Echinacoside Ameliorates UVB-Induced Skin Damage Through Selective Inhibition of the Cutaneous TRPV3 Channel
Abstract
1. Introduction
2. Results
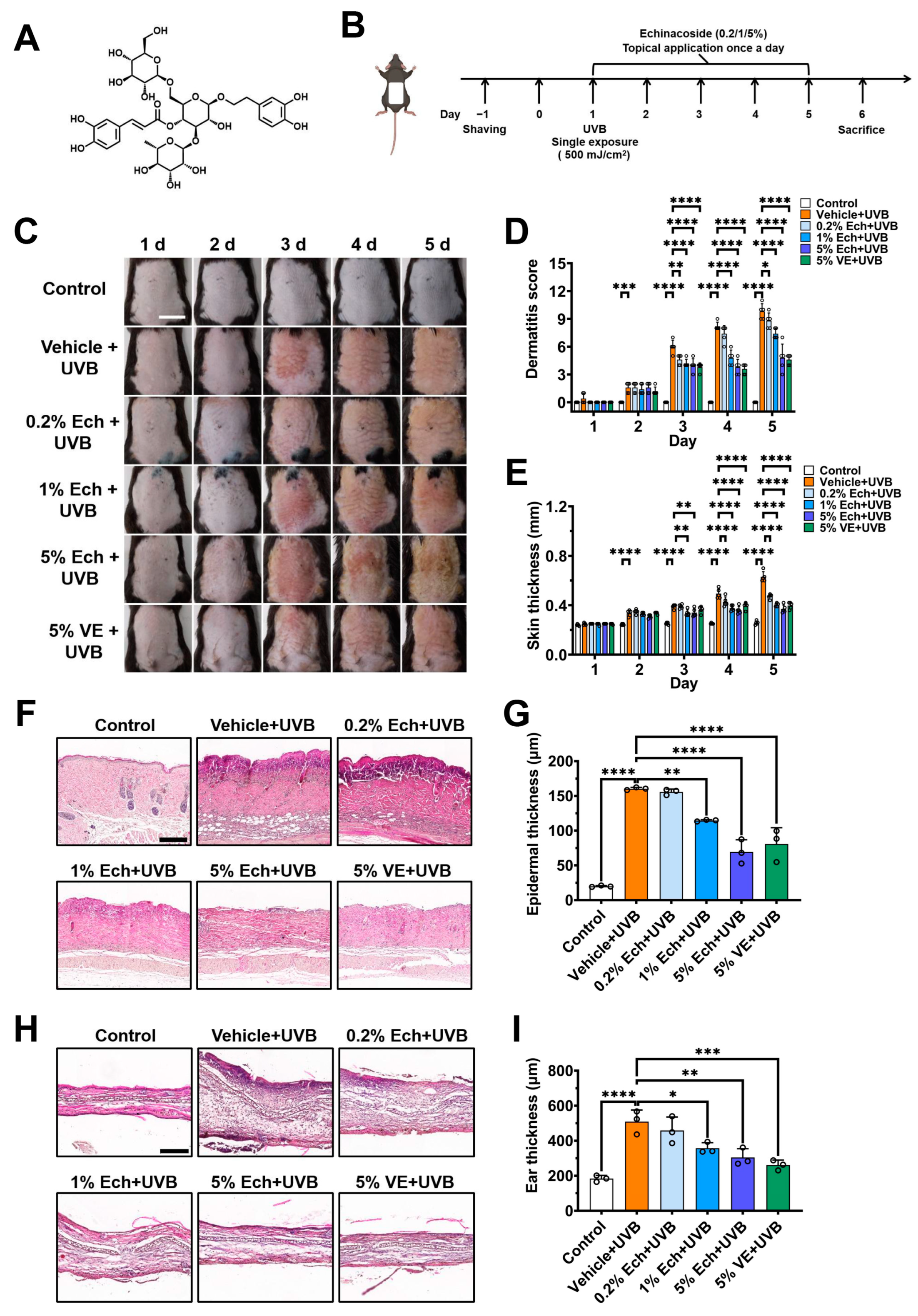
2.1. Echinacoside Dose-Dependently Alleviates UVB-Induced Acute Skin Injury
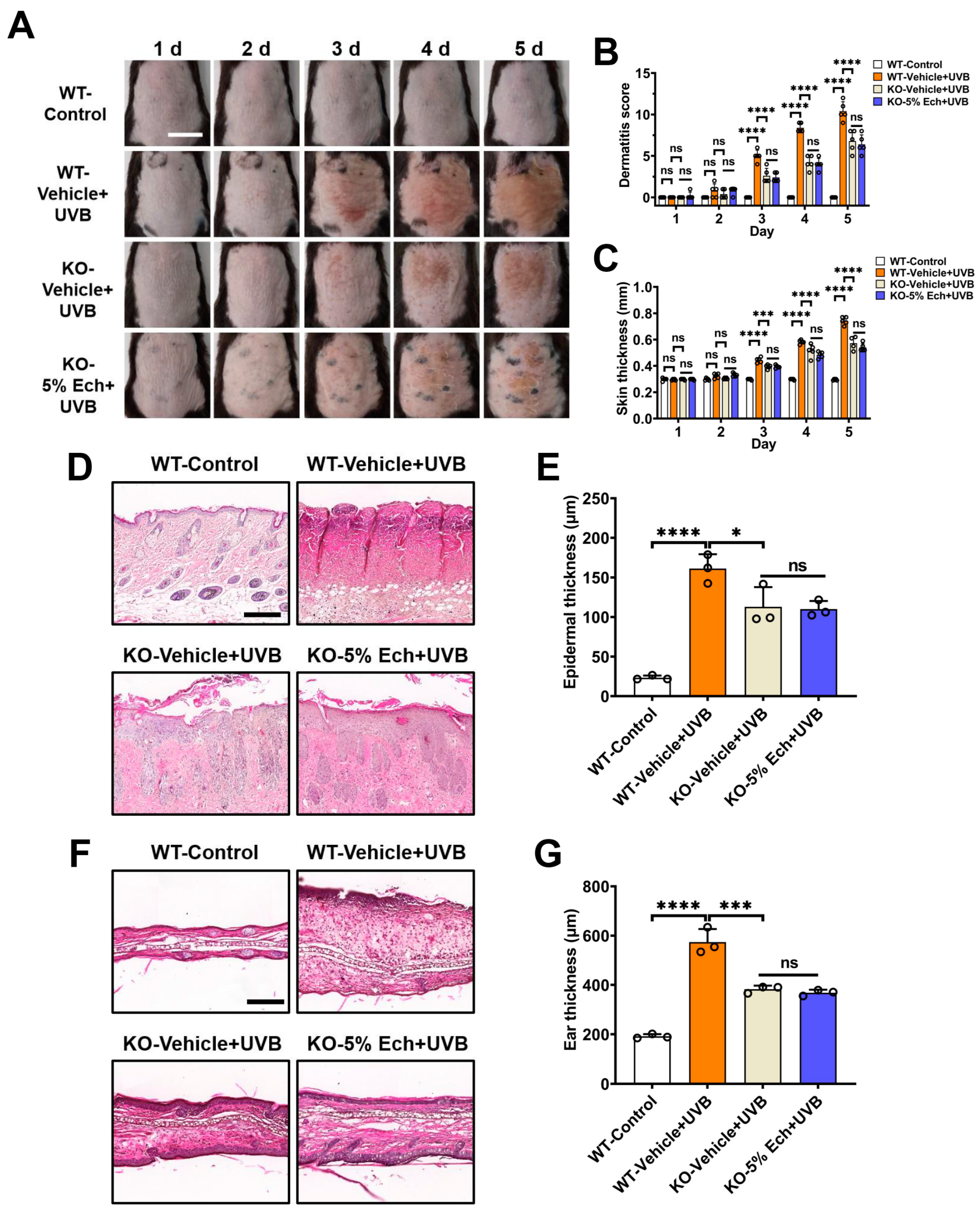
2.2. Attenuation of UVB-Induced Acute Skin Injury by Echinacoside Is Dependent on TRPV3
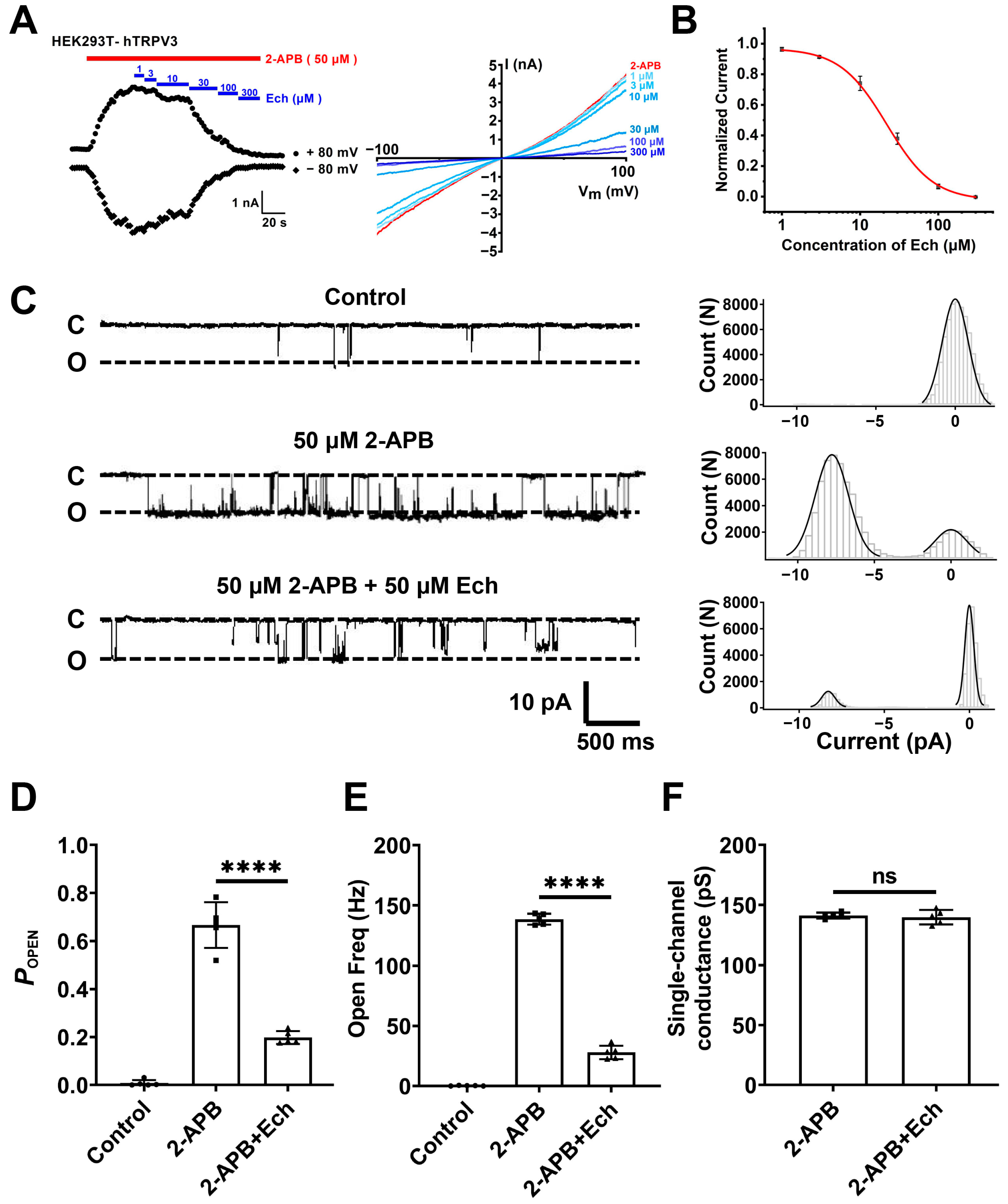
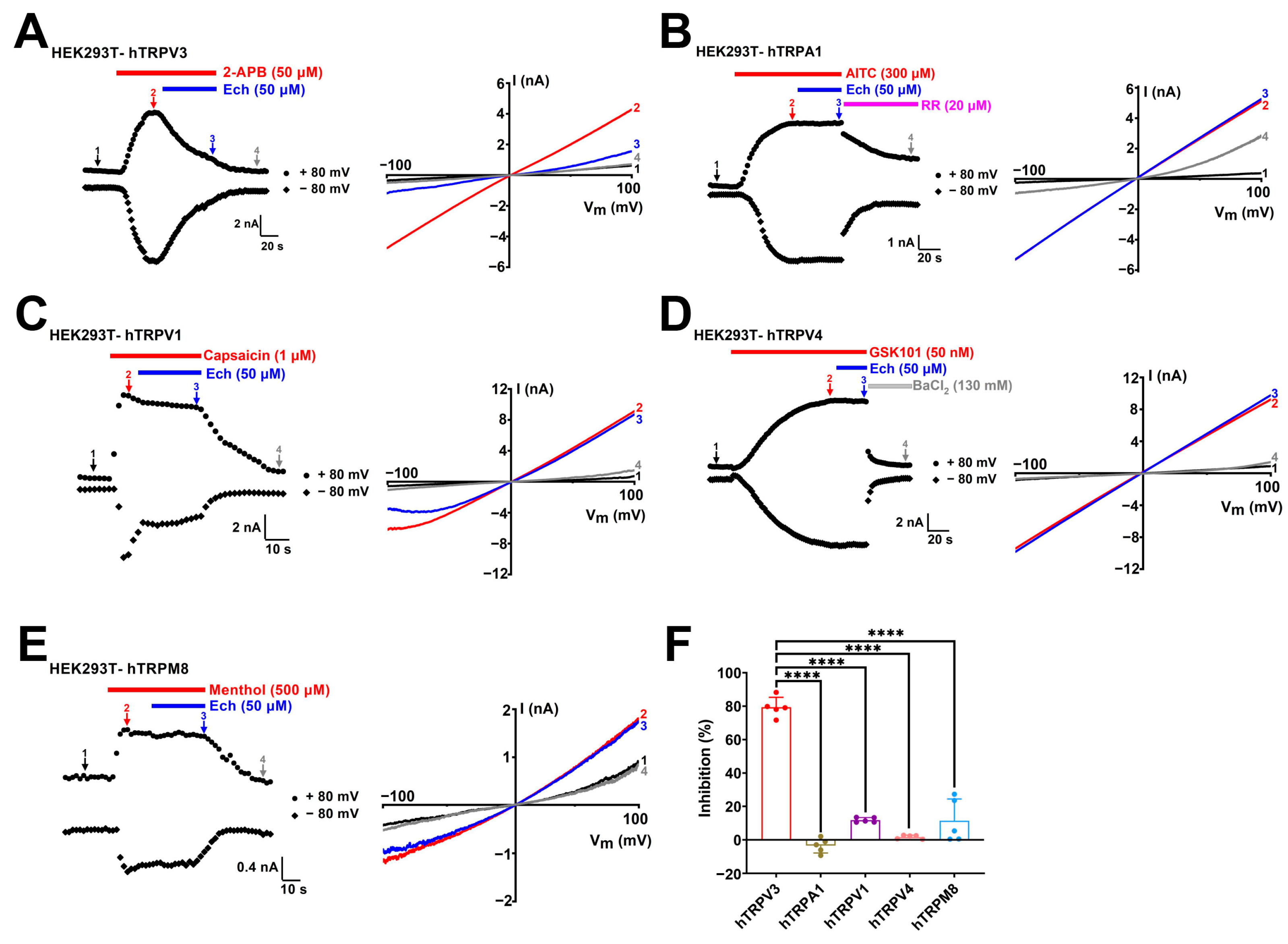
2.3. Echinacoside Is a Selective TRPV3 Inhibitor
2.4. Identification of Residues Critical for Echinacoside Binding to TRPV3
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Compounds and Creams
4.3. Establishment and Treatment of UVB-Induced Acute Skin Injury Mouse Model
4.4. Skin Lesion Scoring and Dorsal Skin Thickness Measurements
4.5. Histology
4.6. Cell Culture and Transient Transfection
4.7. Electrophysiological Recordings
4.8. Molecular Docking and Site-Directed Mutagenesis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hwa, C.; Bauer, E.A.; Cohen, D.E. Skin biology. Dermatol. Ther. 2011, 24, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Q.; Zhi, L.; You, B.; Wang, G.; Wu, N.; Jia, Y. Skin homeostasis: Mechanism and influencing factors. J. Cosmet. Dermatol. 2024, 23, 1518–1526. [Google Scholar] [CrossRef]
- Passeron, T.; Lim, H.W.; Goh, C.L.; Kang, H.Y.; Ly, F.; Morita, A.; Ocampo Candiani, J.; Puig, S.; Schalka, S.; Wei, L.; et al. Photoprotection according to skin phototype and dermatoses: Practical recommendations from an expert panel. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1460–1469. [Google Scholar] [CrossRef]
- Trakatelli, M.; Barkitzi, K.; Apap, C.; Majewski, S.; De Vries, E. Skin cancer risk in outdoor workers: A European multicenter case–control study. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 5–11. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Ananthaswamy, H.N. Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharmacol. 2004, 195, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Gaddameedhi, S. UV-B-Induced Erythema in Human Skin: The Circadian Clock Is Ticking. J. Investig. Dermatol. 2018, 138, 248–251. [Google Scholar] [CrossRef]
- Khalil, C. Human skin explants an in vitro approach for assessing UVB induced damage. Toxicol. In Vitro 2018, 53, 193–199. [Google Scholar] [CrossRef]
- Cavinato, M.; Waltenberger, B.; Baraldo, G.; Grade, C.V.C.; Stuppner, H.; Jansen-Dürr, P. Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontology 2017, 18, 499–516. [Google Scholar] [CrossRef]
- Lei, D.; Ye, L.; Wen, S.; Zhang, J.; Zhang, L.; Man, M.-Q. Preventive and Therapeutic Benefits of Natural Ingredients in Photo-Induced Epidermal Dysfunction. Skin Pharmacol. Physiol. 2024, 37, 1–18. [Google Scholar] [CrossRef]
- Milutinov, J.; Pavlović, N.; Ćirin, D.; Atanacković Krstonošić, M.; Krstonošić, V. The Potential of Natural Compounds in UV Protection Products. Molecules 2024, 29, 5409. [Google Scholar] [CrossRef]
- Song, Y.; Zeng, K.; Jiang, Y.; Tu, P. Cistanches Herba, from an endangered species to a big brand of Chinese medicine. Med. Res. Rev. 2021, 41, 1539–1577. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lin, H.; Gu, L.; Gao, J.; Tzeng, C.-M. Herba Cistanche (Rou Cong-Rong): One of the Best Pharmaceutical Gifts of Traditional Chinese Medicine. Front. Pharmacol. 2016, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Qiao, L.; Mei, S.; Zhang, H.; Ji, S.; Wang, N. Herba Cistanches: Anti-aging. Aging Dis. 2017, 8, 740–759. [Google Scholar]
- Zhou, S.; Feng, D.; Zhou, Y.; Duan, H.; Jiang, Y.; Yan, W. Analysis of the active ingredients and health applications of cistanche. Front. Nutr. 2023, 10, 1101182. [Google Scholar] [CrossRef]
- Trampetti, F.; Pereira, C.; Rodrigues, M.J.; Celaj, O.; D’Abrosca, B.; Zengin, G.; Mollica, A.; Stefanucci, A.; Custódio, L. Exploring the halophyte Cistanche phelypaea (L.) Cout as a source of health promoting products: In vitro antioxidant and enzyme inhibitory properties, metabolomic profile and computational studies. J. Pharm. Biomed. Anal. 2019, 165, 119–128. [Google Scholar] [CrossRef]
- Zou, P.; Song, Y.; Lei, W.; Li, J.; Tu, P.; Jiang, Y. Application of 1 H NMR-based metabolomics for discrimination of different parts and development of a new processing workflow for Cistanche deserticola. Acta Pharm. Sin. B 2017, 7, 647–656. [Google Scholar] [CrossRef]
- Zhang, D.; Lu, C.; Yu, Z.; Wang, X.; Yan, L.; Zhang, J.; Li, H.; Wang, J.; Wen, A.; Singhal, S.S. Echinacoside Alleviates UVB Irradiation-Mediated Skin Damage via Inhibition of Oxidative Stress, DNA Damage, and Apoptosis. Oxid. Med. Cell. Longev. 2017, 2017, 6851464. [Google Scholar] [CrossRef]
- Wen, S.Y.; Ng, S.C.; Noriega, L.; Chen, T.J.; Chen, C.J.; Lee, S.D.; Huang, C.Y.; Kuo, W.W. Echinacoside promotes collagen synthesis and survival via activation of IGF-1 signaling to alleviate UVB-induced dermal fibroblast photoaging. Biofactors 2025, 51, e2152. [Google Scholar] [CrossRef]
- Kalinovskii, A.P.; Utkina, L.L.; Korolkova, Y.V.; Andreev, Y.A. TRPV3 Ion Channel: From Gene to Pharmacology. Int. J. Mol. Sci. 2023, 24, 8601. [Google Scholar] [CrossRef]
- Lei, J.; Tominaga, M. Unlocking the therapeutic potential of TRPV3: Insights into thermosensation, channel modulation, and skin homeostasis involving TRPV3. Bioessays 2024, 46, e2400047. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Qiao, X.; Wang, W.; He, S.; Liang, K.; Hong, X. TRPV3: Structure, Diseases and Modulators. Molecules 2023, 28, 774. [Google Scholar] [CrossRef]
- Xu, H.X.; Ramsey, I.S.; Kotecha, S.A.; Moran, M.M.; Chong, J.H.A.; Lawson, D.; Ge, P.; Lilly, J.; Silos-Santiago, I.; Xie, Y.; et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 2002, 418, 181–186. [Google Scholar] [CrossRef]
- Lai-Cheong, J.E.; Sethuraman, G.; Ramam, M.; Stone, K.; Simpson, M.A.; McGrath, J.A. Recurrent heterozygous missense mutation, p.Gly573Ser, in the TRPV3 gene in an Indian boy with sporadic Olmsted syndrome. Br. J. Dermatol. 2012, 167, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.H.; Kim, S.; Kim, S.-E.; Chung, S.; Lee, S.E. Enhanced Thermal Sensitivity of TRPV3 in Keratinocytes Underlies Heat-Induced Pruritogen Release and Pruritus in Atopic Dermatitis. J. Investig. Dermatol. 2020, 140, 2199–2209.e6. [Google Scholar] [CrossRef]
- Um, J.Y.; Kim, H.B.; Kim, J.C.; Park, J.S.; Lee, S.Y.; Chung, B.Y.; Park, C.W.; Kim, H.O. TRPV3 and Itch: The Role of TRPV3 in Chronic Pruritus according to Clinical and Experimental Evidence. Int. J. Mol. Sci. 2022, 23, 14962. [Google Scholar] [CrossRef] [PubMed]
- Nattkemper, L.A.; Lipman, Z.M.; Ingrasci, G.; Maldonado, C.; Garces, J.C.; Loayza, E.; Yosipovitch, G. Neuroimmune Mediators of Pruritus in Hispanic Scalp Psoriatic Itch. Acta Derm. Venereol. 2023, 103, adv4463. [Google Scholar] [CrossRef]
- Qu, Y.; Sun, X.; Wei, N.; Wang, K. Inhibition of cutaneous heat-sensitive Ca2+-permeable transient receptor potential vanilloid 3 channels alleviates UVB-induced skin lesions in mice. FASEB J. 2023, 37, e23309. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, X.; Qi, H.; Ma, Q.; Zhou, Q.; Wang, W.; Wang, K. Pharmacological Inhibition of the Temperature-Sensitive and Ca2+-Permeable Transient Receptor Potential Vanilloid TRPV3 Channel by Natural Forsythoside B Attenuates Pruritus and Cytotoxicity of Keratinocytes. J. Pharmacol. Exp. Ther. 2019, 368, 21–31. [Google Scholar] [CrossRef]
- Sun, X.; Qi, H.; Wu, H.; Qu, Y.; Wang, K. Anti-pruritic and anti-inflammatory effects of natural verbascoside through selective inhibition of temperature-sensitive Ca2+-permeable TRPV3 channel. J. Dermatol. Sci. 2020, 97, 229–231. [Google Scholar] [CrossRef]
- Camponogara, C.; Brum, E.S.; Pegoraro, N.S.; Brusco, I.; Rocha, F.G.; Brandenburg, M.M.; Cabrini, D.A.; André, E.; Trevisan, G.; Oliveira, S.M. Neuronal and non-neuronal transient receptor potential ankyrin 1 mediates UVB radiation-induced skin inflammation in mice. Life Sci. 2020, 262, 118557. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.F.; Ma, K.H.; Chang, Y.J.; Lo, L.C.; Jhap, T.Y.; Su, Y.H.; Liu, P.S.; Chueh, S.H. Baicalein inhibits matrix metalloproteinase 1 expression via activation ofTRPV1-Ca-ERKpathway in ultraviolet B–irradiated human dermal fibroblasts. Exp. Dermatol. 2019, 28, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Cevikbas, F.; Pasolli, H.A.; Chen, Y.; Kong, W.; Kempkes, C.; Parekh, P.; Lee, S.H.; Kontchou, N.-A.; Yeh, I.; et al. UVB radiation generates sunburn pain and affects skin by activating epidermal TRPV4 ion channels and triggering endothelin-1 signaling. Proc. Natl. Acad. Sci. USA 2013, 110, E3225–E3234. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tan, L.; Jiao, K.; Xue, C.; Tang, Q.; Jiang, S.; Ren, Y.; Chen, H.; El-Aziz, T.M.A.; Abdelazeem, K.N.M.; et al. Scutellarein attenuates atopic dermatitis by selectively inhibiting transient receptor potential vanilloid 3 channels. Br. J. Pharmacol. 2022, 179, 4792–4808. [Google Scholar] [CrossRef]
- Qi, H.; Shi, Y.; Wu, H.; Niu, C.; Sun, X.; Wang, K. Inhibition of temperature-sensitive TRPV3 channel by two natural isochlorogenic acid isomers for alleviation of dermatitis and chronic pruritus. Acta Pharm. Sin. B 2022, 12, 723–734. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, S.; Yue, X.; Qu, Y.; Zhang, G.; Wang, L.; Sun, X. Echinacoside Ameliorates UVB-Induced Skin Damage Through Selective Inhibition of the Cutaneous TRPV3 Channel. Molecules 2025, 30, 2026. https://doi.org/10.3390/molecules30092026
Mo S, Yue X, Qu Y, Zhang G, Wang L, Sun X. Echinacoside Ameliorates UVB-Induced Skin Damage Through Selective Inhibition of the Cutaneous TRPV3 Channel. Molecules. 2025; 30(9):2026. https://doi.org/10.3390/molecules30092026
Chicago/Turabian StyleMo, Shilun, Xinying Yue, Yaxuan Qu, Guoji Zhang, Liqin Wang, and Xiaoying Sun. 2025. "Echinacoside Ameliorates UVB-Induced Skin Damage Through Selective Inhibition of the Cutaneous TRPV3 Channel" Molecules 30, no. 9: 2026. https://doi.org/10.3390/molecules30092026
APA StyleMo, S., Yue, X., Qu, Y., Zhang, G., Wang, L., & Sun, X. (2025). Echinacoside Ameliorates UVB-Induced Skin Damage Through Selective Inhibition of the Cutaneous TRPV3 Channel. Molecules, 30(9), 2026. https://doi.org/10.3390/molecules30092026







