Fate of Dissolved Organic Matter and Cooperation Behavior of Coagulation: Fenton Combined with MBR Treatment for Pharmaceutical Tail Water
Abstract
1. Introduction
2. Results
2.1. Characterization of PTW
2.2. Research on Coagulation for PTW Treatment
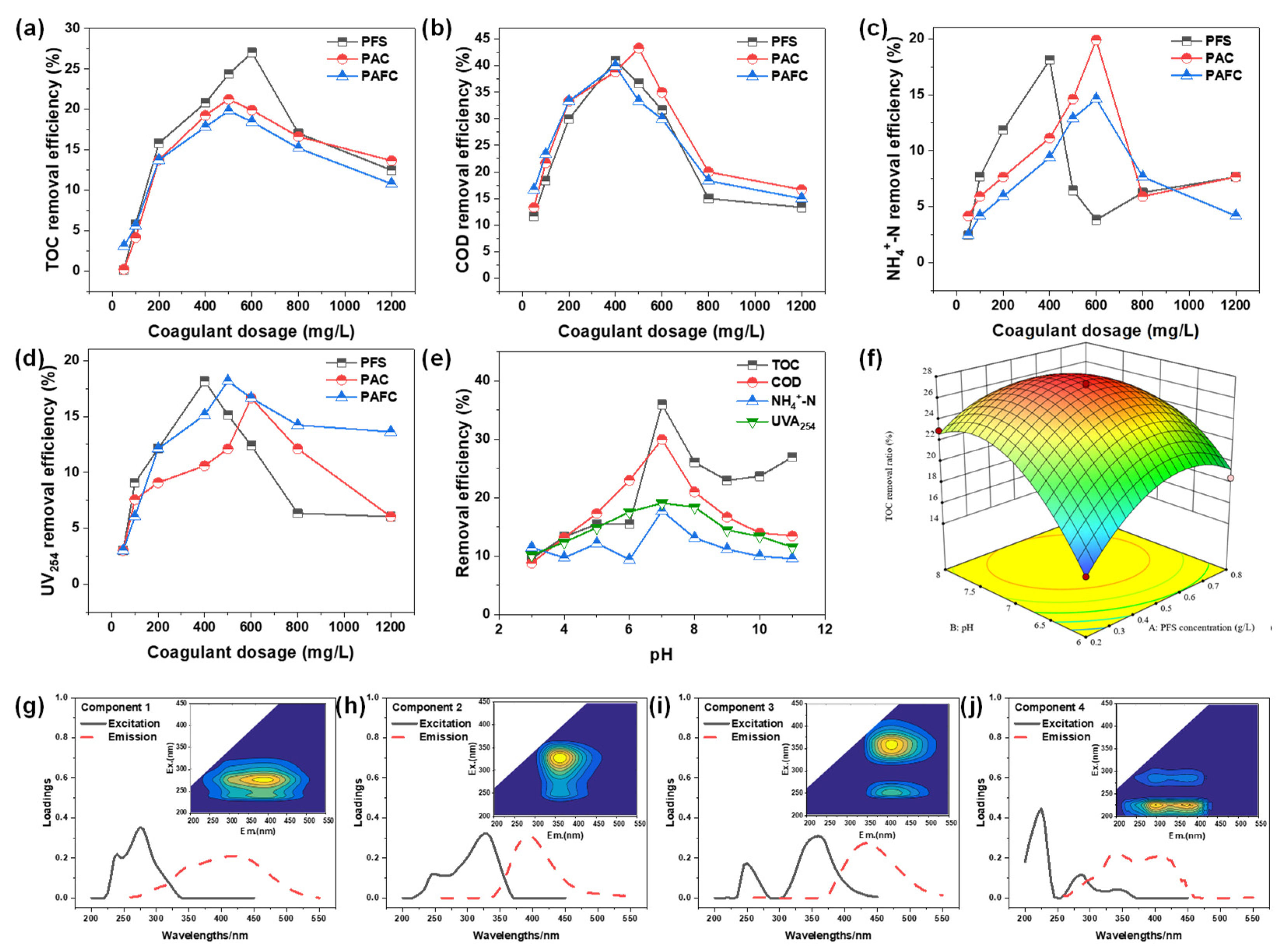
2.2.1. Effect of Coagulation on Treatment
2.2.2. Effect of pH on PTW After PFS Treatment
2.2.3. Optimization and Analysis After Coagulation Treatment
2.2.4. Effect of Stirring Procedure on Coagulation
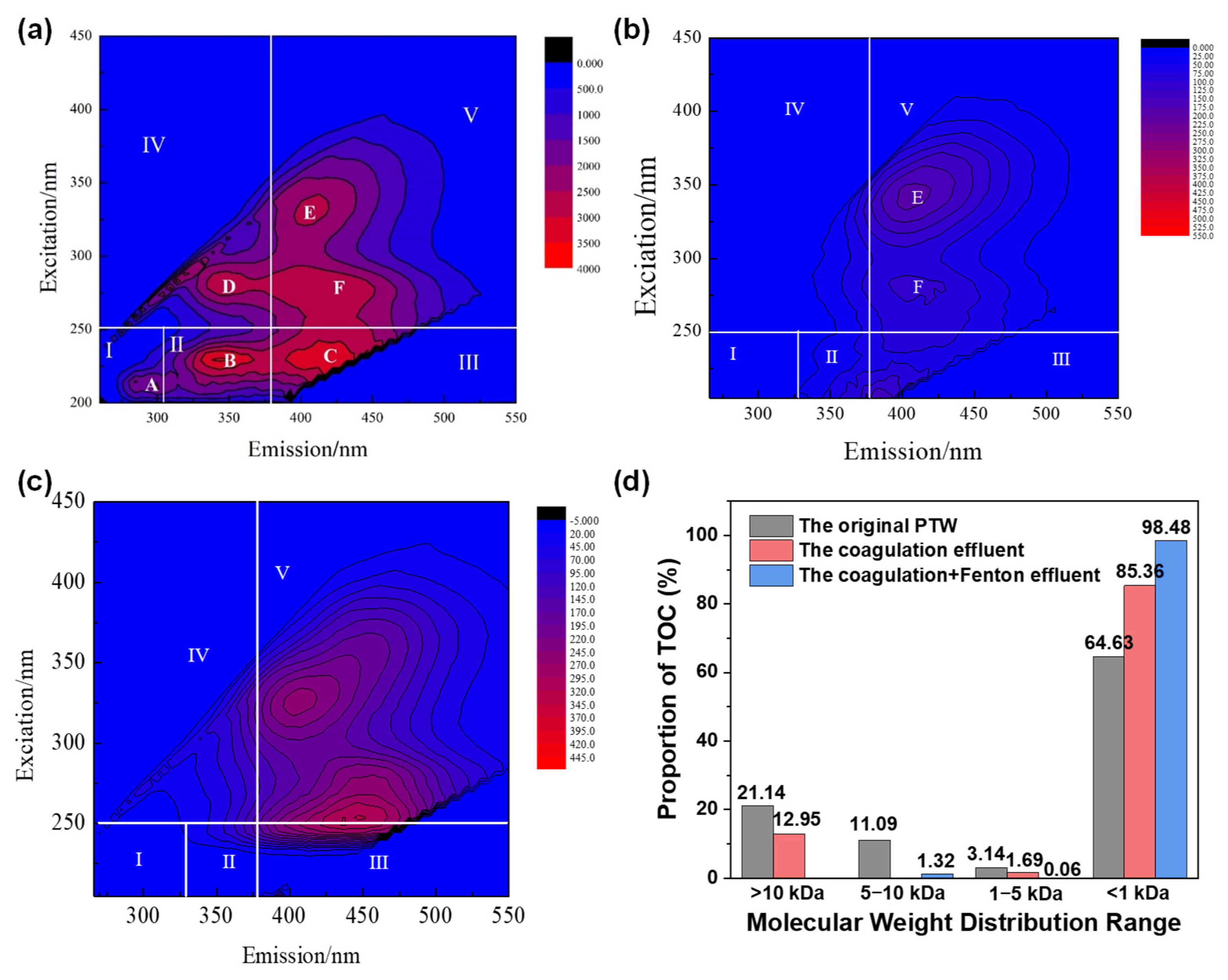
2.2.5. Coagulant on Remove of DOM
- (1)
- Analysis of EEM
- (2)
- PARAFAC analysis of coagulation effluent
- (3)
- Distribution of molecular weight
2.3. The Degradation of Effluent of Coagulation by Fenton Reaction
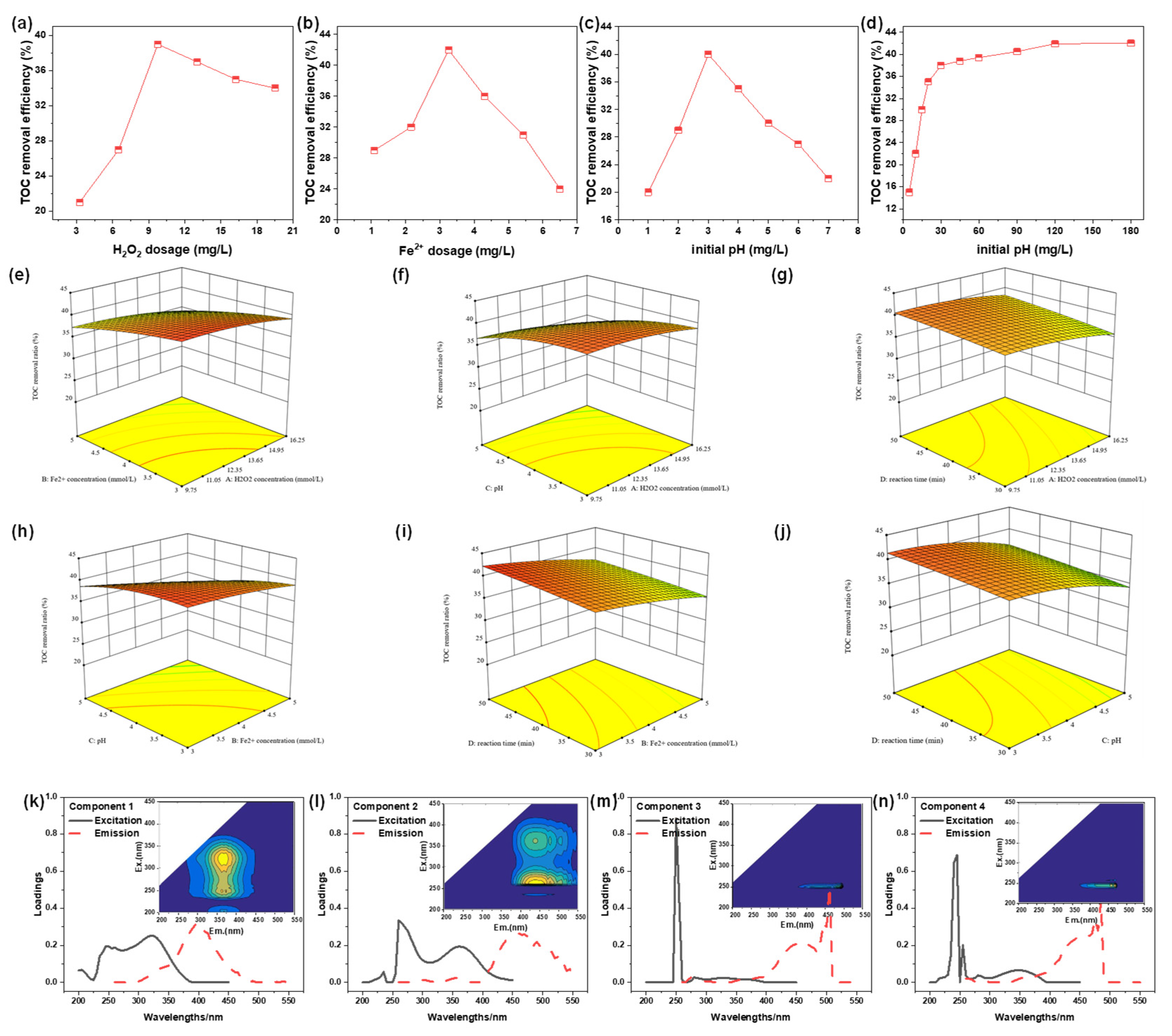
2.3.1. The Effects of Various Parameters on TOC Removal Efficiency of PTW
2.3.2. Optimization and Analysis After Fenton Treatment
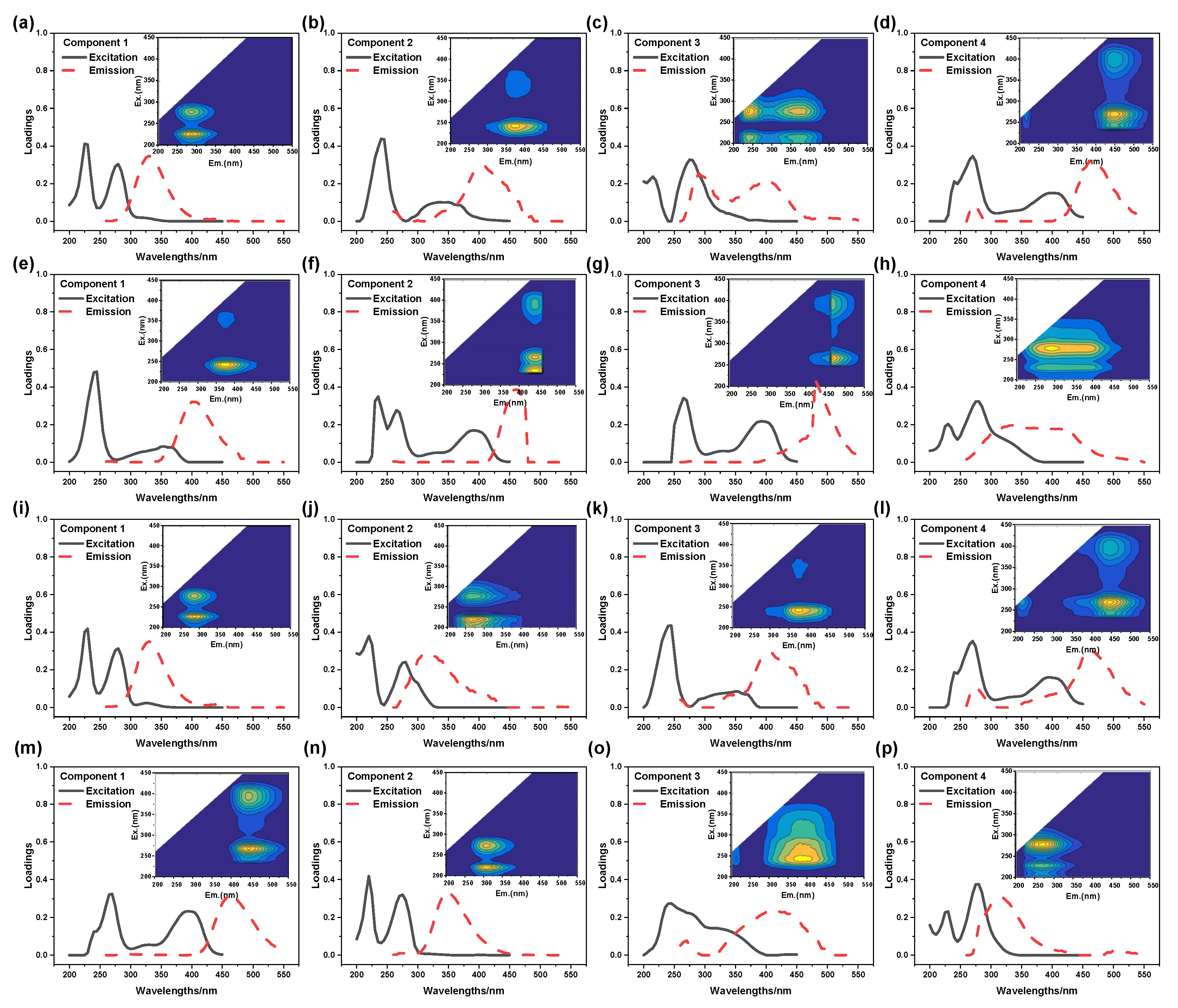
2.3.3. Analysis of DOM After Fenton
- (1)
- Analysis of EEM spectra
- (2)
- PARAFAC analysis of Fenton effluent
- (3)
- Analysis of Molecular Weight Distribution
2.4. Treatment of PTW After MBR
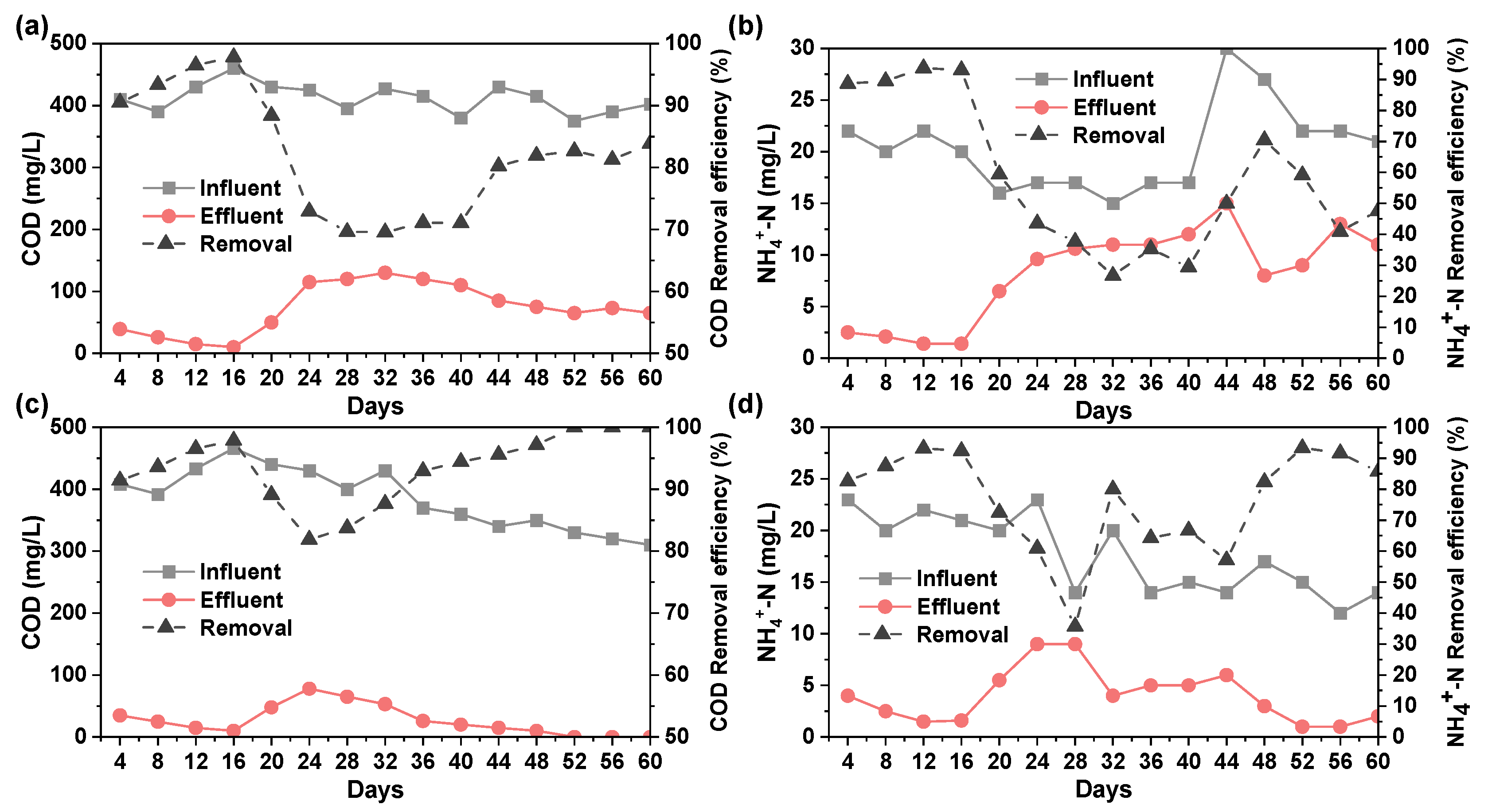
2.4.1. Removal of COD and NH4+-N by MBR
2.4.2. Analysis of DOM After Treatment
- (1)
- PRAFAC analysis of MBR-A influent and effluent
- (2)
- PARAFAC analysis of MBR-B influent and effluent
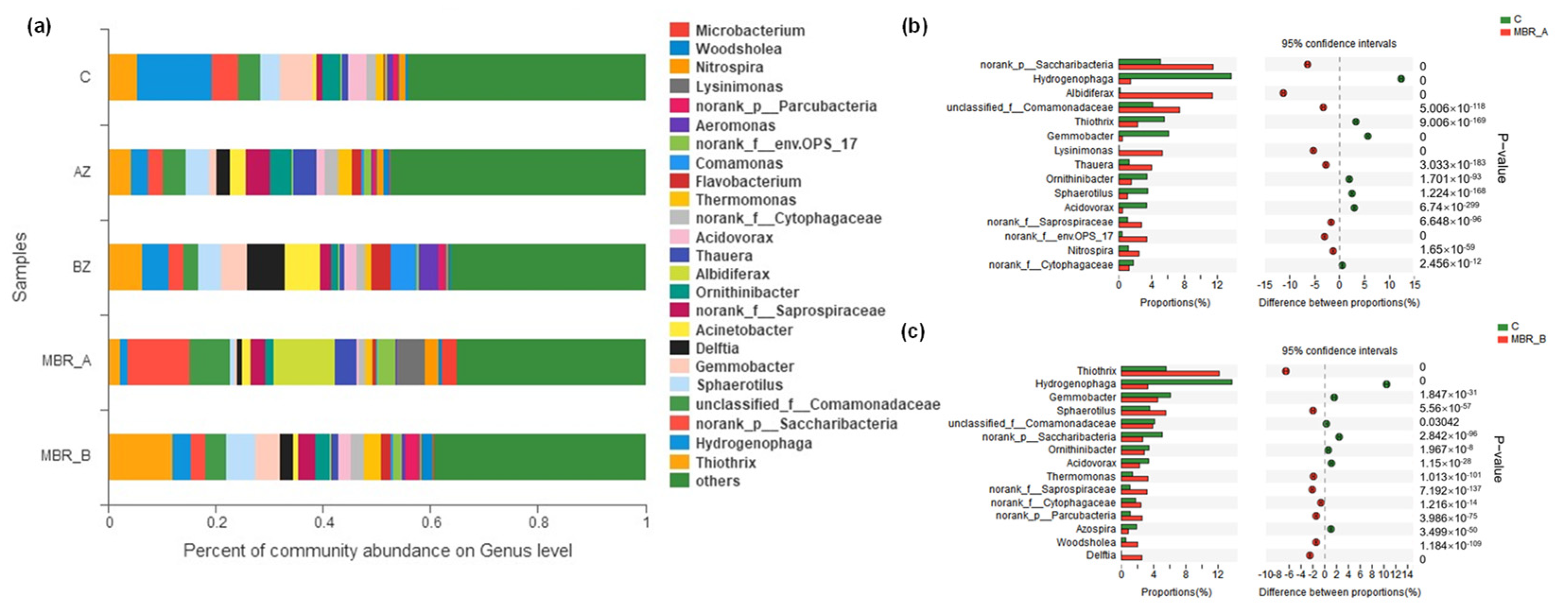
2.4.3. Comparison of the Microorganism Diversity Between MBR A and B
- (1)
- Microorganism diversity index analysis
- (2)
- Analysis of community structure and succession
- (3)
- Comparative analysis of community
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Treatment of PTW by Coagulation
3.2.2. Treatment of Effluent of Coagulation Process by Fenton Reaction
3.2.3. Treatment of Mixture of PTW and Domestic Sewage by MBR
3.2.4. Statistical Analysis
4. Conclusions
- (1)
- Compared to PAC and PAFC, PFS possessed the advantage of lower dosage and higher removal efficiencies to TOC, COD, NH4+-N, and UV254 of the original PTW. The optimum pH and PFS dosage were 7.25 and 0.53 g/L, respectively. Batch experiment indicated that the best performance of coagulation process was achieved when the mixer was stirred at 250 rpm for 3 min and 60 rpm for 10 min, followed by being left for 60 min. According to the difference in molecular weight distribution between the before and after coagulation process, the matter mainly removed by coagulation process was the organics with molecular weight above 10 kDa.
- (2)
- For PTW treated by coagulation process, the best removal efficiency of TOC (43.1%) was obtained by Fenton reaction at 11.6 mmol/L H2O2, 3.0 mmol/L FeSO4, pH = 3.3 and T = 50 min. A kind of common macromolecule aromatic amino acid compounds located at Ex = 250 nm and Em = 500 nm was the main reason that caused the high TOC concentration of the effluent. The decreased percentage of organics with molecular weight above 10 kDa and the increased percentage of organics with molecular weight below 1 kDa meant that some macromolecule compounds were transformed into small molecular weight compounds by Fenton reaction.
- (3)
- Compared with the original PTW, the one treated by coagulation process and Fenton reaction could achieve stable removal efficiency of COD and NH4+-N in a shorter time. More than 95% COD and 50–60% NH4+-N were removed when the mixture of pre-treated PTW and domestic sewage was used as an influent. According to EEM, a kind of amino acid which was similar to tryptophan was the main reason that caused PTW resistance to be degraded. Analysis of the microorganism community in the MBR suggested that norank_f__Saprospiraceae was the key microorganism in the degradation of PTW.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qian, F.; He, M.; Song, Y.; Tysklind, M.; Wu, J. A bibliometric analysis of global research progress on pharmaceutical wastewater treatment during 1994–2013. Environ. Earth Sci. 2015, 73, 4995–5005. [Google Scholar] [CrossRef]
- Khan, A.H.; Khan, N.A.; Zubair, M.; Shaida, M.A.; Manzar, M.S.; Abutaleb, A.; Naushad, M.; Iqbal, J. Sustainable green nanoadsorbents for remediation of pharmaceuticals from water and wastewater: A critical review. Environ. Res. 2022, 204, 112243. [Google Scholar] [CrossRef]
- Phan, H.N.Q.; Leu, H.J.; Nguyen, V.N.D. Enhancing pharmaceutical wastewater treatment: Ozone-assisted electrooxidation and precision optimization via response surface methodology. J. Water Process Eng. 2024, 58, 104782. [Google Scholar] [CrossRef]
- López-Fernández, R.; Martínez, L.; Villaverde, S. Membrane bioreactor for the treatment of pharmaceutical wastewater containing corticosteroids. Desalination 2012, 300, 19–23. [Google Scholar] [CrossRef]
- Tang, C.J.; Zheng, P.; Chen, T.T.; Zhang, J.Q.; Mahmood, Q.; Ding, S.; Chen, X.G.; Wu, D.T. Enhanced nitrogen removal from pharmaceutical wastewater using SBA-ANAMMOX process. Water Res. 2011, 45, 201–210. [Google Scholar] [CrossRef]
- Choudhary, A.K.; Kumar, S.; Sharma, C. Removal of chloro-organics and color from pulp and paper mill wastewater by polyaluminium chloride as coagulant. Desalin. Water Treat. 2015, 53, 697–708. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. Adsorption of phenolic compounds on low-cost adsorbents: A review. Adv. Colloid Interface Sci. 2008, 143, 48–67. [Google Scholar] [CrossRef] [PubMed]
- Gadipelly, C.; Pérez-González, A.; Yadav, G.D.; Ortiz, I.; Ibáñez, R.; Rathod, V.K.; Marathe, K.V. Pharmaceutical Industry Wastewater: Review of the Technologies for Water Treatment and Reuse. Ind. Eng. Chem. Res. 2014, 53, 11571–11592. [Google Scholar] [CrossRef]
- Domingues, E.; Fernandes, E.; Gomes, J.; Martins, R.C.; Quinta-Ferreira, R.M.; Castro, L.M. Olive oil extraction industry wastewater treatment by coagulation and Fenton’s process. J. Water Process Eng. 2021, 39, 101818. [Google Scholar] [CrossRef]
- Ikehata, K.; Jodeiri Naghashkar, N.; Gamal El-Din, M. Degradation of Aqueous Pharmaceuticals by Ozonation and Advanced Oxidation Processes: A Review. Ozone Sci. Eng. 2006, 28, 353–414. [Google Scholar] [CrossRef]
- Sirtori, C.; Zapata, A.; Oller, I.; Gernjak, W.; Agüera, A.; Malato, S. Decontamination industrial pharmaceutical wastewater by combining solar photo-Fenton and biological treatment. Water Res. 2009, 43, 661–668. [Google Scholar] [CrossRef]
- Pandis, P.K.; Kalogirou, C.; Kanellou, E.; Vaitsis, C.; Savvidou, M.G.; Anagnostopoulos, V.A.; Sygellou, L.; Yiantzi, E.; Psillakis, E.; Mantzavinos, D. Key points of advanced oxidation processes (AOPs) for wastewater, organic pollutants and pharmaceutical waste treatment: A mini review. ChemEngineering 2022, 6, 8. [Google Scholar] [CrossRef]
- Lin, R.; Li, Y.; Yong, T.; Chen, L.; Wang, Y.; Huang, H.; Zhang, Y.; Liu, J.; Chen, J.; Crittenden, J. Synergistic effects of oxidation, coagulation and adsorption in the integrated fenton-based process for wastewater treatment: A review. J. Environ. Manag. 2022, 306, 114460. [Google Scholar] [CrossRef] [PubMed]
- Sayın, F.E.; Karatas, O.; Özbay, İ.; Arslan-Alaton, I.; Olmez-Hanci, T.; Tureli, G.; Gurses, A.; Dogar, C. Treatment of real printing and packaging wastewater by combination of coagulation with Fenton and photo-Fenton processes. Chemosphere 2022, 306, 135539. [Google Scholar] [CrossRef] [PubMed]
- Joss, A.; Salzgeber, D.; Eugster, J.; König, R.; Rottermann, K.; Burger, S.; Fabijan, P.; Leumann, S.; Mohn, J.; Siegrist, H. Full-Scale nitrogen removal from digester liquid with partial nitridation and anammox in one SBR. Environ. Sci. Technol. 2009, 43, 5301–5306. [Google Scholar] [CrossRef] [PubMed]
- Monteoliva-García, A.; Martín-Pascual, J.; Muñío, M.D.M.; Poyatos, J.M. Effects of carrier addition on water quality and pharmaceutical removal capacity of a membrane bioreactor–advanced oxidation process combined treatment. Sci. Total Environ. 2020, 708, 135104. [Google Scholar] [CrossRef]
- Sipma, J.; Osuna, B.; Collado, N.; Monclús, H.; Ferrero, G.; Comas, J.; Rodriguez-Roda, I. Comparison of removal of pharmaceuticals in MBR and activated sludge systems. Desalination 2010, 250, 653–659. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Liu, L.; Cheng, S.; Wu, Z.; Yang, L. Using EEM-PARAFAC to identify and trace the pollution sources of surface water with receptor models in Taihu Lake Basin, China. J. Environ. Manag. 2022, 321, 115925. [Google Scholar] [CrossRef]
- Shin, C.; Bae, J. Current status of the pilot-scale anaerobic membrane bioreactor treatments of domestic wastewaters: A critical review. Bioresour. Technol. 2018, 247, 1038–1046. [Google Scholar] [CrossRef]
- Seib, M.D.; Berg, K.J.; Zitomer, D.H. Low energy anaerobic membrane bioreactor for municipal wastewater treatment. J. Membr. Sci. 2016, 514, 450–457. [Google Scholar] [CrossRef]
- Yu, H.; Song, Y.; Tu, X.; Du, E.; Liu, R.; Peng, J. Assessing removal efficiency of dissolved organic matter in wastewater treatment using fluorescence excitation emission matrices with parallel factor analysis and second derivative synchronous fluorescence. Bioresour. Technol. 2013, 144, 595–601. [Google Scholar] [CrossRef]
- Pan, H.; Yu, H.; Wang, Y.; Xie, P.; Liu, Y.; Zhang, Y. Investigating variations of fluorescent dissolved organic matter in wastewater treatment using synchronous fluorescence spectroscopy combined with principal component analysis and two-dimensional correlation. Environ. Technol. 2018, 39, 2495–2502. [Google Scholar] [CrossRef]
- Yu, H.; Song, Y.; Liu, R.; Pan, H.; Xiang, L.; Qian, F. Identifying changes in dissolved organic matter content and characteristics by fluorescence spectroscopy coupled with self-organizing map and classification and regression tree analysis during wastewater treatment. Chemosphere 2014, 113, 79–86. [Google Scholar] [CrossRef]
- Hur, J. Microbial changes in selected operational descriptors of dissolved organic matters from various sources in a watershed. Water Air Soil Pollut. 2011, 215, 465–476. [Google Scholar] [CrossRef]
- Doumic, L.I.; Soares, P.A.; Ayude, M.A.; Cassanello, M.; Boaventura, R.A.R.; Vilar, V.J.P. Enhancement of a solar photo-Fenton reaction by using ferrioxalate complexes for the treatment of a synthetic cotton-textile dyeing wastewater. Chem. Eng. J. 2015, 277, 86–96. [Google Scholar] [CrossRef]
- Mcginnis, B.D.; Adams, V.D.; Middlebrooks, E.J. Degradation of ethylene glycol in photo Fenton systems. Water Res. 2000, 34, 2346–2354. [Google Scholar] [CrossRef]
- Luo, W.; Zhu, L.H.; Wang, N.; Tang, H.Q.; Cao, M.J.; She, Y.B. Efficient removal of organic pollutants with magnetic nanoscale BiFeO3 as a reusable heterogeneous Fenton-like catalyst. Environ. Sci. Technol. 2010, 44, 1786–1791. [Google Scholar] [CrossRef]
- Ortega-Liébana, M.C.; Sánchez-López, E.; Hidalgo-Carrillo, J.; Marinas, A.; Marinas, J.M.; Urbano, F.J. A comparative study of photocatalytic degradation of 3-chloropyridine under UV and solar light by homogeneous (photo-Fenton) and heterogeneous (TiO2) photocatalysis. Appl. Catal. B Environ. 2012, 127, 316–322. [Google Scholar] [CrossRef]
- Jin, Y.; Huang, P.; Chen, X.; Chen, S.; Wang, X.; Peng, J. Ciprofloxacin degradation performances and mechanisms by the heterogeneous electro-Fenton with flocculated fermentation biochar. Environ. Pollut. 2023, 324, 121425. [Google Scholar] [CrossRef]
- Zhou, X.; Feng, J.; Zhang, L.; Wang, Y.; Zhu, X.; Li, Y. Enhanced dewatering of dredging slurry by Fenton preoxidation and composite coagulants: Optimization experiments and dewatering mechanisms. Environ. Sci. Pollut. Res. 2024, 31, 50359–50371. [Google Scholar] [CrossRef]
- Yu, G.H.; Wu, M.J.; Wei, G.R.; Luo, Y.H.; Ran, W.; Wang, B.R.; Zhang, J.C.; Shen, Q.R. Binding of organic ligands with Al (III) in dissolved organic matter from soil: Implications for soil organic carbon storage. Environ. Sci. Technol. 2012, 46, 6102–6109. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Zhao, X.; Zhu, C.; Xi, B.; Liu, Y.; He, X. Assessment of humification degree of dissolved organic matter from different composts using fluorescence spectroscopy technology. Chemosphere 2014, 95, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, J.D.; Perdue, E.M. Proton-binding study of standard and reference fulvic acids, humic acids, and natural organic matter. Geochim. Cosmochim. Acta 2003, 67, 85–96. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, B.; Liu, C.; Xiao, S.; Xia, D.; Zhou, Y. Tracking variation of fluorescent dissolved organic matter during full-scale printing and dyeing wastewater treatment. Chemosphere 2020, 252, 126559. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Teng, C.Y.; Qian, C.; Yu, H.; Li, Y.; Liu, Y. Characterizing properties and environmental behaviors of dissolved organic matter using two-dimensional correlation spectroscopic analysis. Environ. Sci. Technol. 2019, 53, 4683–4694. [Google Scholar] [CrossRef]
- Derrien, M.; Shin, K.H.; Hur, J. Assessment on applicability of common source tracking tools for particulate organic matter in controlled end member mixing experiments. Sci. Total Environ. 2019, 666, 187–196. [Google Scholar] [CrossRef]
- Han, S.; Nam, S.; Kang, J. ·OH radical monitoring technologies for AOP advanced oxidation process. Water Sci. Technol. 2002, 46, 7–12. [Google Scholar] [CrossRef]
- Shang, N.C.; Chen, Y.H.; Ma, H.W.; Lee, C.Y.; Chang, C.H.; Yang, C.W. Oxidation of methyl methacrylate from semiconductor wastewater by O3 and O3/UV processes. J. Hazard. Mater. 2007, 147, 307–312. [Google Scholar] [CrossRef]
- Huber, M.; Göbel, A.; Joss, A.; Hermann, N.; Löffler, D.; McArdell, C.S.; Ried, A.; Siegrist, H.; Ternes, T.A.; von Gunten, U. Oxidation of pharmaceuticals during ozonation of municipal wastewater effluents: A pilot study. Environ. Sci. Technol. 2005, 39, 4290–4299. [Google Scholar] [CrossRef]
- Meyerstein, D. What are the oxidizing intermediates in the fenton and fenton-like reactions? A perspective. Antioxidants 2022, 11, 1368. [Google Scholar] [CrossRef]
- Kida, M.; Kojima, T.; Tanabe, Y.; Hayashi, K.; Kudoh, S.; Maie, N.; Fujitake, N. Origin, distributions, and environmental significance of ubiquitous humic-like fluorophores in Antarctic lakes and streams. Water Res. 2019, 163, 114901. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jiang, L.; Fang, C.; Wu, M.; Guo, J.; Zhang, H. Effects of di-n-butyl phthalate and di-2-ethylhexyl phthalate on pollutant removal and microbial community during wastewater treatment. Ecotoxicol. Environ. Saf. 2020, 198, 110665. [Google Scholar] [CrossRef]
- Zheng, M.; Han, Y.; Han, H.; Wang, H.; Li, X.; Ma, J. Synergistic degradation on phenolic compounds of coal pyrolysis wastewater (CPW) by lignite activated coke-active sludge (LAC-AS) process: Insights into succession of microbial community under selective pressure. Bioresour. Technol. 2019, 281, 126–134. [Google Scholar] [CrossRef]
- Murphy, K.R.; Ruiz, G.M.; Dunsmuir, W.T.M.; Waite, T.D. Optimized parameters for fluorescence-based verification of ballast water exchange by ships. Environ. Sci. Technol. 2006, 40, 2357–2362. [Google Scholar] [CrossRef] [PubMed]
- Moona, N.; Holmes, A.; Wünsch, U.J. Full-scale manipulation of the empty bed contact time to optimize dissolved organic matter removal by drinking water biofilters. ACS EST Water 2021, 1, 1117–1126. [Google Scholar] [CrossRef]
- Lee, S.; Hur, J. Heterogeneous adsorption behavior of landfill leachate on granular activated carbon revealed by fluorescence excitation emission matrix (EEM)-parallel factor analysis (PARAFAC). Chemosphere 2016, 149, 41–48. [Google Scholar] [CrossRef]
- Maqbool, T.; Quang, V.L.; Cho, J.; Hur, J. Characterizing fluorescent dissolved organic matter in a membrane bioreactor via excitation–emission matrix combined with parallel factor analysis. Bioresour. Technol. 2016, 209, 31–39. [Google Scholar] [CrossRef]
- Yu, H.; Song, Y.; Gao, H.; Liu, L.; Yao, L.; Peng, J. Applying fluorescence spectroscopy and multivariable analysis to characterize structural composition of dissolved organic matter and its correlation with water quality in an urban river. Environ. Earth Sci. 2015, 73, 5163–5171. [Google Scholar] [CrossRef]
- Hu, Y.; Lei, D.; Wu, D.; Zhang, X.; Chen, Y.; Li, X. Residual β-lactam antibiotics and ecotoxicity to Vibrio fischeri, daphniamagna of pharmaceutical wastewater in the treatment process. J. Hazard. Mater. 2022, 425, 127840. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, Z.; Wang, X.; Li, J.; Yang, Y.; Zhang, Q. Impact of copper and sulfamethazine stress on microbial community and antibiotic resistance genes in denitrification systems: Comparison between heterotrophic and sulfur autotrophic denitrification processes. J. Water Process Eng. 2024, 66, 106078. [Google Scholar] [CrossRef]
- Ng, K.K.; Shi, X.Q.; Ng, H.Y. Evaluation of system performance and microbial communities of a bioaugmented anaerobic membrane bioreactor treating pharmaceutical wastewater. Water Res. 2015, 81, 311–324. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhao, C.; Qian, F.; Su, J.; Gao, H. Fate of Dissolved Organic Matter and Cooperation Behavior of Coagulation: Fenton Combined with MBR Treatment for Pharmaceutical Tail Water. Molecules 2025, 30, 2520. https://doi.org/10.3390/molecules30122520
Wang J, Zhao C, Qian F, Su J, Gao H. Fate of Dissolved Organic Matter and Cooperation Behavior of Coagulation: Fenton Combined with MBR Treatment for Pharmaceutical Tail Water. Molecules. 2025; 30(12):2520. https://doi.org/10.3390/molecules30122520
Chicago/Turabian StyleWang, Jian, Chunxiao Zhao, Feng Qian, Jie Su, and Hongjie Gao. 2025. "Fate of Dissolved Organic Matter and Cooperation Behavior of Coagulation: Fenton Combined with MBR Treatment for Pharmaceutical Tail Water" Molecules 30, no. 12: 2520. https://doi.org/10.3390/molecules30122520
APA StyleWang, J., Zhao, C., Qian, F., Su, J., & Gao, H. (2025). Fate of Dissolved Organic Matter and Cooperation Behavior of Coagulation: Fenton Combined with MBR Treatment for Pharmaceutical Tail Water. Molecules, 30(12), 2520. https://doi.org/10.3390/molecules30122520




