Validated UHPLC Methods for Melatonin Quantification Reveal Regulatory Violations in EU Online Dietary Supplements Commerce
Abstract
1. Introduction
2. Results and Discussion
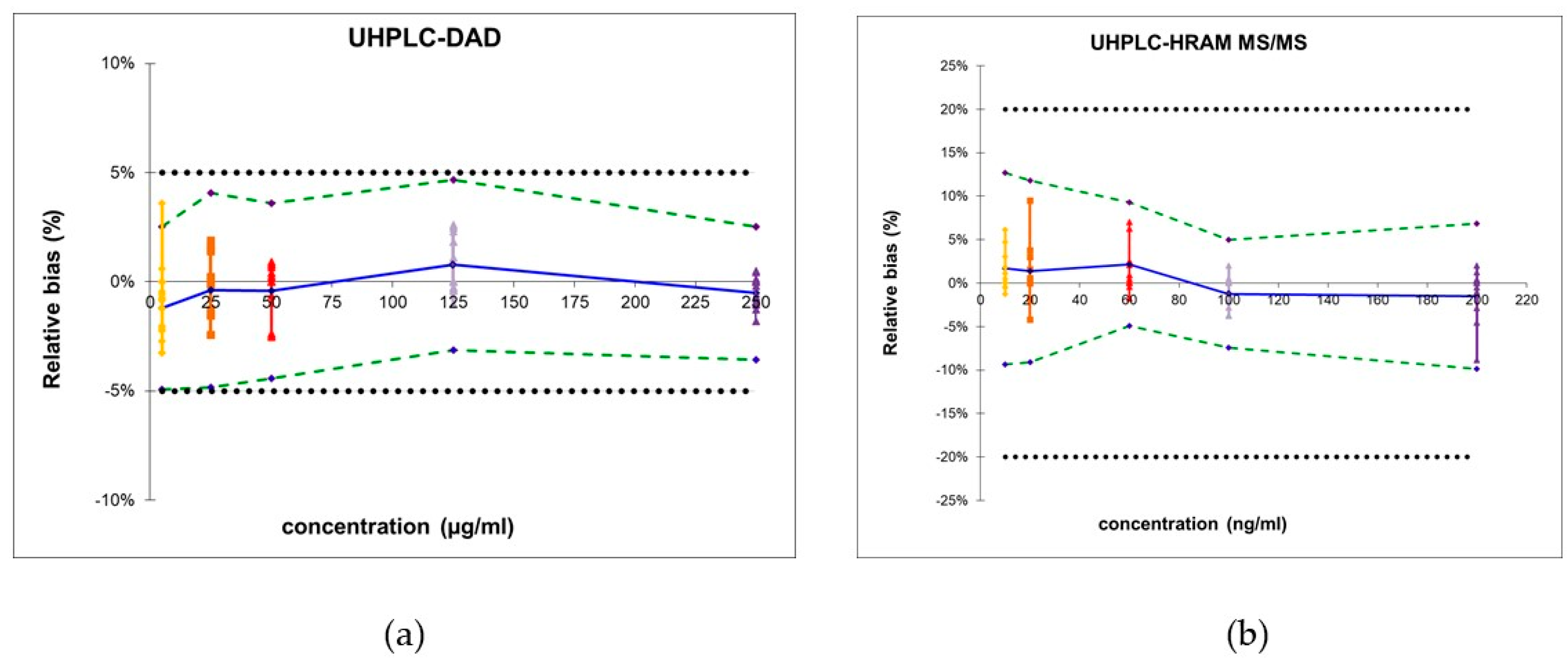
2.1. Development and Validation of the UHPLC-DAD Quantification Method
2.2. Development and Validation of the UHPLC-HRAM MS Quantification Method
2.3. Analysis of Real-Life Samples
2.3.1. Ease of Purchase, Labeling, and Presence of Child-Proof Lid for Gummies
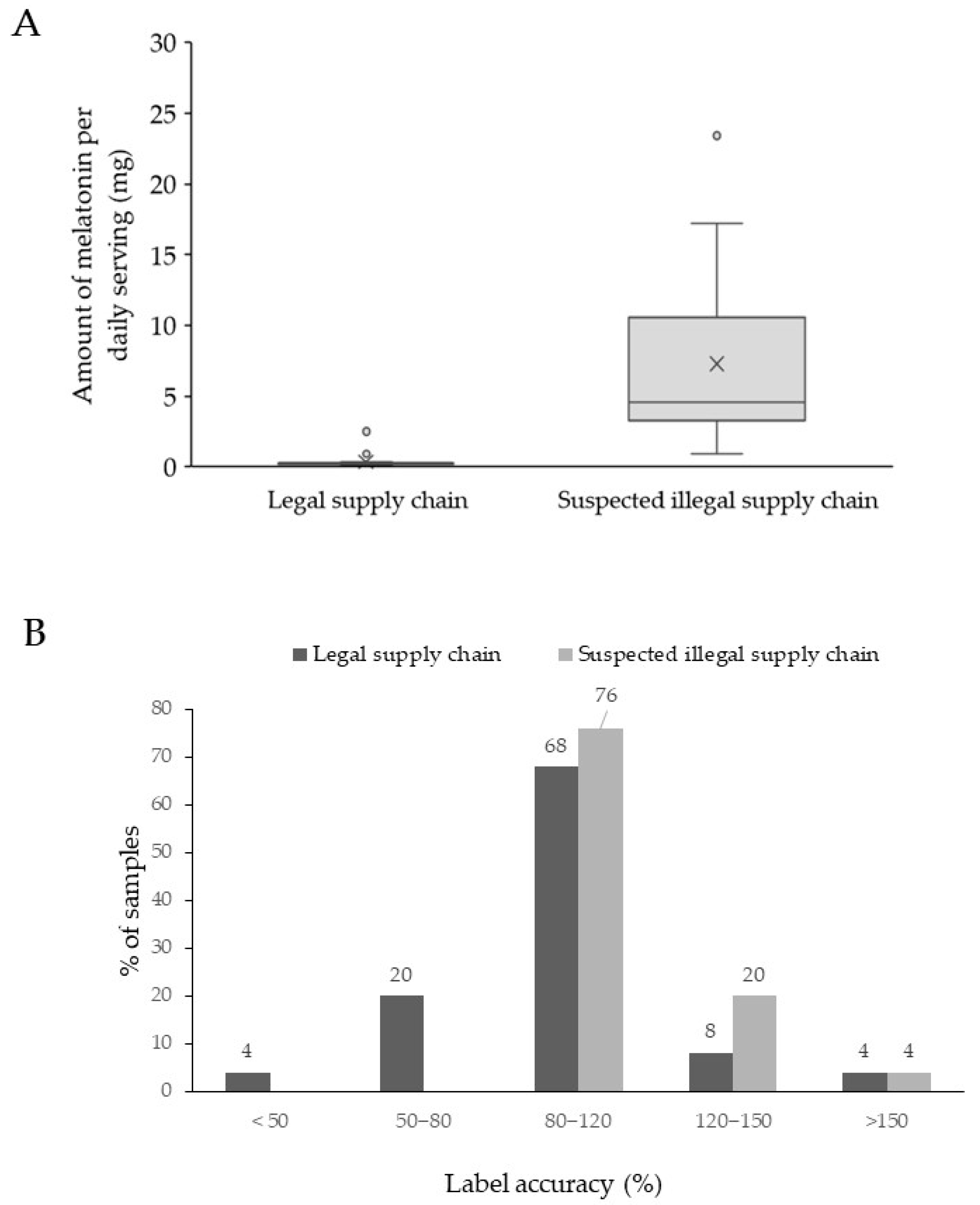
2.3.2. Determination of the Melatonin Content
3. Materials and Methods
3.1. Sample Set
3.2. Quantification of Melatonin
3.2.1. Solvents, Reagents, and Standard Solutions
3.2.2. Instrumental Settings UHPLC-DAD
3.2.3. Instrumental Settings UHPLC-HRAM MS
3.2.4. Validation of the Quantification Methodology
3.3. Sample Preparation and Market Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Somers, V.K.; Xu, H.; Lopez-Jimenez, F.; Covassin, N. Trends in Use of Melatonin Supplements Among US Adults, 1999–2018. JAMA 2022, 327, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Begum, R.; Rashed, A.N. Trends, geographical variation and factors associated with melatonin prescribing in general practices in England: A practice-level analysis. Br. J. Clin. Pharmacol. 2022, 88, 2430–2436. [Google Scholar] [CrossRef]
- Cohen, P.A.; Avula, B.; Wang, Y.H.; Katragunta, K.; Khan, I. Quantity of Melatonin and CBD in Melatonin Gummies Sold in the US. JAMA 2023, 329, 1401–1402. [Google Scholar] [CrossRef] [PubMed]
- Kimland, E.E.; Dahlén, E.; Martikainen, J.; Célind, J.; Kindblom, J.M. Melatonin Prescription in Children and Adolescents in Relation to Body Weight and Age. Pharmaceuticals 2023, 16, 396. [Google Scholar] [CrossRef]
- Bliddal, M.; Kildegaard, H.; Rasmussen, L.; Ernst, M.; Jennum, P.J.; Mogensen, S.H.; Pottegård, A.; Wesselhoeft, R. Melatonin use among children, adolescents, and young adults: A Danish nationwide drug utilization study. Eur. Child Adolesc. Psychiatry 2023, 32, 2021–2029. [Google Scholar] [CrossRef]
- Owens, J. Melatonin use in the pediatric population: An evolving global concern. World J. Pediatr. 2025. [Google Scholar] [CrossRef] [PubMed]
- Valtuille, Z.; Acquaviva, E.; Trebossen, V.; Ouldali, N.; Bourmaud, A.; Sclison, S.; Gomez, A.; Revet, A.; Peyre, H.; Delorme, R.; et al. Prescription Trends of Medications Used to Treat Sleep Disturbances in School-Aged Children: An Interrupted Time-Series Analysis in France, 2016–2023. J. Pediatr. 2025, 280, 114502. [Google Scholar] [CrossRef]
- Andronachi, V.-C.; Simeanu, C.; Matei, M.; Radu-Rusu, R.-M.; Simeanu, D. Melatonin: An Overview on the Synthesis Processes and on Its Multiple Bioactive Roles Played in Animals and Humans. Agriculture 2025, 15, 273. [Google Scholar] [CrossRef]
- Dollins, A.B.; Zhdanova, I.V.; Wurtman, R.J.; Lynch, H.J.; Deng, M.H. Effect of inducing nocturnal serum melatonin concentrations in daytime on sleep, mood, body temperature, and performance. Proc. Natl. Acad. Sci. USA 1994, 91, 1824–1828. [Google Scholar] [CrossRef]
- Zhdanova, I.V.; Wurtman, R.J.; Regan, M.M.; Taylor, J.A.; Shi, J.P.; Leclair, O.U. Melatonin treatment for age-related insomnia. J. Clin. Endocrinol. Metab. 2001, 86, 4727–4730. [Google Scholar] [CrossRef]
- Costello, R.B.; Lentino, C.V.; Boyd, C.C.; O’Connell, M.L.; Crawford, C.C.; Sprengel, M.L.; Deuster, P.A. The effectiveness of melatonin for promoting healthy sleep: A rapid evidence assessment of the literature. Nutr. J. 2014, 13, 106. [Google Scholar] [CrossRef]
- Ferracioli-Oda, E.; Qawasmi, A.; Bloch, M.H. Meta-analysis: Melatonin for the treatment of primary sleep disorders. PLoS ONE 2013, 8, e63773. [Google Scholar] [CrossRef] [PubMed]
- Arendt, J.; Aulinas, A. Physiology of the Pineal Gland and Melatonin. In Endotext; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK550972/ (accessed on 15 May 2025).
- Higueras, T.G.; Cortés, F.C.; Muñoz, A.T.; Forés, S.V.; Alonso, S.S.D.L.C. Attempted suicide by Melatonin overdose: Case report and literature review. Eur. Psychiatry 2022, 65 (Suppl. 1), S836–S837. [Google Scholar] [CrossRef]
- Lelak, K.; Vohra, V.; Neuman, M.I.; Toce, M.S.; Sethuraman, U. Pediatric Melatonin Ingestions—United States, 2012–2021. Mmwr-Morbidity Mortal. Wkly. Rep. 2022, 71, 725–729. [Google Scholar] [CrossRef]
- Freeman, D.I.; Lind, J.N.; Weidle, N.J.; Geller, A.I.; Stone, N.D.; Lovegrove, M.C. Notes from the Field: Emergency Department Visits for Unsupervised Pediatric Melatonin Ingestion—United States, 2019–2022. Mmwr-Morbidity Mortal. Wkly. Rep. 2024, 73, 215–217. [Google Scholar] [CrossRef]
- Yeung, K.W.C.M.; Lee, S.K.M.; Bin, Y.S.; Cheung, J.M.Y. Pharmacists’ perspectives and attitudes towards the 2021 down-scheduling of melatonin in Australia using the Theoretical Domains Framework: A mixed-methods study. Int. J. Clin. Pharm. 2023, 45, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- The National Health Service (NHS). Common Questions about Melatonin. Available online: https://www.nhs.uk/medicines/melatonin/common-questions-about-melatonin (accessed on 15 May 2025).
- Swissmedic Warning About Imports of Melatonin and DHEA by Private Individuals. Available online: https://www.swissmedic.ch/swissmedic/en/medicrime/news/warnings/warnung-importen-melatonin-und-dhea.html (accessed on 15 May 2025).
- Skrzelowski, M.; Brookhaus, A.; Shea, L.A.; Berlau, D.J. Melatonin Use in Pediatrics: Evaluating the Discrepancy in Evidence Based on Country and Regulations Regarding Production. J. Pediatr. Pharmacol. Ther. 2021, 26, 4–20. [Google Scholar] [CrossRef]
- French Agency for Food, Environmental and Occupational Health & Safety (ANSES). ANSES Opinion Request No 2016-SA-0209 on the Risks Associated with the Consumption of Food Supplements Containing Melatonin. Available online: https://www.anses.fr/en/system/files/NUT2016SA0209EN.pdf (accessed on 15 May 2025).
- Vanhee, C.; Deconinck, E.; George, M.; Hansen, A.; Hackl, A.; Wollein, U.; El-Atma, O.; Beerbaum, N.; Aureli, F.; Borioni, A.; et al. The Occurrence of Illicit Smart Drugs or Nootropics in Europe and Australia and Their Associated Dangers: Results from a Market Surveillance Study by 12 Official Medicines Control Laboratories. J. Xenobiot. 2025, 15, 88. [Google Scholar] [CrossRef]
- Cerezo, A.B.; Leal, A.; Álvarez-Fernández, M.A.; Hornedo-Ortega, R.; Troncoso, A.M.; García-Parrilla, C.M. Quality control and determination of melatonin in food supplements. J. Food Compos. Anal. 2016, 45, 80–86. [Google Scholar] [CrossRef]
- Erland, L.A.; Saxena, P.K. Melatonin Natural Health Products and Supplements: Presence of Serotonin and Significant Variability of Melatonin Content. J. Clin. Sleep Med. 2017, 13, 275–281. [Google Scholar] [CrossRef]
- Astray, S.B.; Barbosa-Pereira, L.; Lage-Yusty, M.A.; López-Hernández, J. Comparison of Analytical Methods for the Rapid Determination of Melatonin in Food Supplements. Food Anal. Methods 2021, 14, 734–741. [Google Scholar] [CrossRef]
- Moser, D.; Hussain, S.; Rainer, M.; Jakschitza, T.; Bonn, G.K. A validated method for the rapid quantification of melatonin in over-the-counter hypnotics by the atmospheric pressure solid analysis probe (ASAP). Anal. Methods 2022, 14, 1603. [Google Scholar] [CrossRef]
- Olea, A.M.C.; de Algorta, J.; Arriaga-Ibañez, I.; Villate-Beitia, I.; Gallego, I.; Defterali, Ç.; Pedraz, J.-L. Comparative Quality Analysis of Three Marketed Melatonin Containing Products in Spain for the Improvement of Sleep. J. Pharm. Res. Int. 2023, 35, 17–28. [Google Scholar] [CrossRef]
- Peikova, L.; Tzankova, D.; Stancheva-Zlatkova, M.; Zlatkov, A. Development of RP-HPLC methods for the analysis of melatonin alone and in combination with sleep-enhancing dietary supplements. Pharmacia 2024, 71, 1–7. [Google Scholar] [CrossRef]
- Pawar, R.S.; Coppin, J.P.; Khanna, S.; Parker, C.H. A Survey of Melatonin in Dietary Supplement Products Sold in the United States. Drug Test. Anal. 2024. [Google Scholar] [CrossRef]
- Feinberg, M. Validation of analytical methods based on accuracy profiles. J. Chromatogr. A 2007, 1158, 174–183. [Google Scholar] [CrossRef]
- Gomez-Gomez, A.; Montero-San-Martin, B.; Haro, N.; Pozo, O.J. Nail Melatonin Content: A Suitable Non-Invasive Marker of Melatonin Production. Int. J. Mol. Sci. 2021, 22, 921. [Google Scholar] [CrossRef]
- De Luca, M.; Tauler, R.; Ioele, G.; Ragno, G. Study of photodegradation kinetics of melatonin by multivariate curve resolution (MCR) with estimation of feasible band boundaries. Drug Test. Anal. 2013, 5, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Moussaoui, N.E.; Bendriss, A. The Influence of Storage Conditions on Melatonin Stability. Int. J. Eng. Res. Technol. 2014, 3, 2243–2246. [Google Scholar] [CrossRef]
- Daya, S.; Walker, R.B.; Glass, B.D.; Anoopkumar-Dukie, S. The effect of variations in pH and temperature on stability of melatonin in aqueous solution. J. Pineal Res. 2001, 31, 155–158. [Google Scholar] [CrossRef]
- European Commission. Regulation (EU) No. 1169/2011 on Consumer Information. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32011R1169 (accessed on 15 May 2025).
- European Commission. Directive 2011/91/EU of the European Parliament and of the Council of 13 December 2011 on Indications or Marks Identifying the Lot to Which a Foodstuff Belongs (Codification) Text with EEA Relevance. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32011L0091 (accessed on 15 May 2025).
- EFSA Publication. EFSA Panel on Dietetic Products Nutrition Allergies (NDA) Scientific Opinion on the substantiation of a health claim related to melatonin reduction of sleep onset latency (ID 1698, 1780, 4080) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2241–2257. [Google Scholar] [CrossRef]
- The Council for Responsible Nutrition. CRN Adopts New Guidelines for Melatonin Supplements to Promote Responsible Usage. Available online: https://www.crnusa.org/newsroom/crn-adopts-new-guidelines-melatonin-supplements-promote-responsible-usage (accessed on 15 May 2025).
- Vanhee, C.; Tuenter, E.; Kamugisha, A.; Canfyn, M.; Moens, G.; Courselle, P.; Pieters, L.; Deconinck, E.; Exarchou, V. Identification and Quantification Methodology for the Analysis of Suspected Illegal Dietary Supplements: Reference Standard or no Reference Standard, that’s the Question. J. Forensic. Toxicol. Pharmacol. 2018, 7, 2. [Google Scholar] [CrossRef]
- Vanhee, C.; Barhdadi, S.; Kamugisha, A.; Van Mulders, T.; Vanbrusselen, K.; Willocx, M.; Deconinck, E. The Development and Validation of a Targeted LC-HRAM-MS/MS Methodology to Separate and Quantify p-Synephrine and m-Synephrine in Dietary Supplements and Herbal Preparations. Separations 2023, 10, 444. [Google Scholar] [CrossRef]
- Cohen, P.A.; Jacobs, B.; Van Hoorde, K.; Vanhee, C. Accuracy of Labeling of Galantamine Generic Drugs and Dietary Supplements. JAMA 2024, 331, 974–976. [Google Scholar] [CrossRef]
- European Commission, GUIDANCE DOCUMENT FOR COMPETENT AUTHORITIES FOR THE CONTROL OF COMPLIANCE WITH EU LEGISLATION ON: Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers, Amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and Repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004 and Council Directive 90/496/EEC of 24 September 1990 on Nutrition Labelling of Foodstuffs and Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the Approximation of the Laws of the Member States Relating to Food Supplements with Regard to the Setting of Tolerances for Nutrient Values Declared on a Label. Available online: https://food.ec.europa.eu/system/files/2016-10/labelling_nutrition-vitamins_minerals-guidance_tolerances_1212_en.pdf (accessed on 15 May 2025).
- International Conference on Harmonisation (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use—Validation of Analytical Procedures. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-q2r2-validation-analytical-procedures-step-2b_en.pdf (accessed on 20 May 2025).
- ISO/IEC 17025; General Requirements for the Competence of Testing and Calibration Laboratories. International Organization for Standardization: Geneva, Switzerland, 2017.
- Mandel, J. The Statistical Analysis of Experimental Data; Interscience Publ. J. Wiley & Sons: New York, NY, USA, 1964. [Google Scholar]
| USA | Australia, the UK and Switzerland | Czech Republic, Slovenia and Denmark | Germany | Sweden | Spain and Italy | France | Portugal | Belgium and The Netherlands | Poland |
|---|---|---|---|---|---|---|---|---|---|
| dietary supplement | Prescription-only, not allowed in dietary supplements | Prolonged-release and immediate-release melatonin are prescription-only medicines. Melatonin is not allowed in dietary supplements | Prolonged-release and immediate-release melatonin are prescription-only medicines. Melatonin is allowed in dietary supplements but lacks clear legislation. | Prolonged-release melatonin is a prescription-only medicine. Immediate-release melatonin medicine is available as over-the-counter (OTC) medicine. Melatonin is not allowed in dietary supplements. | Prolonged-release melatonin is a prescription-only medicine. Immediate-release melatonin medicine is available as over-the-counter (OTC) medicine. Melatonin is allowed in dietary supplements up to a daily dose of 1 mg. | Prolonged-release melatonin is a prescription-only medicine. Melatonin is allowed in dietary supplements provided the daily dose does not exceed 2 mg. | Prolonged-release melatonin is a prescription-only medicine. Immediate-release melatonin medicine is available as over-the-counter (OTC) medicine. Melatonin is allowed in dietary supplements provided the daily dose does not exceed 2 mg. | Prolonged-release melatonin is a prescription-only medicine. Immediate-release melatonin medicine is available as over-the-counter (OTC) medicine. Melatonin is allowed in dietary supplements provided that the daily dose does not exceed 0.3 mg. | Over-the-counter (OTC) medicine, no prescription required Melatonin is allowed in dietary supplements up to a daily dose of 1 mg |
| Method | Molecule | RT (min) | Precursor Ion (m/z) | Fragment Ions (m/z) and Their Relative Intensities | SDL | LOQ | Linear Range |
|---|---|---|---|---|---|---|---|
| LC-DAD | Melatonin | 2.5 | n.a. | n.a. | 1 µg/mL (20 µg/g) | 5 µg/mL (100 µg/g) | 5–250 µg/mL |
| LC-MS | Melatonin | 2.7 | 233.128 [M + H]+ | 159.068 (100%) 174.091 (80%) 143.073 (30%) 131.073 (30%) | 0.5 ng/mL (10 ng/g) | 10 ng/mL (200 ng/g) | 10–200 ng/mL |
| Melatonin-D3 | 2.7 | 236.147 [M + H]+ |
| UHPLC-DAD | UHPLC-HRAM MS | ||||
|---|---|---|---|---|---|
| Linearity expressed as R2 | / | 0.99995 | / | 0.99999 | |
| Trueness | Relative bias (%) | 5 µg/mL | 1.3 | 10 ng/mL | 1.6 |
| 25 µg/mL | 3.5 | 20 ng/mL | 0.9 | ||
| 50 µg/mL | 2.9 | 60 ng/mL | 1.0 | ||
| 125 µg/mL | 1.7 | 100 ng/mL | 0.8 | ||
| 250 µg/mL | 3.2 | 200 ng/mL | 0.4 | ||
| Precision | Intermediate precision (%) | 5 µg/mL | 1.7 | 10 ng/mL | −1.2 |
| 25 µg/mL | 1.4 | 20 ng/mL | −0.4 | ||
| 50 µg/mL | 2.2 | 60 ng/mL | −0.4 | ||
| 125 µg/mL | −1.2 | 100 ng/mL | 0.8 | ||
| 250 µg/mL | −1.5 | 200 ng/mL | −0.5 | ||
| Repeatability (%) | 5 µg/mL | 2.8 | 10 ng/mL | 1.7 | |
| 25 µg/mL | 4.0 | 20 ng/mL | 1.6 | ||
| 50 µg/mL | 2.9 | 60 ng/mL | 1.5 | ||
| 125 µg/mL | 2.2 | 100 ng/mL | 1.4 | ||
| 250 µg/mL | 3.4 | 200 ng/mL | 0.8 | ||
| Accuracy | ß-expectation tolerance limits (%) | 5 µg/mL | [−4.9; 2.5] | 10 ng/mL | [−9.4; 12.7] |
| 25 µg/mL | [−4.8; 4.1] | 20 ng/mL | [−9.1: 11.8] | ||
| 50 µg/mL | [−4.4; 3.6] | 60 ng/mL | [−4.9: 9.3] | ||
| 125 µg/mL | [−3.1; 4.7] | 100 ng/mL | [−7.5: 5.0] | ||
| 250 µg/mL | [−3.6;−2.5] | 200 ng/mL | [−9.9: 6.9] | ||
| Uncertainty | Relative expanded uncertainty (%) | 5 µg/mL | 3.5 | 10 ng/mL | 6.4 |
| 25 µg/mL | 3.5 | 20 ng/mL | 8.6 | ||
| 50 µg/mL | 3.4 | 60 ng/mL | 6.1 | ||
| 125 µg/mL | 3.1 | 100 ng/mL | 4.8 | ||
| 250 µg/mL | 1.9 | 200 ng/mL | 7.2 | ||
| n° | Labeled Health Claim | Lot n° and Expiration Date Present | Galenic Form | Amount per Serving Claimed, mg | Amount per Serving Found, mg (% MU) a | % Label Accuracy |
|---|---|---|---|---|---|---|
| Registered online pharmacies and brick-and-mortar drug stores | ||||||
| L1 | No health claim | Yes | Capsules | 0.295 | 0.22 (0.5) | 75 |
| L2 | Sleep support and general relaxant b | Yes | Capsules | 0.29 | 0.31 (2.0) | 105 |
| L3 | Sleep support b | Yes | Tablets | 0.290 | 0.26 (4.2) | 89 |
| L4 | Sleep support b | Yes | Tablets | 0.1 | 0.12 (1.8) | 117 |
| L5 | No health claim | Yes | Tablets | 0.290 | 0.22 (2.2) | 76 |
| L6 | Sleep aid | Yes | Tablets | 0.295 | 0.24 (2.1) | 83 |
| L7 | No health claim labeled | Yes | Tablets | 0.1 | 0.18 (5.2) | 182 |
| L8 | Sleep support b | Yes | Tablets | 0.295 | 0.11 (1.4) | 40 |
| L9 | Sleep support b | Yes | Capsules | 0.298 | 0.24 (0.8) | 81 |
| L10 | Sleep support b | Yes | Capsules | 0.295 | 0.22 (1.5) | 76 |
| L11 | No health claim | Yes | Tablets | 0.145 | 0.14 (2.3) | 97 |
| L12 | Sleep support b | Yes | Tablets | 0.295 | 0.35 (1.2) | 119 |
| L13 | Sleep support and relaxant b | Yes | duocapsule | 0.295 | 0.25 (0.8) | 85 |
| L14 | Sleep support b | Yes | Tablets | 0.29 | 0.31 (3.0) | 107 |
| L15 | No health claim | Yes | Tablets | 0.29 | 0.25 (1.6) | 85 |
| L16 | No health claim | Yes | Tablets | 0.299 | 0.32 (0.3) | 107 |
| L17 | Sleep support b | Yes | Tablets | 0.29 | 0.21 (0.4) | 71 |
| L18 | Sleep support | Yes | Tablets | 1 | 0.90 (0.5) | 91 |
| L19 | Sleep support b | Yes | Oral spray | 0.19 | 0.18 (1.2) | 95 |
| L20 | Sleep support b | Yes | Oral spray | 0.283 | 0.3 (0.5) | 102 |
| L21 | Sleep support b | Yes | Gummies c | 0.290 | 0.295 (2.7) | 102 |
| L22 | Sleep support b | Yes | Gummies c | 0.295 | 0.24 (3.0) | 81 |
| L23 | Sleep support and relaxant b | Yes | Gummies c | 0.295 | 0.38 (1.8) | 129 |
| L24 | Sleep support b | Yes | Soft gel | 0.299 | 0.19 (3.0) | 74 |
| L25 | Sleep support | Yes | Tablet | 3 | 2.5 (1.9) | 83 |
| Rogue online pharmacies and e-commerce sites selling products with a daily melatonin dose > 0.3 mg | ||||||
| I1 | Healthy sleep cycle | Yes | Capsules | 10 | 10.71 (0.2) | 107 |
| I2 | Healthy sleep cycle | Yes | Capsules | 5 | 4.52 (0.2) | 90 |
| I3 | Sleep support | No d | Gummies | 5 | 5.32 (0.1) | 106 |
| I4 | Promotes restful sleep | Yes | Capsules | 1 | 0.93 (0.7) | 93 |
| I5 | Sleep support | Yes | Capsules | 5 | 4.18 (0.2) | 84 |
| I6 | Sleep support | Yes | Capsules | 3 | 3.20 (0.4) | 107 |
| I7 | Sleep support | Yes | Tablets | 3 | 2.91 (0.6) | 97 |
| I8 | Sleep support | Yes | Capsules | 3 | 3.76 (0.5) | 125 |
| I9 | Sleep support | Yes | Capsules | 10 | 10.35 (0.6) | 104 |
| I10 | Promotes a healthy sleep/wake cycle and may reduce the effects of jet lag | Yes | Capsules | 3 | 3.01 (0.4) | 100 |
| I11 | Sleep promotion | Yes | Tablets | 3 | 4.09 (0.4) | 136 |
| I12 | Supports healthy sleep | Yes | Oral drops | 3 | 3.32 (0.2) | 111 |
| I13 | Supports healthy sleep | Yes | Capsules | 1 | 1.43 (2.5) | 143 |
| I14 | Supports healthy sleep | Yes | Capsules | 2 | 2.71 (0.1) | 136 |
| I15 | Supports healthy sleep | Yes | Tablets | 5 | 4.98 (0.1) | 100 |
| I16 | Supports healthy sleep | Yes | Tablets | 10 | 9.72 (0.3) | 97 |
| I17 | Promotes a healthy sleep cycle | Yes | Capsules | 3 | 3.88 (0.1) | 129 |
| I18 | Sleep support | Yes | Capsules | 10 | 17.2 (0.6) | 172 |
| I19 | Sleep support | Yes | Gummies c | 5 | 4.58 (0.6) | 92 |
| I20 | Restful sleep | Yes | Capsules | 10 | 11.54 (0.1) | 115 |
| I21 | Nighttime sleep aid | Yes | Tablets | 12 | 14.36 (0.1) | 120 |
| I22 | Sleep aid | Yes | Soft gels | 10 | 10.31 (0.3) | 103 |
| I23 | Promotes a healthy sleep cycle | Yes | Capsules | 20 | 23.43 (0.2) | 117 |
| I24 | Nighttime sleep aid | Yes | Capsules | 10 | 13.81 (0.5) | 138 |
| I25 | Restful sleep | Yes | Oral patch | 10 | 8.16 (1.7) | 82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanhee, C.; Degrève, C.; Boschmans, N.; Naïmi, Y.; Canfyn, M.; Deconinck, E.; Willocx, M. Validated UHPLC Methods for Melatonin Quantification Reveal Regulatory Violations in EU Online Dietary Supplements Commerce. Molecules 2025, 30, 2647. https://doi.org/10.3390/molecules30122647
Vanhee C, Degrève C, Boschmans N, Naïmi Y, Canfyn M, Deconinck E, Willocx M. Validated UHPLC Methods for Melatonin Quantification Reveal Regulatory Violations in EU Online Dietary Supplements Commerce. Molecules. 2025; 30(12):2647. https://doi.org/10.3390/molecules30122647
Chicago/Turabian StyleVanhee, Celine, Cloë Degrève, Niels Boschmans, Yasmina Naïmi, Michael Canfyn, Eric Deconinck, and Marie Willocx. 2025. "Validated UHPLC Methods for Melatonin Quantification Reveal Regulatory Violations in EU Online Dietary Supplements Commerce" Molecules 30, no. 12: 2647. https://doi.org/10.3390/molecules30122647
APA StyleVanhee, C., Degrève, C., Boschmans, N., Naïmi, Y., Canfyn, M., Deconinck, E., & Willocx, M. (2025). Validated UHPLC Methods for Melatonin Quantification Reveal Regulatory Violations in EU Online Dietary Supplements Commerce. Molecules, 30(12), 2647. https://doi.org/10.3390/molecules30122647







