Chemical Fate of Ascorbic Acid in Wheat Flour Extract: Impact of Dissolved Molecular Oxygen (O2), Metal Ions, Wheat Endogenous Enzymes and Glutathione (GSH)
Abstract
1. Introduction
2. Results and Discussion
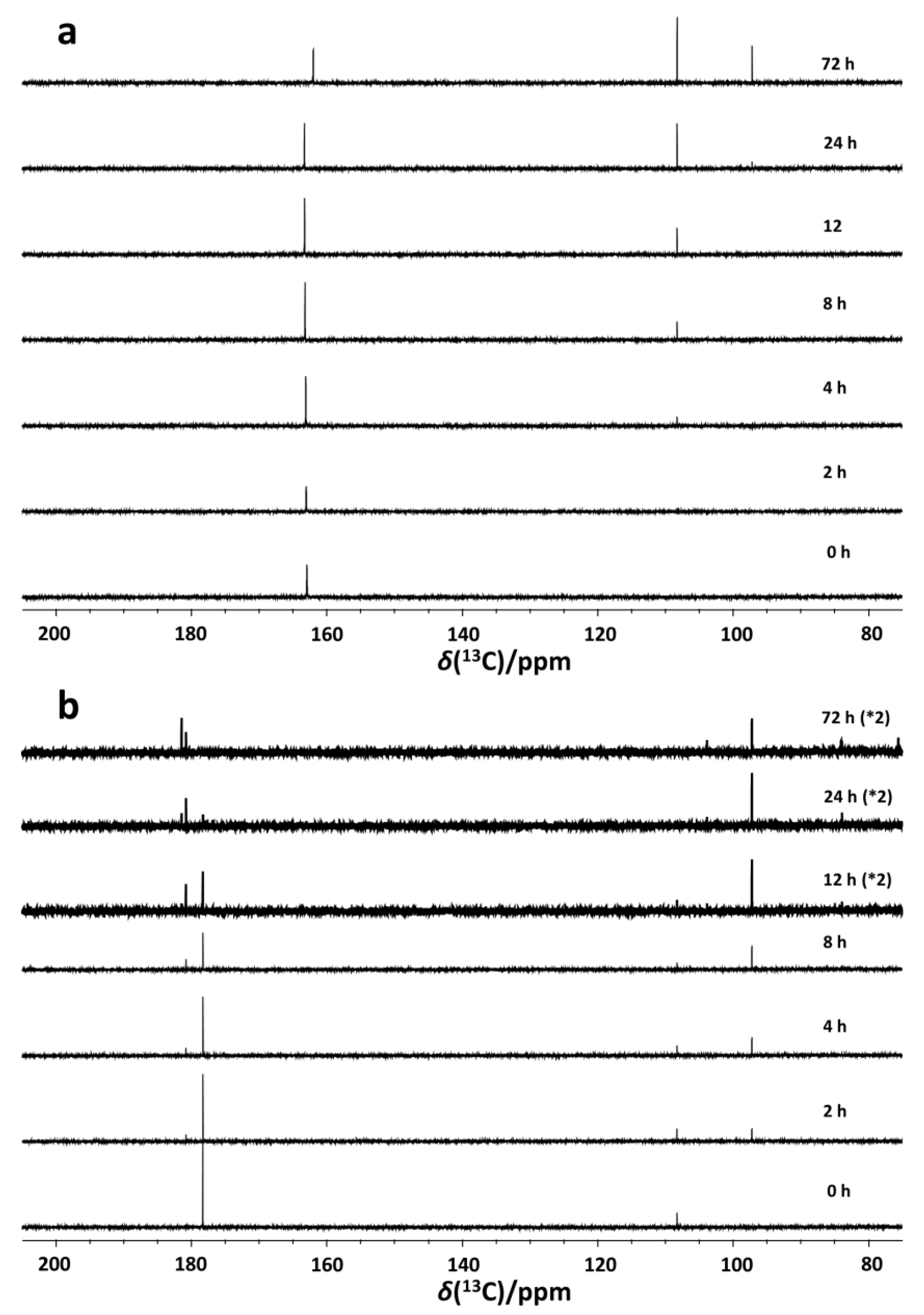
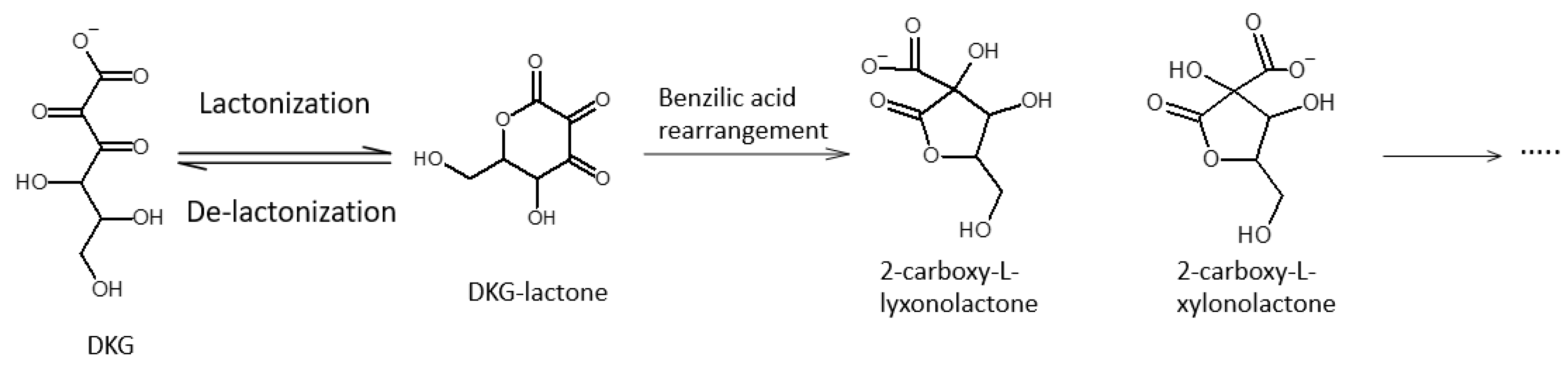
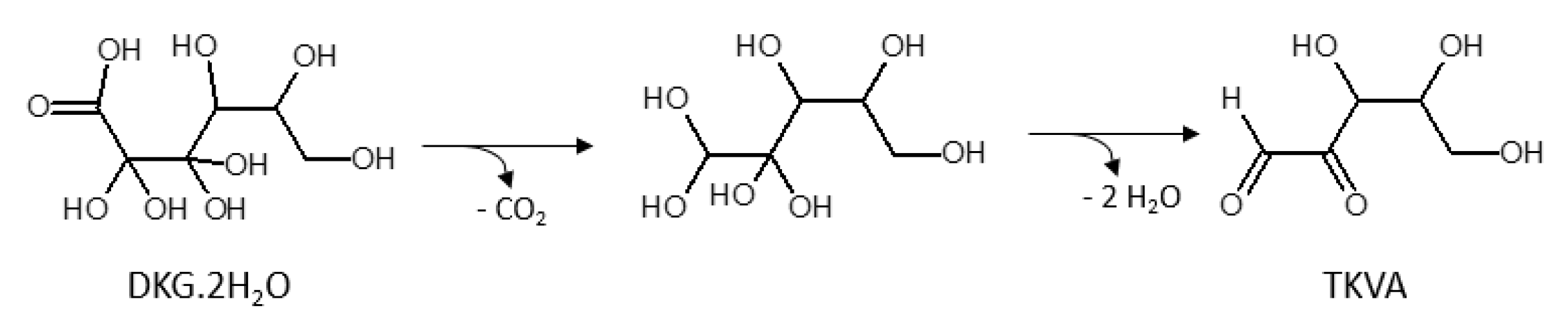
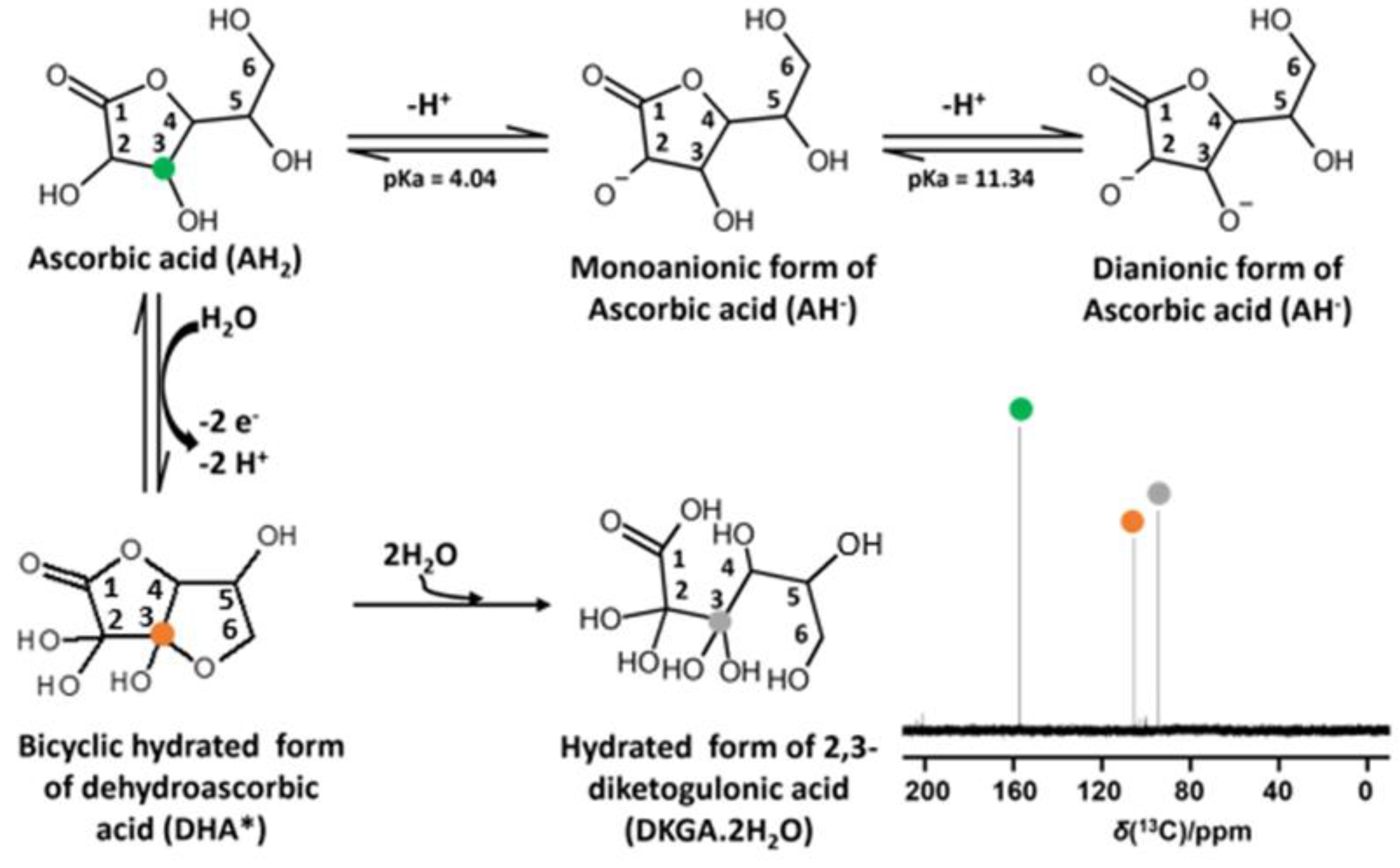
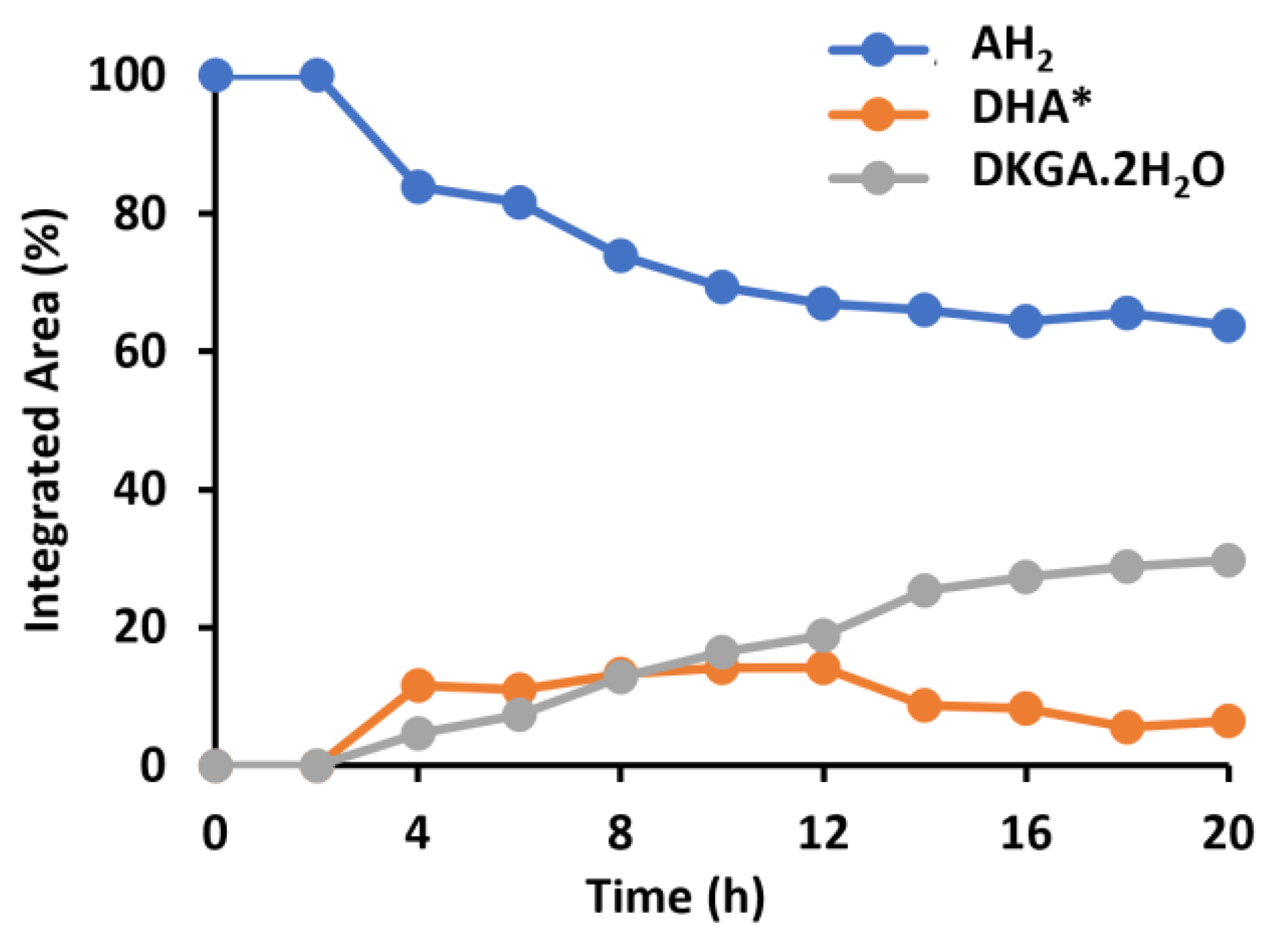
2.1. Identification of Ascorbic Acid and Its Reaction Products in Water at Ambient Conditions
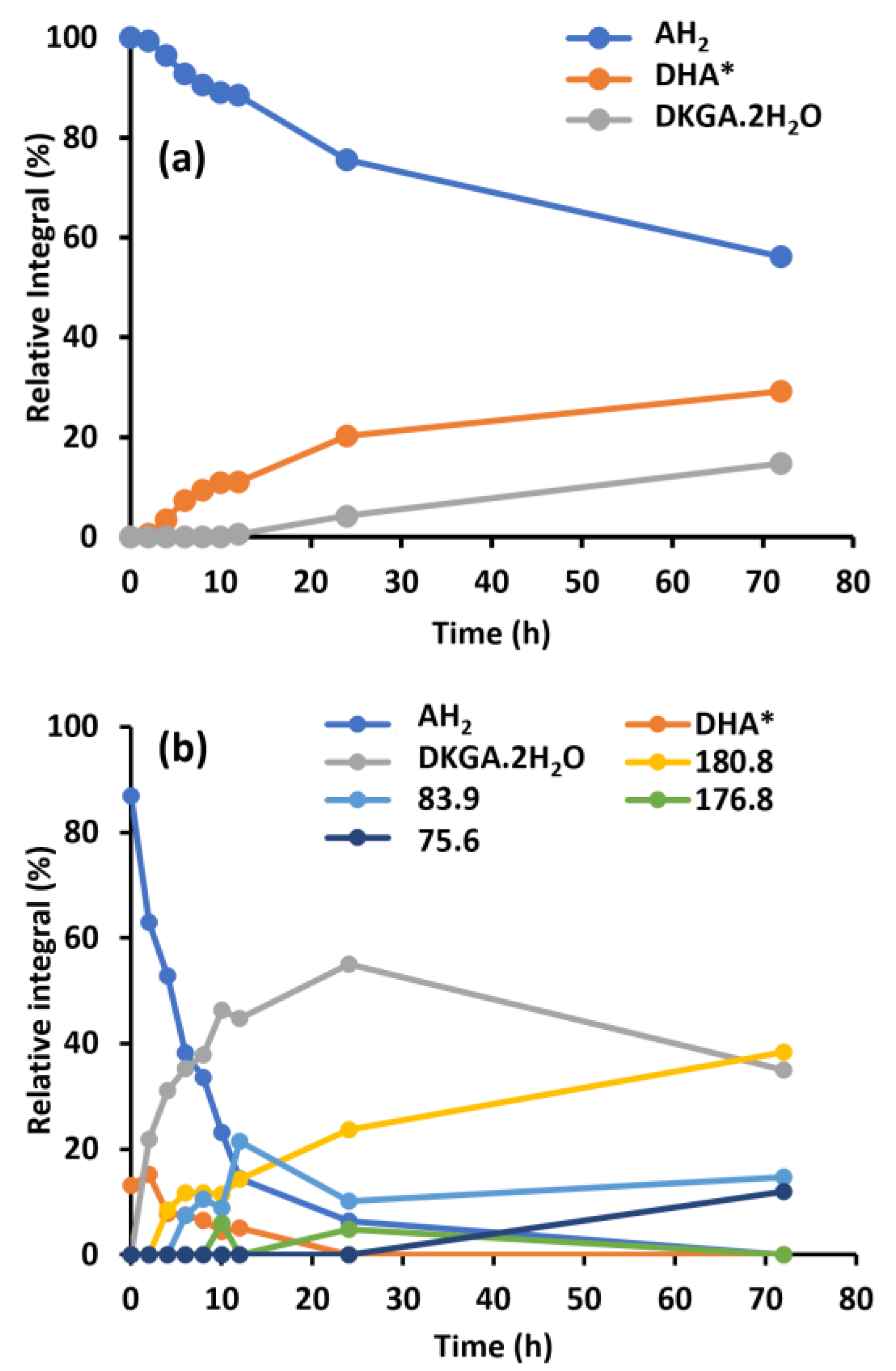
2.2. Impact of Molecular Oxygen on the Chemical Conversion of AH2
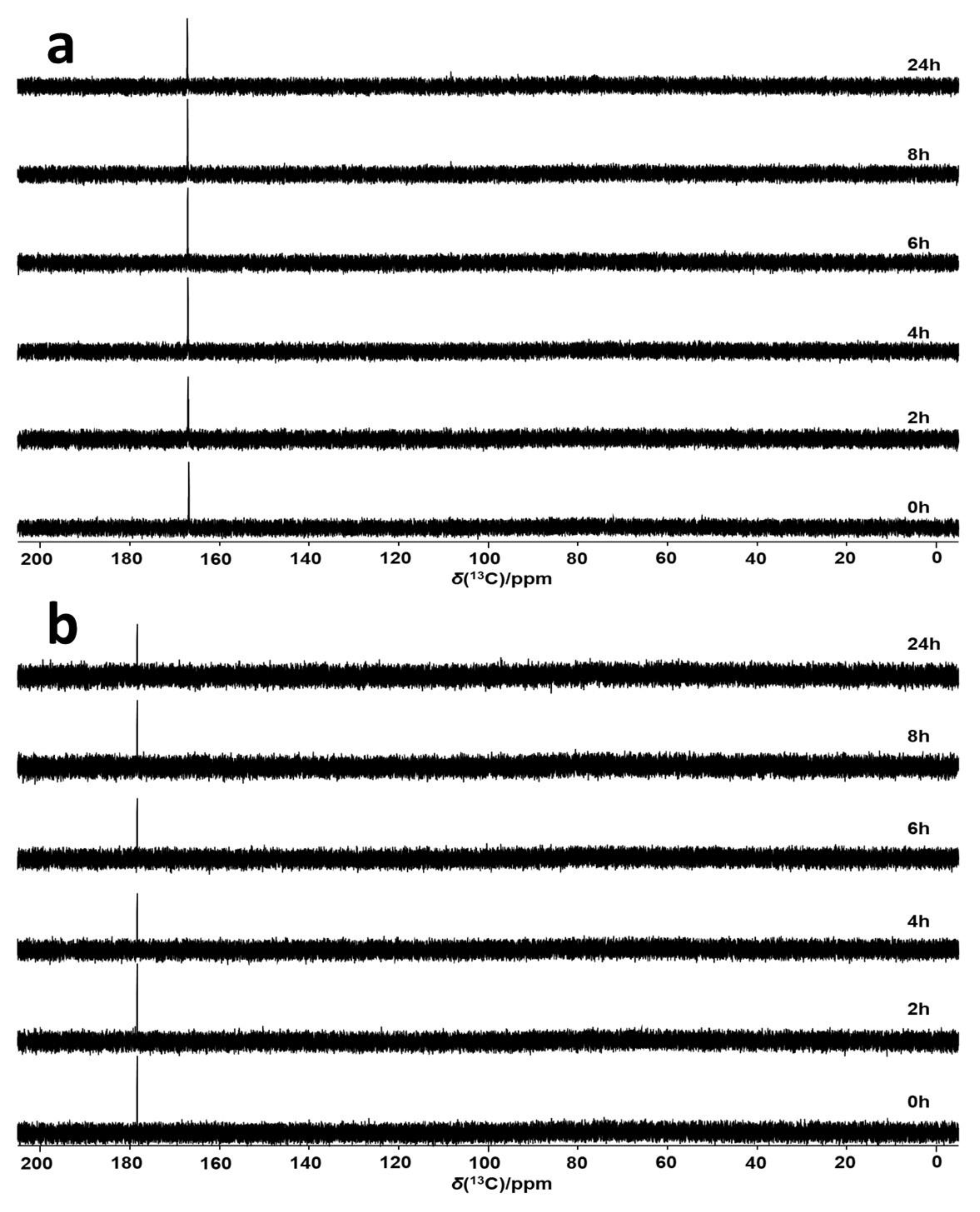
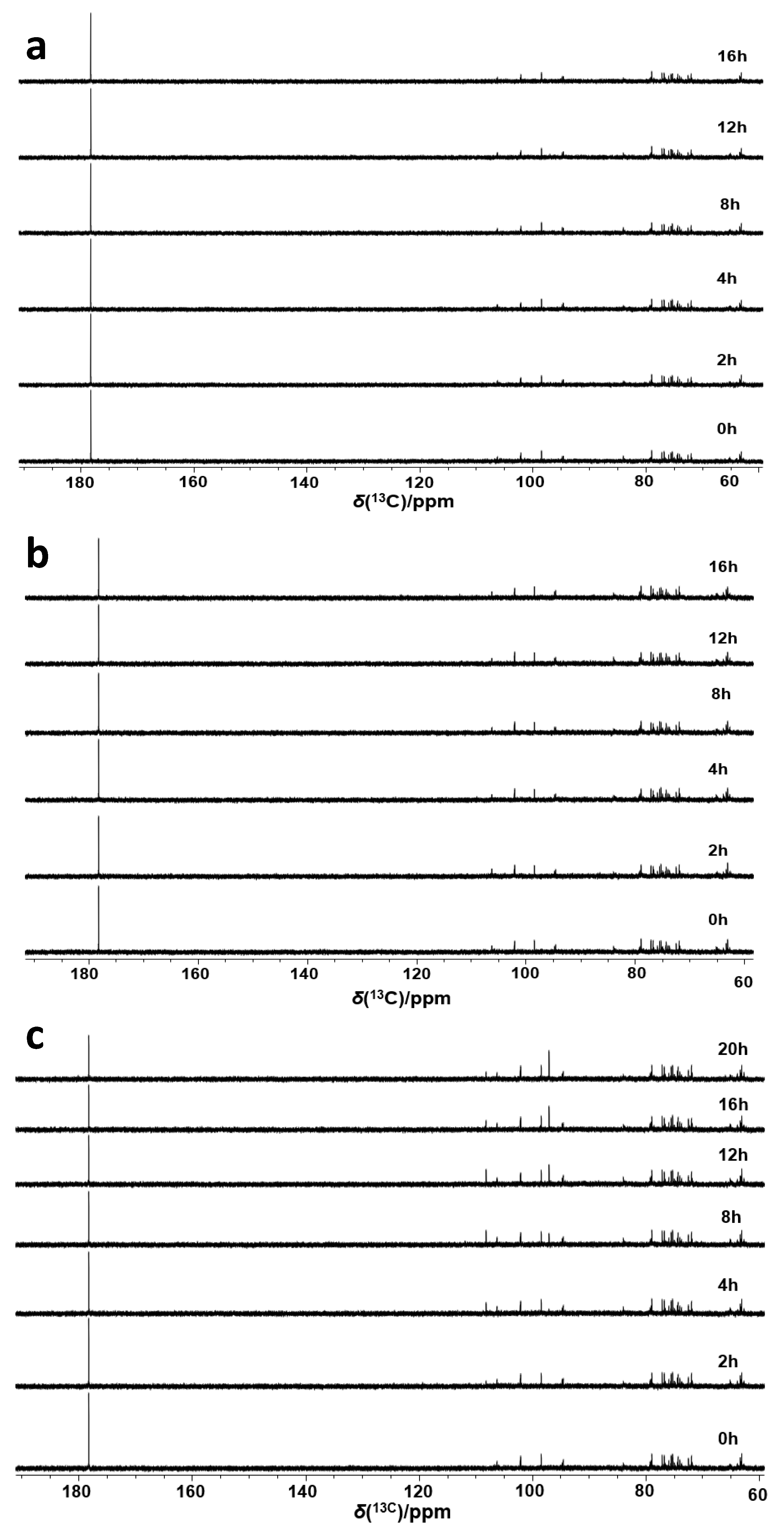
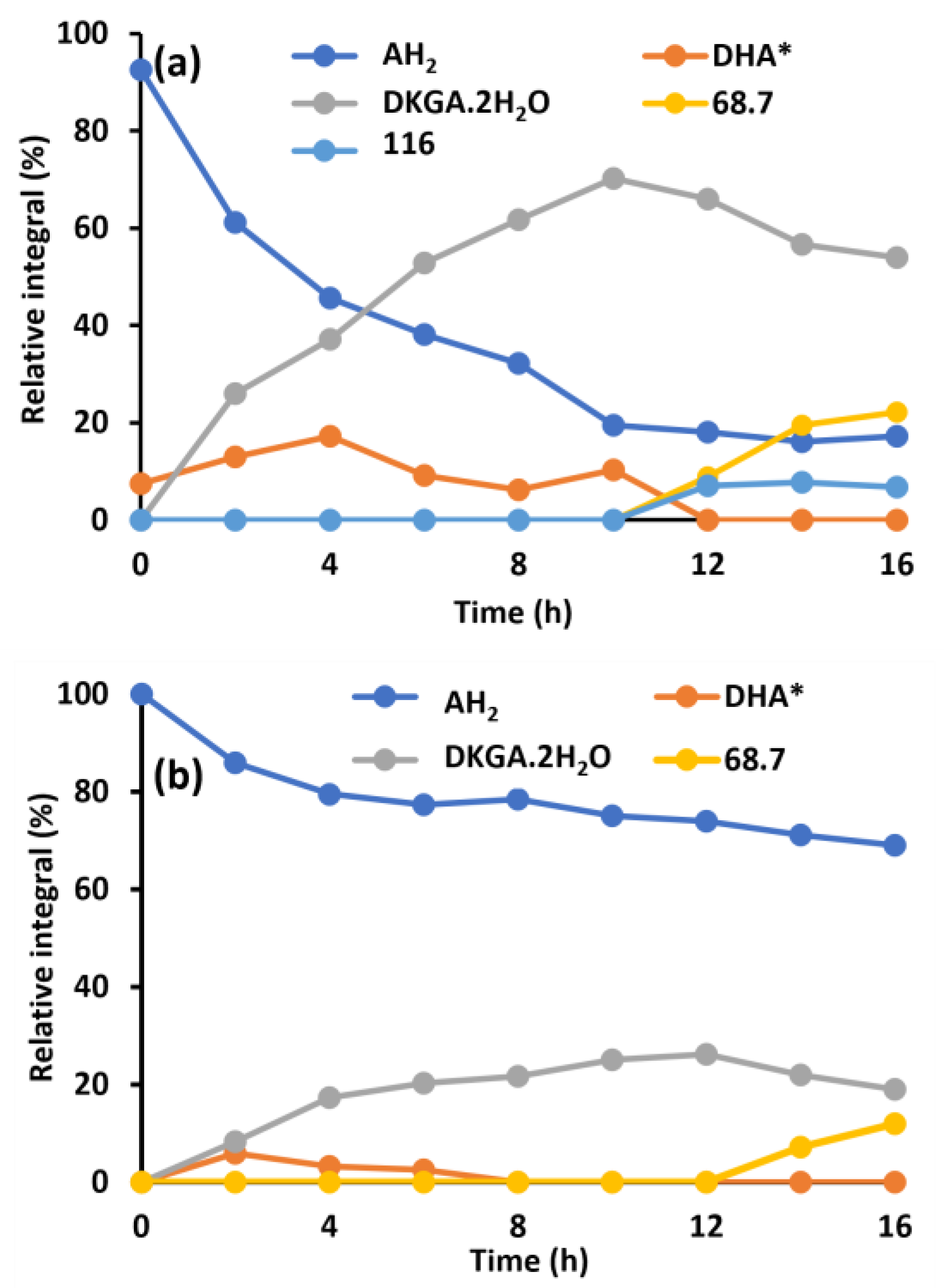
2.3. Enzymatic Oxidation of Ascorbic Acid
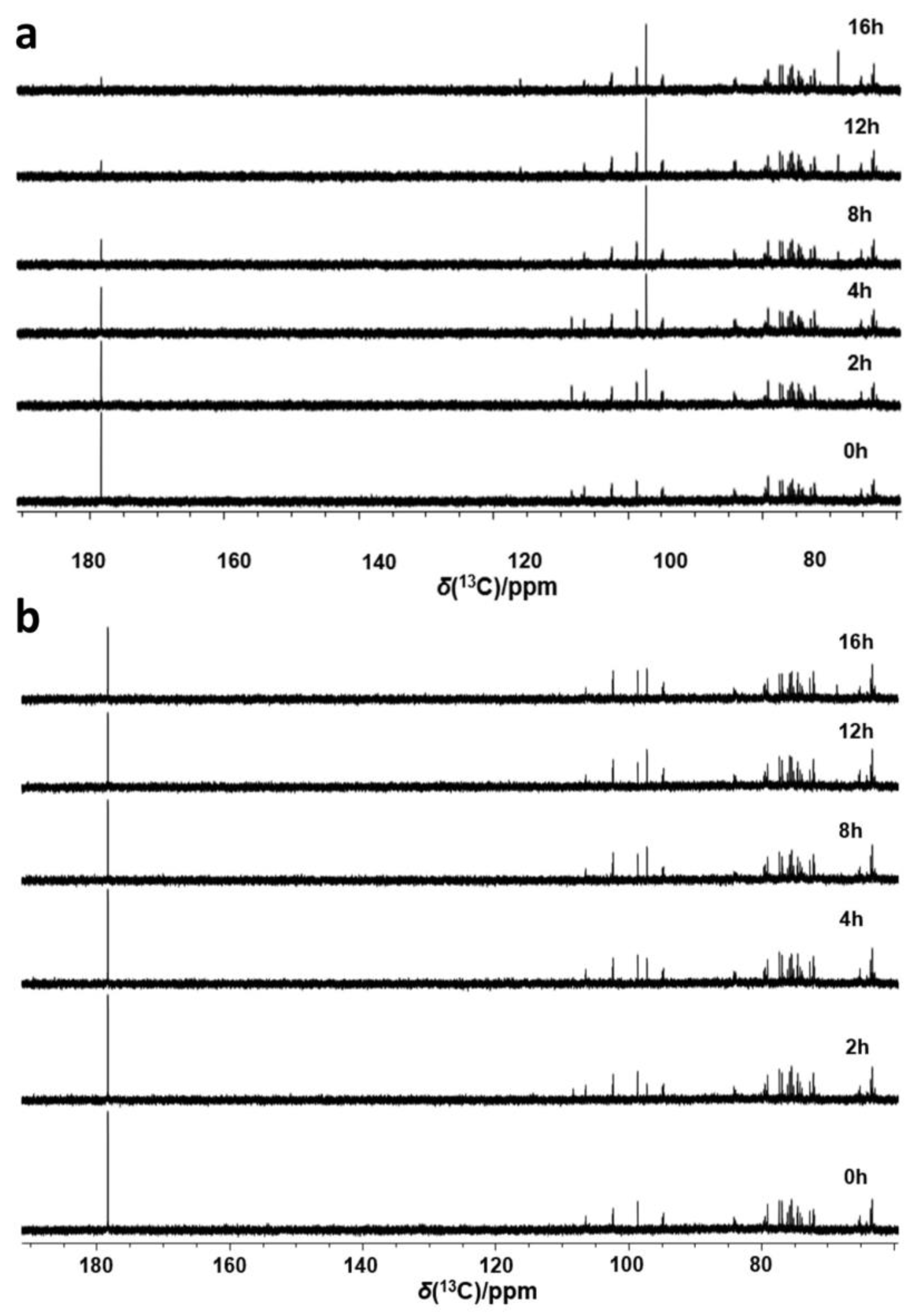
2.4. The Effect of Glutathione on the Conversion of Ascorbic Acid
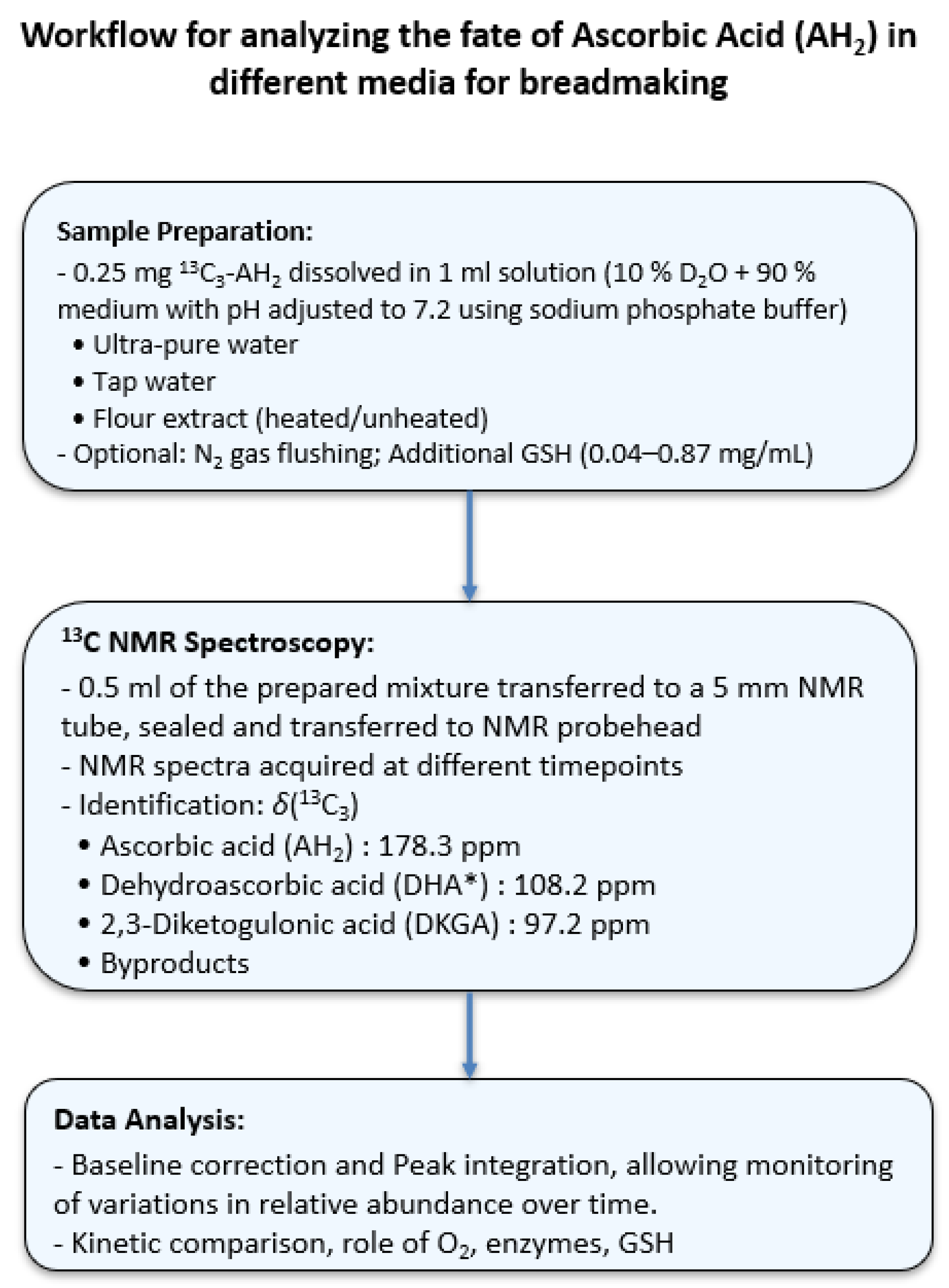
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of the Different Aqueous Solutions
3.3. 13C Liquid-State Nuclear Magnetic Resonance Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sample | Solvent Used | Prepared Under Atmospheric Conditions or Flushed with N2 |
|---|---|---|
| AH2 in Ultra-pure water | Ultra-pure water (MilliQ, Millipore) | Atmospheric conditions |
| AH2 in Tap water | Tap water | Atmospheric conditions |
| AH2 in Ultra-pure water—N2 treated | Ultra-pure water (MilliQ, Millipore) | Flushed with N2 |
| AH2 in Tap water—N2 treated | Tap water | Flushed with N2 |
| AH2 in Flour extract | 1 Flour extract in tap water | Atmospheric conditions |
| AH2 in Heated flour extract | 1 Heat-treated (70 °C—30 min) flour extract | Atmospheric conditions |
| AH2 in Flour extract—N2 treated | 1 Flour extract in tap water | Flushed with N2 |
| AH2-Flour + 0.04 GSH-Atm | 0.04 mg/mL GSH added to the flour extract | Atmospheric conditions |
| AH2-Flour + 0.44 GSH-Atm | 0.44 mg/mL GSH added to the flour extract | Atmospheric conditions |
| AH2-Flour + 0.87 GSH-Atm | 0.87 mg/mL GSH added to the flour extract | Atmospheric conditions |
| Transition Metal Ion | Concentration (µg/L) | |
|---|---|---|
| Pure Water | Tap Water | |
| Zn | 5.71 | 89.76 |
| Fe | 2.91 | 50.88 |
| Cu | 0.30 | 23.54 |
| Cd | 0.30 | 1.17 |
| Ni | 0.14 | 1.20 |
| Mn | 0.05 | 0.78 |
| Mo | 0.03 | 0.96 |
| Co | 0.01 | −0.01 |
References
- Ali, A.; Riaz, S.; Khalid, W.; Fatima, M.; Mubeen, U.; Babar, Q.; Manzoor, M.F.; Zubair Khalid, M.; Madilo, F.K. Potential of Ascorbic Acid in Human Health against Different Diseases: An Updated Narrative Review. Int. J. Food Prop. 2024, 27, 493–515. [Google Scholar] [CrossRef]
- Mittu, B.; Bhat, Z.R.; Chauhan, A.; Kour, J.; Behera, A.; Kaur, M. Ascorbic Acid; Elsevier Inc.: Amsterdam, The Netherlands, 2021; ISBN 9780323897792. [Google Scholar]
- Naidu, K.A. Vitamin C in Human Health and Disease Is Still a Mystery? An Overview. Funct. Foods Connect. Nutr. Health Food Sci. 2013, 10, 145–168. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Levine, M. Vitamin C: The Known and the Unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef] [PubMed]
- Joye, I.J.; Lagrain, B.; Delcour, J.A. Use of Chemical Redox Agents and Exogenous Enzymes to Modify the Protein Network during Breadmaking–A Review. J. Cereal Sci. 2009, 50, 11–21. [Google Scholar] [CrossRef]
- Koehler, P. Effect of Ascorbic Acid in Dough: Reaction of Oxidized Glutathione with Reactive Thiol Groups of Wheat Glutelin. J. Agric. Food Chem. 2003, 51, 4954–4959. [Google Scholar] [CrossRef] [PubMed]
- Beghin, A.S.; Ooms, N.; Brijs, K.; Pareyt, B.; Moldenaers, P.; Delcour, J.A. How Yeast Impacts the Effect of Ascorbic Acid on Wheat Flour Dough Extensional Rheology. Food Biophys. 2021, 16, 406–414. [Google Scholar] [CrossRef]
- Grosch, W.; Wieser, H. Redox Reactions in Wheat Dough as Affected by Ascorbic Acid. J. Cereal Sci. 1999, 29, 1–16. [Google Scholar] [CrossRef]
- Every, D.; Gilpin, M.J.; Larsen, N.G. Continuous Spectrophotometric Assay and Properties of Ascorbic Acid Oxidising Factors in Wheat. J. Cereal Sci. 1995, 21, 231–239. [Google Scholar] [CrossRef]
- Every, D.; Simmons, L.D.; Ross, M.P. Distribution of Redox Enzymes in Millstreams and Relationships to Chemical and Baking Properties of Flour. Cereal Chem. 2006, 83, 62–68. [Google Scholar] [CrossRef]
- Schofield, J.D.; Chen, X. Analysis of Free Reduced and Free Oxidised Glutathione in Wheat Flour. J. Cereal Sci. 1995, 21, 127–136. [Google Scholar] [CrossRef]
- Every, D.; Simmons, L.; Sutton, K.H.; Ross, M. Studies on the Mechanism of the Ascorbic Acid Improver Effect on Bread Using Flour Fractionation and Reconstitution Methods. J. Cereal Sci. 1999, 30, 147–158. [Google Scholar] [CrossRef]
- Kuninori, T.; Matsumoto, H. L-Ascorbic Acid Oxidizing System in Dough and Dough Improvement. Cereal Chem. 1963, 40, 647–657. [Google Scholar]
- Nakamura, M.; Kurata, T. Effect of L-Ascorbic Acid on the Rheological Properties of Wheat Flour-Water Dough. Cereal Chem. 1997, 74, 647–650. [Google Scholar] [CrossRef]
- Li, W.; Bollecker, S.S.; Schofield, J.D. Glutathione and Related Thiol Compounds. I. Glutathione and Related Thiol Compounds in Flour. J. Cereal Sci. 2004, 39, 205–212. [Google Scholar] [CrossRef]
- Dong, W.; Hoseney, R.C. Effects of Certain Breadmaking Oxidants and Reducing Agents on Dough Rheological Properties. Cereal Chem. 1995, 72, 58–64. [Google Scholar]
- Washko, P.W.; Welch, R.W.; Dhariwal, K.R.; Wang, Y.; Levine, M. Ascorbic Acid and Dehydroascorbic Acid Analyses in Biological Samples. Anal. Biochem. 1992, 204, 1–14. [Google Scholar] [CrossRef]
- Paukstelis, J.V.; Mueller, D.D.; Seib, P.A.; Lillard, D.W. NMR Spectroscopy of Ascorbic Acid and Its Derivatives. In Ascorbic Acid: Chemistry, Metabolism, and Uses; ACS Publications: Washington, DC, USA, 1982; pp. 125–151. [Google Scholar]
- Tajmir-Riahi, H.A. Coordination Chemistry of Vitamin C. Part II. Interaction of L-Ascorbic Acid with Zn(II), Cd(II), Hg(II), and Mn(II) Ions in the Solid State and in Aqueous Solution. J. Inorg. Biochem. 1991, 42, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Albertino, A.; Barge, A.; Cravotto, G.; Genzini, L.; Gobetto, R.; Vincenti, M. Natural Origin of Ascorbic Acid: Validation by 13C NMR and IRMS. Food Chem. 2009, 112, 715–720. [Google Scholar] [CrossRef]
- Kerber, R.C. “As Simple as Possible, but Not Simpler”—The Case of Dehydroascorbic Acid. J. Chem. Educ. 2008, 85, 1237. [Google Scholar] [CrossRef]
- Hvoslef, J.; Pedersen, B.; Wennerström, O.; Enzell, C.R.; Åkeson, Å.; Lundquist, G. The Structure of Dehydroascorbic Acid in Solution. Acta Chem. Scand. 1979, 33b, 503–511. [Google Scholar] [CrossRef]
- Tolbert, B.M.; Ward, J.B. Dehydroascorbic Acid. Ascorbic Acid Chem. Metab. Uses 1982, 200, 101–123. [Google Scholar]
- Kang, S.-O.; Sapper, H.; Lohmann, W. The Oxidative Degradation of ʟ-Ascorbic Acid via an α-Ketoaldehyde. Zeitschrift für Naturforsch. C 1982, 37, 1064–1069. [Google Scholar] [CrossRef]
- Dewhirst, R.A.; Murray, L.; Mackay, C.L.; Sadler, I.H.; Fry, S.C. Characterisation of the Non-Oxidative Degradation Pathway of Dehydroascorbic Acid in Slightly Acidic Aqueous Solution. Arch. Biochem. Biophys. 2020, 681, 108240. [Google Scholar] [CrossRef]
- Buettner, G.R.; Jurkiewicz, B.A. Catalytic Metals, Ascorbate and Free Radicals: Combinations to Avoid. Radiat. Res. 1996, 145, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.T.; Martell, A.E. Metal Ion and Metal Chelate Catalyzed Oxidation of Ascorbic Acid by Molecular Oxygen. I. Cupric and Ferric Ion Catalyzed Oxidation. J. Am. Chem. Soc. 1967, 89, 4176–4185. [Google Scholar] [CrossRef]
- Liao, M.L.; Seib, P.A. Chemistry of L-Ascorbic Acid Related to Foods. Food Chem. 1988, 30, 289–312. [Google Scholar] [CrossRef]
- Moya, H.D.; Coichev, N. Kinetic Studies of the Oxidation of L-Ascorbic Acid by Tris(Oxalate)Cobaltate in the Presence of CDTA Metal Ion Complexes. J. Braz. Chem. Soc. 2006, 17, 364–368. [Google Scholar] [CrossRef]
- Jansson, P.J.; Jung, H.R.; Lindqvist, C.; Nordström, T. Oxidative Decomposition of Vitamin C in Drinking Water. Free Radic. Res. 2004, 38, 855–860. [Google Scholar] [CrossRef]
- Legay, C.; Rodriguez, M.J.; Sérodes, J.B.; Levallois, P. Estimation of Chlorination By-Products Presence in Drinking Water in Epidemiological Studies on Adverse Reproductive Outcomes: A Review. Sci. Total Environ. 2010, 408, 456–472. [Google Scholar] [CrossRef]
- Chwastowski, J.; Ciesielski, W.; Khachatryan, K.; Kołoczek, H.; Kulawik, D.; Oszczęda, Z.; Soroka, J.A.; Tomasik, P.; Witczak, M. Water of Increased Content of Molecular Oxygen. Water 2020, 12, 2488. [Google Scholar] [CrossRef]
- Sapper, H.; Institut für Biophysik der Justus-Liebig-Universität Gießen Sa-Ouk; Paul, H.-H.; Lohmann, W. The Reversibility of the Vitamin C Redox System: Electrochemical Reasons and Biological Aspects. Zeitschrift für Naturforschung C 1982, 37, 942–946. [Google Scholar] [CrossRef]
- Winkler, B.S.; Orselli, S.M.; Rex, T.S. The Redox Couple between Glutathione and Ascorbic Acid: A Chemical and Physiological Perspective. Free Radic. Biol. Med. 1994, 17, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Lauwers, K.; Breynaert, E.; Rombouts, I.; Delcour, J.A.; Kirschhock, C.E.A. Water Electrolyte Promoted Oxidation of Functional Thiol Groups. Food Chem. 2016, 197, 1235–1239. [Google Scholar] [CrossRef]
- Touitou, E.; Alkabes, M.; Memoli, A.; Alhaique, F. Glutathione Stabilizes Ascorbic Acid in Aqueous Solution. Int. J. Pharm. 1996, 133, 85–88. [Google Scholar] [CrossRef]
- Beghin, A.S.; Ooms, N.; Brijs, K.; Pareyt, B.; Delcour, J.A. Release of 14C-Labeled Carbon Dioxide from Ascorbic Acid during Straight Dough Wheat Bread Making. Cereal Chem. 2022, 99, 731–736. [Google Scholar] [CrossRef]
- Pfeilsticker, K.; Roeung, S. Charakterisierung Derl-Ascorbinsureoxidase (EC 1.10.3.3.) Aus Weizenmehl. Z. Fur Lebensm. Und-Forschungr Lebensm. Und-Forsch. 1982, 174, 306–308. [Google Scholar] [CrossRef]
- Harris, R.K.; Becker, E.D.; Cabral de Menezes, S.M.; Goodfellow, R.; Granger, P. NMR Nomenclature. Nuclear Spin Properties and Conventions for Chemical Shifts(IUPAC Recommendations 2001). Pure Appl. Chem. 2001, 73, 1795–1818. [Google Scholar] [CrossRef]
- Houlleberghs, M.; Hoffmann, A.; Dom, D.; Kirschhock, C.E.A.; Taulelle, F.; Martens, J.A.; Breynaert, E. Absolute Quantification of Water in Microporous Solids with 1 H Magic Angle Spinning NMR and Standard Addition. Anal. Chem. 2017, 89, 6940–6943. [Google Scholar] [CrossRef]
- Vanderschaeghe, H.; Houlleberghs, M.; Verheyden, L.; Dom, D.; Chandran, C.V.; Radhakrishnan, S.; Martens, J.A.; Breynaert, E. Absolute Quantification of Residual Solvent in Mesoporous Silica Drug Formulations Using Magic-Angle Spinning NMR Spectroscopy. Anal. Chem. 2022, 95, 1880–1887. [Google Scholar] [CrossRef]
- Chamas, A.; Qi, L.; Mehta, H.S.; Sears, J.A.; Scott, S.L.; Walter, E.D.; Hoyt, D.W. High Temperature/Pressure MAS-NMR for the Study of Dynamic Processes in Mixed Phase Systems. Magn. Reson. Imaging 2019, 56, 37–44. [Google Scholar] [CrossRef]
- De Man, W.L.; Chandran, C.V.; Wouters, A.G.B.; Radhakrishnan, S.; Martens, J.A.; Breynaert, E.; Delcour, J.A. Hydration of Wheat Flour Water-Unextractable Cell Wall Material Enables Structural Analysis of Its Arabinoxylan by High-Resolution Solid-State 13C MAS NMR Spectroscopy. J. Agric. Food Chem. 2022, 70, 10604–10610. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beghin, A.S.; Radhakrishnan, S.; Ooms, N.; Chandran, C.V.; Duerinckx, K.; Pareyt, B.; Brijs, K.; Delcour, J.A.; Breynaert, E. Chemical Fate of Ascorbic Acid in Wheat Flour Extract: Impact of Dissolved Molecular Oxygen (O2), Metal Ions, Wheat Endogenous Enzymes and Glutathione (GSH). Molecules 2025, 30, 2582. https://doi.org/10.3390/molecules30122582
Beghin AS, Radhakrishnan S, Ooms N, Chandran CV, Duerinckx K, Pareyt B, Brijs K, Delcour JA, Breynaert E. Chemical Fate of Ascorbic Acid in Wheat Flour Extract: Impact of Dissolved Molecular Oxygen (O2), Metal Ions, Wheat Endogenous Enzymes and Glutathione (GSH). Molecules. 2025; 30(12):2582. https://doi.org/10.3390/molecules30122582
Chicago/Turabian StyleBeghin, Alice S., Sambhu Radhakrishnan, Nand Ooms, C. Vinod Chandran, Karel Duerinckx, Bram Pareyt, Kristof Brijs, Jan A. Delcour, and Eric Breynaert. 2025. "Chemical Fate of Ascorbic Acid in Wheat Flour Extract: Impact of Dissolved Molecular Oxygen (O2), Metal Ions, Wheat Endogenous Enzymes and Glutathione (GSH)" Molecules 30, no. 12: 2582. https://doi.org/10.3390/molecules30122582
APA StyleBeghin, A. S., Radhakrishnan, S., Ooms, N., Chandran, C. V., Duerinckx, K., Pareyt, B., Brijs, K., Delcour, J. A., & Breynaert, E. (2025). Chemical Fate of Ascorbic Acid in Wheat Flour Extract: Impact of Dissolved Molecular Oxygen (O2), Metal Ions, Wheat Endogenous Enzymes and Glutathione (GSH). Molecules, 30(12), 2582. https://doi.org/10.3390/molecules30122582







