Mucoadhesive PVA Film for Sustained Resveratrol Delivery: Formulation, Characterization, and Release Profile
Abstract
1. Introduction
2. Results and Discussion
2.1. Visual Assessment
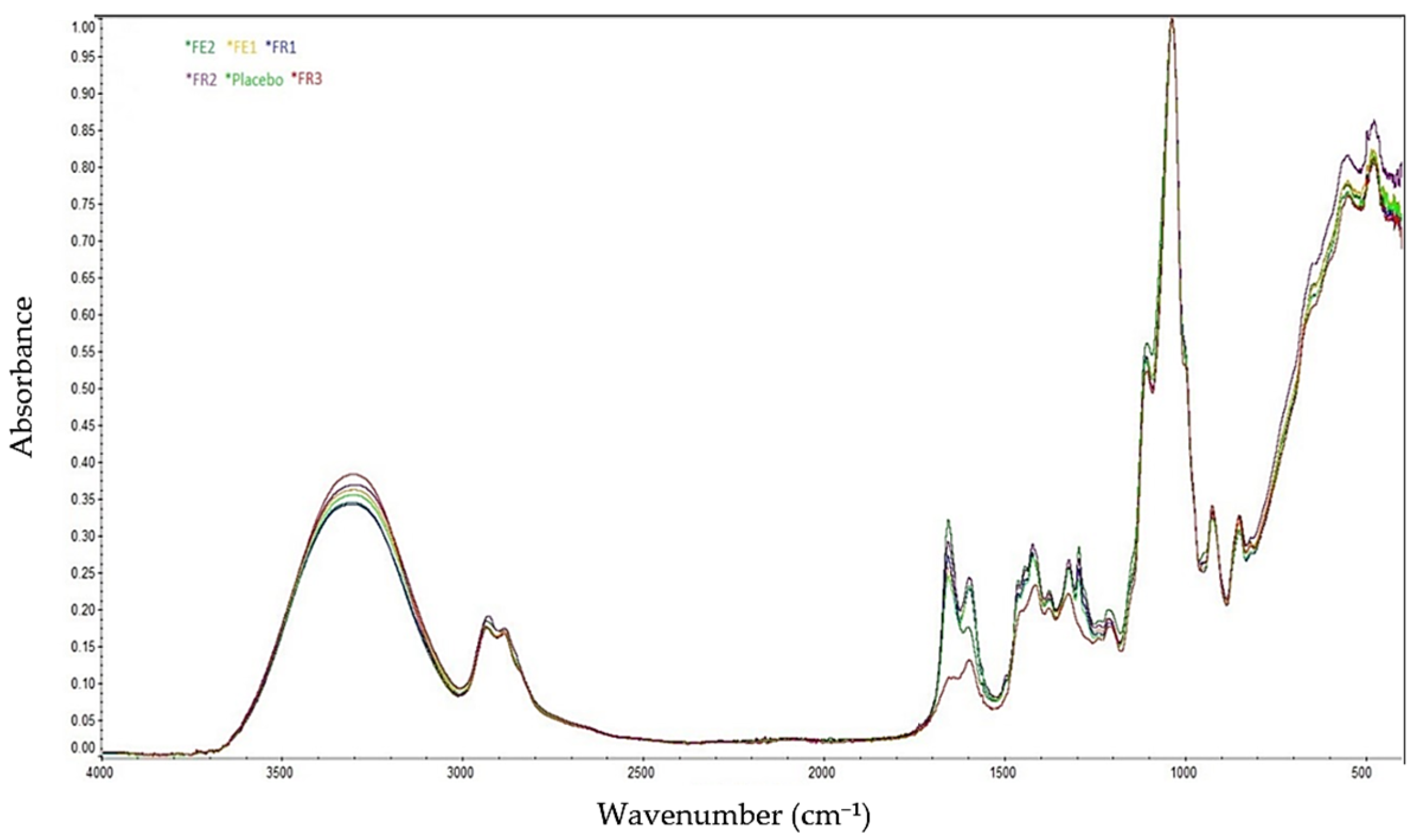
2.2. FT-IR Spectroscopy Analyses
2.3. Contact Angle Measurement (Wettability)
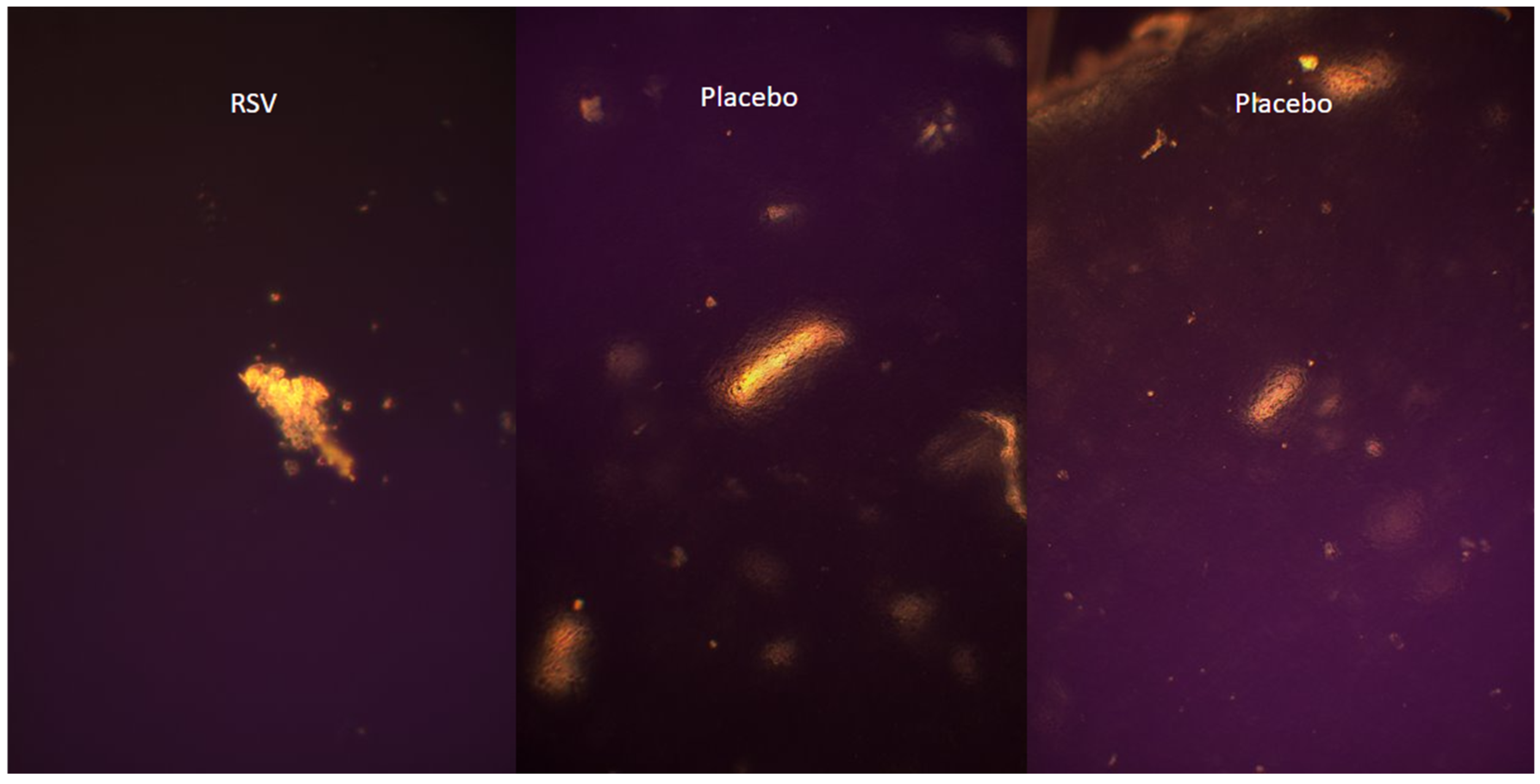
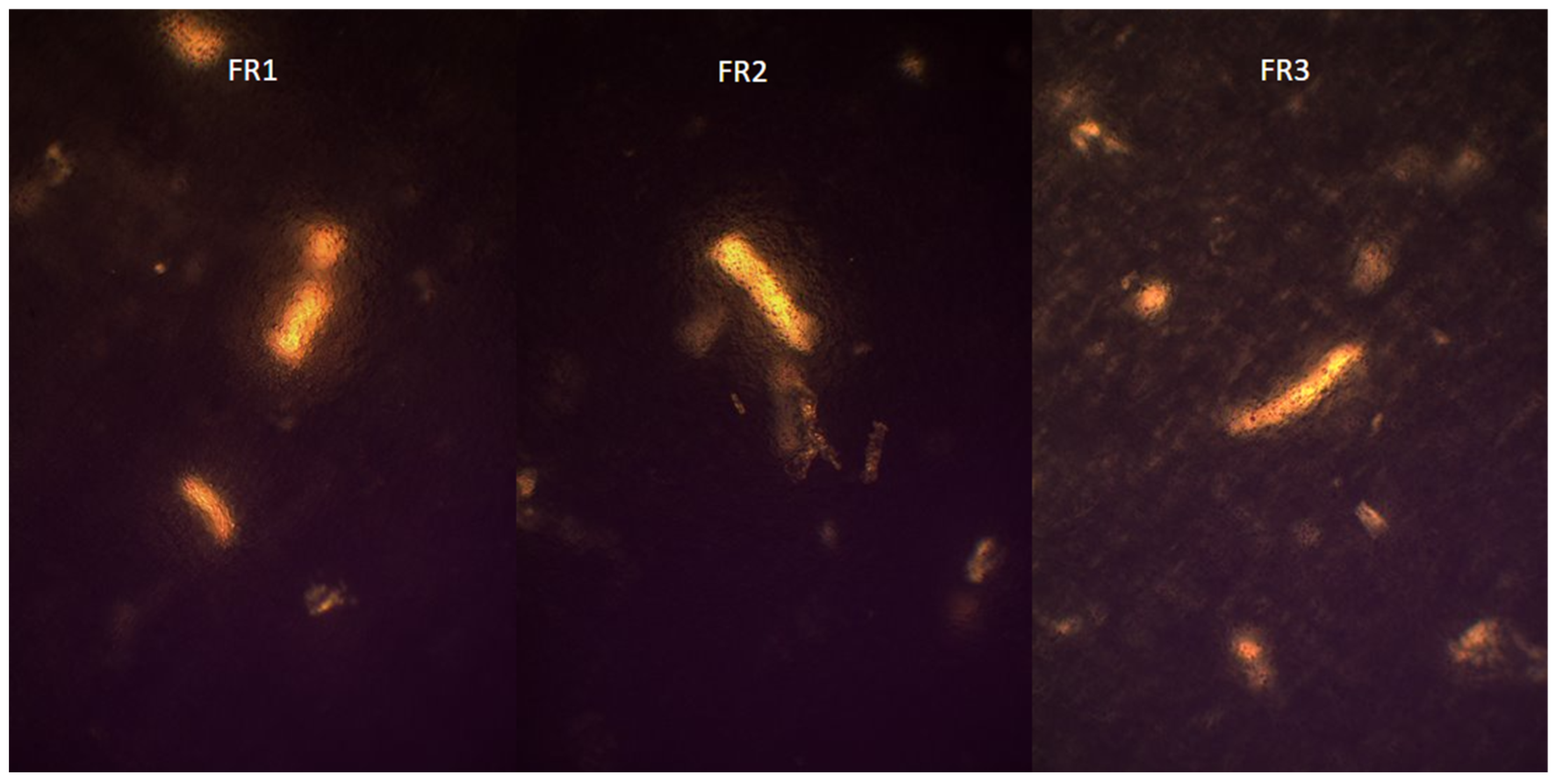
2.4. Visualization of Polymer Crystalline Structure Using Polarized Optical Microscopy
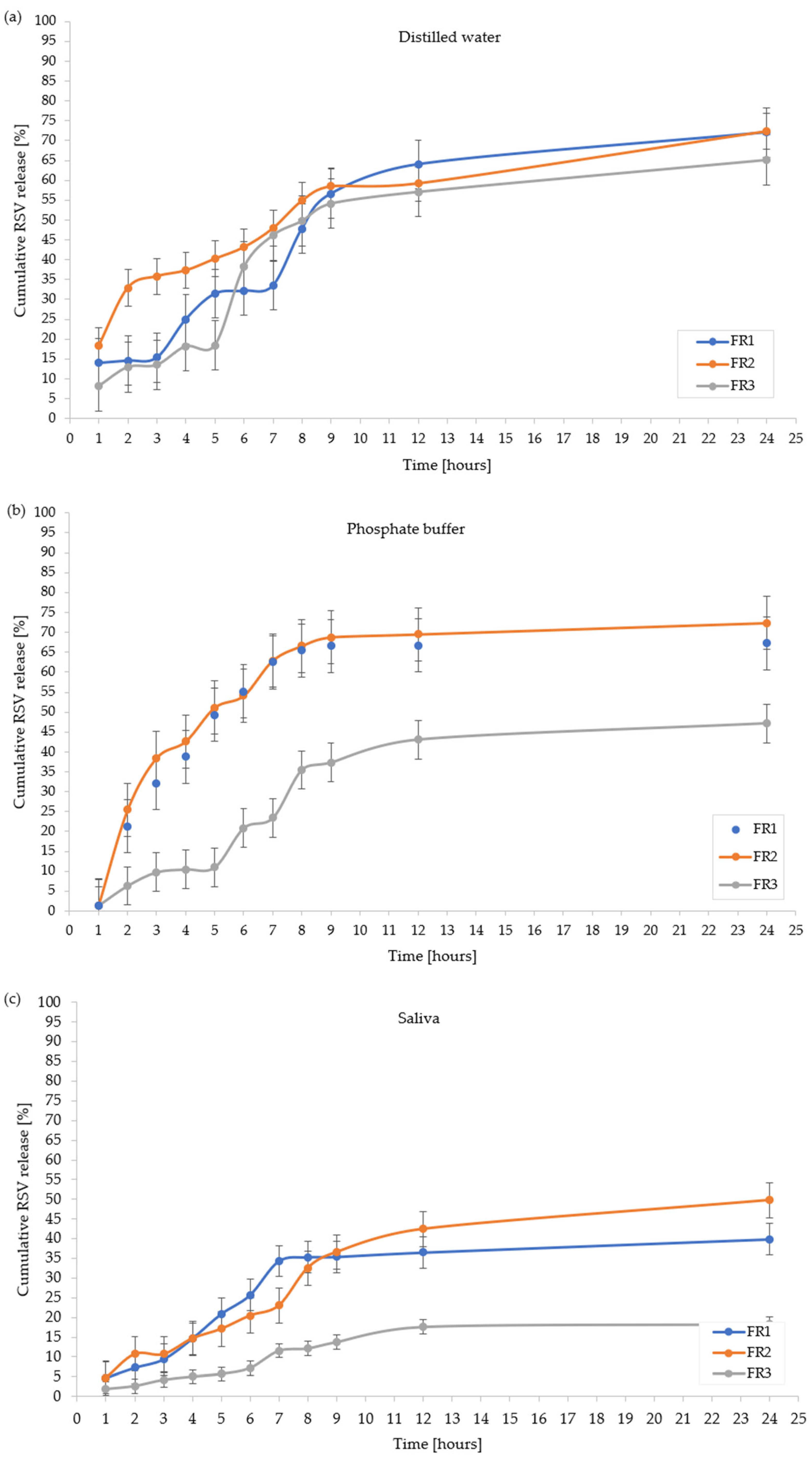
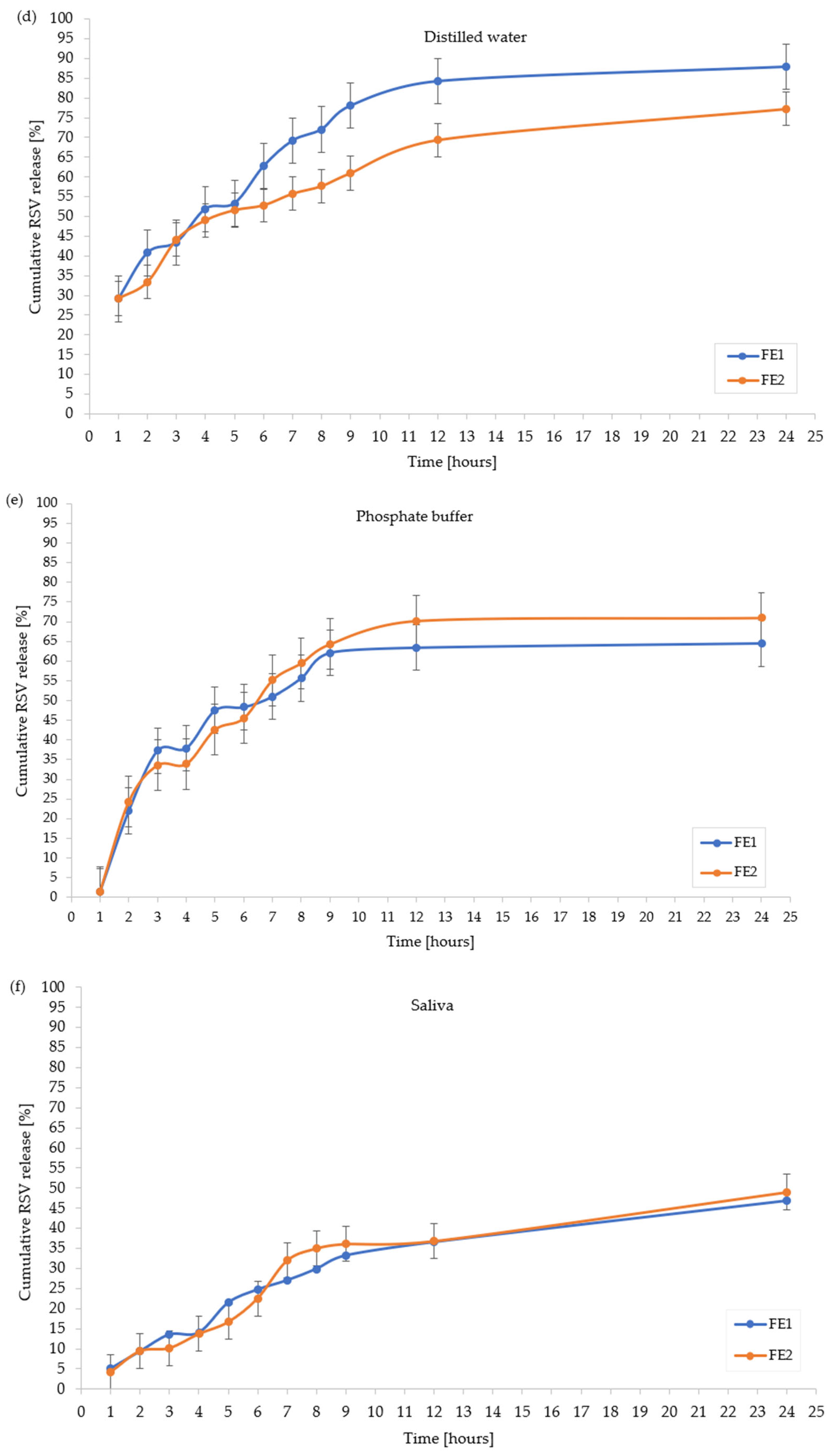
2.5. In Vitro Resveratrol Release Profile
2.6. Disintegration Test
2.7. Summary of Results
3. Materials and Methods
3.1. Materials, Reagents, and Apparatus
3.1.1. Materials and Regents
3.1.2. Apparatus
3.1.3. Preparation of Reynoutria japonica Extract
3.2. Preparation of a Water–Ethanol Solution with RSV
3.3. Preparation of Phosphate Buffer
3.4. Preparation of Artificial Saliva Solution
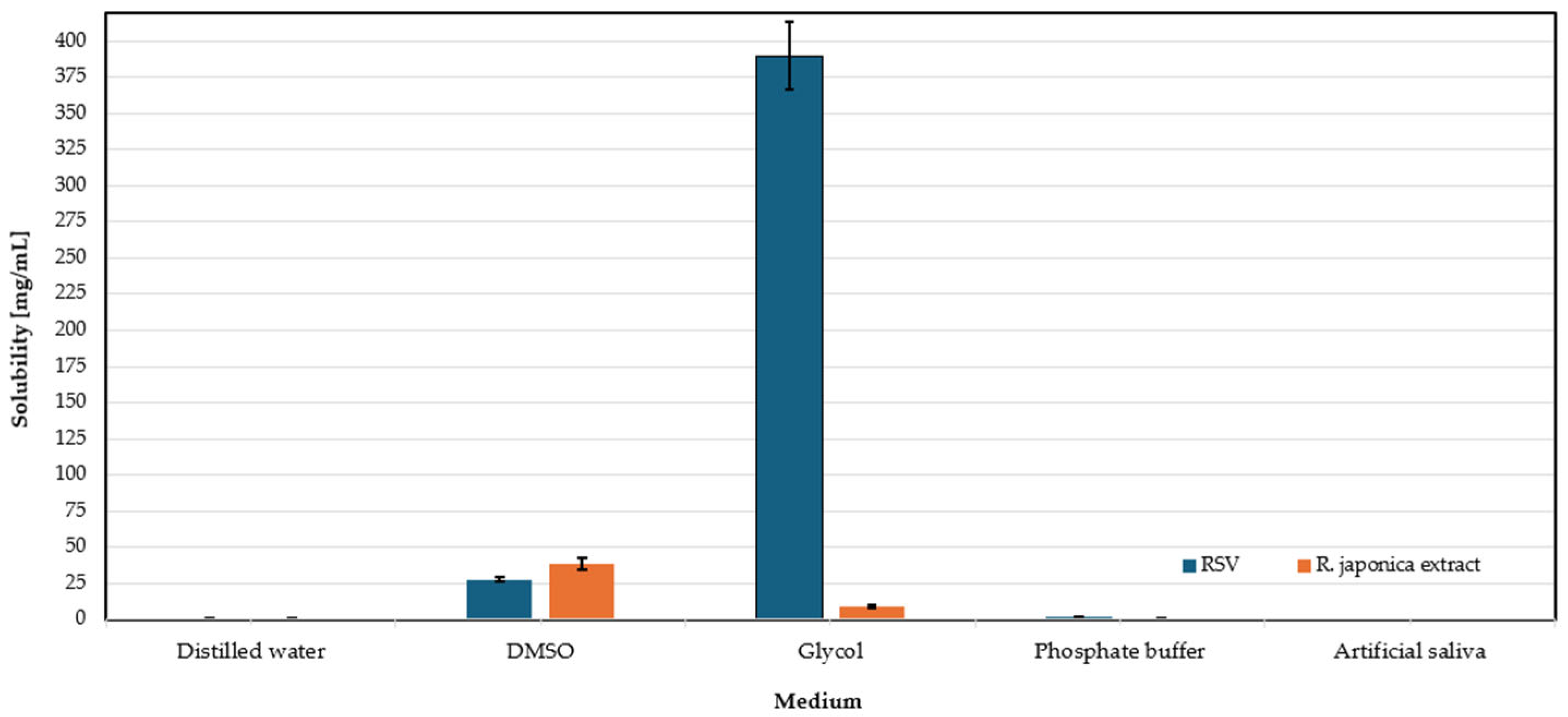
3.5. Solubility Assessment of RSV and Extract of Reynoutria japonica
3.6. Methods
3.6.1. Polymer Film Preparation Technology
3.6.2. Methods of the Assessment of Polymeric Films with Plant Material
- 1.
- Visual Assessment
- 2.
- Measurement of Mass and Thickness
- 3.
- Bending Resistance Test
- 4.
- Mucoadhesion Strength Test
- 5.
- FT-IR Spectroscopy
- 6.
- Visualization of Polymer Crystalline Structure Using Polarized Optical Microscopy
- 7.
- Contact Angle Measurement
- 8.
- Determining the Release Profile of Resveratrol from Film Formulation
- 9.
- Study of the RSV Content in A Single Carrier and pH of Extract Measurement after Film Disintegration
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| API | Active pharmaceutical ingredient |
| DMSO | Dimethyl sulfoxide |
| EtOH | Ethanol |
| MC 400 cP | Methylcellulose, viscosity 400 cP |
| MCA 15C | Methylcellulose, viscosity 15 cP |
| NaCMC | Sodium carboxymethylcellulose |
| PGE | Propylene glycol |
| PVA | Polyvinyl alcohol |
| PVP | Polyvinylpyrrolidone |
| RSV | Resveratrol |
| W | Distilled water |
References
- Yang, T.; Wang, L.; Zhu, M.; Zhang, L.; Yan, L. Properties and Molecular Mechanisms of Resveratrol: A Review. Pharmaize 2015, 70, 501–506. [Google Scholar]
- Robinson, K.; Mock, C.; Liang, D. Pre-Formulation Studies of Resveratrol. Drug Dev. Ind. Pharm. 2015, 41, 1464–1469. [Google Scholar] [CrossRef]
- Shakibaei, M.; Harikumar, K.B.; Aggarwal, B.B. Resveratrol Addiction: To Die or Not to Die. Mol. Nutr. Food Res. 2009, 53, 115–128. [Google Scholar] [CrossRef]
- Mukherjee, S.; Dudley, J.I.; Das, D.K. Dose-Dependency of Resveratrol in Providing Health Benefits. Dose-Response 2010, 8, 478–500. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, R.; Zhang, X.; Zhu, S.; Liu, S.; Yang, J.; Li, Z.; Gao, T.; Liu, F.; Hu, H. The Invasive Species Reynoutria japonica Houtt. As a Promising Natural Agent for Cardiovascular and Digestive System Illness. Front. Pharmacol. 2022, 13, 863707. [Google Scholar] [CrossRef]
- Selvaraj, S.; Sun, Y.; Sukumaran, P.; Singh, B.B. Resveratrol Activates Autophagic Cell Death in Prostate Cancer Cells via Downregulation of STIM1 and the Mtor Pathway. Mol. Carcinog. 2015, 55, 818–831. [Google Scholar] [CrossRef]
- Mosavi, S.S.; Rabizadeh, S.; Yadegar, A.; Seifouri, S.; Mohammadi, F.; Qahremani, R.; Salehi, S.S.; Rajab, A.; Esteghmati, A.; Nakhjavani, M. Therapeutic Effects of Resveratrol and Omega-3 in Mice Atherosclerosis: Focus on Histopathological Changes. BMC Complement. Med. Ther. 2023, 23, 81. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, L.E.; Newton, R.; Kennedy, G.E.; Fenwick, P.S.; Leung, R.H.F.; Ito, K.; Russell, R.E.K.; Barnes, P.J. Anti-Inflammatory Effects of Resveratrol in Lung Epithelial Cells: Molecular Mechanisms. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L774–L783. [Google Scholar] [CrossRef]
- Zhou, H.; Shang, L.; Li, X.; Zhang, X.; Gao, G.; Guo, C.; Chen, B.; Liu, Q.; Gong, Y.; Shao, C. Resveratrol Augments the Canonical Wnt Signaling Pathway in Promoting Osteoblastic Differentiation of Multipotent Mesenchymal Cells. Exp. Cell Res. 2009, 315, 2953–2962. [Google Scholar] [CrossRef]
- Baker, J.; Petersen, R.C. Cellular Senescence in Brain Aging and Neurodegenerative Diseases: Evidence and Perspectives. J. Clin. Investig. 2018, 128, 1208–1216. [Google Scholar] [CrossRef]
- Sun, C.; Hu, Y.; Liu, X.; Wu, T.; Wang, Y.; He, W.; Wei, W. Resveratrol Downregulates the Constitutional Activation of Nuclear Factor-Κb in Multiple Myeloma Cells. Cancer Genet. Cytogenet. 2006, 165, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Golkar, L.; Ding, X.Z.; Ujiki, M.B.; Salabat, M.R.; Kelly, D.L.; Scholtens, D.; Fought, A.J.; Bentrem, D.J.; Talamonti, M.S.; Bell, R.H.; et al. Resveratrol Inhibits Pancreatic Cancer Cell Proliferation Through Transcriptional Induction of Macrophage Inhibitory Cytokine-1. J. Surg. Res. 2007, 138, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Dhir, N. Resveratrol-Mediated Chemoprevention of Diethylnitrosamine-Initiated Hepatocarcinogenesis: Inhibition of Cell Proliferation and Induction of Apoptosis. Chem. Biol. Interact. 2009, 179, 131–144. [Google Scholar] [CrossRef]
- Campagna, M.; Rivas, C. Antiviral Activity of Resveratrol. Biochem. Soc. Trans. 2010, 38, 50–53. [Google Scholar] [CrossRef]
- Dutta, D.; Bagchi, P.; Chatterjee, A.; Nayak, M.K.; Mukherjee, A.; Chattopadhyay, S.; Nagashima, S.; Kobayashi, N.; Komoto, S.; Taniguchi, K.; et al. The Molecular Chaperone Heat Shock Protein-90 Positively Regulates Rotavirus Infection. Virology 2009, 391, 325–333. [Google Scholar] [CrossRef]
- Vestergaard, M.; Ingmer, H. Antibacterial and Antifungal Properties of Resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Huang, Z.; Zhou, W.; Tang, Z.; Ma, R.; Liang, J. Anti-Biofilm Activities from Resveratrol Against Fusobacterium Nucleatum. Front. Microbiol. 2016, 7, 1065. [Google Scholar] [CrossRef]
- Wu, Y.; Li, L.; Wen, S.; Yu, Y.; Zhao, S.; Li, Y.; Liu, Y. Resveratrol: Botanical Origin, Pharmacological Activity, and Applications. Biomed. Pharmacother. 2022, 148, 112990. [Google Scholar] [CrossRef]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The Biology of Streptococcus mutans. Microbiol. Spectr. 2019, 7, 10-1128. [Google Scholar] [CrossRef]
- Chouhan, K.B.; Tewari, S.; Puri, K.K.; Agarwal, P. Dental Caries; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Featherstone, J.D. The caries balance: Contributing Factors and Early Detection. J. Calif. Dent. Assoc. 2003, 31, 129–133. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal Diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal Diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Williams, D.W.; Lewis, M.A.; Samaranayake, L.P. Candida Biofilms and Oral Candidosis: Treatment and Prevention. Periodontology 2000 2011, 55, 250–265. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Oral Health Surveillance Report. Trends in Dental Caries and Sealants, Tooth Retention, and Edentulism; CDC: Atlanta, GA, USA, 2019. [Google Scholar]
- Golshah, A.; Mirzaeei, S.; Nikkerdar, N.; Ghorbani, F. Gingivitis Effectiveness of Emulgel Containing 2% Resveratrol in Orthodontic Patients: An 8-Week Randomized Clinical Trial. Int. J. Dent. 2021, 2021, 6615900. [Google Scholar] [CrossRef] [PubMed]
- Rayyan, M.; Terkawi, T.; Abdo, H.; Azim, D.A.; Khalaf, A.; AlKhouli, Z.; Meziad, M.; Alshamma, M.; Naim, H.A. Efficacy of Grape Seed Extract Gel in the Treatment of Chronic Periodontitis: A Randomized Clinical Study. J. Investig. Clin. Dent. 2018, 9, e12318. [Google Scholar] [CrossRef] [PubMed]
- Pozdnyakov, D.; Ayrapetyan, E.; Konovalov, D. The Study of the Anti-Inflammatory Activity of a Stomatological Gel Based on an Extract of Artemisia Scoparia Waldst. et Kit. J. Res. Pharm. 2022, 26, 189–197. [Google Scholar] [CrossRef]
- Berta, G.N.; Romano, F.; Vallone, R.; Abbadessa, G.; DiScipio, F.; Defabians, P. An Innovative Strategy for Oral Biofilm Control in Early Childhood Based on a Resveratrol-Cyclodextrin Nanotechnology Approach. Materials 2021, 14, 3801. [Google Scholar] [CrossRef]
- Min, K.K.; Neupane, S.; Adhikari, N.; Sohn, W.J.; An, S.-Y.; Kim, J.-Y.; An, C.-H.; Lee, Y.; Kim, Y.-G.; Park, J.-W.; et al. Effects of Resveratrol on Bone-Healing Capacity in the Mouse Tooth Extraction Socket. J. Periodontal Res. 2020, 55, 247–257. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, S.; Xu, W.; Zhou, Y.; Luan, H.; Wang, D. Resveratrol Decreases Local Inflammatory Markers and Systemic Endotoxin in Patients with Aggressive Periodontitis. Medicine 2022, 101, e29393. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Dvořák, J.; Rosiak, N.; Tykarska, E.; Szymańska, E.; Winnickaet, K.; Ruchała, M.A.; Cielecka-Piontek, J. Buccal Resveratrol Delivery System as a Potential New Concept for the Periodontitis Treatment. Pharmaceutics 2021, 13, 417. [Google Scholar] [CrossRef]
- Sterer, N.; Nuas, S.; Mizrahi, B.; Goldenberg, C.; Weiss, E.; Domb, A.; Perez-Davidi, M. Oral Malodor Reduction by a Palatal Mucoadhesive Tablet Containing Herbal Formulation. J. Dent. 2008, 36, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Porto, I.C.C.M.; Rocha, A.B.B.; Ferreira, I.I.S.; Nascimento, T.G.D.; de Freitas, J.M.D.; de Freitas, J.D.; de Freitas, J.D. Polyphenols and Brazilian Red Propolis Incorporated into a Total-Etching Adhesive System Help in Maintaining Bonding Durability. Heliyon 2021, 7, e06237. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Sadarani, B.; Majumdar, A. Optimization and Evaluation of Mucoadhesive Buccal Films Loaded with Resveratrol. J. Drug Deliv. Sci. Technol. 2018, 47, 433–442. [Google Scholar] [CrossRef]
- Vecchi, C.F.; Cesar, G.B.; De Souza, P.R.; Caetano, W.; Bruschi, M.L. Mucoadhesive Polymeric Films Comprising Polyvinyl Alcohol, Polyvinylpyrrolidone, and Poloxamer 407 For Pharmaceutical Applications. Pharm. Dev. Technol. 2021, 26, 138–149. [Google Scholar] [CrossRef]
- Nawrot-Hadzik, I.; Ślusarczyk, S.; Granica, S.; Hadzik, J.; Matkowski, A. Phytochemical Diversity in Rhizomes of Three Reynoutria Species and their Antioxidant Activity Correlations Elucidated by LC-ESI-MS/MS Analysis. Molecules 2019, 24, 1136. [Google Scholar] [CrossRef]
- Dołowacka-Jóźwiak, A.; Nawrot-Hadzik, I.; Matkowski, A.; Nowakowski, P.; Dudek-Wicher, R.; Markowska, D.; Adamski, R.; Krzyżanowska-Gołąb, D.; Karolewicz, B. Optimization of Cellulose Derivative-, PVA-, and PVP-Based Films with Reynoutria Japonica Extract to Improve Periodontal Disease Treatment. Materials 2024, 17, 6205. [Google Scholar] [CrossRef]
- Registration of Medicinal Products, Medical Devices and Biocidal Products. Polish Pharmacopoeia, 13th ed.; Registration of Medicinal Products, Medical Devices and Biocidal Products: Warsaw, Poland, 2023. [Google Scholar]

| Formulation | FR1 | FE1 | FR2 | FE2 | FR3 | FE3 |
|---|---|---|---|---|---|---|
| Average thickness [mm] | 0.14 ± 0.01 | 0.15 ± 0.01 | 0.15 ± 0.01 | 0.15 ± 0.01 | 0.18 ± 0.01 | 0.22 ± 0.02 |
| Average mass [mg] | 157 ± 3.0 | 138 ± 2.5 | 129 ± 1.8 | 138 ± 2.8 | 154 ± 4.0 | 159 ± 2.5 |
| Average mucoadhesiveness [g] | 197.22 ± 0.75 | 197.45 ± 0.5 | 261.11 ± 0.5 | 299.43 ± 0.38 | 175.78 ± 0.36 | 180.33 ± 0.6 |
| Formulation | FR1 | FE1 | FR2 | FE2 | FR3 | FE3 |
|---|---|---|---|---|---|---|
| Average contact angle [°] | 48.76 ± 0.01 | 48.87 ± 0.01 | 55.07 ± 0.02 | 58.28 ± 0.02 | 48.45 ± 0.01 | 49.02 ± 0.01 |
| Test | RSV Release [%] | ||||
|---|---|---|---|---|---|
| FR1 | FR2 | FR3 | FE1 | FE2 | |
| Distilled water | 72.06 ± 2.21 | 72.42± 2.32 | 65.10 ± 1.75 | 87.84 ± 1.97 | 77.23 ± 2.11 |
| Phosphate buffer | 73.15 ± 2.99 | 84.12 ± 2.41 | 57.71 ± 1.32 | 66.70 ± 1.03 | 78.70 ± 2.36 |
| Artificial saliva | 39.86 ± 1.04 | 49.74 ± 0.99 | 18.28 ± 0.87 | 48.80 ± 1.01 | 49.70 ± 1.04 |
| Formulation | Medium | pH | Observed Disintegration Behavior |
|---|---|---|---|
| FR1 | Distilled water | 7.10 | Partially disintegrated |
| Phosphate buffer | 6.94 | Partially disintegrated | |
| Glycol | 8.04 | Intact | |
| DMSO | 11.83 | Intact, rolled | |
| Artificial saliva | 7.60 | Partially disintegrated | |
| FR2 | Distilled water | 7.33 | Partially disintegrated |
| Phosphate buffer | 6.91 | Intact, bulged | |
| Glycol | 7.86 | Intact | |
| DMSO | 11.71 | Intact, rolled | |
| Artificial saliva | 7.36 | Intact, flexible | |
| FR3 | Distilled water | 7.20 | Complete disintegration |
| Phosphate buffer | 6.90 | Partially disintegrated | |
| Glycol | 7.76 | Intact | |
| DMSO | 11.86 | Intact | |
| Artificial saliva | 7.65 | Intact, flexible | |
| FE1 | Distilled water | 7.12 | Partially disintegrated |
| Phosphate buffer | 6.90 | Partially disintegrated | |
| Glycol | 7.98 | Intact | |
| DMSO | 11.38 | Intact, rolled | |
| Artificial saliva | 7.43 | Partially disintegrated | |
| FE2 | Distilled water | 7.43 | Complete disintegration |
| Phosphate buffer | 6.91 | Partially disintegrated | |
| Glycol | 8.05 | Intact | |
| DMSO | 11.31 | Intact, rolled | |
| Artificial saliva | 7.37 | Complete disintegration | |
| FE3 | Distilled water | 6.80 | Complete disintegration |
| Phosphate buffer | 6.88 | Partially disintegrated | |
| Glycol | 7.54 | Intact | |
| DMSO | 11.33 | Intact, transparent | |
| Artificial saliva | 7.42 | Intact, flexible |
| Polymers and Additives | Formulation | |||||
|---|---|---|---|---|---|---|
| FR1 | FR2 | FR3 | FE1 | FE2 | FE3 | |
| PVA | + | + | + | + | + | + |
| PVP | + | + | - | + | + | + |
| MC 400 cP | + | - | + | + | - | + |
| NaCMC | + | + | + | + | + | - |
| MCA 15C | - | + | - | - | + | - |
| Water (W) | + | + | - | + | + | - |
| Propylene Glycol (PGE) | + | + | + | + | + | + |
| Ethanol 25% (EtOH) (aqueous solution) | + | + | + | + | + | + |
| Ingredient | Quantity |
|---|---|
| PVA 2.5% | 14.31 g |
| PVP 5% | 8.45 g |
| RSV | 0.395 mg in 3 mL 25% EtOH |
| MC 400 cP 5% | 22.76 g |
| NaCMC 4% | 19.51 g |
| Water | 2 mL |
| Glycerol 85% | 1.95 g |
| Ingredient | Quantity |
|---|---|
| PVA 2.5% | 14.31 g |
| PVP 5% | 8.45 g |
| R. japonica extract | 200 mg in 3 mL 25% EtOH |
| MC 400 cP 5% | 22.76 g |
| NaCMC 4% | 19.51 g |
| Water | 2 mL |
| Glycerol 85% | 1.95 g |
| Ingredient | Quantity |
|---|---|
| PVA 2.5% | 14.29 g |
| PVP 5% | 8.45 g |
| RSV | 0.395 mg in 3 mL 25% EtOH |
| MCA 15C% | 22.75 g |
| NaCMC 4% | 19.52 g |
| Water | 2 mL |
| Glycerol 85% | 1.95 g |
| Ingredient | Quantity |
|---|---|
| PVA 2.5% | 14.29 g |
| PVP 5% | 8.45 g |
| R. japonica extract | 200 mg in 3 mL 25% EtOH |
| MCA 15C 3% | 22.75 g |
| NaCMC 4% | 19.52 g |
| Water | 2 mL |
| Glycerol 85% | 1.95 g |
| Ingredient | Quantity |
|---|---|
| PVA 2.5% | 22.24 g |
| RSV | 0.395 mg in 3 mL 25% EtOH |
| MC 400 cP 5% | 33.35 g |
| NaCMC 4% | 10 g |
| Glycerol 85% | 2.4 g |
| Ingredient | Quantity |
|---|---|
| PVA 2.5% | 38 g |
| PVP 5% | 10 g |
| R. japonica extract | 200 mg in 3 mL 25% EtOH |
| MC 400 cP 5% | 57 g |
| Glycerol 85% | 5 g |
| FR1/FE1 | FR2/FE2 | FR3 | FE3 |
|---|---|---|---|
| PVA + PVP 🔽 Mixing 30 min Cooling (−18 °C) 18 h 🔽 + Extract/RSV, Mixing 2 h Cooling (−18 °C) 17 h 🔽 Ultrasounds 15 min Freezing 1 h Ultrasounds 15 min Cooling (−18 °C) 19 h Mixing 1.5 h 🔽 + MC 400 cP + NaCMC, Mixing 2 h, Cooling (−18 °C) 19 h 🔽 + Glycerol, Mixing 3 h, Cooling (−18 °C) 16 h 🔽 + Water, Mixing 1.5 h 🔽 5 × freezing and 30 min of mixing after each freezing 🔽 4 × sonification, 30 min after each cooling for 45 min in −18 °C 🔽 Pouring 30 g/vacuum–0.04 Pa 🔽 Drying 48 h at 37 °C | PVA + PVP 🔽 Mixing 30 min Cooling (−18 °C) 18 h 🔽 + Extract/RSV, Mixing 2 h, Cooling (−18 °C) 17 h 🔽 Ultrasounds 15 min Freezing 1 h Ultrasounds 15 min Cooling (−18 °C) 19 h Mixing 1.5 h 🔽 + MC A15C + NaCMC, Mixing 12 h, Cooling 19 h 🔽 + Glycerol, Mixing 3 h, Cooling (−18 °C) 16 h 🔽 + Water, Mixing 1.5 h 🔽 5 × freezing, 30 min of mixing after each freezing 🔽 4 × sonification, 30 min after each cooling for 45 min in −18 °C 🔽 Pouring 30 g/vacuum–0.04 Pa 🔽 Drying 48 h at 37 °C | PVA + RSV, Mixing 2 h Cooling (−18 °C) 17 h 🔽 Ultrasounds 15 min Freezing 1 h Ultrasounds 15 min Cooling (−18 °C) 19 h Mixing 1.5 h 🔽 + NaCMC, Mixing 2 h, Cooling (−18 °C) 19 h 🔽 + Glycerol, Mixing 3 h, Cooling (−18 °C) 16 h 🔽 5 × freezing + 30 min of mixing after each freezing 🔽 4 × sonification, 30 min after each cooling for 45 min in −18 °C 🔽 Pouring 30 g/vacuum–0.04 Pa 🔽 Drying 48 h at 37 °C | PVA + PVP 🔽 Mixing 30 min Cooling (−18 °C) 18 h 🔽 + Extract, Mixing 2 h, Cooling (−18 °C) 17 h 🔽 Ultrasounds 15 min Freezing 1 h Ultrasounds 15 min Cooling (−18 °C) 19 h Mixing 1.5 h 🔽 + MC 400 cP Mixing 2 h, Cooling (−18 °C) 19 h 🔽 + Glycerol, Mixing 3 h, Cooling (−18 °C) 16 h 🔽 5 × freezing and 30 min of mixing after each freezing 🔽 4 × sonification, 30 min after each cooling for 45 min in −18 °C 🔽 Pouring 30 g/vacuum–0.04 Pa 🔽 Drying 48 h at 37 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dołowacka-Jóźwiak, A.; Nawrot-Hadzik, I.; Matkowski, A.; Ciecieląg, T.; Gawin-Mikołajewicz, A.; Dudek-Wicher, R.; Prochoń, M.; Markowska, D.; Adamski, R.; Wiater, A.; et al. Mucoadhesive PVA Film for Sustained Resveratrol Delivery: Formulation, Characterization, and Release Profile. Molecules 2025, 30, 2642. https://doi.org/10.3390/molecules30122642
Dołowacka-Jóźwiak A, Nawrot-Hadzik I, Matkowski A, Ciecieląg T, Gawin-Mikołajewicz A, Dudek-Wicher R, Prochoń M, Markowska D, Adamski R, Wiater A, et al. Mucoadhesive PVA Film for Sustained Resveratrol Delivery: Formulation, Characterization, and Release Profile. Molecules. 2025; 30(12):2642. https://doi.org/10.3390/molecules30122642
Chicago/Turabian StyleDołowacka-Jóźwiak, Arleta, Izabela Nawrot-Hadzik, Adam Matkowski, Tomasz Ciecieląg, Agnieszka Gawin-Mikołajewicz, Ruth Dudek-Wicher, Mirosława Prochoń, Dorota Markowska, Robert Adamski, Adrian Wiater, and et al. 2025. "Mucoadhesive PVA Film for Sustained Resveratrol Delivery: Formulation, Characterization, and Release Profile" Molecules 30, no. 12: 2642. https://doi.org/10.3390/molecules30122642
APA StyleDołowacka-Jóźwiak, A., Nawrot-Hadzik, I., Matkowski, A., Ciecieląg, T., Gawin-Mikołajewicz, A., Dudek-Wicher, R., Prochoń, M., Markowska, D., Adamski, R., Wiater, A., & Karolewicz, B. L. (2025). Mucoadhesive PVA Film for Sustained Resveratrol Delivery: Formulation, Characterization, and Release Profile. Molecules, 30(12), 2642. https://doi.org/10.3390/molecules30122642










