Magnetic Resonance Imaging and X-Ray Imaging Properties of Ultrasmall Lanthanide Oxide (Ln = Eu, Gd, and Tb) Nanoparticles Synthesized via Thermal Decomposition
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties
2.2. Crystallinity
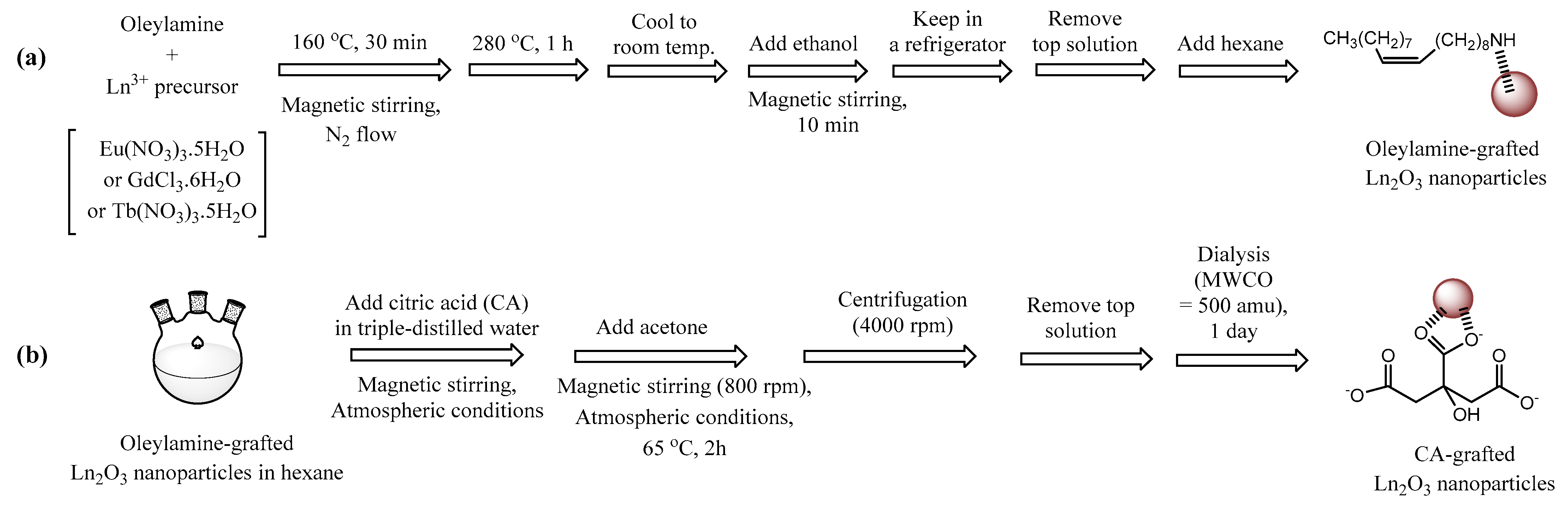
2.3. Surface-Grafting Results
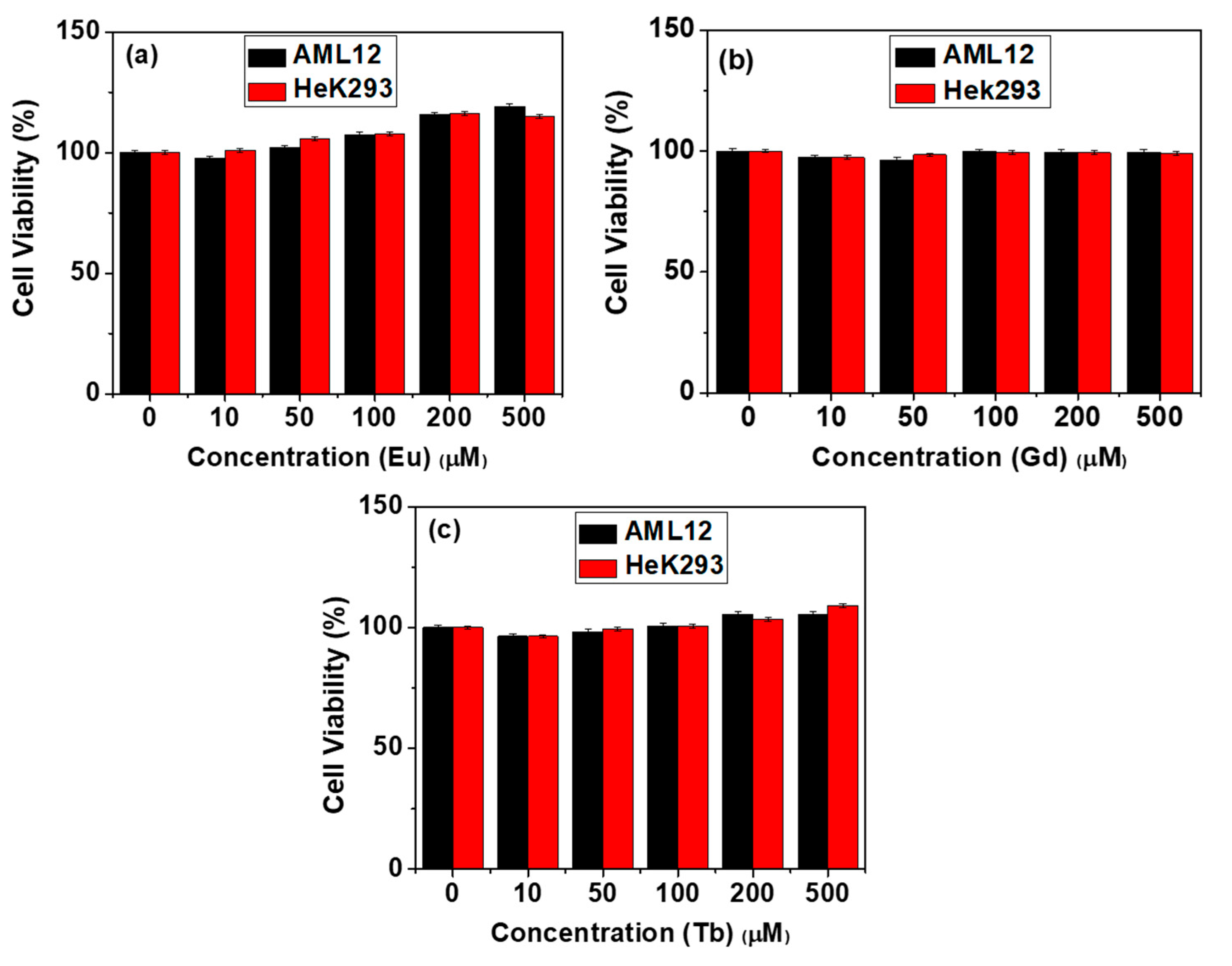
2.4. In Vitro Cytotoxicity
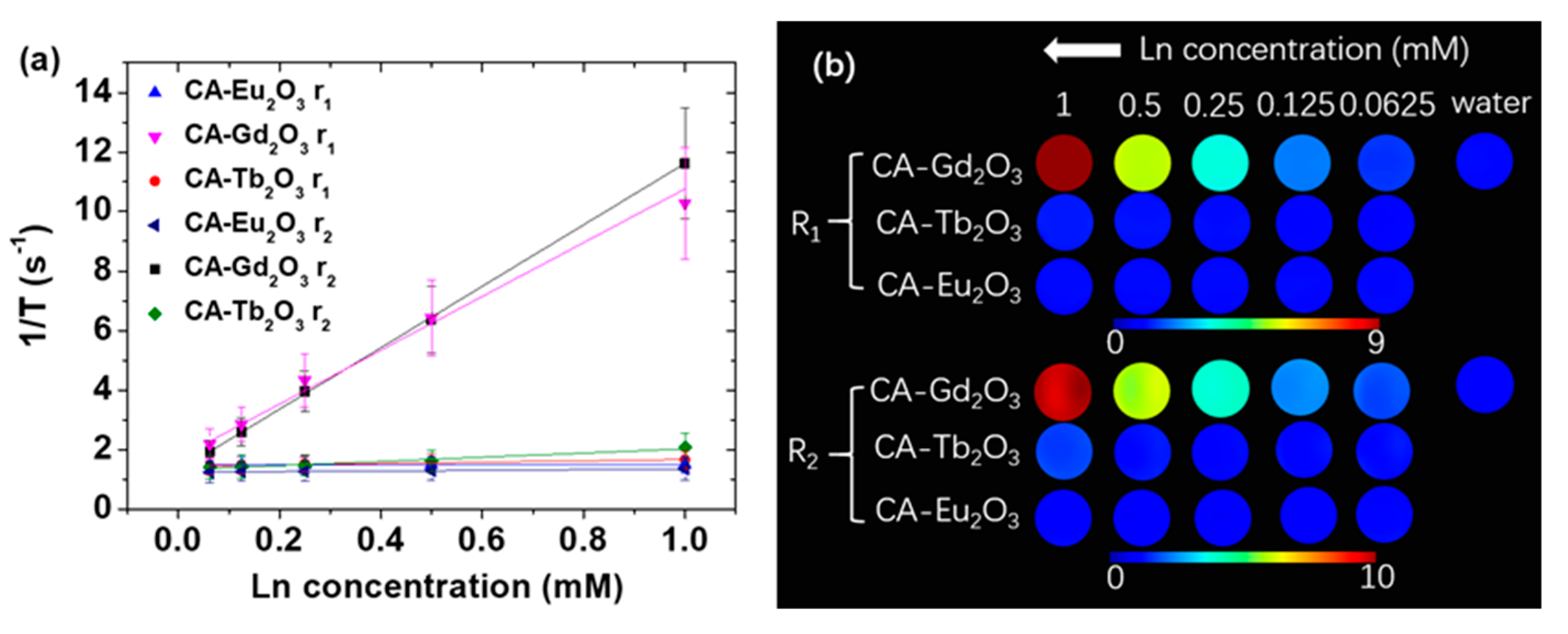
2.5. Magnetic Resonance Imaging Properties
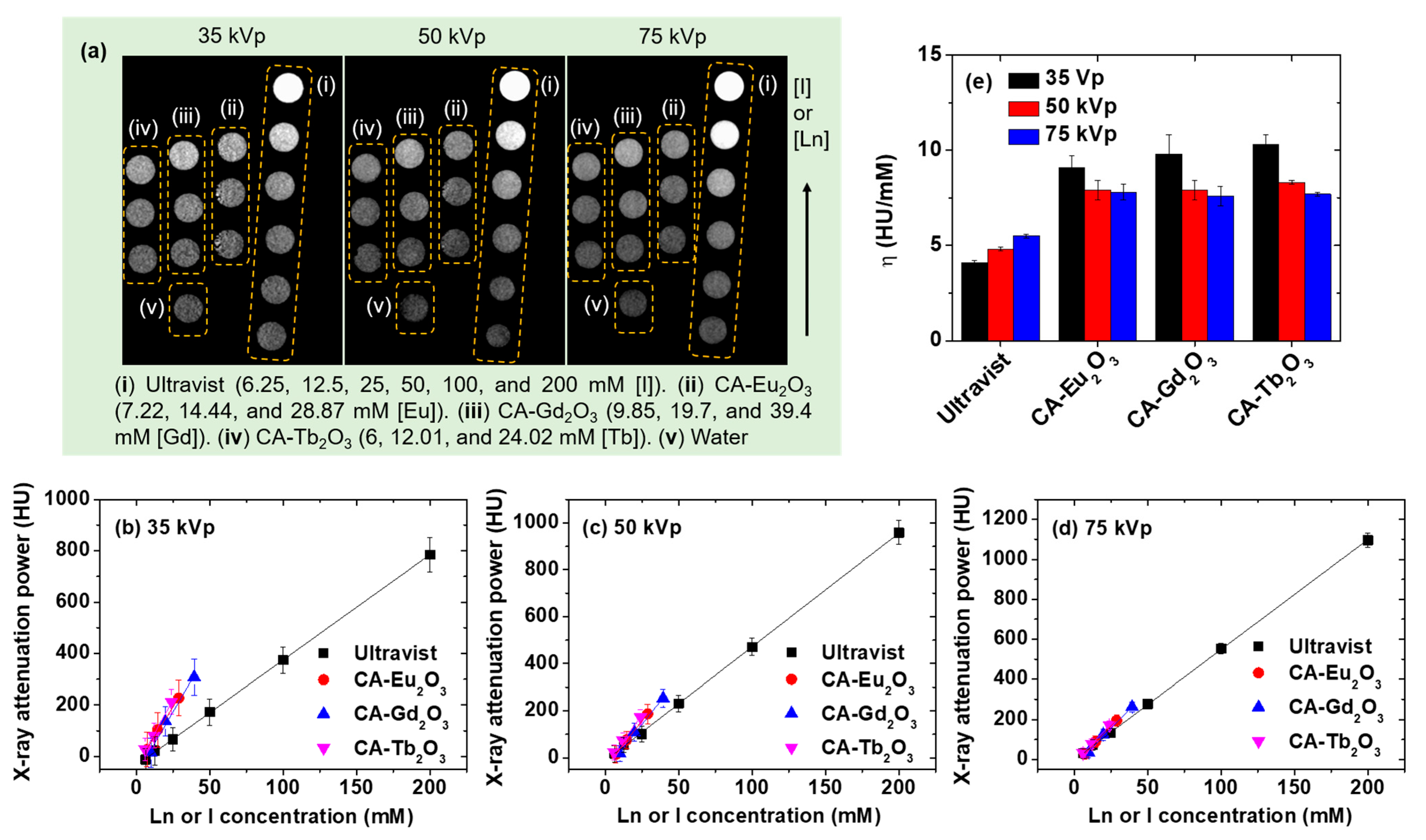
2.6. X-Ray Imaging Properties
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Ln2O3 (Ln = Eu, Gd, and Tb) Nanoparticles via Thermal Decomposition
3.3. Synthesis of CA-Grafted Ln2O3 Nanoparticles via Ligand Exchange
3.4. General Characterization
3.5. MRI Imaging Parameters
3.6. X-Ray Imaging Parameters
3.7. In Vitro Cellular Cytotoxicity Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martí-Bonmatí, L.; Sopena, R.; Bartumeus, P.; Sopena, P. Multimodality imaging techniques. Contrast Media Mol. Imaging 2010, 5, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Davis, J.J. Multimodality and nanoparticles in medical imaging. Dalton Trans. 2011, 40, 6087–6103. [Google Scholar] [CrossRef] [PubMed]
- Frullano, L.; Meade, T.J. Multimodal MRI contrast agents. J. Biol. Inorg. Chem. 2007, 12, 939–949. [Google Scholar] [CrossRef]
- Zheng, J.; Perkins, G.; Kirilova, A.; Allen, C.; Jaffray, D.A. Multimodal contrast agent for combined computed tomography and magnetic resonance imaging applications. Investig. Radiol. 2006, 41, 339–348. [Google Scholar] [CrossRef]
- Koole, R.; van Schooneveld, M.M.; Hilhorst, J.; Castermans, K.; Cormode, D.P.; Strijkers, G.J.; Donega, C.M.; Vanmaekelbergh, D.; Griffioen, A.W.; Nicolay, K.; et al. Paramagnetic lipid-coated silica nanoparticles with a fluorescent quantum dot core: A new contrast agent platform for multimodality imaging. Bioconjug. Chem. 2008, 19, 2471–2479. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zong, H.; Trivedi, E.R.; Vesper, B.J.; Waters, E.A.; Barrett, A.G.M.; Radosevich, J.A.; Hoffman, B.M.; Meade, T.J. Synthesis and characterization of new porphyrazine-Gd (III) conjugates as multimodal MR contrast agents. Bioconjug. Chem. 2010, 21, 2267–2275. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Liu, S.; He, J.; Pan, C.C.; Li, H.; Zhou, Z.Y.; Ding, Y.; Huo, D.; Hu, Y. Synthesis and Application of Strawberry-Like Fe3O4-Au Nanoparticles as CT-MR Dual-Modality Contrast Agents in Accurate Detection of the Progressive Liver Disease. Biomaterials 2015, 51, 194–207. [Google Scholar] [CrossRef]
- Blum, A.; Gillet, R.; Rauch, A.; Urbaneja, A.; Biouichi, H.; Dodin, G.; Germain, E.; Lombard, C.; Jaquet, P.; Louis, M.; et al. 3D reconstructions, 4D imaging and postprocessing with CT in musculoskeletal disorders: Past, present and future. Diagn. Interv. Imaging 2020, 101, 693–705. [Google Scholar] [CrossRef]
- Schaefer, P.W.; Grant, P.E.; Gonzalez, R.G. Diffusion-weighted MR imaging of the brain. Radiology 2000, 217, 331–345. [Google Scholar] [CrossRef]
- Wang, Z.J.; Reddy, G.P.; Gotway, M.B.; Yeh, B.M.; Hetts, S.W.; Higgins, C.B. CT and MR imaging of pericardial disease. RadioGraphics 2003, 23, S167–S180. [Google Scholar] [CrossRef]
- Andersson-Engels, S.; Klinteberg, C.; Svanberg, K.; Svanberg, S. In vivo fluorescence imaging for tissue diagnostics. Phys. Med. Biol. 1997, 42, 815. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Qian, K.; Mei, X. A theranostic nanoplatform: Magneto-gold@fluorescence polymer nanoparticles for tumor targeting T1 & T2-MRI/CT/NIR fluorescence imaging and induction of genuine autophagy mediated chemotherapy. Nanoscale 2018, 10, 10467–10478. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Wang, Y.; Wang, M.; Zhang, L.; Du, X.; Gu, H.; Zhang, C. Iodinated oil-loaded, fluorescent mesoporous silica-coated iron oxide nanoparticles for magnetic resonance imaging/computed tomography/fluorescence trimodal imaging. Int. J. Nanomed. 2014, 9, 2527. [Google Scholar] [CrossRef]
- Zhao, Y.; Hao, X.; Lu, W.; Wang, R.; Shan, X.; Chen, Q.; Sun, G.; Liu, J. Facile preparation of double rare earth-doped carbon dots for MRI/CT/FI multimodal imaging. ACS Appl. Nano Mater. 2018, 1, 2544–2551. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.N.; Li, X.D.; Chang, J. Multimodality imaging in nanomedicine and nanotheranostics. Cancer Biol. Med. 2016, 13, 339. [Google Scholar] [CrossRef]
- Bridot, J.L.; Faure, A.C.; Laurent, S.; Riviere, C.; Billotey, C.; Hiba, B.; Janier, M.; Josserand, V.; Coll, J.-L.; Elst, L.V.; et al. Hybrid gadolinium oxide nanoparticles: Multimodal contrast agents for in vivo imaging. J. Am. Chem. Soc. 2007, 129, 5076–5084. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, M. Multifunctional magnetic nanoparticles for medical imaging applications. J. Mater. Chem. 2009, 19, 6258–6266. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements; Butterworth-Heinemann: New York, NY, USA, 1997; p. 1243. [Google Scholar]
- Cotton, F.A.; Wilkinson, G. Advanced Inorganic Chemistry, 4th ed.; A Wiley-Interscience Publication: New York, NY, USA, 1980; p. 984. [Google Scholar]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Ipe, B.I.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of nanoparticles. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef]
- Kattel, K.; Park, J.Y.; Xu, W.; Kim, H.G.; Lee, E.J.; Bony, B.A.; Heo, W.C.; Lee, J.J.; Jin, S.; Baeck, J.S.; et al. A facile synthesis, in vitro and in vivo MR studies of D-glucuronic acid-coated ultrasmall Ln2O3 (Ln = Eu, Gd, Dy, Ho, and Er) nanoparticles as a new potential MRI contrast agent. ACS Appl. Mater. Interfaces 2011, 3, 3325–3334. [Google Scholar] [CrossRef]
- Miao, X.; Xu, W.; Cha, H.; Chang, Y.; Oh, I.T.; Chae, K.S.; Tegafaw, T.; Ho, S.L.; Kim, S.J.; Lee, G.H. Ultrasmall Gd2O3 nanoparticles surface-coated by polyacrylic acid (PAA) and their PAA-size dependent relaxometric properties. Appl. Surf. Sci. 2019, 477, 111–115. [Google Scholar] [CrossRef]
- Xu, W.; Bony, B.A.; Kim, C.R.; Baeck, J.S.; Chang, Y.; Bae, J.E.; Chae, K.S.; Kim, T.J.; Lee, G.H. Mixed lanthanide oxide nanoparticles as dual imaging agent in biomedicine. Sci. Rep. 2013, 3, 3210. [Google Scholar] [CrossRef]
- Liu, S.; Yue, H.; Ho, S.L.; Kim, S.; Park, J.A.; Tegafaw, T.; Ahmad, M.Y.; Kim, S.; Al Saidi, A.K.A.; Zhao, D.; et al. Enhanced Tumor Imaging Using Glucosamine-Conjugated Polyacrylic Acid-Coated Ultrasmall Gadolinium Oxide Nanoparticles in Magnetic Resonance Imaging. Int. J. Mol. Sci. 2022, 23, 1792. [Google Scholar] [CrossRef]
- Ghazanfari, A.; Marasini, S.; Miao, X.; Park, J.A.; Jung, K.H.; Ahmad, M.Y.; Yue, H.; Ho, S.L.; Liu, S.; Jang, Y.J.; et al. Synthesis, characterization, and X-ray attenuation properties of polyacrylic acid-coated ultrasmall heavy metal oxide (Bi2O3, Yb2O3, NaTaO3, Dy2O3, and Gd2O3) nanoparticles as potential CT contrast agents. Colloids Surf. A Physicochem. Eng. Asp. 2019, 576, 73–81. [Google Scholar] [CrossRef]
- Ucar, Z.G.P.; Akca, S.; Dogan, T.; Halefoglu, Y.Z.; Kaynar, U.H.; Ayvacikli, M.; Guinea, J.G.; Topaksu, M.; Can, N. Comprehensive study of photoluminescence and cathodoluminescence of Eu and Tb doped Mg2SiO4 prepared via a solid-state reaction technique. Opt. Mater. 2020, 100, 109698. [Google Scholar] [CrossRef]
- Chen, F.; Chen, M.; Yang, C.; Liu, J.; Luo, N.; Yang, G.; Chen, D.; Li, L. Terbium-doped gadolinium oxide nanoparticles prepared by laser ablation in liquid for use as a fluorescence and magnetic resonance imaging dual-modal contrast agent. Phys. Chem. Chem. Phys. 2015, 17, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Kim, M.A.; Cho, J.H.; Lee, S.J.; Yang, I.; Cho, J.; Kim, S.K.; Lee, C.; Park, J.K. Europium-doped gadolinium sulfide nanoparticles as a dual-mode imaging agent for T1-weighted MR and photoluminescence imaging. Biomaterials 2012, 33, 5865–5874. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; He, W.; Li, F.; Perera, T.S.H.; Gan, L.; Han, Y.; Wang, X.; Li, S.; Dai, H. Luminescence enhanced Eu3+/Gd3+ co-doped hydroxyapatite nanocrystals as imaging agents in vitro and in vivo. ACS Appl. Mater. Interfaces 2016, 8, 10212–10219. [Google Scholar] [CrossRef]
- Mondal, S.; Nguyen, V.T.; Park, S.; Choi, J.; Vo, T.M.T.; Shin, J.H.; Kang, Y.H.; Oh, J. Rare earth element doped hydroxyapatite luminescent bioceramics contrast agent for enhanced biomedical imaging and therapeutic applications. Ceram. Int. 2020, 46, 29249–29260. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kumar, S.; Umar, A.; Singh, J.; Rawat, M.; Mehta, S.K. Europium-doped gadolinium oxide nanoparticles: A potential photoluminescencent probe for highly selective and sensitive detection of Fe3+ and Cr3+ ions. Sens. Actuators B Chem. 2017, 243, 579–588. [Google Scholar] [CrossRef]
- Fu, R.; Ou, M.; Yang, C.; Hu, Y.; Yin, H. Enhanced luminescence and paramagnetic properties of Gd2O3: Tb3+ multifunctional nanoparticles by K+/Co2+ doping. J. Lumin. 2020, 222, 117154. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Ma, H.; Zhang, L.; Jiang, H.; Song, H.; Zhang, L.; Luo, Y. A nanoscaled lanthanide metal–organic framework as a colorimetric fluorescence sensor for dipicolinic acid based on modulating energy transfer. J. Mater. Chem. C 2016, 4, 7294–7301. [Google Scholar] [CrossRef]
- Binnemans, K.; Van Herck, K.; Görller-Walrand, C. Influence of dipicolinate ligands on the spectroscopic properties of europium (III) in solution. Chem. Phys. Lett. 1997, 266, 297–302. [Google Scholar] [CrossRef]
- Carr, R.; Evans, N.H.; Parker, D. Lanthanide complexes as chiral probes exploiting circularly polarized luminescence. Chem. Soc. Rev. 2012, 41, 7673–7686. [Google Scholar] [CrossRef]
- Bera, D.; Qian, L.; Tseng, T.-K.; Holloway, P.H. Quantum Dots and Their Multimodal Applications: A Review. Materials 2010, 3, 2260–2345. [Google Scholar] [CrossRef]
- Nikam, A.V.; Prasad, B.L.V.; Kulkarni, A.A. Wet chemical synthesis of metal oxide nanoparticles: A review. CrystEngComm 2018, 20, 5091–5107. [Google Scholar] [CrossRef]
- Pan, Y.; Xie, X.; Huang, Q.; Gao, C.; Wang, Y.; Wang, L.; Yang, B.; Su, H.; Huang, L.; Huang, W. Inherently Eu2+/Eu3+ codoped Sc2O3 nanoparticles as high-performance nanothermometers. Adv. Mater. 2018, 30, 1705256. [Google Scholar] [CrossRef]
- Söderlind, F.; Pedersen, H.; Petoral, R.M.; Käll, P.-O.; Uvdal, K. Synthesis and characterization of Gd2O3 nanocrystals functionalized by organic acids. J. Colloid Interface Sci. 2005, 288, 140–148. [Google Scholar] [CrossRef]
- X’Pert High Score, PANalytical Software; Version 1.1; Card No.Gd2O3: 00-012-0797, Tb2O3: 01-074-1986, Eu2O3: 03-065-3182; Malvern Panalytical: Almelo, The Netherlands, 1998.
- Ranoszek-Soliwoda, K.; Tomaszewska, E.; Socha, E.; Krzyczmonik, P.; Ignaczak, A.; Orlowski, P.; Krzyzowska, M.; Celichowski, G.; Grobelny, J. The role of tannic acid and sodium citrate in the synthesis of silver nanoparticles. J. Nanopart. Res. 2017, 19, 273. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, P.; Wang, T. Preparation of superparamagnetic polyaniline hybrid hollow microspheres in oil/water emulsion with magnetic nanoparticles as cosurfactant. Chem. Eng. J. 2011, 171, 711–716. [Google Scholar] [CrossRef]
- Abboud, M.; Youssef, S.; Podlecki, J.; Habchi, R.; Germanos, G.; Foucaran, A. Superparamagnetic Fe3O4 nanoparticles, synthesis and surface modification. Mater. Sci. Semicond. Process 2015, 39, 641–648. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases, HSAB, Part I: Fundamental principles. J. Chem. Educ. 1968, 45, 581–587. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases, HSAB, Part II: Underlying theories. J. Chem. Educ. 1968, 45, 643–648. [Google Scholar] [CrossRef]
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Corbierre, M.K.; Cameron, N.S.; Lennox, R.B. Polymer-stabilized gold nanoparticles with high grafting densities. Langmuir 2004, 20, 2867–2873. [Google Scholar] [CrossRef]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. CRC Handbook of Chemistry and Physics, 96th ed.; CRC Press: Boca Raton, FL, USA, 2015–2016; pp. 4–64. [Google Scholar] [CrossRef]
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI contrast agents: Current challenges and new frontiers. Chem. Rev. 2018, 119, 957–1057. [Google Scholar] [CrossRef]
- Lauffer, R.B. Paramagnetic metal complexes as water proton relaxation agents for NMR imaging: Theory and design. Chem. Rev. 1987, 87, 901–927. [Google Scholar] [CrossRef]
- Marasini, S.; Yue, H.; Ho, S.L.; Jung, K.-H.; Park, J.A.; Cha, H.; Ghazanfari, A.; Ahmad, M.Y.; Liu, S.; Jang, Y.J.; et al. D-Glucuronic Acid-Coated Ultrasmall Paramagnetic Ln2O3 (Ln = Tb, Dy, and Ho) Nanoparticles: Magnetic Properties, Water Proton Relaxivities, and Fluorescence Properties. Eur. J. Inorg. Chem. 2019, 34, 3832–3839. [Google Scholar] [CrossRef]
- LaConte, L.E.W.; Nitin, N.; Zurkiya, O.; Caruntu, D.; O’Conner, C.J.; Hu, X.; Bao, G. Coating Thickness of Magnetic Iron Oxide Nanoparticles Affects R2 Relaxivity. J. Magn. Reson. Imaging 2007, 26, 1634–1641. [Google Scholar] [CrossRef]
- Ahmad, M.Y.; Liu, S.; Tegafaw, T.; Saidi, A.K.A.A.; Zhao, D.; Liu, Y.; Nam, S.-W.; Chang, Y.; Lee, G.H. Heavy Metal-Based Nanoparticles as High-Performance X-ray Computed Tomography Contrast Agents. Pharmaceuticals 2023, 16, 1463. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, J.H.; Seltzer, S.M. Tables of X-Ray Mass Attenuation Coefficients and Mass Energy-Absorption Coefficients from 1 keV to 20 MeV for Elements Z = 1 to 92 and 48 Additional Substances of Dosimetric Interest. Gaithersburg, NIST, USA. 1996. Available online: http://www.nist.gov/pml/data/xraycoef (accessed on 5 June 2025).

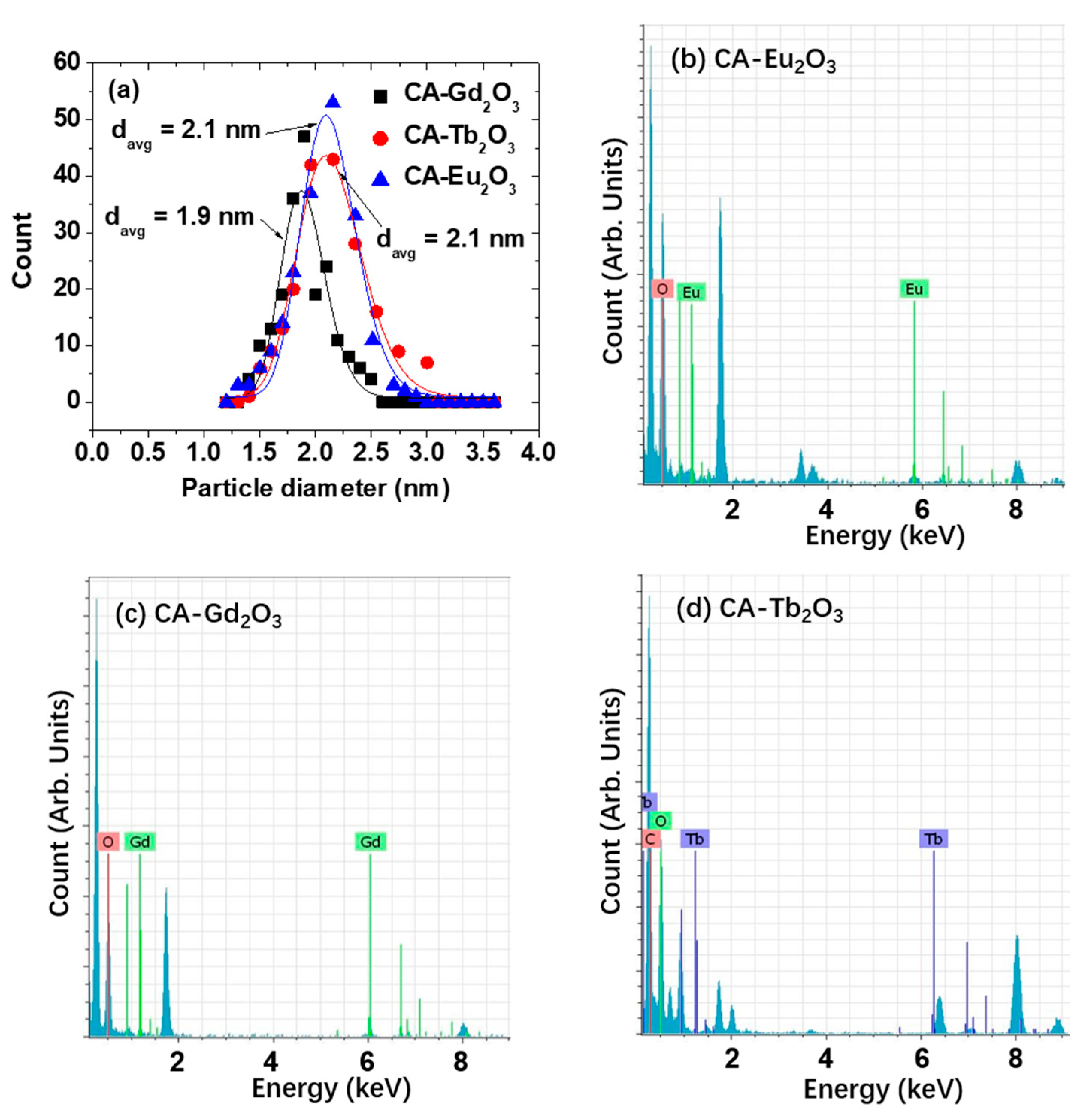


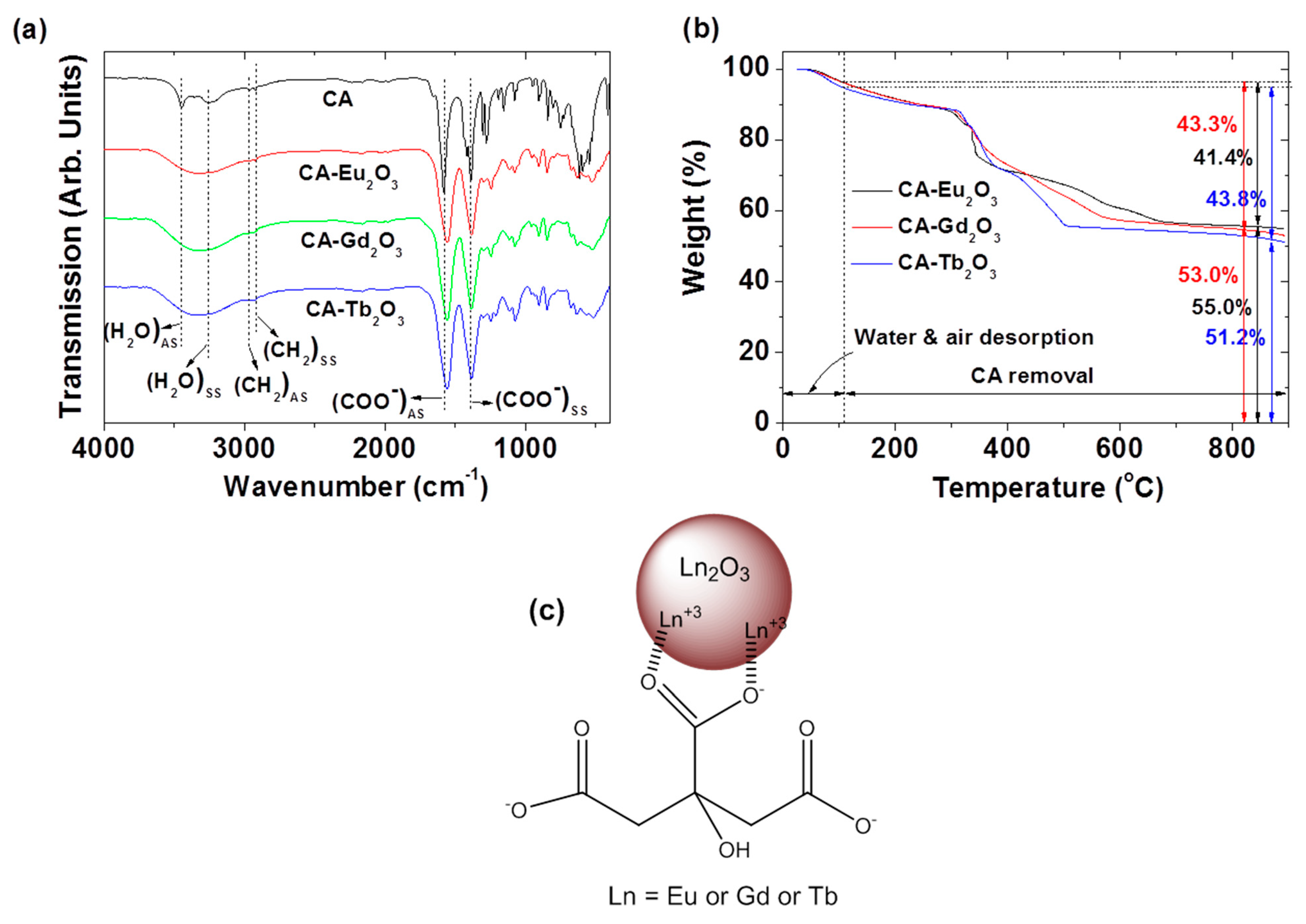
| Nanoparticle | davg (nm) | aavg (nm) | ζ (mV) | Surface-Grafting Amount | ||
|---|---|---|---|---|---|---|
| P a (wt%) | σ b (nm−2) | NNP c | ||||
| Eu2O3 | 2.1 ± 0.1 | 26.4 ± 1.0 | −16.7 ± 0.4 | 41.4 | 4.1 | 58 |
| Gd2O3 | 1.9 ± 0.1 | 23.7 ± 1.0 | −15.9 ± 0.2 | 43.3 | 3.9 | 44 |
| Tb2O3 | 2.1 ± 0.1 | 27.7 ± 1.0 | −11.0 ± 0.3 | 43.8 | 4.9 | 69 |
| Vibration a | CA | CA-Eu2O3 | CA-Gd2O3 | CA-Tb2O3 |
|---|---|---|---|---|
| (COO−) AS | 1582 | 1556 | 1560 | 1556 |
| (COO−) SS | 1388 | 1384 | 1389 | 1384 |
| (CH2) AS | 2966 | ~2966 | ~2966 | ~2966 |
| (CH2) SS | 2922 | 2922 | 2922 | 2922 |
| (H2O) AS | 3446 | ~3318 | ~3318 | ~3318 |
| (H2O) SS | ~3257 |
| Material | davg (nm) | r1 (s−1mM−1) | r2 (s−1mM−1) | η (HU/mM) | Ref. | ||
|---|---|---|---|---|---|---|---|
| 35 kVp | 50 kVp | 75 kVp | |||||
| CA-Eu2O3 | 2.1 | 0.02 ± 0.01 | 0.11 ± 0.01 | 9.1 ± 0.6 | 7.9 ± 0.2 | 7.8 ± 0.4 | This study |
| CA-Gd2O3 | 1.9 | 9.04 ± 0.53 | 10.33 ± 0.14 | 9.8 ± 1.0 | 7.9 ± 0.5 | 7.6 ± 0.5 | This study |
| CA-Tb2O3 | 2.1 | 0.22 ± 0.03 | 0.71 ± 0.09 | 10.3 ± 0.5 | 8.3 ± 0.1 | 7.7 ± 0.1 | This study |
| D-glucuronic acid-Eu2O3 | 2.0 | 0.006 | 3.82 | - | - | - | [22] |
| D-glucuronic acid-Gd2O3 | 2.4 | 4.25 | 27.11 | - | - | - | [22] |
| D-glucuronic acid-Tb2O3 | 2.0 | <1.0 | 7.68 | - | - | - | [53] |
| PAA 1-Gd2O3 | 1.9 | - | - | 5.9 (70 kVp) | [55] | ||
| PAA 1-GdF3 nanoplate | 10.6 × 7.0 × 4.2 2 | - | - | ~7.9 (60 kVp) | [55] | ||
| Ultravist | - | - | - | 4.1 ± 0.1 | 4.8 ± 0.1 | 5.5 ± 0.1 | This study |
| Gadovist | - | 4.4 ± 0.1 | 4.7 ± 0.1 | - | - | - | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Tegafaw, T.; Ho, S.L.; Yue, H.; Zhao, D.; Liu, Y.; Mulugeta, E.; Chen, X.; Lee, H.; Ahn, D.; et al. Magnetic Resonance Imaging and X-Ray Imaging Properties of Ultrasmall Lanthanide Oxide (Ln = Eu, Gd, and Tb) Nanoparticles Synthesized via Thermal Decomposition. Molecules 2025, 30, 2519. https://doi.org/10.3390/molecules30122519
Liu S, Tegafaw T, Ho SL, Yue H, Zhao D, Liu Y, Mulugeta E, Chen X, Lee H, Ahn D, et al. Magnetic Resonance Imaging and X-Ray Imaging Properties of Ultrasmall Lanthanide Oxide (Ln = Eu, Gd, and Tb) Nanoparticles Synthesized via Thermal Decomposition. Molecules. 2025; 30(12):2519. https://doi.org/10.3390/molecules30122519
Chicago/Turabian StyleLiu, Shuwen, Tirusew Tegafaw, Son Long Ho, Huan Yue, Dejun Zhao, Ying Liu, Endale Mulugeta, Xiaoran Chen, Hansol Lee, Dabin Ahn, and et al. 2025. "Magnetic Resonance Imaging and X-Ray Imaging Properties of Ultrasmall Lanthanide Oxide (Ln = Eu, Gd, and Tb) Nanoparticles Synthesized via Thermal Decomposition" Molecules 30, no. 12: 2519. https://doi.org/10.3390/molecules30122519
APA StyleLiu, S., Tegafaw, T., Ho, S. L., Yue, H., Zhao, D., Liu, Y., Mulugeta, E., Chen, X., Lee, H., Ahn, D., Yang, J.-u., Park, J. A., Baek, A., Kim, J., Chang, Y., & Lee, G. H. (2025). Magnetic Resonance Imaging and X-Ray Imaging Properties of Ultrasmall Lanthanide Oxide (Ln = Eu, Gd, and Tb) Nanoparticles Synthesized via Thermal Decomposition. Molecules, 30(12), 2519. https://doi.org/10.3390/molecules30122519










