of Five Mulberry Varieties. Foods 2024, 13, 4088. [Google Scholar] [CrossRef]
- Jia, M.L.; Li, N.; Wang, B.B.; Fan, W.; Xia, A.H.; Li, J.S. Evaluation of nutrition, aroma components and antioxidant activity of mulberry fruits from nine varieties. J. Fruit Sci. 2002, 39, 221–231. [Google Scholar]
- Flores-Sosa, A.R.; Soto-Magaña, D.; Gonzalez-dela Vara, L.E.; Sanchez-Segura, L.; Bah, M.; Rivera-Pastrana, D.; Nava, G.M.; Mercado-Silva, E.M. Red drupelet reversion in blackberries caused by mechanical damage is not linked to a reduction in anthocyanin content. Postharvest Biol. Technol. 2021, 180, 111618. [Google Scholar] [CrossRef]
- Cong, Y.; Liu, Y.; Tang, Y.; Ma, J.; Ma, J.; Liu, Z.; Yang, X.; Zhang, H. Optimization and Testing of the Technological Parameters for the Microwave Vacuum Drying of Mulberry Harvests. Appl. Sci. 2024, 14, 4137. [Google Scholar] [CrossRef]
- Feng, B.S.; Liu, L.X.; Sun, J. Quality analysis of golden peach fruits with different harvest maturity. Food Ferment. Ind. 2023, 49, 175–182. [Google Scholar]
- Morris, D.L. Quantitative determination of carbohydrates with Dreywood’s anthrone reagent. Science 1948, 107, 254–255. [Google Scholar] [CrossRef]
- Mo, R.L.; Zhang, N.; Zhou, Y. Influence of eight rootstocks on fruit quality of Morus multicaulis cv. ‘Zing’ and the comprehensive evaluation of fruit quality traits. Not. Bot. Horti Agrobot. 2022, 50, 12598. [Google Scholar] [CrossRef]
- Xiong, Z.Y.; Liu, L.; Zhang, Z.L.; Cao, L.H.; Cao, D.; Du, Z.X.; Tang, Y. Unravelling the role of surface modification in the dermocompatibility of silver nanoparticles in vitro and in vivo. Chemosphere 2022, 291, 133111. [Google Scholar] [CrossRef]
- Turxun, A.D.; Li, Y.; Liu, M.W. Comparative study on total flavonoid content and antioxidant activity of medicinal mulberries from different producing areas in Xinjiang. J. Health Res. 2024, 53, 465–471. [Google Scholar]
- Gemert, L. Compilations of Flavour Threshold Values in Water and Other Media, 2nd ed.; Oliemans Punter & Partners BV: Utrecht, The Netherlands, 2011. [Google Scholar]

| Group | Fruit Drop (%) | Mold (%) | Marketable Fruit (%) |
|---|---|---|---|
| T1 | 1.30 ± 0.60 d | 0.00 ± 0.00 c | 98.73 ± 0.64 a |
| T2 | 3.30 ± 0.60 c | 0.00 ± 0.00 c | 96.75 ± 0.61 b |
| T3 | 9.30 ± 1.50 b | 1.72 ± 0.16 b | 89.01 ± 1.72 c |
| T4 | 28.71 ± 2.11 a | 5.72 ± 1.20 a | 65.73 ± 3.21 d |
| Indicator | Time | |||
|---|---|---|---|---|
| T1 | T2 | T3 | T4 | |
| Single-fruit weight/g | 11.97 ± 0.80 c | 18.07 ± 0.95 b | 21.39 ± 1.91 a | 22.18 ± 1.45 a |
| Fruit shape index | 10.25 ± 0.92 a | 8.01 ± 0.79 b | 6.75 ± 0.73 c | 6.67 ± 0.63 c |
| Hardness/N | 2.18 ± 0.04 a | 1.48 ± 0.28 b | 0.66 ± 0.07 c | 0.32 ± 0.04 d |
| L* | 25.24 ± 1.03 c | 39.54 ± 2.48 c | 44.82 ± 0.33 a | 44.59 ± 1.16 a |
| a* | −7.52 ± 0.17 b | −9.14 ± 0.10 c | −9.61 ± 0.18 c | −4.77 ± 0.22 a |
| b* | 11.95 ± 0.40 d | 18.10 ± 0.89 b | 19.90 ± 0.41 a | 15.07 ± 0.02 c |
| C* | 14.08 ± 0.44 d | 19.98 ± 0.64 b | 22.04 ± 0.46 a | 15.80 ± 0.09 c |
| Indicator | Time | |||
|---|---|---|---|---|
| T1 | T2 | T3 | T4 | |
| Color | 14.15 ± 0.26 d | 17.38 ± 0.51 c | 18.68 ± 0.77 a | 16.92 ± 0.64 b |
| Juiciness | 2.90 ± 0.31 d | 13.62 ± 0.28 c | 17.45 ± 0.75 b | 19.35 ± 0.23 a |
| Sweetness | 8.43 ± 0.21 d | 12.43 ± 0.47 c | 18.67 ± 0.25 b | 19.27 ± 0.16 a |
| Sourness | 18.38 ± 0.29 a | 14.90 ± 1.27 b | 6.60 ± 0.43 c | 2.27 ± 0.34 d |
| Flavor | 2.98 ± 0.23 d | 9.65 ± 0.23 b | 18.28 ± 0.28 a | 14.93 ± 0.63 b |
| Total score | 46.84 ± 1.99 d | 67.98 ± 1.31 c | 79.68 ± 1.27 a | 73.17 ± 1.91 b |
| Indicator | Time | |||
|---|---|---|---|---|
| T1 | T2 | T3 | T4 | |
| TSSs (%) | 6.04 ± 0.54 d | 14.8 ± 0.67 c | 17.11 ± 0.57 b | 26.19 ± 0.91 a |
| SSs (mg/g) | 113.21 ± 3.15 d | 296.93 ± 5.20 b | 349.79 ± 9.04 a | 282.06 ± 8.04 c |
| TA (mg/g) | 26.62 ± 0.77 a | 19.71 ± 2.95 b | 13.61 ± 1.39 c | 19.93 ± 1.66 b |
| Total phenolic content (mg GAE/g) | 3.75 ± 0.02 a | 3.67 ± 0.02 b | 0.97 ± 0.03 d | 1.24 ± 0.06 c |
| TSSs/TA | 0.25 ± 0.03 c | 0.75 ± 0.01 b | 1.29 ± 0.06 a | 1.32 ± 0.04 a |
| SSs/TA | 4.22 ± 0.06 d | 20.06 ± 0.45 b | 25.70 ± 1.57 a | 14.22 ± 0.19 c |
| Number | Category | Compound Name | CAS | Content/(μg/kg) | |||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | ||||
| 1 | esters | Octanoic acid, methyl ester | 111-11-5 | 77.07 ± 16.70 a | 17.25 ± 0.06 b | 4.69 ± 2.70 b | 6.15 ± 0.23 b |
| 2 | Butyrolactone | 96-48-0 | 60.48 ± 0.00 | - | - | - | |
| 3 | Tetrahydrofurfuryl acrylate | 2399-48-6 | 53.72 ± 0.00 | - | - | - | |
| 4 | 2-Propenoic acid, 2-methyl-, octyl ester | 2157-01-9 | 38.20 ± 1.13 a | - | 7.40 ± 0.00 b | 5.57 ± 2.54 b | |
| 5 | Nonanoic acid, methyl ester | 1731-84-6 | 36.85 ± 2.30 a | 7.61 ± 0.32 b | 5.32 ± 0.95 b | 6.30 ± 0.48 b | |
| 6 | Oxalic acid, diallyl ester | 615-99-6 | 26.68 ± 0.00 b | 5.09 ± 0.00 b | 131.68 ± 1.31 a | - | |
| 7 | n-Caproic acid vinyl ester | 3050-69-9 | 20.75 ± 2.77 a | - | 2.89 ± 0.00 b | 2.88 ± 0.00 b | |
| 8 | Hexadecanoic acid, methyl ester | 112-39-0 | 19.60 ± 0.15 a | 3.82 ± 0.50 b | 1.29 ± 0.00 c | 2.14 ± 0.73 c | |
| 9 | Ethyl (E)-hex-3-enyl carbonate | 175397-71-4 | 186.79 ± 10.24 a | 35.11 ± 8.28 b | 14.82 ± 7.31 b | 10.17 ± 2.03 b | |
| 10 | Heptanoic acid, methyl ester | 106-73-0 | 17.82 ± 0.56 a | 4.01 ± 0.81 b | 1.72 ± 0.88 c | 4.11 ± 0.44 b | |
| 11 | Hexanoic acid, methyl ester | 106-70-7 | 145.22 ± 18.48 a | 47.99 ± 3.85 b | 18.77 ± 12.90 b | 26.41 ± 0.63 b | |
| 12 | 3-Hexen-1-ol, propanoate, (Z)- | 33467-74-2 | - | 25.24 ± 3.62 a | 0.53 ± 0.00 b | 6.52 ± 0.00 b | |
| 13 | alcohols | 4-Heptenal, (Z)- | 6728-31-0 | 37.72 ± 3.81 a | 22.05 ± 0.00 b | - | - |
| 14 | 3-Buten-1-ol, 2-methyl- | 4516-90-9 | 35.49 ± 0.00 | - | - | - | |
| 15 | Cyclohexanol, 1-ethenyl- | 1940-19-8 | 25.42 ± 0.00 a | 3.48 ± 0.00 b | - | - | |
| 16 | Cyclobutene-3,4-diol, tetramethyl- | 90112-64-4 | 15.46 ± 0.00 | - | - | - | |
| 17 | 3-Cyclohexen-1-ol, 4-methyl-1-(1-methylethyl)-, (R)- | 20126-76-5 | 14.00 ± 1.35 a | 3.40 ± 0.00 b | 1.20 ± 0.00 c | 1.02 ± 0.15 c | |
| 18 | 2-Undecen-4-ol | 22381-86-8 | 13.80 ± 4.27 a | 4.89 ± 1.45 b | 2.07 ± 1.14 b | 1.43 ± 0.00 b | |
| 19 | 2-Penten-1-ol, (Z)- | 1576-95-0 | 121.58 ± 6.98 a | 26.48 ± 0.64 b | 7.85 ± 5.85 c | 7.35 ± 0.53 c | |
| 20 | 1-Penten-3-ol | 616-25-1 | 117.32 ± 2.58 a | 29.25 ± 1.08 b | 8.64 ± 7.09 c | 8.89 ± 0.83 c | |
| 21 | 1-Octen-3-ol | 3391-86-4 | 13.62 ± 1.33 b | 8.87 ± 0.93 b | 6.99 ± 5.62 b | 36.38 ± 2.47 a | |
| 22 | 2-Heptanol, (S)- | 6033-23-4 | 102.72 ± 0.00 | - | - | - | |
| 23 | 2-Hepten-3-ol, 4,5-dimethyl- | 55956-37-1 | - | 26.08 ± 0.00 | - | - | |
| 24 | 6,6-Dimethyl-1,3-heptadien-5-ol | 81912-03-0 | - | 15.87 ± 0.00 | - | - | |
| 25 | 1-Hexanol | 111-27-3 | - | - | 48.70 ± 6.22 b | 54.02 ± 1.79 a | |
| 26 | Cyclopentanol, 2-methyl- | 24070-77-7 | - | - | 479.06 ± 0.00 | - | |
| 27 | Cyclohexanol | 108-93-0 | - | - | 8.28 ± 1.36 b | 12.53 ± 0.00 a | |
| 28 | alkanes | Cyclopropane, propyl- | 2415-72-7 | 164.29 ± 6.67 a | 94.09 ± 2.14 b | - | - |
| 29 | 1-Azabicyclo[3.1.0]hexane | 285-76-7 | 149.44 ± 7.07 | - | - | - | |
| 30 | Pentane, 2-methoxy- | 6795-88-6 | 14.03 ± 0.00 a | 4.93 ± 0.16 b | 0.61 ± 0.00 d | 1.16 ± 0.00 c | |
| 31 | Bicyclo[2.1.0]pentane, 1,4-dimethyl- | 17065-18-8 | - | 3.96 ± 0.58 c | 17.62 ± 0.00 a | 14.49 ± 0.00 b | |
| 32 | Pentane, 1-chloro- | 543-59-9 | - | - | 4.63 ± 5.22 | 15.99 ± 1.87 | |
| 33 | ketones | 1-Penten-3-one | 1629-58-9 | 93.46 ± 12.41 a | 19.40 ± 4.27 b | 3.93 ± 1.67 b | 2.30 ± 0.46 b |
| 34 | 3,5-Octadien-2-one | 38284-27-4 | 38.27 ± 4.85 a | 10.09 ± 0.00 b | 10.04 ± 0.00 b | 5.93 ± 0.22 b | |
| 35 | 3-Octen-2-one, (E)- | 18402-82-9 | - | 2.86 ± 0.00 b | 1.45 ± 0.00 b | 23.40 ± 2.31 a | |
| 36 | 2-Methylhept-6-en-3-one | 26144-88-7 | - | - | 22.85 ± 0.00 | - | |
| 37 | aldehydes | 4-Ethyl-2-hexynal | 71932-97-3 | 72.81 ± 0.00 a | - | 5.45 ± 0.00 b | - |
| 38 | Hexanal | 66-25-1 | 6504.75 ± 25.11 a | 2936.55 ± 31.74 b | 730.45 ± 39.90 c | 613.60 ± 7.12 c | |
| 39 | Nonanal | 124-19-6 | 54.53 ± 9.10 a | 30.02 ± 3.06 b | 36.51 ± 34.53 b | 69.90 ± 4.95 a | |
| 40 | 2,4-Hexadienal, (E,E)- | 142-83-6 | 51.48 ± 0.12 a | 12.46 ± 2.40 b | 5.51 ± 0.00 c | ||
| 41 | Pentanal | 110-62-3 | 45.89 ± 3.08 a | 18.87 ± 0.55 b | 14.84 ± 12.50 b | 30.86 ± 2.78 ab | |
| 42 | Heptanal | 111-71-7 | 45.17 ± 48.18 | 39.97 ± 6.05 | 35.71 ± 5.40 | 38.59 ± 0.37 | |
| 43 | 2-Pentenal, (E)- | 1576-87-0 | 40.08 ± 5.37 | - | - | - | |
| 44 | 2-Heptenal, (E)- | 18829-55-5 | 31.82 ± 0.60 a | 18.18 ± 1.47 b | 10.91 ± 8.97 b | 44.21 ± 1.28 a | |
| 45 | 2,2-Dimethyl-4-octenal | 30390-60-4 | 29.05 ± 0.00 | - | - | - | |
| 46 | 2-Hexenal | 505-57-7 | 2807.95 ± 57.59 a | 2138.64 ± 35.97 a | 844.93 ± 43.86 b | 881.93 ± 8.41 b | |
| 47 | 1-Cyclohexene-1-carboxaldehyde, 2,6,6-trimethyl- | 432-25-7 | 25.72 ± 1.54 a | 6.51 ± 0.18 b | 2.37 ± 0.00 c | 1.86 ± 0.10 c | |
| 48 | 2-Octenal, (E)- | 2548-87-0 | 203.26 ± 14.91 a | 56.55 ± 51.25 b | 21.96 ± 8.12 b | 17.34 ± 0.84 b | |
| 49 | 2-Hexenal, (E)- | 6728-26-3 | 16.95 ± 0.00 b | 38.97 ± 0.00 a | 11.15 ± 5.09 b | 10.95 ± 0.00 b | |
| 50 | 2,6-Nonadienal, (E,Z)- | 557-48-2 | 140.95 ± 14.00 a | 17.53 ± 0.22 b | 8.27 ± 1.63 b | 4.07 ± 0.01 b | |
| 51 | 2,4-Heptadienal, (E,E)- | 4313-03-5 | 139.46 ± 16.53 a | 27.96 ± 1.30 b | 4.01 ± 0.50 b | - | |
| 52 | 2-Nonenal, (E)- | 18829-56-6 | 100.98 ± 0.16 a | 30.24 ± 2.17 b | 17.35 ± 3.30 c | 10.73 ± 0.63 d | |
| 53 | acids | Propanoic acid, anhydride | 123-62-6 | 27.71 ± 10.85 | - | - | - |
| 54 | Pentanoic acid, 2-methyl-, anhydride | 63169-61-9 | - | 4.59 ± 0.00 a | 3.60 ± 0.00 b | - | |
| 55 | Acetic acid, hydroxy- | 79-14-1 | 18.42 ± 0.00 a | - | - | 1.86 ± 0.00 b | |
| 56 | Others | 1,3-Hexadiene, 3-ethyl-2-methyl- | 61142-36-7 | 187.92 ± 10.01 a | 98.36 ± 2.56 b | 24.14 ± 17.84 c | - |
| 57 | 7-Oxabicyclo[4.1.0]heptane | 286-20-4 | - | 32.45 ± 0.00 a | - | 11.73 ± 0.16 b | |
| 58 | 3-Methoxyhex-1-ene | 108811-41-2 | - | 20.68 ± 0.00 | - | - | |
| 59 | Cyanamide, dimethyl- | 1467-79-4 | - | 364.72 ± 0.00 | - | - | |
| 60 | Furan, 2-pentyl- | 3777-69-3 | 54.98 ± 7.84 a | 21.25 ± 2.06 b | 9.60 ± 10.43 b | 3.55 ± 0.23 b | |
| 61 | Furan, 2-butyltetrahydro- | 1004-29-1 | 52.40 ± 1.21 a | 25.50 ± 0.00 b | 5.37 ± 0.00 c | 9.20 ± 7.97 c | |
| 62 | trans-2-(2-Pentenyl)furan | 70424-14-5 | 23.25 ± 0.00 | - | - | - | |
| 63 | Furan, 2-ethyl- | 3208-16-0 | 20.09 ± 0.77 a | 5.08 ± 0.12 b | 1.34 ± 0.54 c | 6.23 ± 0.26 b | |
| 64 | 1,1′-Biphenyl, 4-methoxy- | 613-37-6 | 12.76 ± 0.33 a | 4.32 ± 0.36 b | 0.95 ± 0.34 c | 1.04 ± 0.13 c | |
| 65 | Butane, 1-(2-propenyloxy)- | 3739-64-8 | 202.81 ± 9.94 a | 20.46 ± 0.00 b | 3.62 ± 1.98 c | 4.69 ± 0.58 c | |
| 66 | 1H-1,2,4-Triazole | 288-88-0 | 12.43 ± 0.00 | - | - | - | |
| 67 | 1-Methyl-1H-1,2,4-triazole | 6086-21-1 | - | - | 15.67 ± 0.00 a | 0.84 ± 0.00 b | |
| Marking Scheme | Color and Luster | Juicy | Sweet | Sour | Flavor |
|---|---|---|---|---|---|
| 0–4.9 | Light green, indicating lack of luster | Largely absent | Astringent | Sour and astringent | No fruity flavor |
| 5.0–9.9 | Greenish, dull surface | Not much | Slightly sweet | Slightly acidic | Lightly fruity |
| 10–14.9 | Greenish yellow, brightly colored | More | Sweet | suitably sweet and sour | Strong flavor, milk flavor |
| 15–20 | Yellowish white, bright surface | Rich | Very sweet | No acidity | Stronger aroma, sweeter |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Tan, W.; Huang, L.; Tian, J. Physicochemical Properties and Aroma Profiles of Golden Mulberry Fruits at Different Harvesting Stages. Molecules 2025, 30, 2717. https://doi.org/10.3390/molecules30132717
Li K, Tan W, Huang L, Tian J. Physicochemical Properties and Aroma Profiles of Golden Mulberry Fruits at Different Harvesting Stages. Molecules. 2025; 30(13):2717. https://doi.org/10.3390/molecules30132717
Chicago/Turabian StyleLi, Kunfeng, Wen Tan, Lingxia Huang, and Jinhu Tian. 2025. "Physicochemical Properties and Aroma Profiles of Golden Mulberry Fruits at Different Harvesting Stages" Molecules 30, no. 13: 2717. https://doi.org/10.3390/molecules30132717
APA StyleLi, K., Tan, W., Huang, L., & Tian, J. (2025). Physicochemical Properties and Aroma Profiles of Golden Mulberry Fruits at Different Harvesting Stages. Molecules, 30(13), 2717. https://doi.org/10.3390/molecules30132717







