Abstract
Cationic surfactants, accounting for approximately 7% of the global surfactant market, are widely used in applications such as fabric softeners, biocides, and corrosion inhibitors. Recently, gemini surfactants—comprising two amphiphilic units linked by a spacer—have attracted significant interest due to their superior surface activity, lower critical micelle concentrations, and strong antimicrobial properties. However, their poor biodegradability, resulting from their complex molecular structure, has raised environmental concerns. To address this, researchers have developed ester-based gemini surfactants incorporating biodegradable bonds. This study aimed to investigate the relationship between the structure of ester-based gemini surfactants (hydrophobic chain length and spacer type) and their antimicrobial activity against bacteria and fungi. Three series of compounds featuring different functional groups in the spacer were synthesized, along with a trimeric surfactant for comparative purposes. The results demonstrated that both the hydrophobic chain length and the presence of additional cationic groups significantly influence the CMC and antimicrobial performance. Quantum mechanical calculations were also performed to search for correlations between electronic properties and chemical reactivity of compounds. These findings highlight that ester-based gemini surfactants combine high surface and antimicrobial activity with the potential for improved biodegradability, making them promising candidates for use in environmentally friendly applications.
1. Introduction
Cationic surfactants, which constitute nearly 7% of the global surfactant market, are utilized in a wide range of applications, including fabric softeners, asphalt modifiers, corrosion inhibitors, biocides, and textile processing agents [1]. In recent years, gemini surfactants—a new class of cationic surface-active agents—have garnered significant interest. These compounds are generally composed of two amphiphilic units linked by a spacer molecule [2,3,4,5]. There is a growing body of research focused on their synthesis and the exploration of their remarkable and often unconventional properties. Compared to traditional monomeric surfactants, gemini surfactants are much more efficient at reducing interfacial tension and can form micelles at notably low critical micelle concentrations (CMC) [6,7,8,9]. Additionally, they offer enhanced wetting characteristics and exhibit unique rheological and aggregation behavior. Owing to these advantageous features, gemini surfactants are being investigated for a wide range of potential applications, including enhanced oil recovery [10,11,12], gene delivery systems [13,14,15], corrosion prevention in iron-based materials [16,17,18], and environmental remediation [19,20].
Moreover, gemini surfactants exhibit strong antimicrobial activity due to their unique molecular structure, which enhances their ability to disrupt microbial cell membranes. The presence of two cationic head groups and hydrophobic tails enables more effective interaction with negatively charged bacterial surfaces, leading to increased membrane permeability and cell lysis. These compounds have shown broad-spectrum activity against both Gram-positive and Gram-negative bacteria, as well as fungi [16,21,22,23,24]. Their high surface activity and low CMC contribute to their efficiency at low dosages, making them promising candidates for use in disinfectants, personal care products, and biomedical applications [15].
Gemini surfactants are generally considered to be poorly biodegradable, largely due to their complex molecular structure and the presence of multiple hydrophobic and cationic groups. This resistance to microbial degradation poses environmental concerns, especially for applications involving large-scale or long-term use [25,26]. To address this issue, researchers are developing modified gemini surfactants incorporating biodegradable linkers or more environmentally friendly components to enhance their breakdown in natural environments. Nonetheless, environmental considerations are becoming increasingly important in the development of new surfactant systems. With stricter environmental policies and growing ecological awareness, the demand for eco-friendly alternatives to conventional surfactants is on the rise. One promising approach to improving biodegradability is the incorporation of labile bonds, such as ester linkages, into surfactant molecules [27,28,29]. Ester-based gemini surfactants can be designed with various structural configurations to tailor their properties and biodegradability. Ester bonds may be introduced into the hydrophobic tails [26,30], the spacer linking the two head groups, or as part of side chains [31,32,33] (Figure 1). Compounds containing an ester group in both the linker and the substituents are also described in the literature [34], but they are much more difficult to obtain and, for now, have no major practical significance. Each arrangement influences the surfactant’s stability, hydrolysis rate, and surface activity.
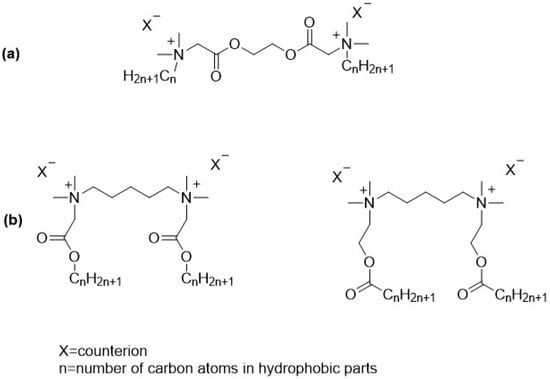
Figure 1.
Example structures of ester-based gemini surfactants: (a) ester bonds in spacer; (b) ester bonds in hydrophobic parts of the molecules (first one—betainate, second—esterquat gemini).
The aim of the present work was to investigate the relationship between the structure of the ester-based gemini surfactants (hydrophobic chain length) and antimicrobial activity against bacteria and fungi. Another criterion differentiating the classes of tested gemini surfactants is the structure of the linker. In this article, we present the antimicrobial activity of compounds containing a functionalized spacer with an amine or ether group. Additionally, for comparative purposes, a compound containing three ammonium groups was obtained and analyzed.
2. Results
2.1. Synthesis
We obtained three series of ester-based (betainate type) gemini surfactants: PMTH2 compounds possess an additional tertiary amino group in the linker, TMTH compounds have additional secondary amino group in the linker and N2OE compounds possess additional ether group in the spacer. Additionally, for comparative purposes, a trimeric compound was obtained, designated as PMTH3. All gemini surfactants were obtained in our laboratory by quaternization of the appropriate tertiary diamines with the appropriate acyl chlorides (Figure 2).
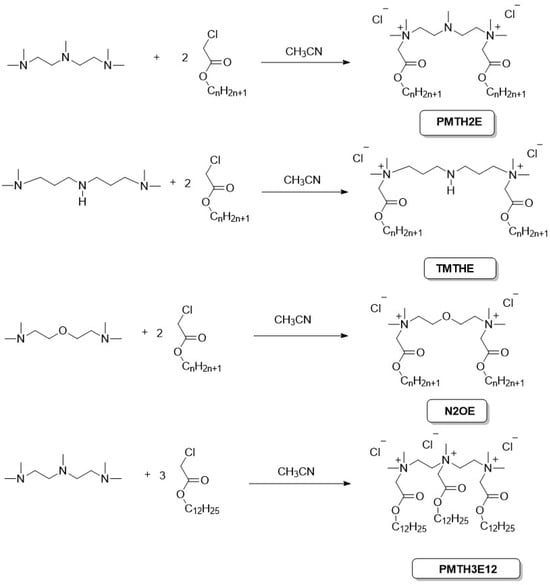
Figure 2.
Synthesis of obtained surfactants.
All compounds were obtained with a purity above 90%. The synthetic details and spectroscopic data were described earlier: PMTH2 and TMTH [27,35], N2OE [36]. The formulas and abbreviations of compounds analyzed in this article are presented in Figure 3.
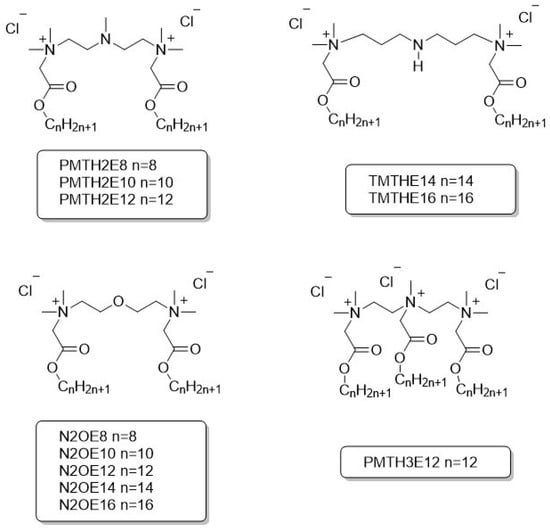
Figure 3.
Structures and abbreviations of ester-based gemini and trimeric surfactants.
2.2. Surface Properties of Gemini Surfactants
Gemini surfactants exhibit significantly higher surface activity than their monomeric counterparts. Due to their dual hydrophilic head groups and hydrophobic tails connected by a spacer, they adsorb more efficiently at interfaces, reduce surface and interfacial tension more effectively, and form micelles at much lower CMCs [2,37,38]. Ester-based gemini surfactants are an attractive alternative to classic gemini surfactants because they have lower CMC values [39]. In the case of gemini ester derivatives, one can also observe their significant advantage in surface properties in relation to monomeric surfactants with the same length of hydrophobic substituent [40,41].
The CMC values for all synthesized surfactants were obtained by conductometric titration. The exemplary relationship of the specific conductivity (k) on the concentration is shown in Figure 4.
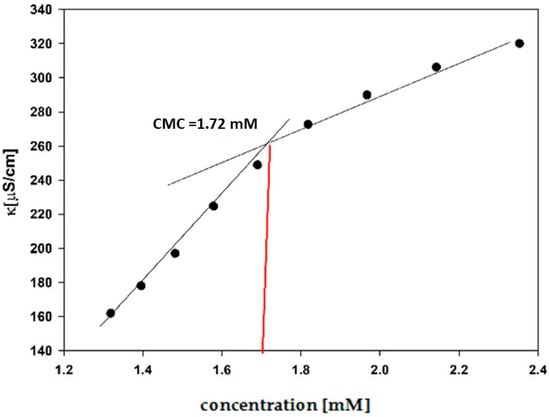
Figure 4.
A plot of specific conductivity (k) for the aqueous solution of N2OE10 versus its concentration.
The values of CMC, degree of counterion binding (β), and standard Gibbs energy of micellization (ΔG°mic) for all of obtained surfactants are listed in Table 1. The CMC values of ester-based gemini surfactants, similar to other gemini surfactants, decrease with the increase in the number of methylene groups in the hydrocarbon chain and the increase in the number of positively charged nitrogen atoms [42].

Table 1.
Aggregation parameters for ester-based gemini and trimeric surfactants.
The aggregation parameters in Table 1 illustrate clear trends in the self-assembly behavior of ester-based gemini and trimeric surfactants. For the PMTH2En series, a gradual decrease in the CMC is observed with increasing hydrophobic chain length: from 2.1 mM (PMTH2E8) to 1.6 mM (PMTH2E12). This indicates enhanced micelle formation as the hydrophobic chain becomes longer. The counterion binding parameter (β) increases from 0.20 to 0.53, suggesting that longer chains promote stronger counterion association. The ΔG°mic becomes more negative, shifting from −23.09 to −34.56 kJ/mol, confirming that micellization is thermodynamically more favorable for surfactants with longer hydrophobic chains. The trimeric surfactant PMTH3E12 shows a remarkable reduction in CMC (0.013 mM), highlighting the significant impact of an additional cationic head group. Its ΔG°mic is also more negative (−51.86 kJ/mol), indicating highly spontaneous micelle formation. The TMTHE series follows the expected pattern: CMC decreases from 0.08 mM (TMTHE14) to 0.03 mM (TMTHE16), while ΔG°mic becomes more negative, demonstrating stronger micellization with longer chains. For the N2OEn series, the initial CMC is relatively high at 8.50 mM (N2OE8), but decreases significantly to 0.05 mM (N2OE16). The β values are higher across this series (up to 0.63), suggesting strong counterion binding. The ΔG°mic values also become more negative as the chain length increases from −34.77 to −57.14 kJ/mol. The results confirm that both increasing hydrophobic chain length and introducing additional cationic head groups improve micellization efficiency, reduce CMC, and enhance thermodynamic favorability of micelle formation.
2.3. Antimirobial Activity
Gemini surfactants with ester bonds are also known from their antimicrobial activity [43,44,45]. All obtained surfactants were subjected to tests to determine antimicrobial activity. The obtained values of the minimum inhibitory concentration (MIC) are listed in Table 2.

Table 2.
MIC values of ester-bonded gemini and trimeric surfactants.
Taking into account bacteria and microscopic fungi as a common pool of microorganisms, it can be stated that the most effective antimicrobial compounds in the group tested are PMTH2E8 and PMTH2E10. For all tested microorganisms, the MIC value is equal to or lower than 156 mM. The highest MIC values were obtained for compounds containing 14 and 16 methylene groups (TMTHE14, TMTHE16, N2OE14, N2OE16). Gemini surfactants with shorter hydrophobic chains, where the number of -CH2- groups is between 8 and 12, show better biocidal efficacy due to the optimal combination of hydrophobicity, solubility, and flexibility. These features allow for effective adsorption and destabilization of microbial membranes, which translates into lower concentrations inhibiting the growth of microorganisms. The authors have already proven the relationship between the MIC value and the length of the alkyl chain in previous studies [37,46], which was also shown by [47,48,49]. The length of the alkyl chain is only one of the elements influencing antimicrobial efficacy. An additional element, presented for the first time in this study, is the insertion of ester groups into the chain structure, which strongly influence value of minimal concentration, inhibiting growth of microorganisms.
However, it should be remembered that bacteria and microscopic fungi are microorganisms with two completely different cell structures. Without a doubt, such a difference between the prokaryotic cell in bacteria and the eukaryotic cell in fungi influences the different effects of biocides. For this reason, the values of the MIC of individual groups of microorganisms are also different. All compounds containing twelve-carbon chains are characterized by lower MIC values for microscopic fungi compared to bacteria. The sensitivity range of the tested microorganisms for the compounds PMTH2E12, PMTH3E12, and N2OE12 is as follows: Candida albicans < Aspergillus brasiliensis < Staphylococcus aureus < Escherichia coli. The least sensitive to the action of these compounds are therefore Gram-negative bacteria E. coli. In turn, compounds containing 8 and 10 carbon atoms in the aliphatic chains PMTH2E8, PMTH2E10, N2OE8, and N2OE10, as well as 14 and 16 carbon atoms—TMTHE14, TMTHE16—at the lowest concentrations acted on Gram-positive bacteria Staphylococcus aureus and yeast Candida albicans. The least sensitive to these compounds were the molds, Aspergillus brasiliensis. The sensitivity series is slightly different for compounds N2OE14 and N2OE16 (Figure 5), which contain oxygen in the spacer instead of a nitrogen atom. In this case, the most sensitive are Gram-positive bacteria, followed by microscopic fungi, and the least sensitive are Gram-negative bacteria.
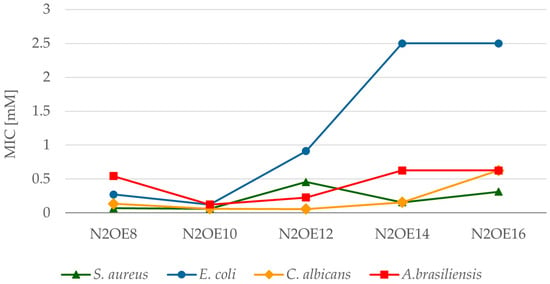
Figure 5.
The relationships between MIC and the number of carbon atoms in the alkyl substituent for N2OEn (n = 8, 10, 12, 14, 16).
In the obtained results, the compound PMTH3E12 deserves attention, as it contains three hydrophobic chains in the molecule. This compound acts at a concentration over 16 times lower on A. brasiliensis molds compared to E. coli bacteria. This is influenced, among other factors, by the structure of external structures—the cell wall and membrane, which are the first place of contact of biocidal compounds with the cell. Gram-positive bacteria such as S. aureus have a thick layer of peptidoglycan in their cell wall, and Gram-negative bacteria such as E. coli—additionally an external lipid membrane. These structures may hinder the penetration of large, strongly hydrophobic molecules, such as three-chain surfactants. Fungi, on the other hand, contain membranes in their cellular structure, which contain more lipids, including sterols with the main compound—ergosterol. Due to such a cellular structure, microscopic fungi are more susceptible to the effects of hydrophobic surfactants. Additionally, the antifungal activity of the PMTH3E12 compound is better compared to the PMTH2E12 analog, containing two hydrophobic chains. Three-chain surfactants have a larger hydrophobic surface, which allows for a stronger effect and better penetration of the lipid cell membrane of fungi. This leads to much greater destabilization of the membrane, disruption of its integrity, lysis or ion efflux [49,50,51,52].
In the presented work, ester bonds -COO- were introduced into the structure of the hydrophilic chain, which are connected to the nitrogen atom by a methylene group -CH2-. Such gemini surfactants with betaine groups have a changed structure of the polar head, which affects their ability to aggregate and interact with cell membranes. These compounds are more hydrophilic, compared to classic gemini alkyl surfactants, which reduces their ability to interact with lipid cell membranes of microorganisms. As a result, betaine surfactants may show a weaker ability to destabilize bacterial and fungal membranes, which translates into a decrease in antimicrobial activity. However, comparing the MIC values obtained in the presented work with the values obtained in earlier studies [37,46], where compounds containing simple alkyl chains were used, the described trend is variable. Compounds containing only 8 to 10 carbon atoms in the alkyl chain show lower MIC values of ester-based gemini surfactants. This tendency changes when the chain length increases to 14–16 methylene groups. The MIC value of compounds containing the simple alkyl chain 14-O-14 increases for E. coli by up to more than 80-fold compared to N2OE14 (from a value of 0.0293 mM to 2.5 mM, respectively). In turn, for compounds containing 12 methylene groups in the chain, the MIC value for the tested microorganisms is variable and strictly depends on the compound—its linker structure and counterions. In the presented work, classical gemini surfactants containing only alkyl chains were replaced with ester derivatives because they are more easily biodegradable [27], which is extremely important from the point of view of environmental protection and the quantities in which biocides are currently used.
2.4. Quantum Mechanical Calculations
We have analyzed the frontier molecular orbitals (Figure 6), as they play an important role in the chemical reactivity of molecules. Representatives of surfactant groups with a twelve-carbon chain and different linker structures were selected for analysis using quantum mechanical calculations to estimate the dependencies resulting from the linker structure: with an ether group (N2OE12), an additional tertiary (PMTH2E12), and an additional quaternary amine group in the linker (PMTH3E12). These orbitals participate in chemical reactions or interactions with other species and indicate the active site of the compound.
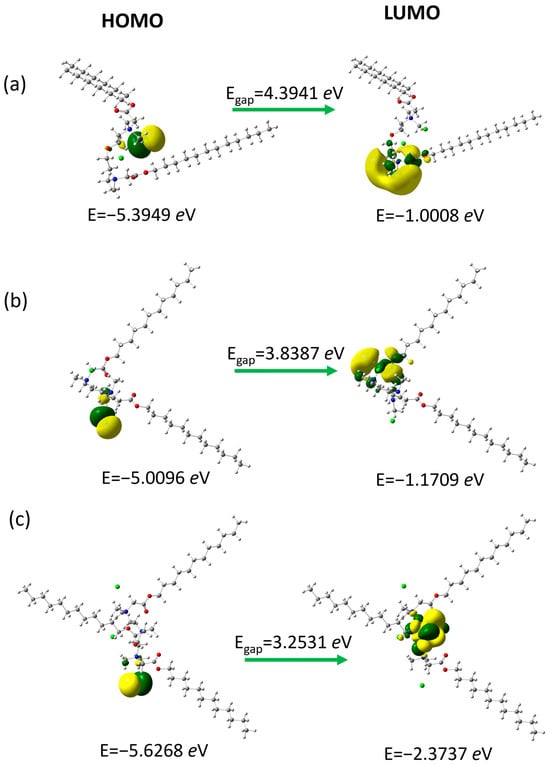
Figure 6.
Frontier molecular orbitals for (a) N2OE12, (b) PMTH2E12, and (c) PMTH3E12. Green and yellow colors represent opposite signs of the orbital values.
The highest occupied molecular orbital (HOMO) with higher energy is an electron-rich molecular orbital. It acts primarily as an electron donor and is responsible for the nucleophilicity of the molecule. In studied compounds, it is located around the chloride anion. The lowest unoccupied molecular orbital (LUMO) with lower energy is characterized by electron deficiency and the ability to accept electrons, which is related to the electrophilicity of the molecule. It is distributed over the fragments of the linker near the nitrogen atoms. The so-called energy gap (Egap), which is the difference between ELUMO-EHOMO is related to the chemical reactivity and kinetic stability of the molecules [53]. Moreover, it explains the charge transfer interaction within the molecular moieties, which influences the biological activity of compounds [54,55]. The calculated energy gap increases in the order PMTH3E12 < PMTH2E12 < N2OE12 (Table 3).

Table 3.
Global reactivity parameters (in eV) of the N2OE12, PMTH2E12, and PMTH3E12.
Additionally, based on the values of the HOMO and LUMO energies, the global reactivity parameters related to the chemical reactivity and stability of the compounds were calculated, i.e., the chemical potential (μ = (ELUMO + EHOMO)/2), global hardness (η = (ELUMO − EHOMO), global softness (σ = 1/2η), global electrophilicity index (ω = μ2/2η) and the maximum electronic charge (ΔNmax = −μ/η) [56,57,58]. Electronic chemical potential μ and global hardness η tend to increase while global softness σ, global electrophilicity ω, and global maximum electron transfer ΔNmax tend to decrease in the order, PMTH3E12, PMTH2E12, and N2OE12. Hardness and softness are related to the reactivity and stability of compounds. The lower value of hardness in a molecule refers to a soft molecule and is related to higher chemical reactivity. The lowest hardness value is shown by PMTH3E12, which also has the best antifungal properties. As already mentioned, bacteria and microscopic fungi have different cell structures, so inverse correlations between global reactivity parameters and antibacterial and antifungal properties can be expected.
Based on the electrostatic potential maps, trends in electrostatic interactions of surfactant molecules can be indicated (Figure 7). The negative electrostatic potential, marked in red, corresponds to concentrated electron density (Cl− counterions) in the molecules. In contrast, the positive electrostatic potential, the bluest area, indicates strongly positive fragments of the molecules (quaternary nitrogen atoms). Light green color indicates the neutral electrostatic potential of hydrocarbon chains, which is associated with the dominant role of the van der Waals effect in interactions, including dispersion and exchange-repulsion potentials [59].
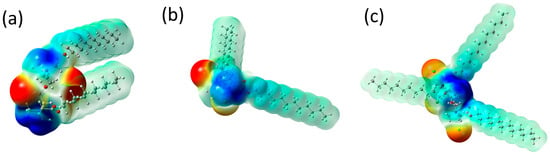
Figure 7.
Molecular electrostatic potential maps for (a) N2OE12, (b) PMTH2E12, and (c) PMTH3E12. Red and blue colors denote to the negative and positive electrostatic potential, respectively. Light green color indicates the neutral electrostatic potential.
3. Materials and Methods
Gemini and trimeric surfactants were obtained according to the procedure development in our laboratory [27,35,36]. All reagents and solvents were obtained from Merck (Poznan, Poland).
CMC values were obtained conductometrically by using a Conductivity Meter Elmetron CC-505 (Zabrze, Poland). The apparatus was calibrated by using a standard (147 μS/cm in 298.15 K). All the solutions were prepared using double-distilled water. Conductivity measurements were carried out at a temperature of 298.15 K. The conductometric titration was repeated at least three times for each gemini surfactant, and CMC was calculated as the mean value of three measurements. The CMCs of synthesized compounds were obtained by conductometric titration, creating relationship graphs of the characteristic conductivity in water of the surfactants as a function of the concentration [37]. The graphs consist of two lines with differing slopes. The line with higher inclination shows behavior before micellization, and the second line illustrates the process of micellization. The CMC values are at the intersection of the linear regressions of these lines. The degree of counterion binding (β) was calculated according to Frahm’s method [60] as (1 − α), where α = Smicellar/Spremicellar, i.e., the ratio of the slope after and before CMC. The ΔG°mic values were calculated by using Equation (1) [37]:
where R is the gas constant, T is the temperature in K, and the CMC is in mol/L.
The ester-based gemini and trimeric surfactants were tested for antimicrobial activity against bacteria: Escherichia coli ATCC 10536, Staphylococcus aureus ATCC 6538, yeast Candida albicans ATCC10231, and molds Aspergillus niger ATCC 16401. The MIC values for all microorganisms were determined by a tube standard two-fold dilution method [21]. Each of microorganisms was resuspended in physiological salt solution (molds in water with Tween 80 addition) and diluted to 107 cfu/mL for bacteria and 106 cfu/mL for yeast and molds. In the next step, 1 mL of microorganism suspension was mixed with 1 mL of media: TSB (Merck Poznan, Poland) for bacteria/MEB (Merck Poznan, Poland) for microscopic fungi containing serial dilutions of the tested compounds. All samples were incubated at 37 °C for 24 h bacteria, 48 h yeast, and 28 °C for 48 h molds. As a growth control, a suspension of microorganisms in a medium without the biocides was used. The MICs were defined as the lowest concentration of the compounds in which there was no visible growth.
The DFT calculations were performed using the GAUSSIAN16 program package [61]. The calculations employed the B3LYP exchange–correlation functional, which combines the hybrid exchange functional of Becke [62,63] with the gradient-correlation functional of Lee et al. [64] in conjunction with the 6–311 ++ G(d,p) basis set [55]. The HOMO and LUMO energies were calculated and the energy value from a.u. was converted to eV (1 a.u. = 27.2114 eV).
4. Conclusions
In this study, a series of ester-based gemini and trimeric surfactants with varying hydrophobic chain lengths and spacer structures were synthesized and characterized. The aggregation behavior revealed that increasing the alkyl chain length and the number of cationic head groups significantly enhances micellization efficiency, as evidenced by lower CMC, higher counterion binding parameters, and more negative ΔG°mic. The antimicrobial activity tests demonstrated that these surfactants possess broad-spectrum efficacy against Gram-positive and Gram-negative bacteria as well as fungi, with notable differences depending on both chain length and molecular architecture. Interestingly, shorter-chain derivatives generally exhibited stronger antimicrobial effects at lower concentrations, whereas trimeric and long-chain surfactants showed enhanced surface activity but slightly reduced antimicrobial potency. Quantum mechanical calculations provided insights into the electronic structure and reactivity of selected compounds, highlighting how molecular features influence their physicochemical and biological properties. The data suggest that compounds with smaller energy gaps and lower global hardness exhibit higher chemical reactivity, which may correlate with their biological activity. Overall, this work underscores the potential of ester-based gemini and trimeric surfactants as multifunctional agents with tunable properties, suitable for various industrial and biomedical applications. Future research should focus on further improving their biodegradability to align with environmental sustainability goals.
Author Contributions
Conceptualization, B.B. and I.K.; methodology, I.K.; software, A.S.; validation, A.K. (Anna Koziróg), A.K. (Anna Komasa) and I.K.; formal analysis, A.K. (Anna Koziróg) and A.K. (Anna Komasa); investigation, B.B.; resources, I.K.; data curation, A.S.; writing—original draft preparation, A.S., A.K. (Anna Komasa) and A.K. (Anna Koziróg); writing—review and editing, B.B. and I.K.; visualization, A.K. (Anna Komasa); supervision, I.K.; project administration, B.B.; funding acquisition, I.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the subsidy from the Faculty of Chemistry, Adam Mickiewicz University for maintaining research potential.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CMC | critical micelle concentration |
| MIC | minimum inhibitory concentration |
| FMOs | frontier molecular orbitals |
| HOMO | highest occupied molecular orbital |
| LUMO | lowest unoccupied molecular orbital |
References
- Tehrani-Bagha, A.R.; Holmberg, K. Cationic Ester-Containing Gemini Surfactants: Adsorption at Tailor-Made Surfaces Monitored by SPR and QCM. Langmuir 2008, 24, 6140–6145. [Google Scholar] [CrossRef]
- Brycki, B.E.; Kowalczyk, I.H.; Szulc, A.; Kaczerewska, O.; Pakiet, M. Multifunctional Gemini Surfactants: Structure, Synthesis, Properties and Applications. In Application and Characterization of Surfactants; Najjar, R., Ed.; InTech: Rijeka, Croatia, 2017; ISBN 978-953-51-3325-4. [Google Scholar]
- Menger, F.M.; Keiper, J.S.; Azov, V. Gemini Surfactants with Acetylenic Spacers. Langmuir 2000, 16, 2062–2067. [Google Scholar] [CrossRef]
- Menger, F.M.; Littau, C.A. Gemini-Surfactants: Synthesis and Properties. J. Am. Chem. Soc. 1991, 113, 1451–1452. [Google Scholar] [CrossRef]
- Sharma, T.; Dohare, N.; Kumari, M.; Singh, U.K.; Khan, A.B.; Borse, M.S.; Patel, R. Comparative Effect of Cationic Gemini Surfactant and Its Monomeric Counterpart on the Conformational Stability and Activity of Lysozyme. RSC Adv. 2017, 7, 16763–16776. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.B.; Kudryavtseva, L.A.; Pankratov, V.A.; Lukashenko, S.S.; Rizvanova, L.Z.; Konovalov, A.I. Geminal Alkylammonium Surfactants: Aggregation Properties and Catalytic Activity. Russ. J. Gen. Chem. 2006, 76, 1625–1631. [Google Scholar] [CrossRef]
- Kuperkar, K.; Modi, J.; Patel, K. Surface-Active Properties and Antimicrobial Study of Conventional Cationic and Synthesized Symmetrical Gemini Surfactants. J. Surfactants Deterg. 2012, 15, 107–115. [Google Scholar] [CrossRef]
- Chang, H.; Cui, Y.; Wang, Y.; Li, G.; Gao, W.; Li, X.; Zhao, X.; Wei, W. Wettability and Adsorption of PTFE and Paraffin Surfaces by Aqueous Solutions of Biquaternary Ammonium Salt Gemini Surfactants with Hydroxyl. Colloids Surf. A 2016, 506, 416–424. [Google Scholar] [CrossRef]
- Bhadani, A.; Singh, S. Novel Gemini Pyridinium Surfactants: Synthesis and Study of Their Surface Activity, DNA Binding, and Cytotoxicity. Langmuir 2009, 25, 11703–11712. [Google Scholar] [CrossRef]
- Kamal, M.S. A Review of Gemini Surfactants: Potential Application in Enhanced Oil Recovery. J. Surfactants Deterg. 2016, 19, 223–236. [Google Scholar] [CrossRef]
- Pal, N.; Saxena, N.; Mandal, A. Studies on the Physicochemical Properties of Synthesized Tailor-Made Gemini Surfactants for Application in Enhanced Oil Recovery. J. Mol. Liq. 2018, 258, 211–224. [Google Scholar] [CrossRef]
- Han, X.; Lu, M.; Fan, Y.; Li, Y.; Holmberg, K. Recent Developments on Surfactants for Enhanced Oil Recovery. Tenside Surfactants Deterg. 2021, 58, 164–176. [Google Scholar] [CrossRef]
- Cardoso, A.M.S.; Faneca, H.; Almeida, J.A.S.; Pais, A.A.C.C.; Marques, E.F.; de Lima, M.C.P.; Jurado, A.S. Gemini Surfactant Dimethylene-1,2-Bis(Tetradecyldimethylammonium Bromide)-Based Gene Vectors: A Biophysical Approach to Transfection Efficiency. Biochim. Biophys. Acta 2011, 1808, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Pisárčik, M.; Devínsky, F. Surface Tension Study of Cationic Gemini Surfactants Binding to DNA. Cent. Eur. J. Chem. 2014, 12, 577–585. [Google Scholar] [CrossRef]
- Ahmady, A.R.; Hosseinzadeh, P.; Solouk, A.; Akbari, S.; Szulc, A.M.; Brycki, B.E. Cationic Gemini Surfactant Properties, Its Potential as a Promising Bioapplication Candidate, and Strategies for Improving Its Biocompatibility: A Review. Adv. Colloid Interface Sci. 2022, 299, 102581. [Google Scholar] [CrossRef]
- Brycki, B.; Szulc, A. Gemini Surfactants as Corrosion Inhibitors. A Review. J. Mol. Liq. 2021, 344, 117686. [Google Scholar] [CrossRef]
- Mahdavian, M.; Tehrani-Bagha, A.R.; Alibakhshi, E.; Ashhari, S.; Palimi, M.J.; Farashi, S.; Javadian, S.; Ektefa, F. Corrosion of Mild Steel in Hydrochloric Acid Solution in the Presence of Two Cationic Gemini Surfactants with and without Hydroxyl Substituted Spacers. Corros. Sci. 2018, 137, 62–75. [Google Scholar] [CrossRef]
- Mao, T.; Huang, H.; Liu, D.; Shang, X.; Wang, W.; Wang, L. Novel Cationic Gemini Ester Surfactant as an Efficient and Eco-Friendly Corrosion Inhibitor for Carbon Steel in HCl Solution. J. Mol. Liq. 2021, 339, 117174. [Google Scholar] [CrossRef]
- Rosen, M.J.; Li, F. The Adsorption of Gemini and Conventional Surfactants onto Some Soil Solids and the Removal of 2-Naphthol by the Soil Surfaces. J. Colloid Interface Sci. 2001, 234, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Duan, H.; Jiang, R. Synergistic Corrosion Inhibition Effect of Quinoline Quaternary Ammonium Salt and Gemini Surfactant in H2S and CO2 Saturated Brine Solution. Corros. Sci. 2015, 91, 108–119. [Google Scholar] [CrossRef]
- Brycki, B.; Kowalczyk, I.; Kozirog, A. Synthesis, Molecular Structure, Spectral Properties and Antifungal Activity of Polymethylene-α,ω-Bis(N,N-Dimethyl-N-Dodecyloammonium Bromides). Molecules 2011, 16, 319–335. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, S.; Yu, J.; Chen, X.; Lei, Q.; Fang, W. Antibacterial Activity, in Vitro Cytotoxicity, and Cell Cycle Arrest of Gemini Quaternary Ammonium Surfactants. Langmuir 2015, 31, 12161–12169. [Google Scholar] [CrossRef] [PubMed]
- Minbiole, K.P.C.; Jennings, M.C.; Ator, L.E.; Black, J.W.; Grenier, M.C.; LaDow, J.E.; Caran, K.L.; Seifert, K.; Wuest, W.M. From Antimicrobial Activity to Mechanism of Resistance: The Multifaceted Role of Simple Quaternary Ammonium Compounds in Bacterial Eradication. Tetrahedron 2016, 72, 3559–3566. [Google Scholar] [CrossRef]
- Dani, U.; Bahadur, A.; Kuperkar, K. Micellization, Antimicrobial Activity and Curcumin Solubilization in Gemini Surfactants: Influence of Spacer and Non-Polar Tail. Colloid Interface Sci. Commun. 2018, 25, 22–30. [Google Scholar] [CrossRef]
- Brycki, B.; Waligórska, M.; Szulc, A. The Biodegradation of Monomeric and Dimeric Alkylammonium Surfactants. J. Hazard. Mater. 2014, 280, 797–815. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.R.; Oskarsson, H.; van Ginkel, C.G.; Holmberg, K. Cationic Ester-Containing Gemini Surfactants: Chemical Hydrolysis and Biodegradation. J. Colloid Interface Sci. 2007, 312, 444–452. [Google Scholar] [CrossRef]
- Garcia, M.T.; Ribosa, I.; Kowalczyk, I.; Pakiet, M.; Brycki, B. Biodegradability and Aquatic Toxicity of New Cleavable Betainate Cationic Oligomeric Surfactants. J. Hazard. Mater. 2019, 371, 108–114. [Google Scholar] [CrossRef]
- Akram, M.; Anwar, S.; Ansari, F.; Bhat, I.A.; Kabir-ud-Din, K.-D. Bio-Physicochemical Analysis of Ethylene Oxide-Linked Diester-Functionalized Green Cationic Gemini Surfactants. RSC Adv. 2016, 6, 21697–21705. [Google Scholar] [CrossRef]
- Brycki, B.; Szulc, A. Gemini Alkyldeoxy-D-Glucitolammonium Salts as Modern Surfactants and Microbiocides: Synthesis, Antimicrobial and Surface Activity, Biodegradation. PLoS ONE 2014, 9, e84936. [Google Scholar] [CrossRef]
- Javadian, S.; Aghdastinat, H.; Tehrani-Bagha, A.; Gharibi, H. Self-Assembled Nano Structures of Cationic Ester-Containing Gemini Surfactants: The Surfactant Structure and Salt Effects. J. Chem. Thermodyn. 2013, 62, 201–210. [Google Scholar] [CrossRef]
- Akram, M.; Lal, H.; Kabir-ud-Din. Exploring the Binding Mode of Ester-Based Cationic Gemini Surfactants with Calf Thymus DNA: A Detailed Physicochemical, Spectroscopic and Theoretical Study. Bioorganic Chem. 2022, 119, 105555. [Google Scholar] [CrossRef]
- Pisárčik, M.; Polakovičová, M.; Markuliak, M.; Lukáč, M.; Devinsky, F. Self-Assembly Properties of Cationic Gemini Surfactants with Biodegradable Groups in the Spacer. Molecules 2019, 24, 1481. [Google Scholar] [CrossRef] [PubMed]
- Abdul Rub, M. Investigation of Micellar and Interfacial Phenomenon of Amitriptyline Hydrochloride with Cationic Ester-Bonded Gemini Surfactant Mixture in Different Solvent Media. PLoS ONE 2020, 15, e0241300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, C.; Sang, J.; Zhang, L.; Xiao, Y.; Lin, W. A Novel Ester-Bonded Gemini Quaternary Ammonium Salt with Good Antimicrobial Activity and Anti-Mold Performance for Wet Blue Leather. J. Am. Leather Chem. Assoc. 2022, 117, 131–140. [Google Scholar] [CrossRef]
- Pakiet, M.; Kowalczyk, I.; Leiva Garcia, R.; Akid, R.; Brycki, B. Cationic Clevelable Surfactants as Highly Efficient Corrosion Inhibitors of Stainless Steel AISI 304: Electrochemical Study. J. Mol. Liq. 2020, 315, 113675. [Google Scholar] [CrossRef]
- Pakiet, M.; Tedim, J.; Kowalczyk, I.; Brycki, B. Functionalised Novel Gemini Surfactants as Corrosion Inhibitors for Mild Steel in 50 mM NaCl: Experimental and Theoretical Insights. Colloids Surf. Physicochem. Eng. Asp. 2019, 580, 123699. [Google Scholar] [CrossRef]
- Brycki, B.E.; Szulc, A.; Kowalczyk, I.; Koziróg, A.; Sobolewska, E. Antimicrobial Activity of Gemini Surfactants with Ether Group in the Spacer Part. Molecules 2021, 26, 5759. [Google Scholar] [CrossRef]
- Brycki, B.; Szulc, A.; Brycka, J.; Kowalczyk, I. Properties and Applications of Quaternary Ammonium Gemini Surfactant 12-6-12: An Overview. Molecules 2023, 28, 6336. [Google Scholar] [CrossRef]
- Liu, D.; Yang, X.; Liu, P.; Mao, T.; Shang, X.; Wang, L. Synthesis and characterization of gemini ester surfactant and its application in efficient fabric softening. J. Mol. Liq. 2020, 299, 112236. [Google Scholar] [CrossRef]
- Tatsumi, T.; Zhang, W.; Kida, T.; Nakatsuji, Y.; Ono, D.; Takeda, T.; Ikeda, I. Novel Hydrolyzable and Biodegradable Cationic Gemini Surfactants: 1,3-bis[(Acyloxyalkyl)-dimethylammonio]-2-hydroxypropane Dichloride. J. Surfactants Deterg. 2000, 3, 167–172. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.R.; Holmberg, K. Cationic Ester-Containing Gemini Surfactants: Physical−Chemical Properties. Langmuir 2010, 26, 9276–9282. [Google Scholar] [CrossRef]
- Kaczerewska, O.; Leiva-Garcia, R.; Akid, R.; Brycki, B.; Kowalczyk, I.; Pospieszny, T. Effectiveness of O -Bridged Cationic Gemini Surfactants as Corrosion Inhibitors for Stainless Steel in 3 M HCl: Experimental and Theoretical Studies. J. Mol. Liq. 2018, 249, 1113–1124. [Google Scholar] [CrossRef]
- Obłąk, E.; Piecuch, A.; Rewak-Soroczyńska, J.; Paluch, E. Activity of Gemini Quaternary Ammonium Salts against Microorganisms. Appl. Microbiol. Biotechnol. 2019, 103, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Obłąk, E.; Piecuch, A.; Krasowska, A.; Łuczyński, J. Antifungal Activity of Gemini Quaternary Ammonium Salts. Microbiol. Res. 2013, 168, 630–638. [Google Scholar] [CrossRef]
- Allen, R.A.; Jennings, M.C.; Mitchell, M.A.; Al-Khalifa, S.E.; Wuest, W.M.; Minbiole, K.P.C. Ester- and Amide-Containing multiQACs: Exploring Multicationic Soft Antimicrobial Agents. Bioorg. Med. Chem. Lett. 2017, 27, 2107–2112. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, I.; Pakiet, M.; Szulc, A.; Koziróg, A. Antimicrobial Activity of Gemini Surfactants with Azapolymethylene Spacer. Molecules 2020, 25, 4054. [Google Scholar] [CrossRef]
- Balgavý, P.; Devinsky, F. Cut-off Effects in Biological Activities of Surfactants. Adv. Colloid Interface Sci. 1996, 66, 23–63. [Google Scholar] [CrossRef]
- Mechken, K.A.; Menouar, M.; Talbi, Z.; Saidi-Besbes, S.; Belkhodja, M. Self-Assembly and Antimicrobial Activity of Cationic Gemini Surfactants Containing Triazole Moieties. RSC Adv. 2024, 14, 19185–19196. [Google Scholar] [CrossRef]
- Hafidi, Z.; García, M.T.; Vazquez, S.; Martinavarro-Mateos, M.; Ramos, A.; Pérez, L. Antimicrobial and Biofilm-Eradicating Properties of Simple Double-Chain Arginine-Based Surfactants. Colloids Surf. B Biointerfaces 2025, 253, 114762. [Google Scholar] [CrossRef] [PubMed]
- Wani, F.A.; Amaduddin; Aneja, B.; Sheehan, G.; Kavanagh, K.; Ahmad, R.; Abid, M.; Patel, R. Synthesis of Novel Benzimidazolium Gemini Surfactants and Evaluation of Their Anti-Candida Activity. ACS Omega 2019, 4, 11871–11879. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y. Structure–Activity Relationship of Cationic Surfactants as Antimicrobial Agents. Curr. Opin. Colloid Interface Sci. 2020, 45, 28–43. [Google Scholar] [CrossRef]
- Neubauer, D.; Jaśkiewicz, M.; Bauer, M.; Olejniczak-Kęder, A.; Sikorska, E.; Sikora, K.; Kamysz, W. Biological and Physico-Chemical Characteristics of Arginine-Rich Peptide Gemini Surfactants with Lysine and Cystine Spacers. Int. J. Mol. Sci. 2021, 22, 3299. [Google Scholar] [CrossRef] [PubMed]
- Fleming, I. Frontier Orbitals and Organic Chemical Reactions; John Wiley & Sons: New York, NY, USA, 1976. [Google Scholar]
- Nagabalasubramanian, P.B.; Karabacak, M.; Periandy, S. Vibrational Frequencies, Structural Confirmation Stability and HOMO–LUMO Analysis of Nicotinic Acid Ethyl Ester with Experimental (FT-IR and FT-Raman) Techniques and Quantum Mechanical Calculations. J. Mol. Struct. 2012, 1017, 1–13. [Google Scholar] [CrossRef]
- Kirishnamaline, G.; Magdaline, J.D.; Chithambarathanu, T.; Aruldhas, D.; Anuf, A.R. Theoretical Investigation of Structure, Anticancer Activity and Molecular Docking of Thiourea Derivatives. J. Mol. Struct. 2021, 1225, 129118. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute Hardness: Companion Parameter to Absolute Electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Pearson, R.G. Chemical Hardness and Density Functional Theory. J. Chem. Sci. 2005, 117, 369–377. [Google Scholar] [CrossRef]
- Chattaraj, P.K.; Sarkar, U.; Roy, D.R. Electrophilicity Index. Chem. Rev. 2006, 106, 2065–2091. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Zhao, S.; Hong, K.H.; Zhang, M.-Y.; Song, L.; Yu, F.; Luo, G.; He, Y.-P. Pyromellitic-Based Low Molecular Weight Gelators and Computational Studies of Intermolecular Interactions: A Potential Additive for Lubricant. Langmuir 2021, 37, 2954–2962. [Google Scholar] [CrossRef]
- Hajy Alimohammadi, M.; Javadian, S.; Gharibi, H.; Tehrani-Bagha, A.r.; Alavijeh, M.R.; Kakaei, K. Aggregation Behavior and Intermicellar Interactions of Cationic Gemini Surfactants: Effects of Alkyl Chain, Spacer Lengths and Temperature. J. Chem. Thermodyn. 2012, 44, 107–115. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01 2016; Gaussian, Inc.: Wallingford, CT, USA, 2016.
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. V. Systematic Optimization of Exchange-Correlation Functionals. J. Chem. Phys. 1997, 107, 8554–8560. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).