Tripterhyponoid A from Tripterygium hypoglaucum Inhibiting MRSA by Multiple Mechanisms
Abstract
1. Introduction
2. Results and Discussion
2.1. New Structure Elucidation
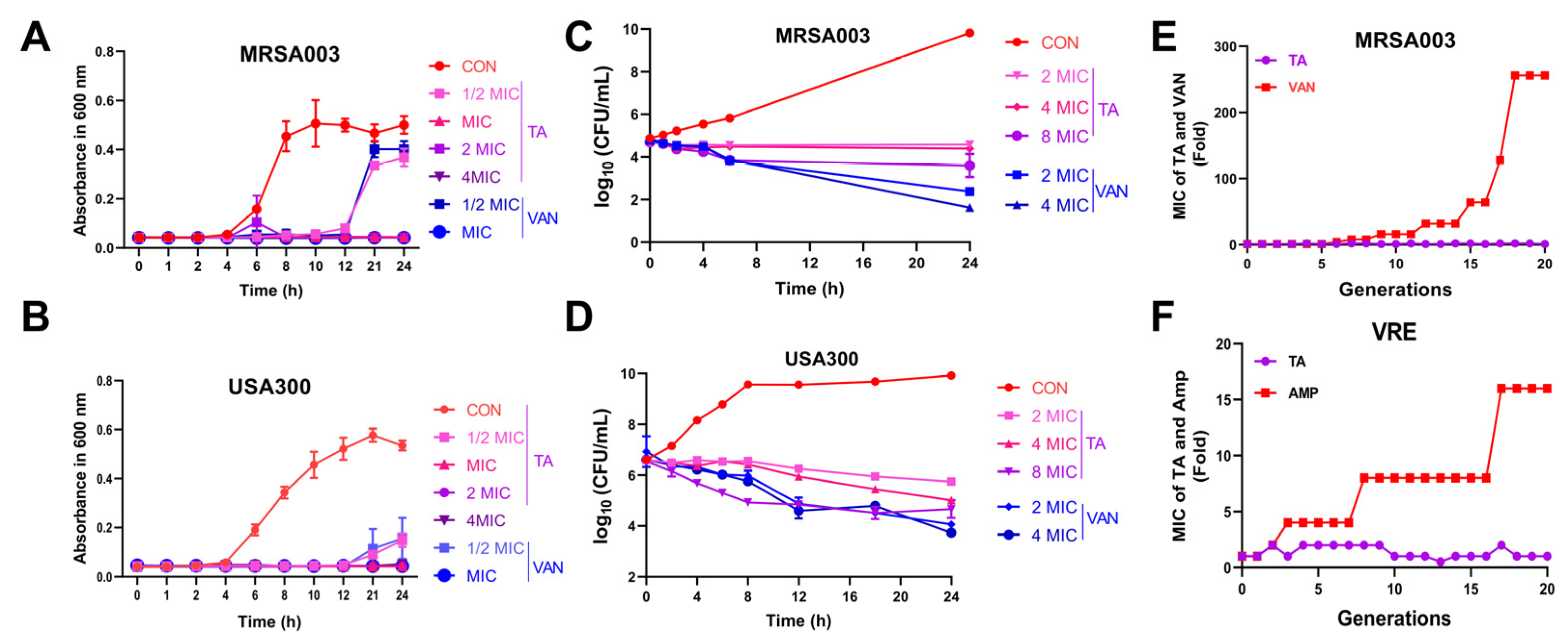
2.2. Tripterhyponoid A Significantly Inhibited MRSA In Vitro
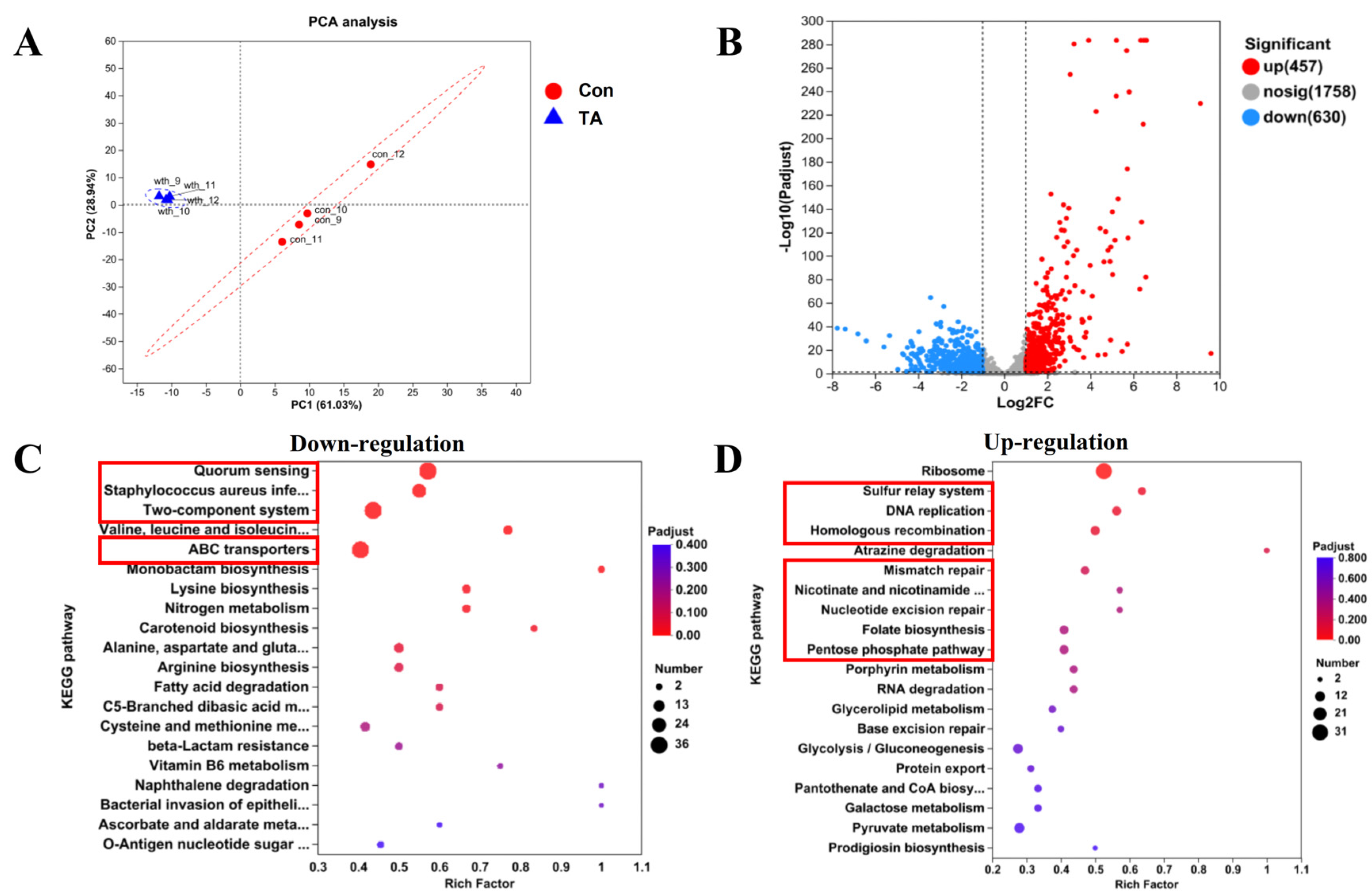
2.3. Exploration of Anti-MRSA Pathways Through Transcriptomics Analysis
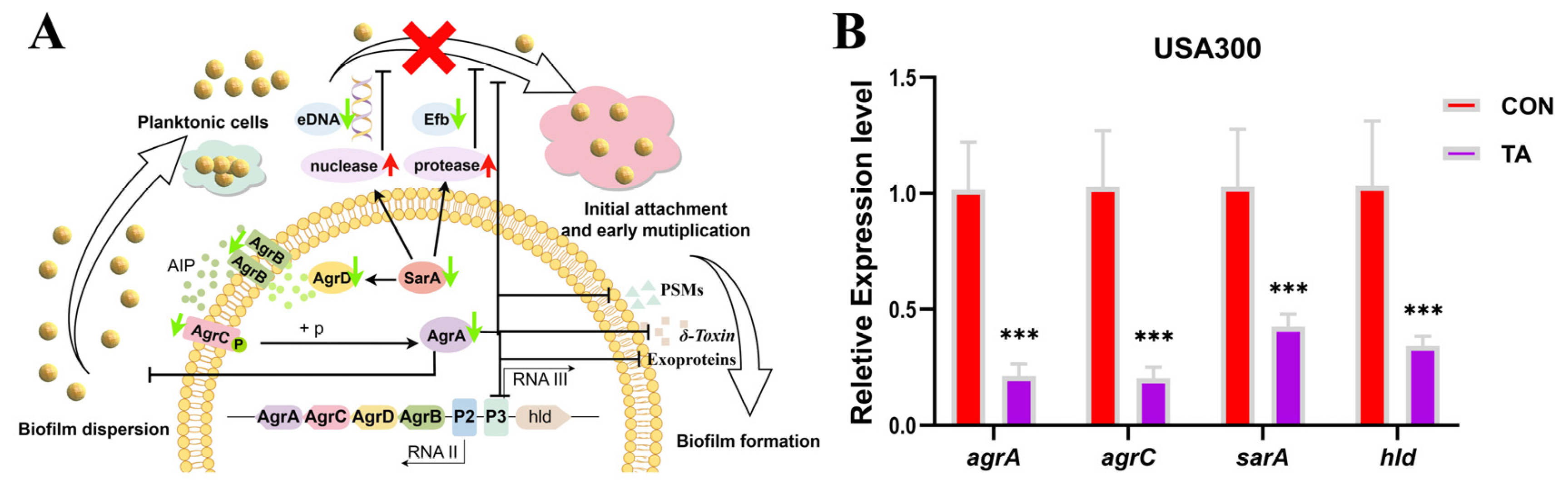
2.4. Tripterhyponoid A Repressed the Expression of Biofilm- and Virulence-Associated Genes
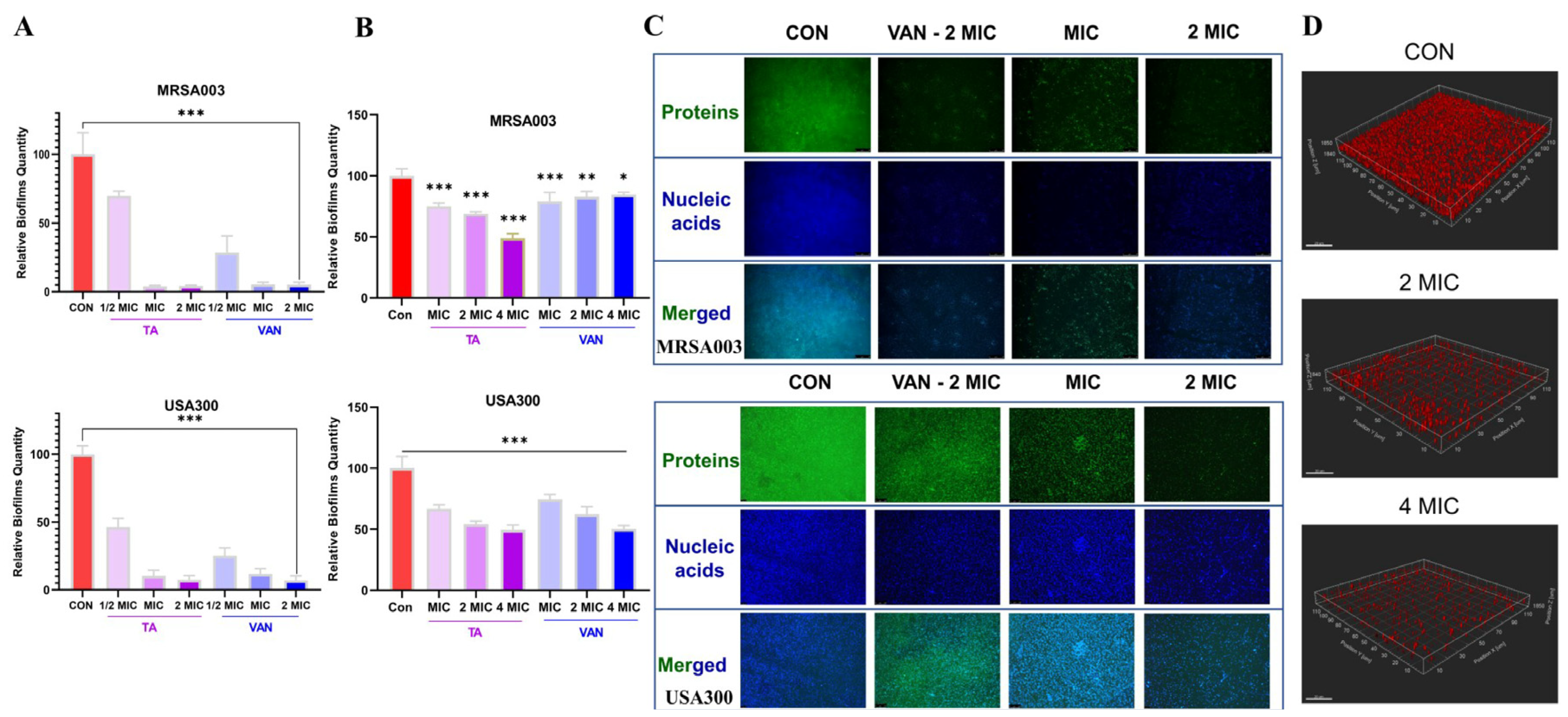
2.5. Tripterhyponoid A Inhibited Biofilm Formation and Eradicated Mature Biofilms
2.6. Tripterhyponoid A Upregulated the Expression of Genes Associated with DNA Replication and Repair
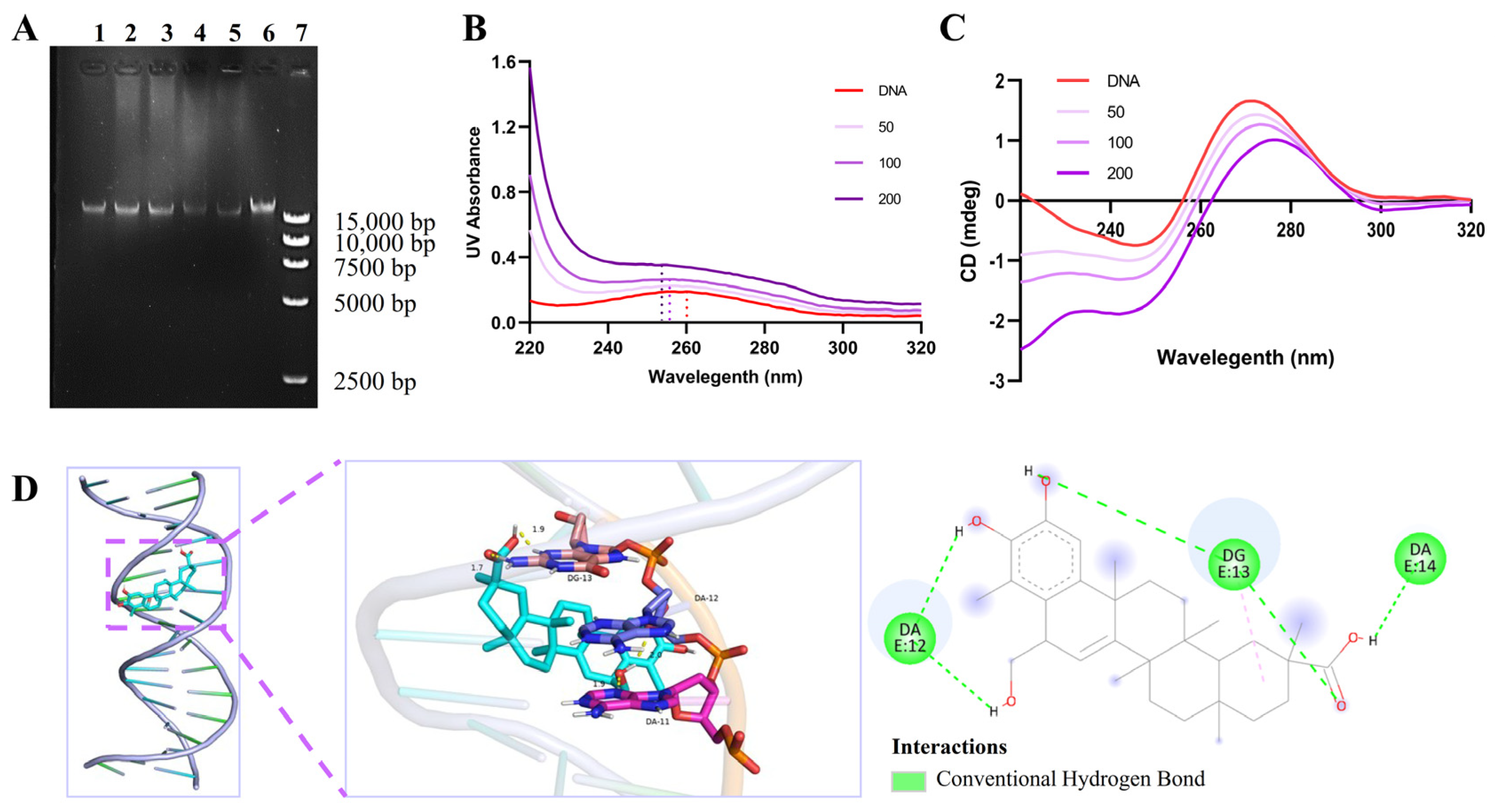
2.7. Tripterhyponoid A Interacted with USA300 Genomic DNA
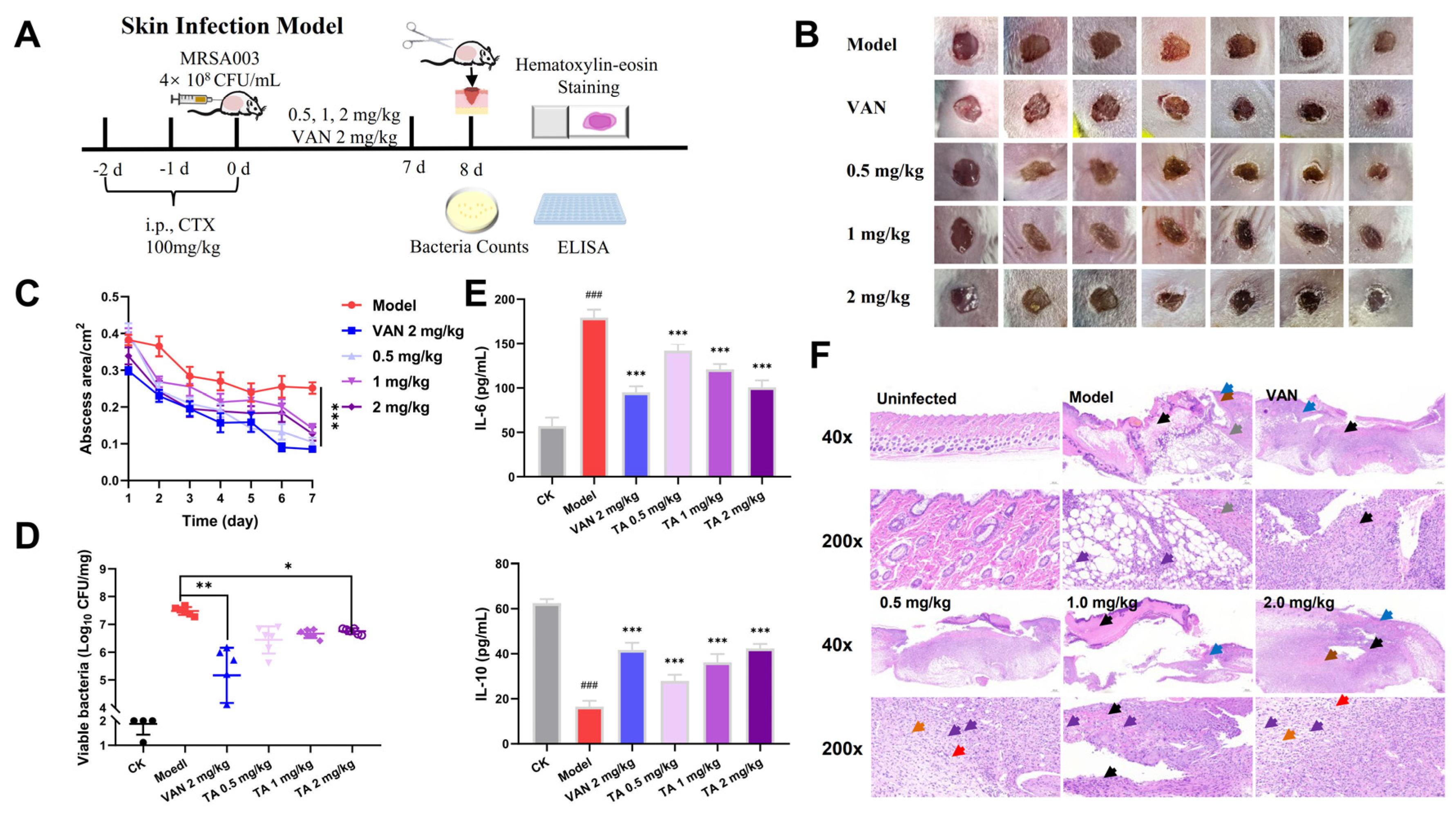
2.8. Tripterhyponoid A Showed Potential Therapeutic Capabilities in a Mouse Skin Infection Model
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Materials
3.3. Extraction and Isolation
3.4. Bacterial Strains and Reagents
3.5. Antibacterial Testing
3.6. Growth Curve
3.7. Time-Kill Curve
3.8. Bacterial Resistance Study
3.9. USA300 Treatment and Collection
3.10. Real-Time Quantitative PCR (RT-qPCR) Validation
3.11. Crystal Violet Assay
3.12. Qualitative Analysis of Mature Biofilm
3.13. Imaging of 3D Biofilm
3.14. DNA Gel Migration Assay
3.15. Ultraviolet–Visible (UV–Vis) Spectroscopy and Circular Dichroism (CD) Assay
3.16. Molecular Docking
3.17. Mouse Skin MRSA Infection Model
3.18. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Ge, J.P.; Liu, B.; Hu, Y.L.; Yang, M.J. Structures of SdrD from Staphylococcus aureus reveal the molecular mechanism of how the cell surface receptors recognize their ligands. Protein Cell 2013, 4, 277–285. [Google Scholar] [CrossRef]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Aguilar, G.R.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global burden of bacterial antimicrobial resistance 1990-2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- Schilcher, K.; Horswill, A.R. Staphylococcal Biofilm Development Structure, Regulation, and Treatment Strategies. Microbiol. Mol. Biol. Rev. 2020, 84, e00026-19. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.L.; Jefferson, K.K. Phase Variation of Poly-N-Acetylglucosamine Expression in Staphylococcus aureus. PLoS Pathog. 2014, 10, e1004292. [Google Scholar] [CrossRef]
- Wang, J.H.; Lu, X.Y.; Wang, C.J.; Yue, Y.J.; Wei, B.; Zhang, H.W.; Wang, H.; Chen, J.W. Research Progress on the Combination of Quorum-Sensing Inhibitors and Antibiotics against Bacterial Resistance. Molecules 2024, 29, 1674. [Google Scholar] [CrossRef]
- Jia, J.; Zheng, M.X.; Zhang, C.W.; Li, B.L.; Lu, C.; Bai, Y.F.; Tong, Q.; Hang, X.D.; Ge, Y.X.; Zeng, L.P.; et al. Killing of Staphylococcus aureus persisters by a multitarget natural product chrysomycin A. Sci. Adv. 2023, 9, eadg5995. [Google Scholar] [CrossRef]
- Zhou, C.L.; Zhou, Y.; Zheng, Y.Q.; Yu, Y.; Yang, K.L.; Chen, Z.Y.; Chen, X.H.; Wen, K.; Chen, Y.J.; Bai, S.L.; et al. Amphiphilic Nano-Swords for Direct Penetration and Eradication of Pathogenic Bacterial Biofilms. ACS Appl. Mater. Interfaces 2023, 15, 20458–20473. [Google Scholar] [CrossRef]
- Bai, S.L.; Wang, J.X.; Yang, K.L.; Zhou, C.L.; Xu, Y.F.; Song, J.F.; Gu, Y.X.; Chen, Z.; Wang, M.; Shoen, C.; et al. A polymeric approach toward resistance-resistant antimicrobial agent with dual-selective mechanisms of action. Sci. Adv. 2021, 7, eabc9917. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.B.; Zeng, F.; Wang, M.X.; Guo, S.; Tang, Z.J.; Itagaki, K.; Lin, Y.J.; Shen, X.C.; Cao, Y.Q.; Duan, J.A.; et al. Antimicrobial activities of lavandulylated flavonoids in Sophora flavences against methicillin-resistant Staphylococcus aureus via membrane disruption. J. Adv. Res. 2024, 57, 197–212. [Google Scholar] [CrossRef]
- Hamion, G.; Aucher, W.; Mercier, A.; Tewes, F.; Bertaux, J.; Girardot, M.; Imbert, C. Insights into betulinic acid as a promising molecule to fight the interkingdom biofilm Staphylococcus aureus-Candida albicans. Int. J. Antimicrob. Agents 2024, 63, 107166. [Google Scholar] [CrossRef]
- Gao, Y.D.; Liu, S.S.; Ma, Y.Z.; Li, C.Z.; Xiao, Z.H.; Nie, S.L.; Tu, J. Effects of camellia saponins on biofilm formation and virulence factor genes of Bacillus cereus. LWT-Food Sci. Technol. 2024, 198, 116023. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Wang, Z.J.; Zhu, M.; Zhou, Z.S.; Hu, B.Y.; Wei, M.Z.; Zhao, Y.L.; Dai, Z.; Luo, X.D. A dual mechanism with H2S inhibition and membrane damage of morusin from Morus alba Linn. against MDR-MRSA. Bioorg. Med. Chem. 2024, 97, 117544. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.M.; Wang, Z.J.; Zu, W.B.; Jiang, Y.M.; Zhu, Y.Y.; Wei, M.Z.; Luo, X.D. Activity of the Caged Xanthone Morellic Acid against Vancomycin Resistant Enterococcus Infection by Targeting the Bacterial Membrane. J. Nat. Prod. 2024, 87, 2366–2375. [Google Scholar] [CrossRef]
- Wang, Z.J.; Zhu, Y.Y.; Bai, L.Y.; Tang, D.M.; Zhou, Z.S.; Wei, M.Z.; He, J.B.; Yu-Duan; Luo, X.D. A new therapeutic strategy for infectious diseases against intracellular multidrug-resistant bacteria. J. Control. Release 2024, 375, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.Y.; Wang, Z.J.; Bai, L.Y.; Zu, W.B.; Zhou, Z.S.; Zhu, Y.Y.; Zhao, Y.L.; Luo, X.D. Diterpenoids of Caryopteris trichosphaera W. W. Sm. inhibiting MRSA and VRE in vitro and in vivo. J. Ethnopharmacol. 2025, 337, 118805. [Google Scholar] [CrossRef]
- Bai, L.Y.; Wang, Z.J.; Lu, Q.Y.; Huang, H.; Zhu, Y.Y.; Zhao, Y.L.; Luo, X.D. 6-Methoxyldihydrochelerythrine Chloride Inhibiting Intra and Extracellular Drug-Resistant Bacteria. ACS Infect. Dis. 2024, 10, 3430–3439. [Google Scholar] [CrossRef]
- Shen, J.S.; Wang, Z.J.; Zhu, Y.Y.; Wei, M.Z.; Wang, X.H.; Luo, X.D. Antifungal bioactivity of Sarcococca hookeriana var. digyna Franch. against fluconazole-resistant Candida albicans in vitro and in vivo. J. Ethnopharmacol. 2024, 333, 118473. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.Z.; Wang, Z.J.; Shi, N.; Bai, L.Y.; Jiang, Y.M.; Jiang, L.; Liu, T.; Wei, M.Z.; Qin, M.L.; Luo, X.D. Anti-MRSA mechanism of spirostane saponin in Rohdea pachynema FT Wang & tang. J. Ethnopharmacol. 2024, 331, 118327. [Google Scholar]
- Liu, T.; Wang, Z.J.; Shi, Y.Z.; Tao, R.; Huang, H.; Zhao, Y.L.; Luo, X.D. Curcusinol from the fruit of Carex baccans with antibacterial activity against multidrug-resistant strains. J. Ethnopharmacol. 2024, 318, 116892. [Google Scholar] [CrossRef]
- Zheng, J.P.; Hu, J.H.; Yang, Y.; Xiong, L.; Yang, H.B.; Zhang, Z.G.; Jiang, N.; Liu, H.T. Suppressive effect of Tripterygium hypoglaucum (Levl.) Hutch extract on rheumatoid arthritis in mice by modulating inflammasome and bile acid metabolism. Biomed. Pharmacother. 2023, 167, 115494. [Google Scholar] [CrossRef]
- Hu, J.H.; Ni, J.M.; Zheng, J.P.; Guo, Y.L.; Yang, Y.; Ye, C.; Sun, X.J.; Xia, H.; Liu, Y.J.; Liu, H.T. Tripterygium hypoglaucum extract ameliorates adjuvant-induced arthritis in mice through the gut microbiota. Chin. J. Nat. Med. 2023, 21, 730–744. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.Z.; Xiang, J.; Gui, W.Y.; Gong, J.H.; Zou, J.D.; Li, C.Y. Chemical Screening, Identification, and Comparison of Tripterygium Hypoglaucum Hutch Preparations by Ultra-High-Performance Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometry Combined with Multivariate Statistical Analysis. J. Sep. Sci. 2024, 47, e70023. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.Q.; Zhang, F.L.; Xiao, X.L.; Wu, Z.; Hu, Q.C.; Jiang, Y.X.; Zhang, W.W.; Wei, S.Z.; Ma, X.; Zhang, X.M. Tripterygium hypoglaucum (Lévl.) Hutch and Its Main Bioactive Components: Recent Advances in Pharmacological Activity, Pharmacokinetics and Potential Toxicity. Front. Pharmacol. 2021, 12, 715359. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.S.; Zhao, Y.L.; Hu, B.Y.; Wang, B.; Liu, Y.P.; Zhu, Y.Y.; He, Y.J.; Wang, Z.J.; Dai, Z.; Zhao, L.X.; et al. Steroidal alkaloid with unprecedented triheterocyclic architecture. Chem. Commun. 2023, 59, 326–329. [Google Scholar] [CrossRef]
- Duan, H.G.; Kawazoe, K.; Bando, M.; Kido, M.; Takaishi, Y. Di- and triterpenoids from Tripterygium hypoglaucum. Phytochemistry 1997, 46, 535–543. [Google Scholar] [CrossRef]
- Li, K.H.; Duan, H.Q.; Kawazoe, K.; Takaishi, Y. Terpenoids from Tripterygium wilfordii. Phytochemistry 1997, 45, 791–796. [Google Scholar] [CrossRef]
- Lindsey, K.L.; Budesinsky, M.; Kohout, L.; van Staden, J. Antibacterial activity of maytenonic acid isolated from the root-bark of Maytenus senegalensis. S. Afr. J. Bot. 2006, 72, 473–477. [Google Scholar] [CrossRef]
- Kim, W.; Zou, G.J.; Hari, T.P.A.; Wilt, I.K.; Zhu, W.P.; Galle, N.; Faizi, H.A.; Hendricks, G.L.; Tori, K.; Pan, W.; et al. A selective membrane-targeting repurposed antibiotic with activity against persistent methicillin-resistant Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2019, 116, 16529–16534. [Google Scholar] [CrossRef]
- Yuan, Z.W.; Wang, J.; Qu, Q.W.; Zhu, Z.X.; Xu, M.R.; Zhao, M.M.; Sun, C.X.; Peng, H.X.; Huang, X.Y.; Dong, Y.; et al. Celastrol Combats Methicillin-Resistant Staphylococcus aureus by Targeting Δ1-Pyrroline-5-Carboxylate Dehydrogenase. Adv. Sci. 2023, 10, 2302459. [Google Scholar] [CrossRef]
- Chrysouli, M.P.; Banti, C.N.; Kourkoumelis, N.; Moushi, E.E.; Tasiopoulos, A.J.; Douvalis, A.; Papachristodoulou, C.; Hatzidimitriou, A.G.; Bakas, T.; Hadjikakou, S.K. Ciprofloxacin conjugated to diphenyltin(iv): A novel formulation with enhanced antimicrobial activity. Dalton Trans. 2020, 49, 11522–11535. [Google Scholar] [CrossRef]
- Chen, S.; Liu, D.; Zhang, Q.; Guo, P.; Ding, S.Y.; Shen, J.Z.; Zhu, K.; Lin, W.H. A Marine Antibiotic Kills Multidrug-Resistant Bacteria without Detectable High-Level Resistance. ACS Infect. Dis. 2021, 7, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Vinodhini, V.; Kavitha, M. Deciphering agr quorum sensing in Staphylococcus aureus: Insights and therapeutic prospects. Mol. Biol. Rep. 2024, 51, 155. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Qiu, H.D.; Yang, N.; Xie, H.J.; Liang, W.D.; Lin, J.Y.; Zhu, H.F.; Zhou, Y.; Wang, N.; Tan, X.Y.; et al. Fascaplysin derivatives binding to DNA via unique cationic five-ring coplanar backbone showed potent antimicrobial/antibiofilm activity against MRSA in vitro and in vivo. Eur. J. Med. Chem. 2022, 230, 114099. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Gupta, J.; Sharma, S.; Sharma, D.; Sharma, S. Focused review on dual inhibition of quorum sensing and efflux pumps: A potential way to combat multi drug resistant Staphylococcus aureus infections. Int. J. Biol. Macromol. 2021, 190, 33–43. [Google Scholar] [CrossRef]
- Wang, G.Z.; Li, L.; Wang, X.K.; Li, X.; Zhang, Y.W.; Yu, J.; Jiang, J.D.; You, X.F.; Xiong, Y.Q. Hypericin enhances β-lactam antibiotics activity by inhibiting sarA expression in methicillin-resistant Staphylococcus aureus. Acta Pharm. Sin. B 2019, 9, 1174–1182. [Google Scholar] [CrossRef]
- Bai, J.R.; Zhong, K.; Wu, Y.P.; Elena, G.; Gao, H. Antibiofilm activity of shikimic acid against Staphylococcus aureus. Food Control 2019, 95, 327–333. [Google Scholar] [CrossRef]
- Bai, J.R.; Wu, Y.P.; Elena, G.; Zhong, K.; Gao, H. Insight into the effect of quinic acid on biofilm formed by Staphylococcus aureus. RSC Adv. 2019, 9, 3938–3945. [Google Scholar] [CrossRef]
- Peschel, A.; Otto, M. Phenol-soluble modulins and staphylococcal infection. Nat. Rev. Microbiol. 2013, 11, 667–673. [Google Scholar] [CrossRef]
- Tomlinson, B.R.; Malof, M.E.; Shaw, L.N. A global transcriptomic analysis of Staphylococcus aureus biofilm formation across diverse clonal lineages. Microb. Genomics 2021, 7, 000598. [Google Scholar] [CrossRef]
- Gowrishankar, S.; Kamaladevi, A.; Balamurugan, K.; Pandian, S.K. In Vitro and In Vivo Biofilm Characterization of Methicillin-Resistant Staphylococcus aureus from Patients Associated with Pharyngitis Infection. Biomed. Res. Int. 2016, 2016, 1289157. [Google Scholar] [CrossRef]
- Wu, X.Y.; Ma, G.L.; Chen, H.W.; Zhao, Z.Y.; Zhu, Z.P.; Xiong, J.; Yang, G.X.; Hu, J.F. Antibacterial and antibiofilm efficacy of the preferred fractions and compounds from Euphorbia humifusa (herba euphorbiae humifusae) against Staphylococcus aureus. J. Ethnopharmacol. 2023, 306, 116177. [Google Scholar] [CrossRef]
- Kong, C.; Chee, C.F.; Richter, K.; Thomas, N.; Abd Rahman, N.; Nathan, S. Suppression of Staphylococcus aureus biofilm formation and virulence by a benzimidazole derivative, UM-C162. Sci. Rep. 2018, 8, 2758. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Tan, X.J.; Jiao, Y.M.; Liu, L.; Zhao, W.S.; Yang, S.; Jia, A.Q. RNA-Seq-based transcriptome analysis of methicillin-resistant Staphylococcus aureus biofilm inhibition by ursolic acid and resveratrol. Sci. Rep. 2014, 4, 5467. [Google Scholar] [CrossRef]
- Shi, C.; Liu, X.F.; Chen, Y.Y.; Dai, J.M.; Li, C.Z.; Felemban, S.; Khowdiary, M.M.; Cui, H.Y.; Lin, L. Inhibitory effects of citral on the production of virulence factors in Staphylococcus aureus and its potential application in meat preservation. Int. J. Food Microbiol. 2024, 413, 110581. [Google Scholar] [CrossRef]
- Fedorowicz, J.; Cruz, C.D.; Morawska, M.; Ciura, K.; Gilbert-Girard, S.; Mazur, L.; Makkyla, H.; Ilina, P.; Savijoki, K.; Fallarero, A.; et al. Antibacterial and antibiofilm activity of permanently ionized quaternary ammonium fluoroquinolones. Eur. J. Med. Chem. 2023, 254, 115373. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Barman, R.; Das, B.; Ghosh, S. Highly Efficient Biofilm Eradication by Antibacterial Two-Dimensional Supramolecular Polymers. Chem. Mat. 2021, 33, 8656–8665. [Google Scholar] [CrossRef]
- Đukanović, S.; Ganić, T.; Lončarević, B.; Cvetković, S.; Nikolić, B.; Tenji, D.; Randjelović, D.; Mitić-Ćulafić, D. Elucidating the antibiofilm activity of Frangula emodin against Staphylococcus aureus biofilms. J. Appl. Microbiol. 2022, 132, 1840–1855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Battini, N.; Ou, J.M.; Zhang, S.L.; Zhang, L.; Zhou, C.H. New Efforts toward Aminothiazolylquinolones with Multitargeting Antibacterial Potential. J. Agric. Food Chem. 2023, 71, 2322–2332. [Google Scholar] [CrossRef]
- Lee, E.H.; Jeon, Y.H.; An, S.J.; Deng, Y.H.; Kwon, H.B.; Lim, Y.J.; Kong, H.; Kim, M.J. Removal effect of Candida albicans biofilms from the PMMA resin surface by using a manganese oxide nanozyme-doped diatom microbubbler. Heliyon 2022, 8, e12290. [Google Scholar] [CrossRef]
- Li, J.Q.; Yu, Y.; Zhou, Y.; Song, J.F.; Yang, A.M.; Wang, M.; Li, Y.Z.; Wan, M.Y.; Zhang, C.H.; Yang, H.; et al. Multi-targeting oligopyridiniums: Rational design for biofilm dispersion and bacterial persister eradication. Bioorg. Chem. 2024, 144, 107163. [Google Scholar] [CrossRef]
- Cheng, F.; Mo, Y.A.; Chen, K.Y.; Shang, X.Y.; Yang, Z.; Hao, B.C.; Shang, R.F.; Liang, J.P.; Liu, Y. Integration of metabolomics and transcriptomics indicates changes in MRSA exposed to terpinen-4-ol. BMC Microbiol. 2021, 21, 305. [Google Scholar] [CrossRef]
- He, R.R.; Zhong, Q.P.; Chen, W.J.; Zhang, M.; Pei, J.F.; Chen, H.M.; Chen, W.X. Transcriptomic and proteomic investigation of metabolic disruption in Listeria monocytogenes triggered by linalool and its application in chicken breast preservation. LWT-Food Sci. Technol. 2023, 176, 114492. [Google Scholar] [CrossRef]
- Putnam, C.D. Strand discrimination in DNA mismatch repair. DNA Repair 2021, 105, 103161. [Google Scholar] [CrossRef]
- Yang, S.; Li, J.Q.; Aweya, J.J.; Yuan, Z.J.; Weng, W.Y.; Zhang, Y.L.; Liu, G.M. Antimicrobial mechanism of Larimichthys crocea whey acidic protein-derived peptide (LCWAP) against Staphylococcus aureus and its application in milk. Int. J. Food Microbiol. 2020, 335, 108891. [Google Scholar] [CrossRef]
- Xie, Y.P.; Ansari, M.F.; Zhang, S.L.; Zhou, C.H. Novel carbazole-oxadiazoles as potential Staphylococcus aureus germicides. Pest. Biochem. Physiol. 2021, 175, 104849. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.Z.; Zhu, Y.Y.; Zu, W.B.; Wang, H.; Bai, L.Y.; Zhou, Z.S.; Zhao, Y.L.; Wang, Z.J.; Luo, X.D. Structure optimizing of flavonoids against both MRSA and VRE. Eur. J. Med. Chem. 2024, 271, 116401. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.Z.; Wang, Z.J.; Zhu, Y.Y.; Zu, W.B.; Zhao, Y.L.; Luo, X.D. Oleanolic acid derivatives against drug-resistant bacteria and fungi by multi-targets to avoid drug resistance. Eur. J. Med. Chem. 2024, 280, 116940. [Google Scholar] [CrossRef] [PubMed]
- Yehia, F.A.A.; Yousef, N.; Askoura, M. Celastrol mitigates staphyloxanthin biosynthesis and biofilm formation in Staphylococcus aureus via targeting key regulators of virulence; in vitro and in vivo approach. BMC Microbiol. 2022, 22, 106. [Google Scholar] [CrossRef]
- Fedorowicz, J.; Saczewski, J.; Konopacka, A.; Waleron, K.; Lejnowski, D.; Ciura, K.; Tomasic, T.; Skok, Z.; Savijoki, K.; Morawska, M.; et al. Synthesis and biological evaluation of hybrid quinolone-based quaternary ammonium antibacterial agents. Eur. J. Med. Chem. 2019, 179, 576–590. [Google Scholar] [CrossRef]


| Position | δCa, Type | δHb (J in Hz) | Position | δCa, Type | δHb (J in Hz) |
|---|---|---|---|---|---|
| 1 | 110.0, CH | 6.69, s | 16 | 38.1, CH2 | 1.90, m 1.43, m |
| 2 | 142.2, C | 17 | 31.6, C | ||
| 3 | 144.7, C | 18 | 45.9, CH | 1.58, overlap | |
| 4 | 121.8 C | 19 | 30.9, CH2 | 2.12, overlap 1.38, d, (4.6) | |
| 5 | 125.2, C | 20 | 41.3, C | ||
| 6 | 42.0, CH | 3.58, m | 21 | 31.7, CH2 | 1.67, m |
| 7 | 122.6, CH | 6.02, d (6.1) | 22 | 36.0, CH2 | 2.12, overlap 0.92, m |
| 8 | 151.2, C | 23 | 11.8, CH3 | 2.21, s | |
| 9 | 38.5, C | 24 | 69.2, CH2 | 3.71, dd, (10.3, 4.1) 3.26, t (10.3) | |
| 10 | 142.9, C | 25 | 37.7, CH3 | 1.47, s | |
| 11 | 37.1, CH2 | 2.09, m 1.83, m | 26 | 22.7, CH3 | 1.28, s |
| 12 | 31.6, CH2 | 2.47, d (15.6) 1.75, d (15.6) | 27 | 19.2, CH3 | 0.75, s |
| 13 | 39.1, C | 28 | 32.1, CH3 | 1.10, s | |
| 14 | 45.0, C | 29 | 33.3, CH3 | 1.17, s | |
| 15 | 30.1, CH2 | 1.61, overlap | 30 | 182.8, C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.-Y.; Jin, Q.; Wang, Z.-J.; Wei, M.-Z.; Zu, W.-B.; Zhou, Z.-S.; Hu, B.-Y.; Zhao, Y.-L.; Qin, X.-J.; Luo, X.-D. Tripterhyponoid A from Tripterygium hypoglaucum Inhibiting MRSA by Multiple Mechanisms. Molecules 2025, 30, 2539. https://doi.org/10.3390/molecules30122539
Zhu Y-Y, Jin Q, Wang Z-J, Wei M-Z, Zu W-B, Zhou Z-S, Hu B-Y, Zhao Y-L, Qin X-J, Luo X-D. Tripterhyponoid A from Tripterygium hypoglaucum Inhibiting MRSA by Multiple Mechanisms. Molecules. 2025; 30(12):2539. https://doi.org/10.3390/molecules30122539
Chicago/Turabian StyleZhu, Yan-Yan, Qiong Jin, Zhao-Jie Wang, Mei-Zhen Wei, Wen-Biao Zu, Zhong-Shun Zhou, Bin-Yuan Hu, Yun-Li Zhao, Xu-Jie Qin, and Xiao-Dong Luo. 2025. "Tripterhyponoid A from Tripterygium hypoglaucum Inhibiting MRSA by Multiple Mechanisms" Molecules 30, no. 12: 2539. https://doi.org/10.3390/molecules30122539
APA StyleZhu, Y.-Y., Jin, Q., Wang, Z.-J., Wei, M.-Z., Zu, W.-B., Zhou, Z.-S., Hu, B.-Y., Zhao, Y.-L., Qin, X.-J., & Luo, X.-D. (2025). Tripterhyponoid A from Tripterygium hypoglaucum Inhibiting MRSA by Multiple Mechanisms. Molecules, 30(12), 2539. https://doi.org/10.3390/molecules30122539






