Chemical Properties, Preparation, and Pharmaceutical Effects of Cyclic Peptides from Pseudostellaria heterophylla
Abstract
1. Introduction
2. Chemical Features of Cyclic Peptides from P. heterophylla
| No. | Compound | Ring Size (aa) | Structure | Mw (m/z) | Ref. |
|---|---|---|---|---|---|
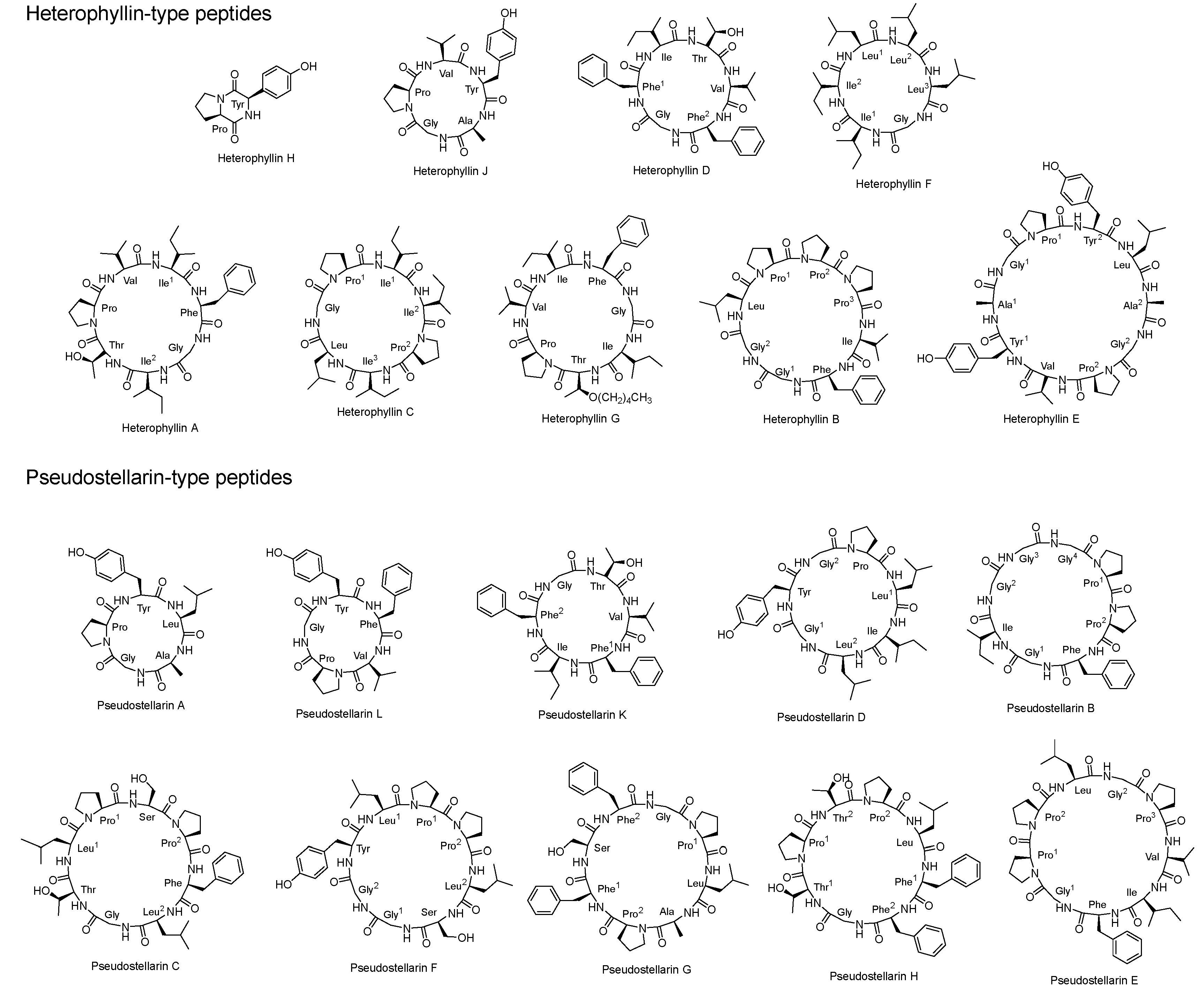
| 1 | Heterophyllin A | 7 | Cyclo(Thr-Pro-Val-Ile-Phe-Gly-Ile) | 727.9 | [16] |
| 2 | Heterophyllin B | 8 | Cyclo(Gly-Gly-Leu-Pro-Pro-Pro-Ile-Phe) | 778.9 | [16] |
| 3 | Heterophyllin C | 7 | Cyclo(Gly-Pro-Ile-Ile-Pro-Ile-Leu) | 703.9 | [17] |
| 4 | Heterophyllin D | 6 | Cyclo(Gly-Phe-Ile-Thr-Val-Phe) | 664.8 | [23] |
| 5 | Heterophyllin E | 10 | Cyclo(Val-Tyr-Ala-Gly-Pro-Tyr-Leu-Ala-Gly-Pro) | 989.1 | [23] |
| 6 | Heterophyllin F | 6 | Cyclo(Ile-Ile-Leu-Leu-Leu-Gly) | 622.8 | [23] |
| 7 | Heterophyllin G | 7 | Cyclo(Pro-Val-Ile-Phe-Gly-Ile-[Thr-O(CH2)4CH3]) | 798.0 | [23] |
| 8 | Heterophyllin H | 2 | Cyclo(Tyr-Pro) | 260.3 | [23] |
| 9 | Heterophyllin J | 5 | Cyclo(Ala-Gly-Pro-Val-Tyr) | 487.6 | [18] |
| 10 | Pseudostellarin A | 5 | Cyclo(Gly-Pro-Tyr-Leu-Ala) | 501.6 | [19] |
| 11 | Pseudostellarin B | 8 | Cyclo(Gly-Ile-Gly-Gly-Gly-Pro-Pro-Phe) | 682.8 | [19] |
| 12 | Pseudostellarin C | 8 | Cyclo(Gly-Thr-Leu-Pro-Ser-Pro-Phe-Leu) | 812.9 | [19] |
| 13 | Pseudostellarin D | 7 | Cyclo(Gly-Gly-Tyr-Pro-Leu-Ile-Leu) | 713.9 | [20] |
| 14 | Pseudostellarin E | 9 | Cyclo(Gly-Pro-Pro-Leu-Gly-Pro-Val-Ile-Phe) | 878.1 | [20] |
| 15 | Pseudostellarin F | 8 | Cyclo(Gly-Gly-Tyr-Leu-Pro-Pro-Leu-Ala-Pro) | 784.9 | [20] |
| 16 | Pseudostellarin G | 8 | Cyclo(Phe-Ser-Phe-Gly-Pro-Leu-Ala-Pro) | 816.9 | [21] |
| 17 | Pseudostellarin H | 8 | Cyclo(Gly-Thr-Pro-Thr-Pro-Leu-Phe-Phe) | 861.0 | [21] |
| 18 | Pseudostellarin K | 6 | Cyclo(Ile-Phe-Gly-Thr-Val-Phe) | 664.8 | [3] |
| 19 | Pseudostellarin L | 5 | Cyclo(Pro-Gly-Tyr-Phe-Val) | 563.7 | [22] |
3. Preparation for Cyclic Peptides from P. heterophylla
3.1. Direct Extraction and Separation
3.2. Biosynthesis
3.3. Chemical Synthesis
4. Pharmacokinetic Characteristics
5. Biological Activities of Cyclic Peptides from P. heterophylla
5.1. Anti-Tumor Activity
5.2. Anti-Inflammatory Activity
5.3. Antioxidant Activity
5.4. Anti-Tussive Activity
5.5. Hypoglycemic Activity
5.6. Promoting Angiogenic Activity
5.7. Modulating Gut Microbiota
5.8. Enhancing Cognitive Function
5.9. Inhibiting Tyrosinase Activity
5.10. Anti-Fibrotic Effects
6. Conclusions and Prospectives
Author Contributions
Funding
Conflicts of Interest
References
- Ni, J.C.; Fan, Y.F.; Ye, Z.Y. Research progress on chemical constituents, pharmacological effects and application of Pseudostellariae Radix. Chin. Tradit. Herb. Drugs 2023, 54, 1963–1977. [Google Scholar] [CrossRef]
- Hu, D.J.; Shakerian, F.; Zhao, J.; Li, S.P. Chemistry, pharmacology and analysis of Pseudostellaria heterophylla: A mini-review. Chin. Med. 2019, 23, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.F.; Zhang, Q.; Zhao, H.T.; Zhang, Y.D.; Liu, H.R.; Yuan, L.J.; Chen, Q.F. A new cyclic peptide from the fibrous root of Pseudostellaria heterophylla. Nat. Prod. Res. 2022, 36, 3368–3374. [Google Scholar] [CrossRef]
- Qin, T.; Zhang, J. Research progress on biological activities and action mechanisms of cyclotides in plants. Chin. Tradit. Herb. Drugs 2021, 5, 255–266. [Google Scholar] [CrossRef]
- Ji, X.; Nielsen, A.L.; Heinis, C. Cyclic peptides for drug development. Angew. Chem. Int. Ed. Engl. 2024, 63, e202308251. [Google Scholar] [CrossRef]
- Simonsen, S.M.; Sando, L.; Ireland, D.C.; Colgrave, M.L.; Bharathi, R.; Göransson, U.; Craik, D.J. A continent of plant defense peptide diversity: Cyclotides in Australian Hybanthus (Violaceae). Plant Cell 2005, 17, 3176–3189. [Google Scholar] [CrossRef] [PubMed]
- Ovesen, R.G.; Brandt, K.K.; Göransson, U.; Nielsen, J.; Hansen, H.C.; Cedergreen, N. Biomedicine in the environment: Cyclotides constitute potent natural toxins in plants and soil bacteria. Environ. Toxicol. Chem. 2011, 30, 1190–1196. [Google Scholar] [CrossRef]
- He, W.J.; Chan, L.Y.; Zeng, G.Z.; Daly, N.L.; Craik, D.J.; Tan, N.H. Isolation and characterization of cytotoxic cyclotides from Viola philippica. Peptides 2011, 32, 1719–1723. [Google Scholar] [CrossRef]
- Gran, L.; Sletten, K.; Skjeldal, L. Cyclic peptides from Oldenlandia affinis DC. molecular and biological properties. Chem. Biodivers. 2008, 5, 2014–2022. [Google Scholar] [CrossRef]
- Nguyen, K.N.T.; Nguyen, G.K.T.; Nguyen, P.Q.T.; Ang, K.H.; Dedon, P.C.; Tam, J.P. Immunostimulating and Gram-negative-specific antibacterial cyclotides from the butterfly pea (Clitoria ternatea). FEBS J. 2016, 283, 2067–2090. [Google Scholar] [CrossRef]
- Daly, N.L.; Wilson, D.T. Plant derived cyclic peptides. Biochem. Soc. Trans. 2021, 49, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.H. Cyclic peptides as therapeutic agents and biochemical tools. Biomol. Ther. 2012, 20, 19–26. [Google Scholar] [CrossRef]
- Buchanan, D.; Mori, S.; Chadli, A.; Panda, S.S. Natural cyclic peptides: Synthetic strategies and biomedical applications. Biomedicines 2025, 13, 240. [Google Scholar] [CrossRef] [PubMed]
- Linker, S.M.; Schellhaas, C.; Kamenik, A.S.; Veldhuizen, M.M.; Waibl, F.; Roth, H.J.; Fouché, M.; Rodde, S.; Riniker, S. Lessons for oral bioavailability: How conformationally flexible cyclic peptides enter and cross lipid membranes. J. Med. Chem. 2023, 66, 2773–2788. [Google Scholar] [CrossRef]
- Dougherty, P.G.; Sahni, A.; Pei, D. Understanding cell penetration of cyclic peptides. Chem. Rev. 2019, 119, 10241–10287. [Google Scholar] [CrossRef]
- Tan, N.H.; Zhou, J.; Chen, C.X.; Zhao, S.X. Cyclopeptides from the roots of Pseudostellaria heterophylla. Phytochemistry 1993, 32, 1327–1330. [Google Scholar] [CrossRef]
- Tan, N.H.; Zhou, J. Heterophyllin C, a new cyclopeptide from Pseudostellaria heterophylla. Acta Bot. Yunnanica 1995, 17, 60. [Google Scholar]
- Yang, Y.B.; Tan, N.H.; Zhang, F.; Lu, Y.Q.; He, M.; Zhou, J. Cyclopeptides and amides from Pseudostellaria heterophylla (Caryophyllaceae). Helv. Chim. Acta 2003, 86, 3376–3379. [Google Scholar] [CrossRef]
- Morita, H.; Kayashita, T.; Kobata, H.; Gonda, A.; Takeya, K.; Itokawa, H. Pseudostellarins A–C, new tyrosinase inhibitory cyclic peptides from Pseudostellaria heterophylla. Tetrahedron 1994, 50, 6797–6804. [Google Scholar] [CrossRef]
- Morita, H.; Kayashita, T.; Kobata, H.; Gonda, A.; Takeya, K.; Itokawa, H. Pseudostellarins D–F, new tyrosinase inhibitory cyclic peptides from Pseudostellaria heterophylla. Tetrahedron 1994, 50, 9975–9982. [Google Scholar] [CrossRef]
- Morita, H.; Kobata, H.; Takeya, K.; Itokawa, H. Pseudostellarin G, a new tyrosinase inhibitory cyclic octapeptide from Pseudostellaria heterophylla. Tetrahedron 1994, 35, 3563–3564. [Google Scholar] [CrossRef]
- Chen, Q.F.; Zhao, X.F.; Zhao, H.T.; Yuan, L.J. Chemical constituents of cyclic peptides from fibrous roots of Pseudostellaria heterophylla. China J. Chin. Mater. Med. 2022, 47, 122–126. [Google Scholar] [CrossRef]
- Ding, Z.T.; Zhou, J.; Tan, N.H. Progress in chemistry of cyclic peptides from plants of caryophyllaceae. Chem. Res. Chin. Univ. 1999, 05, 492–494. [Google Scholar]
- Wang, M.C.; Ren, Z.Q.; Lin, J.; Zhu, X.Q. Research progress of natural antiviral cyclic peptides. Shandong Chem. Ind. 2023, 52, 96–97+108. [Google Scholar] [CrossRef]
- Xu, G.B.; Zhu, Q.F.; Wang, Z.; Zhang, C.L.; Yang, X.; Zhang, J.J.; Wang, F.R.; Liu, J.; Zhou, M.; Wang, Y.L.; et al. Pseudosterins A–C, three 1-ethyl-3-formyl-β-carbolines from Pseudostellaria heterophylla and their cardioprotective effects. Molecules 2021, 26, 5045. [Google Scholar] [CrossRef]
- He, L.H.; Zhong, Z.H.; Zhang, L.J.; Bai, X. Research progress in the separation of chemical components from essential oils by high-speed countercurrent chromatography. Separations 2024, 11, 152. [Google Scholar] [CrossRef]
- Han, C.; Chen, J.H.; Liu, J.; Lee, F.S.C.; Wang, X.R. Isolation and purification of Pseudostellarin B (cyclic peptide) from Pseudostellaria heterophylla (Miq.) Pax by high-speed counter-current chromatography. Talanta 2007, 71, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.P.; Abel-Santos, E.; Wall, M.; Wahnon, D.C.; Benkovic, S.J. Production of cyclic peptides and proteins in vivo. Proc. Natl. Acad. Sci. USA 1999, 96, 13638–13643. [Google Scholar] [CrossRef]
- Jia, A.Q.; Li, X.; Tan, N.H.; Liu, X.Z.; Shen, Y.M.; Zhou, J. Enzymatic cyclization of linear peptide to plant cyclopeptide heterophyllin B. Sci. China Ser. B 2006, 49, 63–66. [Google Scholar] [CrossRef]
- Xu, W.Y.; Zhu, H.T.; Tan, N.H.; Tang, J.; Zhang, Y.J.; Cerny, R.L.; Du, L.C. An in vitro system to study cyclopeptide heterophyllin B biosynthesis in the medicinal plant Pseudostellaria heterophylla. Plant Cell Tissue Organ Cult. 2012, 108, 137–145. [Google Scholar] [CrossRef]
- Zheng, W.; Zhou, T.; Li, J.; Jiang, W.K.; Zhang, J.Q.; Xiao, C.H.; Wei, D.Q.; Yang, C.G.; Xu, R.; Gong, A.H.; et al. The biosynthesis of heterophyllin B in Pseudostellaria heterophylla from prePhHB-encoded precursor. Front. Plant Sci. 2019, 10, 1259. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.Q.; Shu, G.P.; Yang, Y.; Yang, C.G.; Guo, L.P.; Zhou, T. Modulation of heterophyllin B biosynthesis and transcription of key transcription factor genes in Pseudostellaria heterophylla by methyl jasmonate. Plant Growth Regul. 2024, 104, 843–854. [Google Scholar] [CrossRef]
- Himaja, M.; Harish Kumar, K.; Ramana, M.V.; Belagali, S.L. Synthesis and biological evaluation of pseudostellarin D. Eur. J. Med. Chem. 1999, 34, 525–529. [Google Scholar] [CrossRef]
- Poojary, B.; Kumar, K.H.; Belagali, S.L. Synthesis and biological evaluation of pseudostellarin B. Farmaco 2001, 56, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Poojary, B.; Kumar, H.; Belagali, S.L. Synthesis of a new cyclic peptide, pseudostellarin G. Z. Naturforschung 2004, 59, 817–820. [Google Scholar] [CrossRef]
- Zhang, S.P.; Amso, Z.; De Leon Rodriguez, L.M.; Kaur, H.; Brimble, M.A. Synthesis of natural cyclopentapeptides isolated from Dianthus chinensis. J. Nat. Prod. 2016, 79, 1769–1774. [Google Scholar] [CrossRef]
- Liu, D.; Guo, Y.L.; Qu, J.; Zhang, C. Recyclable hypervalent-iodine-mediated solid-phase peptide synthesis and cyclic peptide synthesis. Beilstein J. Org. Chem. 2018, 14, 1112–1119. [Google Scholar] [CrossRef]
- Zhang, J.W.; Zhou, F.; Lu, M.; Ji, W.; Niu, F.; Zha, W.B.; Wu, X.L.; Hao, H.P.; Wang, G.J. Pharmacokinetics-pharmacology disconnection of herbal medicines and its potential solutions with cellular pharmacokinetic-pharmacodynamic strategy. Curr. Drug Metab. 2012, 13, 558–576. [Google Scholar] [CrossRef]
- Zhao, W.O.; Pang, L.; Dong, N.; Yang, S. LC-ESI-MS/MS analysis and pharmacokinetics of heterophyllin B, a cyclic octapeptide from Pseudostellaria heterophylla in rat plasma. Biomed. Chromatogr. 2015, 29, 1693–1699. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, H.; Jiang, C.; Kan, Y.J.; Hu, J.; Pang, W.S. Studies on the oral absorption of cyclic peptides from Pseudostellaria heterophylla and interacting with membrane receptors. Fitoterapia 2022, 156, 105072. [Google Scholar] [CrossRef]
- Huang, Q.Z.; Feng, G.; Su, H.M.; Wang, W.J.; Peng, L.Z.; Ren, C.C. The pharmacokinetics of heterophyllin B in Pseudostellaria heterophylla in Rats analyzed by UPLC-MS/MS. J. Guizhou Univ. Tradit. Chin. Med. 2022, 44, 15–20. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Chen, Y.; Liang, C.L.; Liu, H.; Qiu, F.; Dai, Z. Antitumor effects of immunity-enhancing traditional Chinese medicine. Biomed. Pharmacother. 2020, 121, 109570. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.Y.; Zhang, X.Y.; Mao, L.M.; Zhang, Y.Q. Anti-neoplastic effect of heterophyllin B on ovarian cancer via the regulation of NRF2/HO-1 in vitro and in vivo. Tissue Cell 2024, 91, 102566. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.L.; Sun, R.; Hao, Z.; Xing, Z.Z.; Cheng, H.J.; Shao, L. Heterophyllin B ameliorates gastric cancer tumor growth through activating ER stress. Tissue Cell 2023, 83, 102129. [Google Scholar] [CrossRef]
- Wei, Y.H.; Yin, L.; Zhang, J.Y.; Tang, J.N.; Yu, X.F.; Wu, Z.X.; Gao, Y.H. Heterophyllin B inhibits the malignant phenotypes of gastric cancer cells via CXCR4. Hum. Cell 2023, 36, 676–688. [Google Scholar] [CrossRef]
- Tantai, J.C.; Zhang, Y.; Zhao, H. Heterophyllin B inhibits the adhesion and invasion of ECA-109 human esophageal carcinoma cells by targeting PI3K/AKT/β-catenin signaling. Mol. Med. Rep. 2016, 13, 1097–1104. [Google Scholar] [CrossRef]
- Yang, C.J.; You, L.T.; Yin, X.B.; Liu, Y.; Leng, X.; Wang, W.P.; Sai, N.; Ni, J. Heterophyllin B ameliorates lipopolysaccharide-induced inflammation and oxidative stress in RAW 264.7 macrophages by suppressing the PI3K/Akt pathways. Molecules 2018, 23, 717. [Google Scholar] [CrossRef]
- Zhang, H.J.; Wang, W.; Hu, X.L.; Wang, Z.; Lou, J.S.; Cui, P.; Zhao, X.; Wang, Y.; Chen, X.L.; Lu, S.B. Heterophyllin B enhances transcription factor EB-mediated autophagy and alleviates pyroptosis and oxidative stress after spinal cord injury. Int. J. Biol. Sci. 2024, 20, 5415–5435. [Google Scholar] [CrossRef]
- Chen, C.; Liang, H.; Wang, J.Y.; Ren, G.Q.; Li, R.S.; Cui, Z.G.; Zhang, C.F. Heterophyllin B an active cyclopeptide alleviates dextran sulfate sodium-induced colitis by modulating gut microbiota and repairing intestinal mucosal barrier via AMPK activation. Mol. Nutr. Food Res. 2022, 66, e2101169. [Google Scholar] [CrossRef]
- Bhimaraj, A.; Tang, W.H.W. Role of oxidative stress in disease progression in Stage B, a pre-cursor of heart failure. Heart Fail. Clin. 2012, 8, 101–111. [Google Scholar] [CrossRef]
- Kibel, A.; Lukinac, A.M.; Dambic, V.; Juric, I.; Selthofer-Relatic, K. Oxidative stress in ischemic heart disease. Oxidative Med. Cell. Longev. 2020, 2020, 6627144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liao, S.G.; He, Y.; Li, J.; Zhong, R.F.; He, X.; Liu, Y.; Xiao, T.T.; Lan, Y.Y.; Long, Q.D.; et al. Protective effects of fractions from Pseudostellaria heterophylla against cobalt chloride-induced hypoxic injury in H9c2 cell. J. Ethnopharmacol. 2013, 147, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Bakke, P.S.; Annesi-Maesani, I.; Janson, C.; Nieminen, M.; Vestbo, J.; Viegi, G. The international journal of tuberculosis and lung disease: A home for respiratory epidemiology. Int. J. Tuberc. Lung Dis. 1998, 2, 969–970. Available online: https://www.ingentaconnect.com/content/iuatld/ijtld/1998/00000002/00000012/art00002 (accessed on 5 March 2025).
- Yang, H.; Kan, Y.J.; Zeng, J.; Hu, J.; Zhao, L.; Pang, W.S. Intervention effect of Pseudostellaria heterophylla cyclic peptide extract on LQIS-COPD rat model. Chin. J. Ethnomedicine Ethnopharmacy 2019, 28, 25–27. [Google Scholar]
- Lu, F.; Yang, H.; Lin, S.D.; Zhao, L.; Jiang, C.; Chen, Z.B.; Liu, Y.Y.; Kan, Y.J.; Hu, J.; Pang, W.S. Cyclic peptide extracts derived from Pseudostellaria heterophylla ameliorates COPD via regulation of the TLR4/MyD88 pathway proteins. Front. Pharmacol. 2020, 11, 850. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Yao, F.H.; Wang, Q.; Wen, Z.X.; Chen, Q.C. Effect of pseudostellarin E from Pseudostellaria heterophylla (Miq.) Pax et Hoffm. on differentiation in 3T3-L1 pre-adipocytes and glucose uptake. Lishizhen Med. Mater. Medica Res. 2018, 29, 1028–1030. [Google Scholar]
- Liao, H.J.; Tzen, J.T.C. The potential role of cyclopeptides from Pseudostellaria heterophylla, Linum usitatissimum and Drymaria diandra, and peptides derived from heterophyllin B as dipeptidyl peptidase IV inhibitors for the treatment of type 2 diabetes: An in silico study. Metabolites 2022, 12, 387. [Google Scholar] [CrossRef]
- Liao, H.J.; Tzen, J.T.C. Investigating potential GLP-1 receptor agonists in cyclopeptides from Pseudostellaria heterophylla, Linum usitatissimum, and Drymaria diandra, and peptides derived from heterophyllin B for the treatment of type 2 diabetes: An in silico study. Metabolites 2022, 12, 549. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.B.; Jiang, Z.S.; Ruan, J.S.; Wang, S.M. Radix Pseudostellariae promotes the proliferation of human umbilical vein endothelial cells via MAPK signaling pathway. Fujian J. Tradit. Chin. Med. 2017, 48, 16–19. [Google Scholar]
- Chen, C.; Wang, J.L.; Cheng, M.Q.; Xie, H.F.; Li, W.; Zhang, C. Muribaculum intestinale-derived 3-hydroxybutyric acid from heterophyllin B attenuated pulmonary fibrosis through IDO1-mediated ferroptosis. Pharmacol. Res. 2025, 212, 107587. [Google Scholar] [CrossRef]
- Deng, J.H.; Feng, X.Y.; Zhou, L.J.; He, C.T.; Li, H.L.; Xia, J.; Ge, Y.W.; Zhao, Y.T.; Song, C.; Chen, L.; et al. Heterophyllin B, a cyclopeptide from Pseudostellaria heterophylla, improves memory via immunomodulation and neurite regeneration in i.c.v. Aβ-induced mice. Food Res. Int. 2022, 158, 111576. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.Y.; Wang, Y.F.; Deng, J.H.; He, C.T.; Jiang, J.H.; Yang, Z.Y. Improving effect of Pseudostellaria heterophylla on learning and memory of zebrafish and APP/PS1 mice. Food Ferment. Ind. 2024, 50, 55–61. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Zhang, C.; Li, X.H.; Ma, Z.H.; Ge, Y.W.; Qian, Z.J.; Song, C. Heterophyllin B, a cyclopeptide from Pseudostellaria heterophylla, enhances cognitive function via neurite outgrowth and synaptic plasticity. Phytother. Res. 2021, 35, 5318–5329. [Google Scholar] [CrossRef]
- Li, J.; Zhou, T.; Zheng, W.; Shao, Z. Analysis of the inhibitory activity of different extracts of Radix Pseudostellariae and cyclic peptide HB on tyrosinase. Lishizhen Med. Mater. Medica Res. 2019, 30, 1100–1102. [Google Scholar]
- Morita, H.; Kayashita, T.; Takeya, K.; Itokawa, H. Cyclic peptides from higher plants, part 15, pseudostellarin H, a new cyclic octapeptide from Pseudostellaria heterophylla. J. Nat. Prod. 1995, 58, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Hao, J.T.; Wu, Y.L.; Liu, C.; Shimizu, K.; Li, R.S.; Zhang, C. Protective effects of heterophyllin B against bleomycin-induced pulmonary fibrosis in mice via AMPK activation. Eur. J. Pharmacol. 2022, 921, 174825. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.; Ren, Y.; Zeng, Y.; Huang, Q.; Wang, C. Antihypertensive effect of soybean bioactive peptides: A review. Curr. Opin. Pharmacol. 2022, 62, 74–81. [Google Scholar] [CrossRef]
- Zhang, L.M.; Yang, M.; Zhou, S.W.; Zhang, H.; Feng, Y.; Shi, L.; Li, D.S.; Lu, Q.M.; Zhang, Z.H.; Zhao, M. Blapstin, a diapause-specific peptide-like peptide from the Chinese medicinal beetle Blaps rhynchopetera, has antifungal function. Microbiol. Spectr. 2023, 11, e0308922. [Google Scholar] [CrossRef]
- Zhang, L.M.; Zhou, S.W.; Huang, X.S.; Chen, Y.F.; Mwangi, J.; Fang, Y.Q.; Du, T.; Zhao, M.; Shi, L.; Lu, Q.M. Blap-6, a novel antifungal peptide from the Chinese medicinal beetle Blaps rhynchopetera against Cryptococcus neoformans. Int. J. Mol. Sci. 2024, 25, 5336. [Google Scholar] [CrossRef]
| Parameters | 2.08 mg/kg [39] | 4.16 mg/kg [39] | 8.32 mg/kg [39] | 20 mg/kg [40] |
|---|---|---|---|---|
| Cmax (ng/mL) | 1372 ± 148 | 2446 ± 242 | 4666 ± 192 | 4082.76 ± 892.20 |
| Tmax (min) | 5 | 5 | 10 | 3.0 ± 1.8 |
| T1/2 (min) | 39.9 ± 14.6 | 48.6 ± 11.2 | 64.8 ± 14.5 | 574.2 ± 160.2 |
| MRT0–t (min) | 23.1 ± 3.5 21 | 23.3 ± 3.7 | 21.8 ± 2.2 | 54.0 ± 16.2 |
| MRT0–∞ (min) | 26.5 ± 3.6 | 24.6 ± 4.3 | 22.6 ± 2.6 | 92.4 ± 25.8 |
| AUC0–t (ng·min/mL) | 29,043 ± 5221 | 48,312 ± 2747 | 116,838 ± 12,018 | 42,100 ± 1170 |
| AUC0–∞ (ng·min/mL) | 29,451 ± 5033 | 48,489 ± 2811 | 117,006 ± 11,947 | 718.02 ± 19.77 |
| Vd (L/kg) | 4.38 ± 2.35 | 5.99 ± 1.18 | 6.79 ± 1.94 | 383,460.70 ± 1003.09 |
| CL (L/min/kg) | 0.072 ± 0.013 | 0.086 ± 0.005 | 0.071 ± 0.007 | 464.48 ± 12.99 |
| Ke (1/h) | 0.08 ± 0.02 | |||
| Compound | Types | Testing Subjects | Doses/Duration/Route | Effects | Mechanism | Ref. |
|---|---|---|---|---|---|---|
| HB | In vitro | OVCAR-8 andSKOV3 cells | 0, 25, 50, 75, 100 μM for 24 h | Cell proliferation ↓; Colony formation↓; Apoptosis ↑; | NRF2 and HO-1 expression ↓; Involved in apoptosis induction. | [43] |
| MKN-4 and BGC-823 cells | 0, 10, 25, and 50 μM for 24–48 h | Cell proliferation ↓; Apoptosis ↑; Arrested cell cycle at G0/G1 phase; | Activates ER stress by upregulating IRE1, CHOP, GRP78, downregulating Bcl-2, and facilitating caspase-3 expression. | [44] | ||
| ECA-109 cells | 10, 25, 50 μM, 24 h | Adhesion and invasion ↓; Cell proliferation ↓; E-cadherin expression ↑; | PI3K/AKT/β-catenin pathway ↓; Snail, Vimentin, MMP-2/9 ↓; E-cadherin ↑; | [45] | ||
| HGC-27 and AGS cells | 0, 10, 20, 40, 60, 80, 100 μM for 24 h | Cell viability, colony formation, migration, and invasion ↓; | Binds to CXCR4, PI3K/AKT signaling pathway; PD-L1 expression, metastasis ↓; | [46] | ||
| In vivo | OVCAR8 xenografted nude mice | 20 mg/kg/day, i.p., 35 days | Tumor growth ↓; Tumor volume ↓; Ki67 expression ↑; | Tumor proliferation via NRF2/HO-1 inhibition; Apoptosis in tumor tissues ↑; | [43] | |
| MKN-45 cells-induced tumor model in nude mice | 10, 15 mg/kg/2 days, i.p., 28 days | Tumor growth, tumor weight ↓; IRE1, CHOP, GRP78 ↑; Bcl-2 ↓; | ER stress activation through IRE1, CHOP, GRP78, Bcl-2 suppression, leading to apoptosis and inhibited tumor growth. | [44] | ||
| HGC-27 cells-induced tumor metastasis model in nude mice | 500 mg/kg/day, i.g., 14 days | Tumor metastasis ↓; Lung metastatic nodules ↓; | Targets CXCR4 and modulates PI3K/AKT signaling; PD-L1 expression, tumor cell migration/invasion ↓; | [46] |
| Compound | Types | Testing Subjects | Doses/Duration/Route | Effects | Mechanism | Ref. |
|---|---|---|---|---|---|---|
| HB | In vitro | LPS-stimulated RAW 264.7 cells | 25, 50, 100 μM, 1 h | LPS-induced NO, IL-6, and IL-1β production ↓; ROS generation ↓; Apoptosis ↓; | Suppression of inflammation and oxidative stress through the PI3K/Akt pathway; p-AKT/AKT and p-PI3K/PI3K ratios ↑; | [47] |
| In vivo | SCI contusion model in C57BL/6J mice | 20 mg/kg/day, i.p., 3 days | Motor function ↑; Axonal regeneration ↑; Bladder recovery ↑; | Activates autophagy by enhancing TFEB translocation; Suppresses oxidative stress and pyroptosis by the AMPK-TRPML1-calcineurin pathway. | [48] | |
| DSS (4% Dextran Sulfate Sodium)-induced colitis in C57BL/6J mice | 20, 80 mg/kg/day, i.g., 7 days | Disease activity index↓; Colon length ↑; Histological damage, and inflammation ↓; | Restored the intestinal mucosal barrier, improved microbiota composition, and reduced inflammatory cytokines. | [49] |
| Extract | Types | Testing Subjects | Doses/Duration/Route | Effects | Mechanism | Ref. |
|---|---|---|---|---|---|---|
| CPE | In vitro | Alveolar macrophages | 50, 100, 200, 500, 1000, and 2000 μg/mL for 12, 24, and 48 h | TNF-α release ↓; IL-10 release ↑; Inflammation ↓; | TLR4, MyD88, and AP-1 mRNA levels ↓; JNK/p38 signaling pathways ↓; Pro-inflammatory cytokine release ↑; | [55] |
| In vivo | Smoky environment + Papain aerosol to induce LQIS-COPD rats | 100, 200, 400 mg/kg/d, i.g., 30 days | Cough, shortness of breath, wheezing ↓; RL ↓; Balanced Cdyn; | Improved lung function by reducing RL and enhancing Cdyn; effects may be due to the restoration of lung qi and inhibition of inflammation. | [54] | |
| SCS-induced COPD model rats | 200, 400, 500 mg/kg/day, p.o., 15 days | Lung function ↑; Alveolar destruction ↓; Alveolar space ↑; | TLR4-MyD88 signaling pathway ↓; Inflammatory cytokines (TNF-α, IL-10) ↓; Lung tissue morphology and function ↑; | [55] |
| Compound | Types | Testing Subjects | Doses/Duration/Route | Effects | Mechanism | Ref. |
|---|---|---|---|---|---|---|
| HB | In vitro | HUVEC cells | 0–200 μg/mL for 48 h | Cell proliferation ↑ (0–100 μg/mL HB); Cell proliferation ↓ (150–200 μg/mL HB); | Promoted cell proliferation through increased VEGF expression and activation of the MAPK signaling pathway (Ras/Raf/Mek/Erk). | [58] |
| HB | In vivo | CAM | 0–10 mg/mL/day, 3 days | Vascular proliferation (5 mg/mL/day) ↓; Blood vessel growth (10 mg/mL/da) ↑; | Promoted angiogenesis by influencing growth factors (e.g., VEGF) and their signaling pathways. | [58] |
| Compound | Types | Testing Subjects | Doses/Duration/Route | Effects | Mechanism | Ref. |
|---|---|---|---|---|---|---|
| HB | In vitro | NCM460 cells | 0.1–10 μM, 24 h | Occludin and ZO-1 expression ↑; Disruption of the epithelial barrier induced by TNF-α ↓; | AMPK signaling ↑; Maintain intestinal epithelial barrier function. | [49] |
| HB | In vivo | DSS-induced colitis in mice. | 20, 80 mg/kg/day, i.g., 7 days | Colon length ↑; Inflammation ↓; Deterioration of the intestinal mucosal barrier. | Protects beneficial intestinal bacteria, restores gut health, and protects against colitis. | [49] |
| Compound | Types | Testing Subjects | Doses/Duration/Route | Effects | Mechanism | Ref. |
|---|---|---|---|---|---|---|
| HB | In vitro | Aβ25-35-induced primary cortical neurons | 1, 10 μM, 4 days | Neuron death ↓; | Apoptosis markers (Bax) ↓; Anti-apoptotic markers (Bcl-2) ↑; Synaptic protein levels were preserved; | [61] |
| SH-SY5Y cells | 0.1–10 μM, 1–3 days | Aβ-induced cell damage ↓; | Aβ-induced neuronal apoptosis ↓; Synaptic regeneration↑; | [61] | ||
| Aβ25-35-induced primary cortical neurons | 1–100 μM | Neuronal survival rate ↑; Aβ25-35-induced cell damage ↓; | Expression of apoptosis-related genes (P53 and Caspase3) ↓; β3-tubulin and MAP2-positive neurite density ↑; Synaptic plasticity ↑; | [62] | ||
| Primary cortical neurons | 0.011 μM, 4 days | β3-tubulin-positive neurite density ↑; Neurite outgrowth ↑; | Promotes cognitive enhancement and neuroprotection through neuronal growth. | [63] | ||
| In vivo | Aβ1-42 i.c.v.-induced AD mice | 1, 10 μM/kg/day, i.p., 16 days | Memory deficits ↓; Cognitive performance ↑; Neuroinflammation. ↓; | Microglial activation ↓; Axonal regeneration ↑; Modulation of inflammatory cytokines. | [61] | |
| APP/PS1 transgenic mice | 10 μM/kg/day, i.p., 60 days | Novel object recognition and spatial memory ↑; Exploration of new locations; | Expression of apoptosis-related genes (P53 and Caspase3) ↓; Neuronal survival rate, neurite density, neural connectivity ↑; | [62] | ||
| ICR mice | 1 and 10 μM/kg, i.p., 10 days | Object recognition and location memory ↑; Neurite outgrowth ↑; | Penetrates the blood–brain barrier and regulates dopamine turnover, leading to cognitive improvements and enhanced memory function. | [63] |
| Compounds | Type | Testing Subjects | Effects | Ref. |
|---|---|---|---|---|
| PA | In vitro | Tyrosinase solution | IC50: 187 μM | [20] |
| PB | Tyrosinase solution | 43% tyrosinase inhibition at 4.98 μg/mL | [65] | |
| PB | Tyrosinase solution | IC50: 187 μM | [20] | |
| PC | Tyrosinase solution | IC50: 63 μM | [20] | |
| PC | Mouse B16 melanoma cells | IC50: 134 μM | [20] | |
| PD | Tyrosinase solution | IC50: 100 μM | [21] | |
| PD | Mouse B16 melanoma cells | IC50: 49 μM | [21] | |
| PE | Tyrosinase solution | IC50: 175 μM | [21] | |
| PF | Tyrosinase solution | IC50: 50 μM | [21] | |
| PG | Tyrosinase solution | IC50: 75 μM | [3] | |
| PH | Tyrosinase solution | 15% tyrosinase inhibition at 800 μM | [66] |
| Compound | Types | Testing Subjects | Doses/Duration/Route | Effects | Mechanism | Ref. |
|---|---|---|---|---|---|---|
| HB | In vitro | MLE-12 cells | 0.5 mg/mL, 1 day | ROS ↓; Ferroptosis ↓; Preserved mitochondrial mass; | Suppressed ferroptosis via IDO1-mediated Fe2+ accumulation and oxidative stress reduction. | [60] |
| A549 cells | 0.5 mg/mL, 1 day | Lung fibrosis ↓; ROS and collagen deposition ↓; | IDO1-mediated ferroptosis suppression. | [60] | ||
| NCM460 cells | 0.5 mg/mL, 1 day | Restored colonic epithelial integrity; Oxidative damage ↓; | Modulated ferroptosis and re-established intestinal mucosal barrier. | [60] | ||
| MLE-12 cells | 1, 10, 20, 50, 100 μM, 1 day | Inhibited TGF-β1-induced EMT in alveolar cells; | Inhibited the expression of Vimentin and promoted E-cadherin expression; AMPK activation reduced STING expression. | [66] | ||
| Primary lung fibroblasts | 1, 10, 20, 50, 100 μM, 2 days | Fibroblast transdifferentiation ↓; ECM deposition ↓; | AMPK pathway ↑; STING expression ↓; TGF-β1 signaling in fibroblasts ↓; | [66] | ||
| In vivo | BLM-induced PF in mice | 20 mg/kg/day, i.g., 21 days | Collagen I deposition ↓; Restored intestinal barrier; | Enriched M. intestinale and promoted the production of 3-HA, which modulates IDO1-mediated ferroptosis. | [60] | |
| Antibiotic-induced microbiota depletion in mice | 20 mg/kg/day, i.g., 7 days | Restored intestinal mucosal barrier; PF symptoms↓; | Increased 3-HA production and reprogramed the intestinal ecosystem to modulate the gut microbiota and alleviate PF. | [60] | ||
| C57BL/6 Mice (PF model induced by BLM): | 5 and 20 mg/kg/day, p.o., 14 days | Fibrosis and collagen deposition in lungs ↓; | AMPK ↑; STING expression ↓; TGF-β1/Smad2/3 signaling ↓; ECM deposition and fibroblast accumulation ↓. | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Wen, L.; Jiang, Z.-Z.; Chan, B.C.-L.; Leung, P.-C.; Wong, C.-K.; Tan, N.-H. Chemical Properties, Preparation, and Pharmaceutical Effects of Cyclic Peptides from Pseudostellaria heterophylla. Molecules 2025, 30, 2521. https://doi.org/10.3390/molecules30122521
Yang Y, Wen L, Jiang Z-Z, Chan BC-L, Leung P-C, Wong C-K, Tan N-H. Chemical Properties, Preparation, and Pharmaceutical Effects of Cyclic Peptides from Pseudostellaria heterophylla. Molecules. 2025; 30(12):2521. https://doi.org/10.3390/molecules30122521
Chicago/Turabian StyleYang, Yue, Luan Wen, Zhuang-Zhuang Jiang, Ben Chung-Lap Chan, Ping-Chung Leung, Chun-Kwok Wong, and Ning-Hua Tan. 2025. "Chemical Properties, Preparation, and Pharmaceutical Effects of Cyclic Peptides from Pseudostellaria heterophylla" Molecules 30, no. 12: 2521. https://doi.org/10.3390/molecules30122521
APA StyleYang, Y., Wen, L., Jiang, Z.-Z., Chan, B. C.-L., Leung, P.-C., Wong, C.-K., & Tan, N.-H. (2025). Chemical Properties, Preparation, and Pharmaceutical Effects of Cyclic Peptides from Pseudostellaria heterophylla. Molecules, 30(12), 2521. https://doi.org/10.3390/molecules30122521






