Protective Role of Whey Protein Isolate on MPP+-Induced Differentiation of SH-SY5Y Cells by Modulating the Nrf2 Antioxidant Pathway
Abstract
1. Introduction
2. Results
2.1. Total Amino Acids Contained in WPI
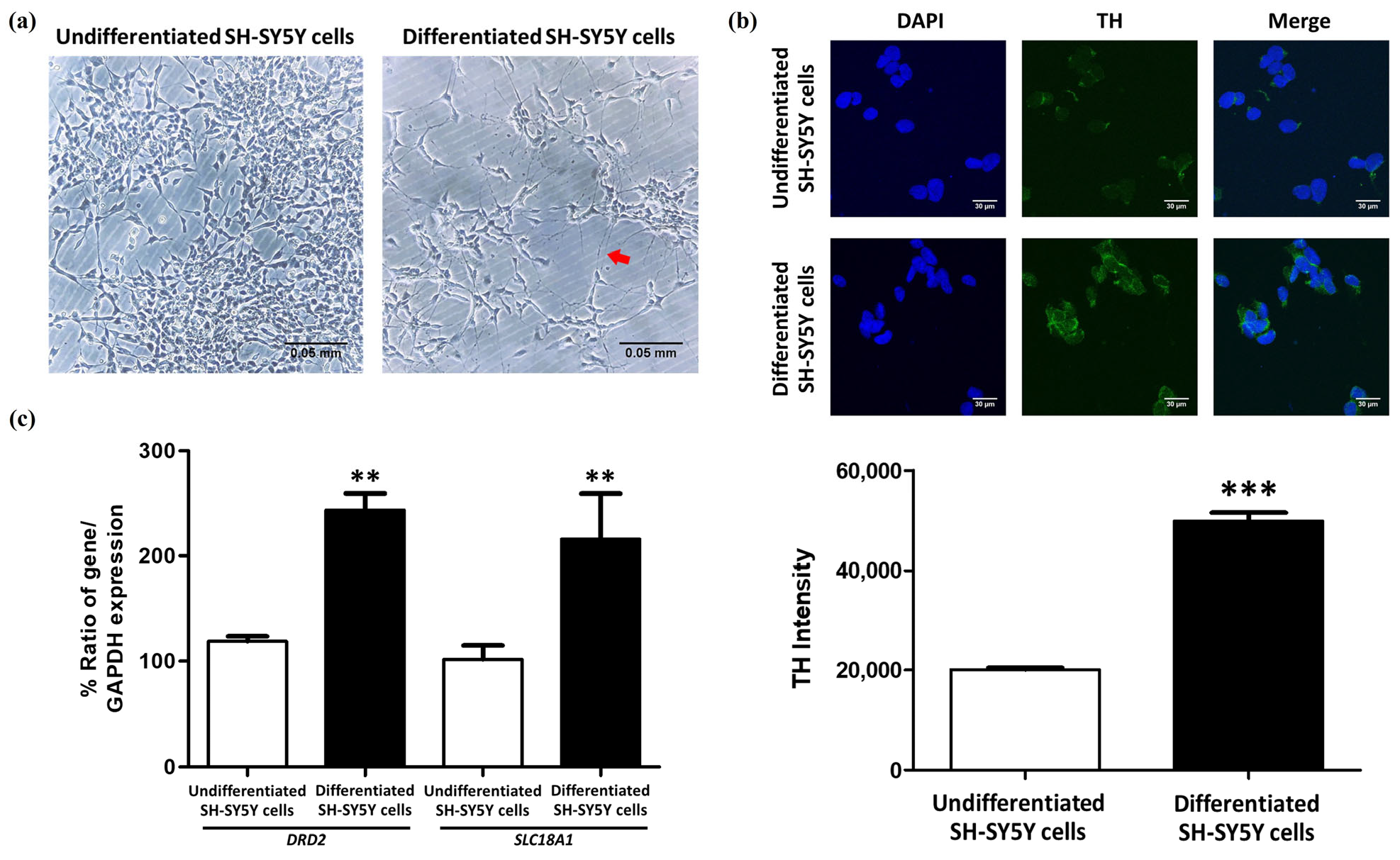
2.2. The Differentiation of SH-SY5Y Cells into Dopaminergic-like Neurons Using RA and TPA
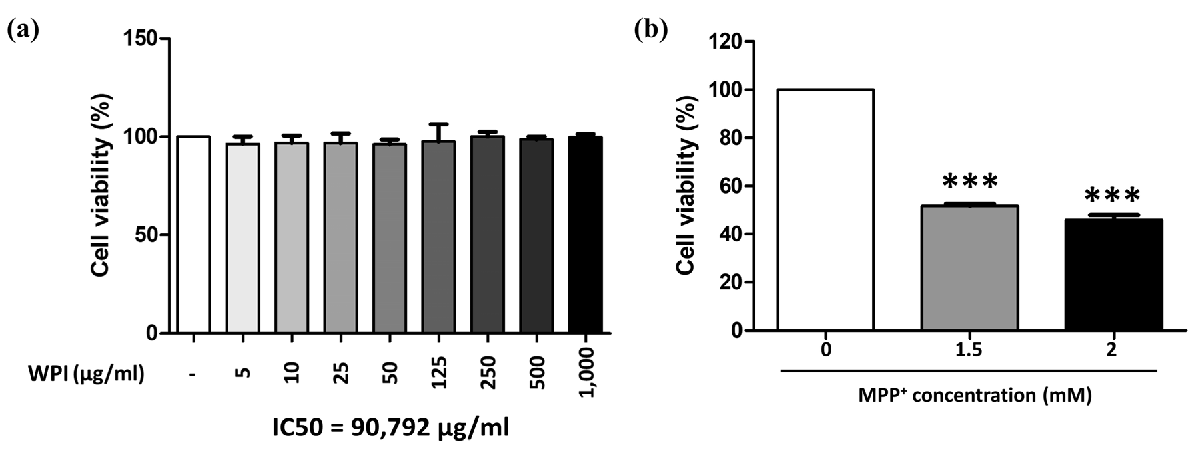
2.3. WPI Has Non-Toxic Effects on Differentiated SH-SY5Y Cells
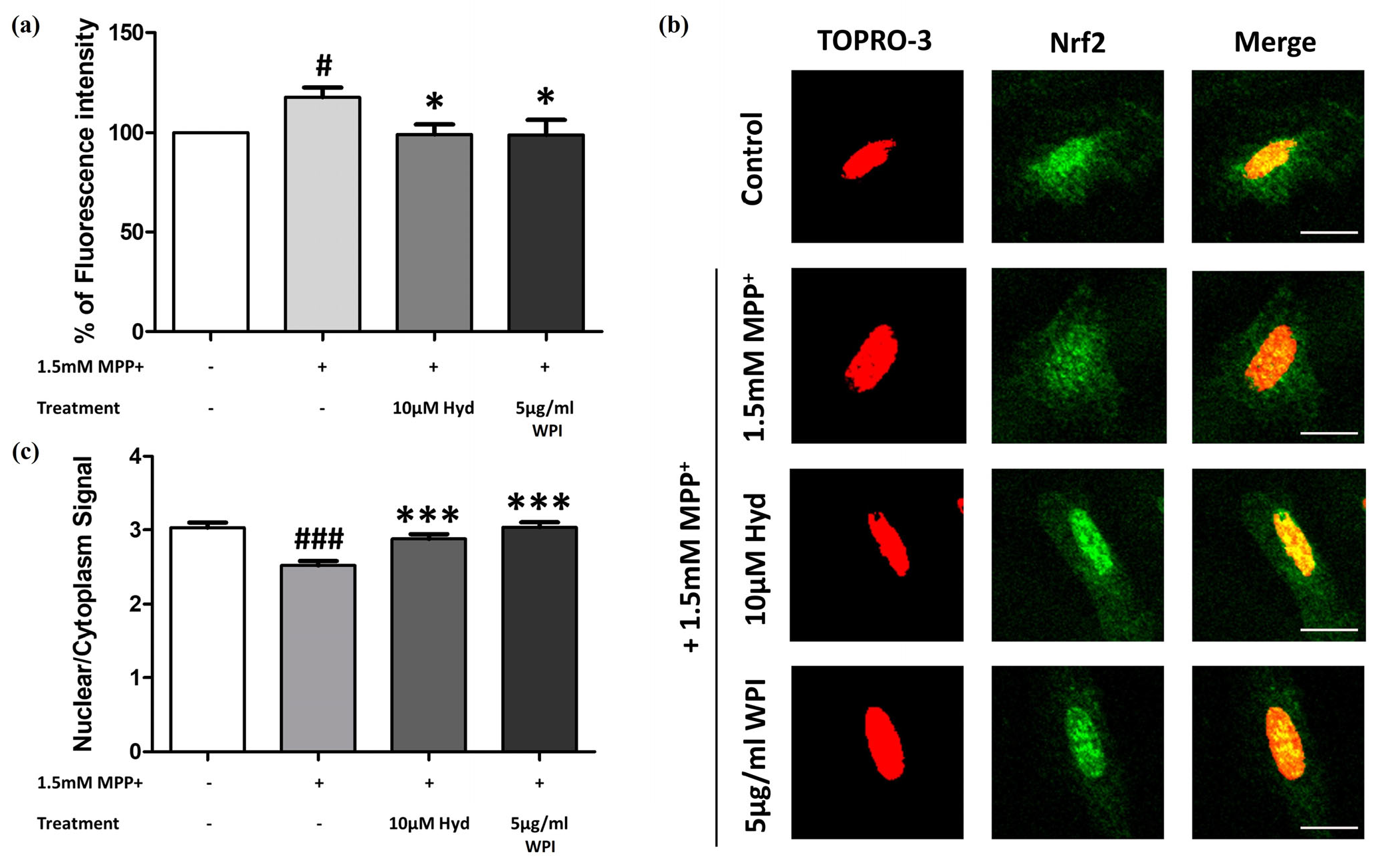
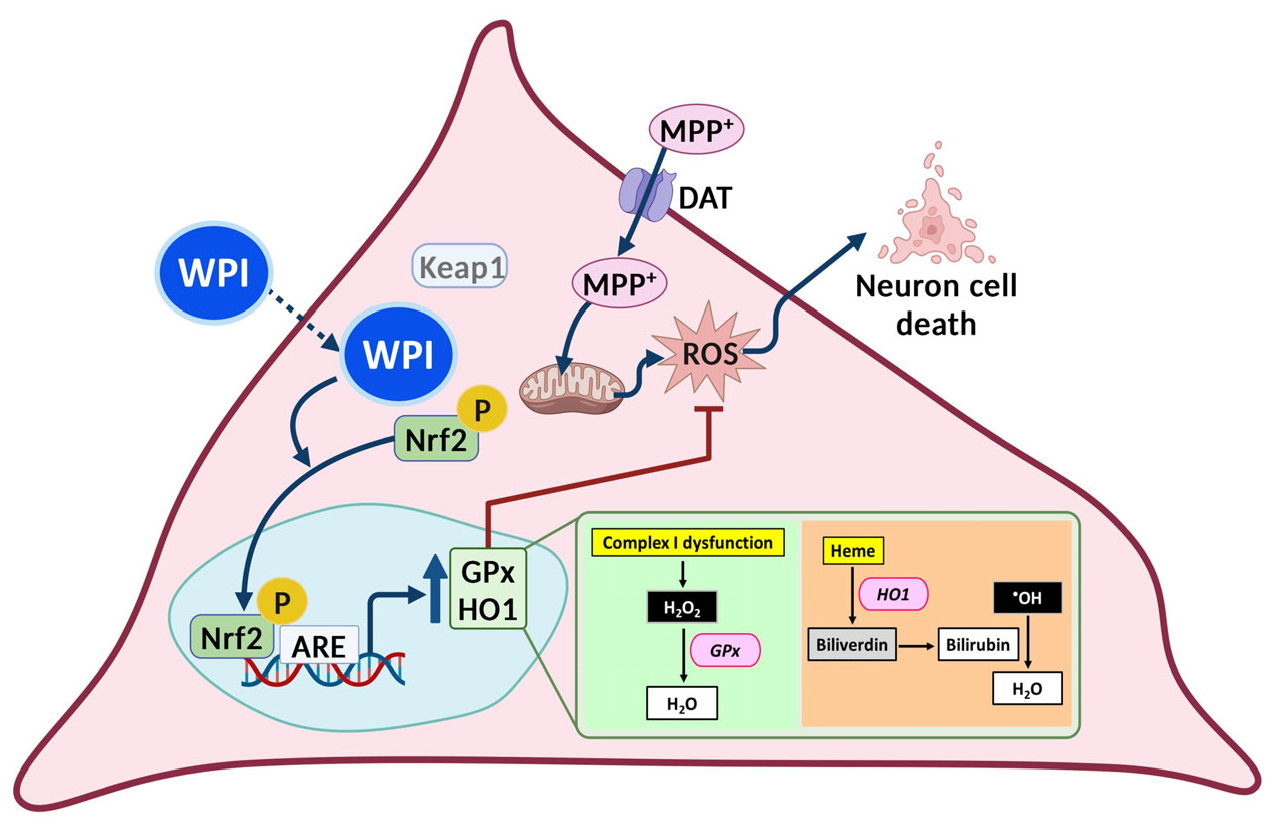
2.4. WPI Reduces the Intracellular ROS in MPP+-Induced Differentiated SH-SY5Y Cells by Inducing Nrf2 Translocation into the Nucleus
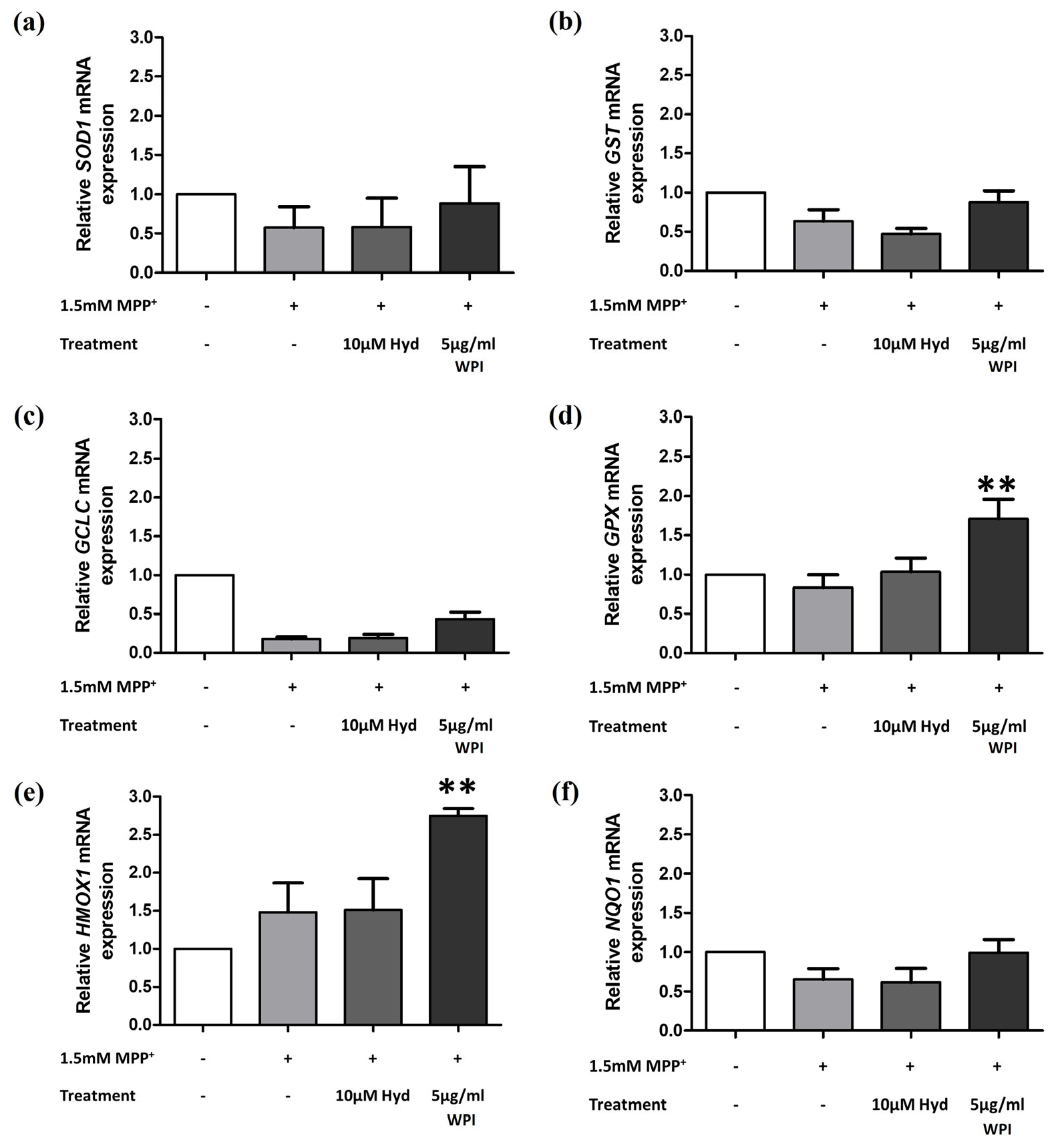
2.5. WPI Increases the Expression of Antioxidants Through the Nrf2 Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Total Amino Acid Analysis
4.3. SH-SY5Y Cell Culture and Differentiation
4.4. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.4.1. RNA Isolation
4.4.2. cDNA Synthesis
4.4.3. RT-PCR Performing
4.4.4. Agarose Gel Electrophoresis
4.5. Preparation of WPI Solution
4.6. Cell Viability Measurement
4.7. Cytotoxicity Test of WPI in Differentiated-SH-SY5Y Cells
4.8. Intracellular ROS Measurement
4.9. Immunofluorescent Staining for Investigating TH and Nfr2 Translocation into the Nucleus
4.9.1. TH Staining for Determining the Increase of Dopaminergic-like Neurons After Differentiation
4.9.2. Nfr2 Translocation into the Nucleus Investigation
4.10. Quantitative Real-Time PCR (RT-qPCR)
4.11. Statistics Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WPI | Whey protein isolate |
| MPP+ | 1-methyl-4-phenylpyridinium |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| Keap1 | Kelch-like ECH-associated protein 1 |
References
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s Disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Li, X.; Li, X.; Liu, Q.; Cheng, Y. Oxidative Stress in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Mol. Neurosci. 2018, 11, 236. [Google Scholar] [CrossRef]
- Afzal, S.; Manap, A.S.A.; Attiq, A.; Albokhadaim, I.; Kandeel, M.; Alhojaily, S.M. From Imbalance to Impairment: The Central Role of Reactive Oxygen Species in Oxidative Stress-Induced Disorders and Therapeutic Exploration. Front. Pharmacol. 2023, 14, 1269581. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Buendia, I.; Michalska, P.; Navarro, E.; Gameiro, I.; Egea, J.; León, R. Nrf2-ARE Pathway: An Emerging Target Against Oxidative Stress and Neuroinflammation in Neurodegenerative Diseases. Pharmacol. Ther. 2016, 157, 84–104. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules. 2020, 25, 5474. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Brandes, M.S.; Gray, N.E. NRF2 as a Therapeutic Target in Neurodegenerative Diseases. ASN Neuro 2020, 12, 1–23. [Google Scholar] [CrossRef]
- Minj, S.; Anand, S. Whey Proteins and Its Derivatives: Bioactivity, Functionality, and Current Applications. Dairy 2020, 1, 233–258. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, Z.; Qiu, C.; Wen, J. A Review of Whey Protein-Based Bioactive Delivery Systems: Design, Fabrication, and Application. Foods 2024, 13, 2453. [Google Scholar] [CrossRef]
- Zang, Z.; Chou, S.; Tian, J.; Lang, Y.; Shen, Y.; Ran, X.; Gao, N.; Li, B. Effect of Whey Protein Isolate on the Stability and Antioxidant Capacity of Blueberry Anthocyanins: A Mechanistic and In Vitro Simulation Study. Food Chem. 2021, 336, 127700. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, L.; Zhou, W.; Zhang, H.; Qin, X.; Liu, G. Whey Protein Isolate and Inulin-Glycosylated Conjugate Affect the Physicochemical Properties and Oxidative Stability of Pomegranate Seed Oil Emulsion. Food Chem. 2024, 444, 138649. [Google Scholar] [CrossRef]
- Zhang, K.; Li, N.; Wang, Z.; Feng, D.; Liu, X.; Zhou, D.; Li, D. Recent Advances in the Color of Aquatic Products: Evaluation Methods, Discoloration Mechanism, and Protection Technologies. Food Chem. 2024, 434, 137495. [Google Scholar] [CrossRef]
- Peng, X.; Kong, B.; Xia, X.; Liu, Q. Reducing and radical-scavenging activities of whey protein hydrolysates prepared with Alcalase. Int. Dairy J. 2010, 20, 360–365. [Google Scholar] [CrossRef]
- Adjonu, R.; Doran, G.; Torley, P.; Agboola, S. Screening of whey protein isolate hydrolysates for their dual functionality: Influence of heat pre-treatment and enzyme specificity. Food Chem. 2013, 136, 1435–1443. [Google Scholar] [CrossRef]
- Mohan, A.; Udechukwu, M.C.; Rajendran, S.R.C.K.; Udenigwe, C.C. Modification of peptide functionality during enzymatic hydrolysis of whey proteins. RSC Adv. 2015, 5, 97400–97407. [Google Scholar] [CrossRef]
- Grey, V.; Mohammed, S.R.; Smountas, A.A.; Bahlool, R.; Lands, L.C. Improved Glutathione Status in Young Adult Patients with Cystic Fibrosis Supplemented with Whey Protein. J. Cyst. Fibros. 2003, 2, 195–198. [Google Scholar] [CrossRef]
- Kong, X.Z.; Guo, M.M.; Hua, Y.F.; Cao, D.; Zhang, C.M. Enzymatic Preparation of Immunomodulating Hydrolysates from Soy Proteins. Bioresour. Technol. 2008, 99, 8873–8879. [Google Scholar] [CrossRef]
- Ahmed, R.; El-Din, E.; Eldenshary, S.; Nada, S.; Asaad, G.F. Pharmacological Study of the Possible Antidepressant Activity of Whey Protein Isolate in Mice. Aust. J. Basic Appl. Sci. 2011, 5, 2649–2659. [Google Scholar]
- Douglas, T.E.L.; Vandrovcová, M.; Kročilová, N.; Keppler, J.K.; Zárubová, J.; Skirtach, A.G.; Bačáková, L. Application of Whey Protein Isolate in Bone Regeneration: Effects on Growth and Osteogenic Differentiation of Bone-Forming Cells. J. Dairy Sci. 2018, 101, 28–36. [Google Scholar] [CrossRef]
- Genç, H.; Friedrich, B.; Alexiou, C.; Pietryga, K.; Cicha, I.; Douglas, T.E.L. Endothelialization of Whey Protein Isolate-Based Scaffolds for Tissue Regeneration. Molecules 2023, 28, 7052. [Google Scholar] [CrossRef] [PubMed]
- Adler, S.; Olsen, W.; Rackerby, B.; Spencer, R.; Dallas, D.C. Effects of Whey Protein Supplementation on Inflammatory Marker Concentrations in Older Adults. Nutrients 2023, 15, 4081. [Google Scholar] [CrossRef]
- Derosa, G.; D’Angelo, A.; Maffioli, P. Change of some oxidative stress parameters after supplementation with whey protein isolate in patients with type 2 diabetes. Nutrition 2020, 73, 110700. [Google Scholar] [CrossRef] [PubMed]
- Boscaini, S.; Cabrera-Rubio, R.; Golubeva, A.; Nychyk, O.; Fülling, C.; Speakman, J.R.; Cotter, P.D.; Cryan, J.F.; Nilaweera, K.N. Depletion of the gut microbiota differentially affects the impact of whey protein on high-fat diet-induced obesity and intestinal permeability. Physiol. Rep. 2021, 9, e14867. [Google Scholar] [CrossRef]
- Xie, H.R.; Hu, L.S.; Li, G.Y. SH-SY5Y Human Neuroblastoma Cell Line: In Vitro Cell Model of Dopaminergic Neurons in Parkinson’s Disease. Chin. Med. J. 2010, 123, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Bell, N.; Hann, V.; Redfern, C.P.F.; Cheek, T.R. Store-operated Ca2+ entry in proliferating and retinoic acid-differentiated N- and S-type neuroblastoma cells. Biochim. Biophys. Acta-Mol. Cell Res. 2013, 1833, 643–651. [Google Scholar] [CrossRef]
- Lopes, F.M.; da Motta, L.L.; De Bastiani, M.A.; Pfaffenseller, B.; Aguiar, B.W.; de Souza, L.F.; Zanatta, G.; Vargas, D.M.; Schönhofen, P.; Londero, G.F.; et al. Differentiation Enhances Dopaminergic Features, Changes Redox Parameters, and Increases Dopamine Transporter Dependency in 6-Hydroxydopamine-Induced Neurotoxicity in SH-SY5Y Cells. Neurotox. Res. 2017, 31, 545–559. [Google Scholar] [CrossRef]
- Schober, A. Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res. 2004, 318, 215–224. [Google Scholar] [CrossRef]
- Presgraves, S.P.; Ahmed, T.; Borwege, S.; Joyce, J.N. Terminally differentiated SH-SY5Y cells provide a model system for studying neuroprotective effects of dopamine agonists. Neurotox. Res. 2003, 5, 579–598. [Google Scholar] [CrossRef]
- Noonong, K.; Sobhon, P.; Sroyraya, M.; Chaithirayanon, K. Neuroprotective and Neurorestorative Effects of Holothuria Scabra Extract in the MPTP/MPP+-Induced Mouse and Cellular Models of Parkinson’s Disease. Front. Neurosci. 2020, 14, 575459. [Google Scholar] [CrossRef]
- Barbosa, D.J.; Capela, J.P.; Silva, R.; Vilas-Boas, V.; Ferreira, L.M.; Branco, P.S.; Fernandes, E.; Bastos, M.D.L.; Carvalho, F. The Mixture of “Ecstasy” and Its Metabolites is Toxic to Human SH-SY5Y Differentiated Cells at In Vivo Relevant Concentrations. Arch. Toxicol. 2014, 88, 455–473. [Google Scholar] [CrossRef] [PubMed]
- Coccini, T.; Vecchio, S.; Crevani, M.; De Simone, U. Cytotoxic Effects of 3,4-Catechol-PV (One Major MDPV Metabolite) on Human Dopaminergic SH-SY5Y Cells. Neurotox. Res. 2019, 35, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Athauda, D.; Foltynie, T. The ongoing pursuit of neuroprotective therapies in Parkinson disease. Nat. Rev. Neurol. 2014, 11, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Hua, Z.; Li, Z. The role of glutamate and glutamine metabolism and related transporters in nerve cells. CNS Neurosci. Ther. 2024, 30, e14617. [Google Scholar] [CrossRef] [PubMed]
- Egbujor, M.C.; Olaniyan, O.T.; Emeruwa, C.N.; Saha, S.; Saso, L.; Tucci, P. An Insight into Role of Amino Acids as Antioxidants via NRF2 Activation. Amino Acids 2024, 56, 23. [Google Scholar] [CrossRef]
- Perlikowska, R. Whether Short Peptides are Good Candidates for Future Neuroprotective Therapeutics? Peptides 2021, 140, 170528. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Dinesh Kumar, B.; Hemalatha, R.; Jyothirmayi, T. Fish Protein Hydrolysates: Proximate Composition, Amino Acid Composition, Antioxidant Activities and Applications: A Review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef]
- Gao, R.; Yu, Q.; Shen, Y.; Chu, Q.; Chen, G.; Fen, S.; Yang, M.; Yuan, L.; McClements, D.J.; Sun, Q. Production, Bioactive Properties, and Potential Applications of Fish Protein Hydrolysates: Developments and Challenges. Trends Food Sci. Technol. 2021, 110, 687–699. [Google Scholar] [CrossRef]
- Huang, D.; Maulu, S.; Ren, M.; Liang, H.; Ge, X.; Ji, K.; Yu, H. Dietary Lysine Levels Improved Antioxidant Capacity and Immunity via the TOR and p38 MAPK Signaling Pathways in Grass Carp, Ctenopharyngodon idellus Fry. Front. Immunol. 2021, 12, 635015. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, Q.; Zhou, X.Q.; Xu, S.X.; Feng, L.; Liu, Y.; Jiang, W.D.; Wu, P.; Zhao, J.; Jiang, J. Effect of Dietary Threonine on Growth Performance and Muscle Growth, Protein Synthesis and Antioxidant-Related Signalling Pathways of Hybrid Catfish Pelteobagrus Vachelli♀ × Leiocassis Longirostris♂. Br. J. Nutr. 2020, 123, 121–134. [Google Scholar] [CrossRef]
- Paul, B.D.; Sbodio, J.I.; Snyder, S.H. Cysteine Metabolism in Neuronal Redox Homeostasis. Trends Pharmacol. Sci. 2018, 39, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Masaldan, S.; Callegari, S.; Dewson, G. Therapeutic Targeting of Mitophagy in Parkinson’s Disease. Biochem. Soc. Trans. 2022, 50, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.N.; Ross, E.K.; Daliparthi, V.; Sumner, W.A.; Kirchhof, D.M.; Manning, E.; Wilkins, H.M.; Linseman, D.A. A Cystine-Rich Whey Supplement (Immunocal®) Provides Neuroprotection from Diverse Oxidative Stress-Inducing Agents In Vitro by Preserving Cellular Glutathione. Oxid. Med. Cell. Longev. 2017, 2017, 3103272. [Google Scholar] [CrossRef]
- Corrochano, A.R.; Buckin, V.; Kelly, P.M.; Giblin, L. Invited Review: Whey Proteins as Antioxidants and Promoters of Cellular Antioxidant Pathways. J. Dairy Sci. 2018, 101, 4747–4761. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.H.; Zhang, J.T.; Yang, G.J.; Liu, H.; Han, Q.B.; Ma, D.L. Emerging Screening Approaches in the Development of Nrf2–Keap1 Protein–Protein Interaction Inhibitors. Int. J. Mol. Sci. 2019, 20, 4445. [Google Scholar] [CrossRef]
- Culletta, G.; Buttari, B.; Arese, M.; Brogi, S.; Almerico, A.M.; Saso, L.; Tutone, M. Natural Products as Non-Covalent and Covalent Modulators of the KEAP1/NRF2 Pathway Exerting Antioxidant Effects. Eur. J. Med. Chem. 2024, 270, 116355. [Google Scholar] [CrossRef]
- Haines, D.D.; Tosaki, A. Heme Degradation in Pathophysiology of and Countermeasures to Inflammation-Associated Disease. Int. J. Mol. Sci. 2020, 21, 9698. [Google Scholar] [CrossRef]
- Kerasioti, E.; Stagos, D.; Tzimi, A.; Kouretas, D. Increase in Antioxidant Activity by Sheep/Goat Whey Protein through Nuclear Factor-Like 2 (Nrf2) is Cell Type Dependent. Food Chem. Toxicol. 2016, 97, 47–56. [Google Scholar] [CrossRef]
- Pyo, M.C.; Yang, S.Y.; Chun, S.H.; Oh, N.S.; Lee, K.W. Protective Effects of Maillard Reaction Products of Whey Protein Concentrate against Oxidative Stress through an Nrf2-Dependent Pathway in HepG2 Cells. Biol. Pharm. Bull. 2016, 39, 1437–1447. [Google Scholar] [CrossRef]
- Cheng, S.H.; Tseng, Y.M.; Wu, S.H.; Tsai, S.M.; Tsai, L.Y. Whey Protein Concentrate Renders MDA-MB-231 Cells Sensitive to Rapamycin by Altering Cellular Redox State and Activating GSK3β/mTOR Signaling. Sci Rep. 2017, 7, 15976. [Google Scholar] [CrossRef]
- Tosukhowong, P.; Boonla, C.; Dissayabutra, T.; Kaewwilai, L.; Muensri, S.; Chotipanich, C.; Joutsa, J.; Rinne, J.; Bhidayasiri, R. Biochemical and Clinical Effects of Whey Protein Supplementation in Parkinson’s Disease: A Pilot Study. J. Neurol. Sci. 2016, 367, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Ross, E.K.; Gray, J.J.; Winter, A.N.; Linseman, D.A. Immunocal® and Preservation of Glutathione as a Novel Neuroprotective Strategy for Degenerative Disorders of the Nervous System. Recent Pat. CNS Drug Discov. 2012, 7, 230–235. [Google Scholar] [CrossRef]
- Georgakopoulos, N.D.; Talapatra, S.K.; Gatliff, J.; Kozielski, F.; Wells, G. Modified Peptide Inhibitors of the Keap1–Nrf2 Protein–Protein Interaction Incorporating Unnatural Amino Acids. Chembiochem 2018, 19, 1810. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, J.; Nie, S.; Ding, L.; Wang, L.; Liu, J.; Liu, W.; Zhang, T. Direct Inhibition of Keap1–Nrf2 Interaction by Egg-Derived Peptides DKK and DDW Revealed by Molecular Docking and Fluorescence Polarization. RSC Adv. 2017, 7, 34963–34971. [Google Scholar] [CrossRef]
- Official Journal of the European Journal of Communities, Determination of Amino Acids. 1998. Available online: https://op.europa.eu/en/publication-detail/-/publication/856db9b7-6f1d-4d6e-a778-c766c9fa1776 (accessed on 28 April 2025).
- Çevikkalp, S.A.; Löker, G.B.; Yaman, M.; Amoutzopoulos, B. A Simplified HPLC Method for Determination of Tryptophan in Some Cereals and Legumes. Food Chem. 2016, 193, 26–29. [Google Scholar] [CrossRef]
- Liu, L.; Yuan, G.; Cheng, Z.; Zhang, G.; Liu, X.; Zhang, H. Identification of the mRNA Expression Status of the Dopamine D2 Receptor and Dopamine Transporter in Peripheral Blood Lymphocytes of Schizophrenia Patients. PLoS ONE 2013, 8, e75259. [Google Scholar] [CrossRef] [PubMed]
- OriGene Technologies, VMAT1 (SLC18A1), (n.d.). Available online: https://www.origene.com/catalog/gene-expression/qpcr-primer-pairs/hp206646-vmat1-slc18a1-human-qpcr-primer-pair-nm-003053 (accessed on 11 May 2025).
- Guo, X.; Han, C.; Ma, K.; Xia, Y.; Wan, F.; Yin, S.; Kou, L.; Sun, Y.; Wu, J.; Hu, J.; et al. Hydralazine Protects Nigrostriatal Dopaminergic Neurons From MPP+ and MPTP Induced Neurotoxicity: Roles of Nrf2-ARE Signaling Pathway. Front. Neurol. 2019, 10, 271. [Google Scholar] [CrossRef]
- Rungruang, P.; Rodthayoy, D.; Hawangjoo, M.; Klaypradit, W.; Chonpathompikunlert, P.; Sansri, V.; Uthayopas, C.; Sroyraya, M. Alleviative and Anti-Inflammatory Effects of Tuna Blood Hydrolysates on MPP+ and TNF-α -Induced Parkinson-Like Disease Model Through the Regulation of Keap1-Nrf2 Antioxidant Pathway and Apoptosis. J. Funct. Foods 2024, 116, 106134. [Google Scholar] [CrossRef]
- Kraokaew, P.; Manohong, P.; Prasertsuksri, P.; Jattujan, P.; Niamnont, N.; Tamtin, M.; Sobhon, P.; Meemon, K. Ethyl Acetate Extract of Marine Algae, Halymenia durvillei, Provides Photoprotection against UV-Exposure in L929 and HaCaT Cells. Mar. Drugs 2022, 20, 707. [Google Scholar] [CrossRef]
- Akimov, M.G.; Fomina-Ageeva, E.V.; Dudina, P.V.; Andreeva, L.A.; Myasoyedov, N.F.; Bezuglov, V.V. ACTH (6–9)PGP peptide protects SH-SY5Y cells from H2O2, tert-butyl hydroperoxide, and cyanide cytotoxicity via stimulation of proliferation and induction of prosurvival-related genes. Molecules 2021, 26, 1878. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, A.Z. DJ-1 plays a neuroprotective role in SH-SY5Y cells by modulating Nrf2 signaling in response to lidocaine-mediated oxidative stress and apoptosis. Kaohsiung J. Med. Sci. 2020, 36, 630–639. [Google Scholar] [CrossRef] [PubMed]
| Amino Acid Profiles | Concentration (mg/100 g) |
|---|---|
| Glutamic acid | 17,457.40 |
| Aspartic acid | 10,920.43 |
| Leucine | 10,441.27 |
| Lysine | 8342.23 |
| Threonine | 7006.69 |
| Isoleucine | 5808.87 |
| Proline | 5747.21 |
| Valine | 5319.86 |
| Alanine | 4955.06 |
| Serine | 4510.00 |
| Cystine | 3323.42 |
| Phenylalanine | 3206.23 |
| Tyrosine | 2957.29 |
| Methionine | 2205.17 |
| Arginine | 2109.95 |
| Histidine | 1872.83 |
| Glycine | 1705.66 |
| Tryptophan | 1536.06 |
| Hydroxylysine | 574.38 |
| Hydroxyproline | Not detected |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rungruang, P.; Sroyraya, M.; Sansri, V. Protective Role of Whey Protein Isolate on MPP+-Induced Differentiation of SH-SY5Y Cells by Modulating the Nrf2 Antioxidant Pathway. Molecules 2025, 30, 2207. https://doi.org/10.3390/molecules30102207
Rungruang P, Sroyraya M, Sansri V. Protective Role of Whey Protein Isolate on MPP+-Induced Differentiation of SH-SY5Y Cells by Modulating the Nrf2 Antioxidant Pathway. Molecules. 2025; 30(10):2207. https://doi.org/10.3390/molecules30102207
Chicago/Turabian StyleRungruang, Panlekha, Morakot Sroyraya, and Veerawat Sansri. 2025. "Protective Role of Whey Protein Isolate on MPP+-Induced Differentiation of SH-SY5Y Cells by Modulating the Nrf2 Antioxidant Pathway" Molecules 30, no. 10: 2207. https://doi.org/10.3390/molecules30102207
APA StyleRungruang, P., Sroyraya, M., & Sansri, V. (2025). Protective Role of Whey Protein Isolate on MPP+-Induced Differentiation of SH-SY5Y Cells by Modulating the Nrf2 Antioxidant Pathway. Molecules, 30(10), 2207. https://doi.org/10.3390/molecules30102207








