Anticancer Effects of Withanolides: In Silico Prediction of Pharmacological Properties
Abstract
1. Introduction
2. Results
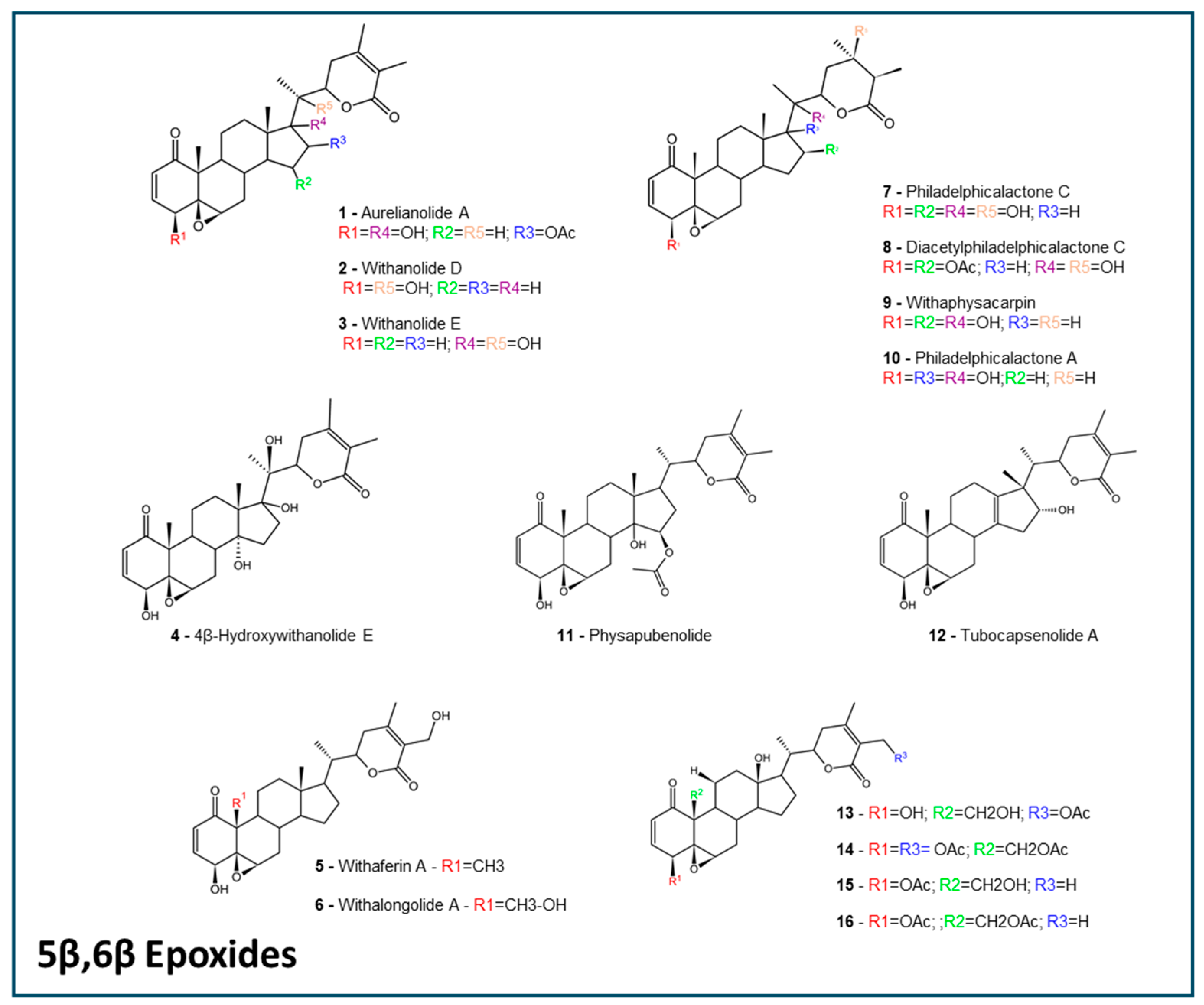
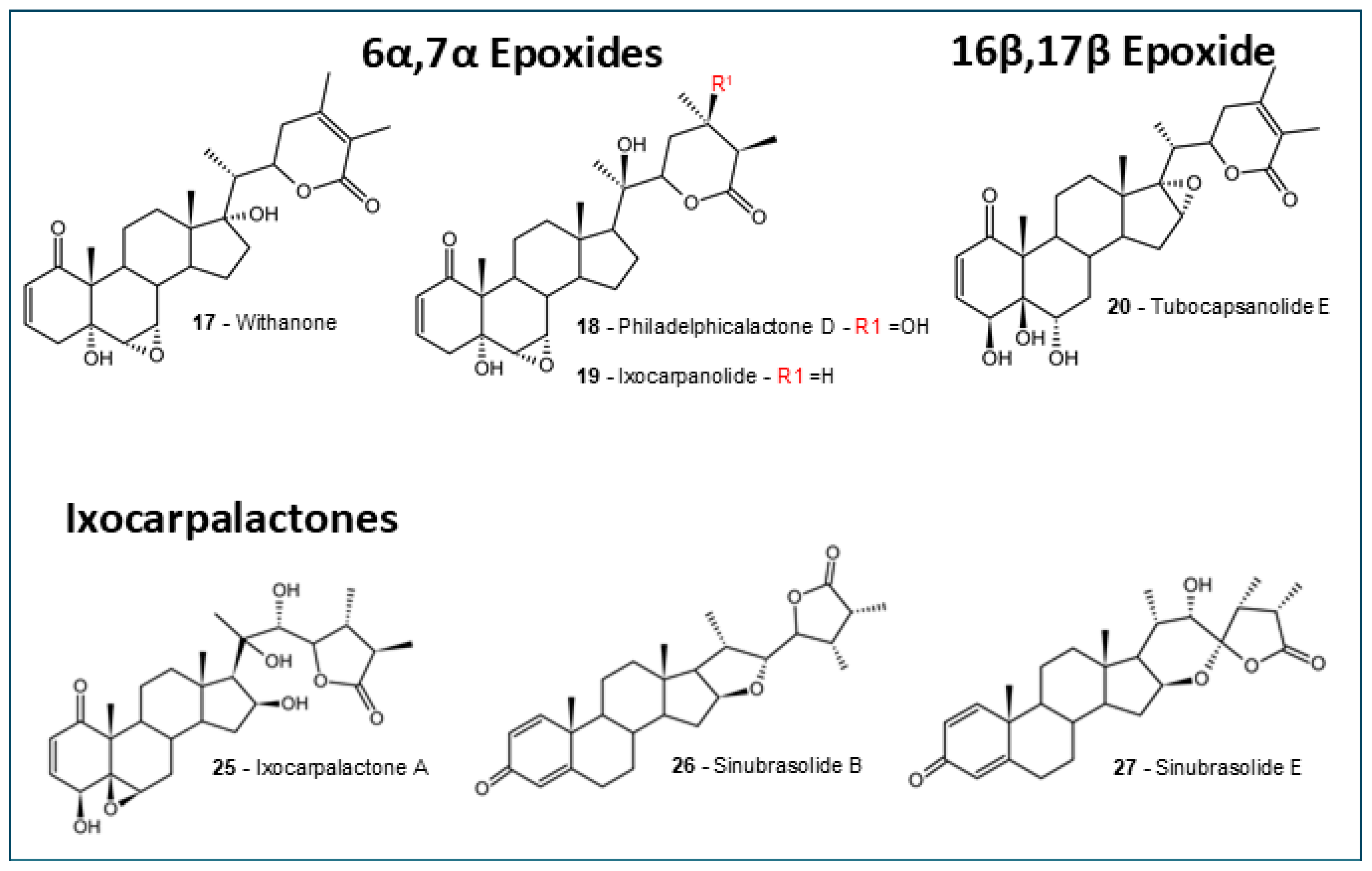
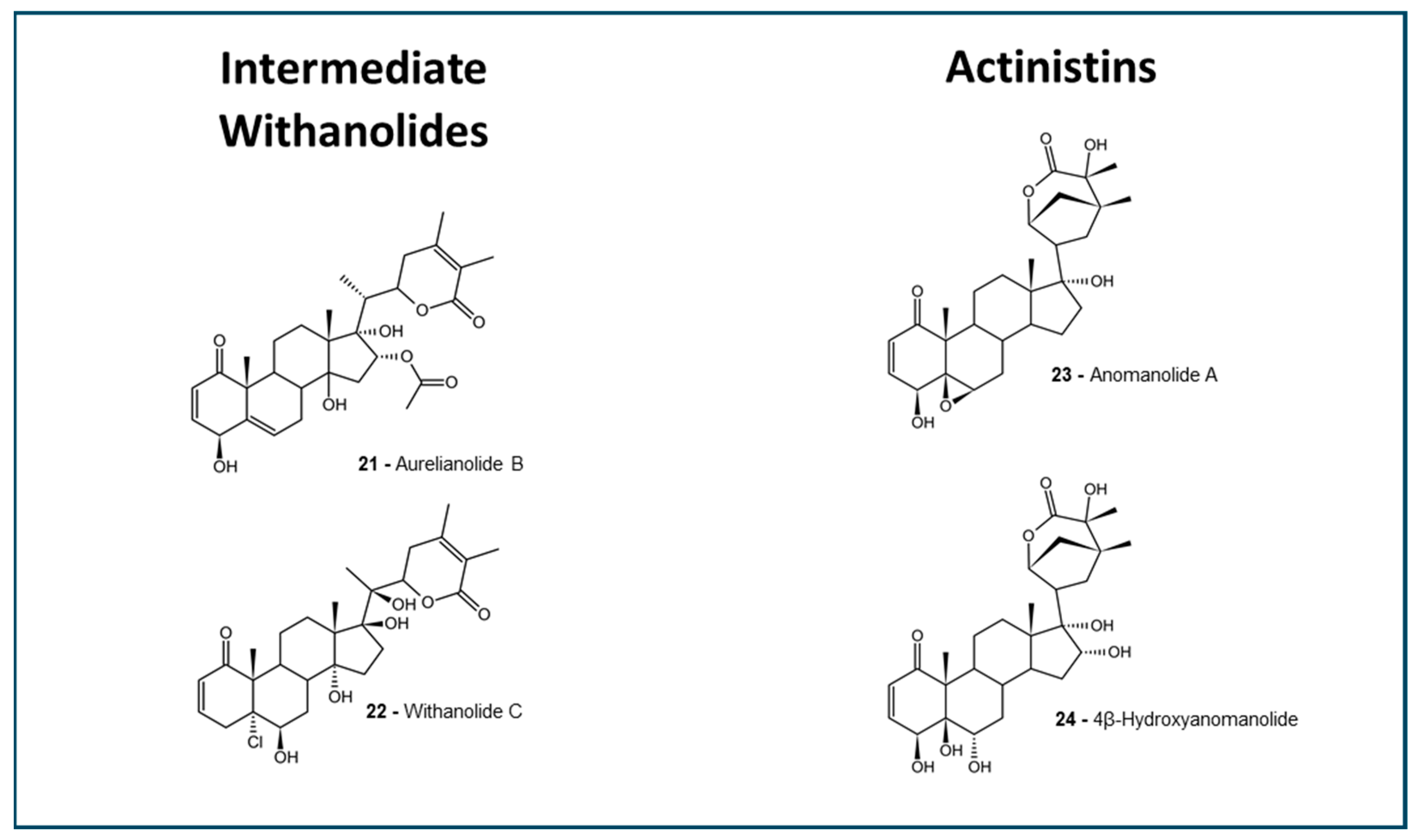
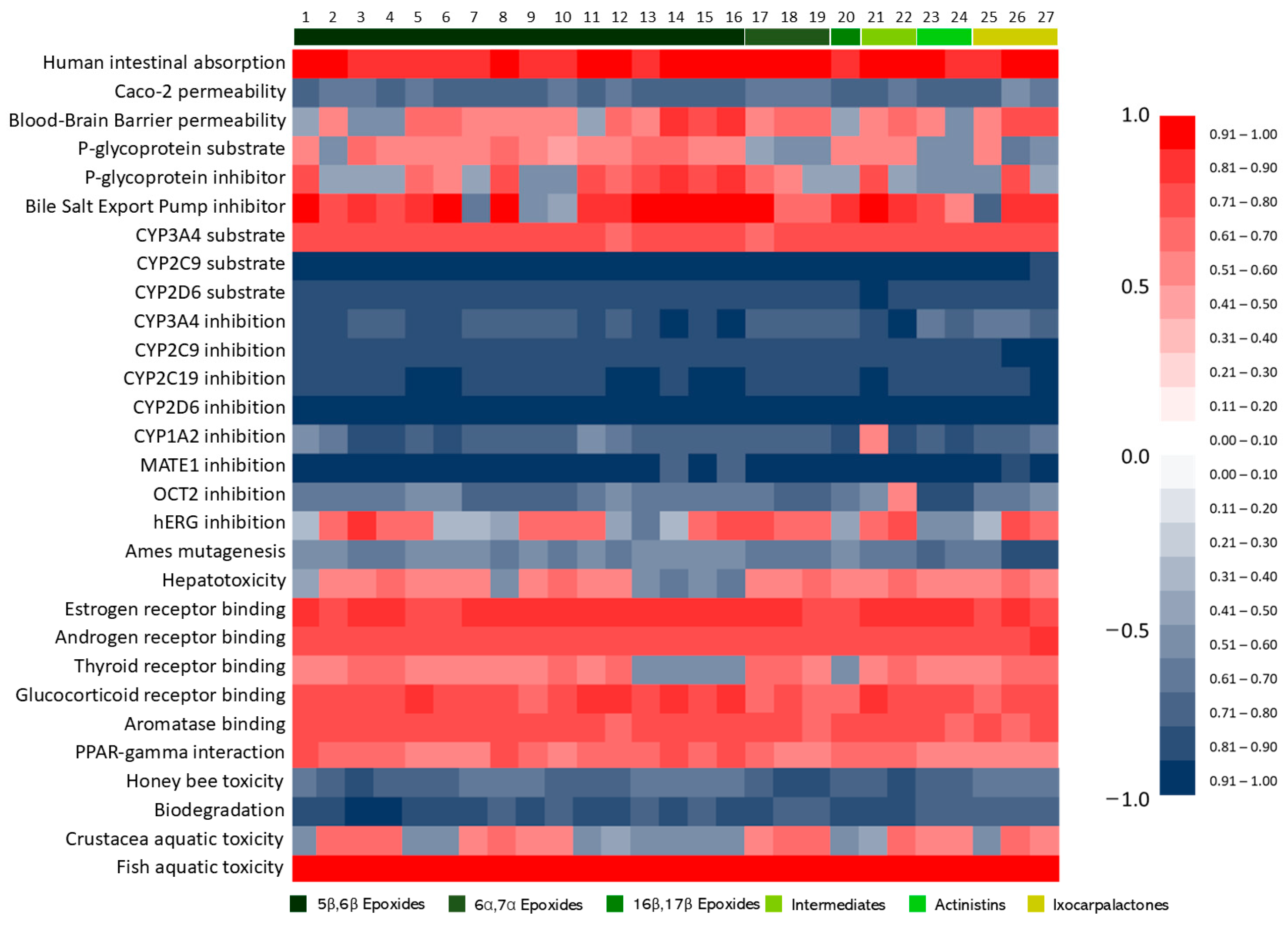
2.1. Withanolides Structures and ADMET Studies
2.2. Oral Absorption
2.2.1. Lipinski’s Rule of Five
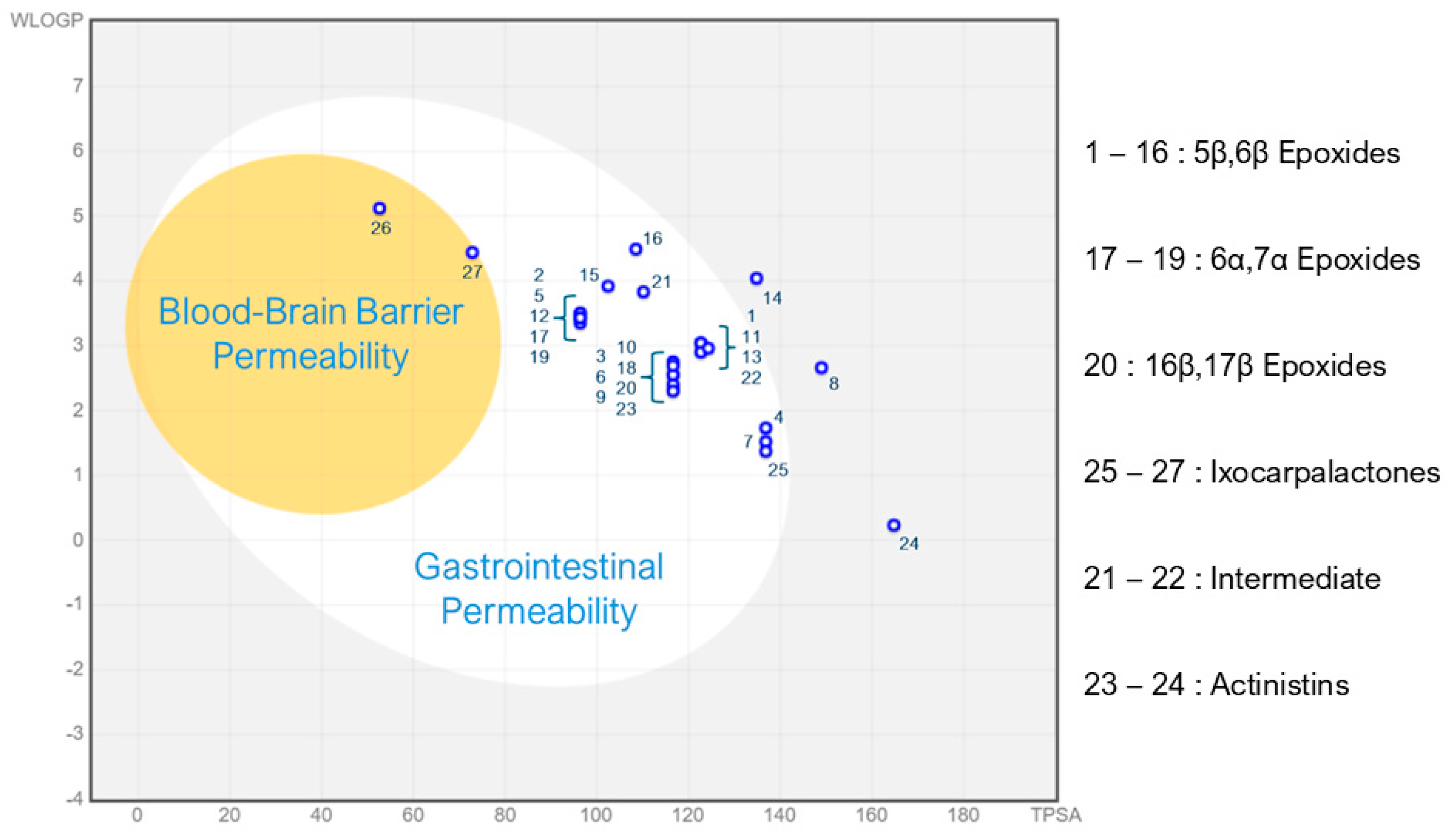
2.2.2. BOILED-Egg Prediction
2.2.3. AdmetSAR Barrier Predictions
2.3. Toxicity Predictions
2.3.1. Hepatotoxicity
2.3.2. Cardiotoxicity
2.3.3. Ames Mutagenesis
2.4. Metabolism and Excretion Predictions
2.4.1. Cytochrome Interactions
2.4.2. P-Glycoprotein Interaction
2.4.3. OCT-2 and MATE-1 Inhibition
2.4.4. BSEP Inhibition
2.5. Nuclear Receptor Binding
2.6. Ecotoxicity Predictions
3. Discussion
3.1. Absorption and Distribution
3.2. Toxicity
3.3. Metabolism and Excretion
3.4. Ecotoxicity
4. Materials and Methods
4.1. Databases
4.2. Withanolides Selection Criteria
4.3. Antineoplastic Drugs
4.4. ADMET: Physicochemical Descriptors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | ATP-binding cassette |
| ADMET | Absorption, Distribution, Metabolism, Excretion and Toxicity |
| BBB | Blood–Brain Barrier |
| BSEP | Bile Salt Export Pump |
| MATE-1 | Multidrug and Toxin Extrusion protein 1 |
| NR | Nuclear Receptors |
| OCT-2 | Organic Cation Transporter 2 |
| P-gp | P-glycoprotein |
| TPSA | Topological Polar Surface Area |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Luo, Q.; Zhang, Y.; Jia, F.; Zhao, Y.; Wang, F. Advances in Toxicological Research of the Anticancer Drug Cisplatin. Chem Res. Toxicol. 2019, 32, 1469–1486. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Miao, J.; Wang, Y.; Luo, W.; Ji, Z.; Lai, H.; Zhang, M.; Cheng, X.; Wang, J.; Fang, Y.; et al. Single-Cell Analysis Supports a Luminal-Neuroendocrine Transdifferentiation in Human Prostate Cancer. Commun. Biol. 2020, 3, 778. [Google Scholar] [CrossRef]
- Magalhães, H.; Fontes-Sousa, M.; Machado, M. Immunotherapy in Advanced Gastric Cancer: An Overview of the Emerging Strategies. Can. J. Gastroenterol. Hepatol. 2018, 2018, 2732408. [Google Scholar] [CrossRef]
- Dzobo, K. The Role of Natural Products as Sources of Therapeutic Agents for Innovative Drug Discovery. In Comprehensive Pharmacology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 408–422. [Google Scholar]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences Taskforce; Supuran, C.T. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.-J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Gielecińska, A.; Kciuk, M.; Mujwar, S.; Celik, I.; Kołat, D.; Kałuzińska-Kołat, Ż.; Kontek, R. Substances of Natural Origin in Medicine: Plants vs. Cancer. Cells 2023, 12, 986. [Google Scholar] [CrossRef]
- Hussain, H.; Csuk, R.; Green, I.R.; Ur Rehman, N.; Abbas, G.; Hussain, W. Journey Describing the Cytotoxic Potential of Withanolides: A Patent Review. Recent. Pat. Anticancer Drug Discov. 2018, 13, 411–421. [Google Scholar] [CrossRef]
- Chen, L.-X.; Xia, G.-Y.; He, H.; Huang, J.; Qiu, F.; Zi, X.-L. New Withanolides with TRAIL-Sensitizing Effect from Physalis pubescens L. RSC Adv. 2016, 6, 52925–52936. [Google Scholar] [CrossRef]
- Huang, M.; He, J.-X.; Hu, H.-X.; Zhang, K.; Wang, X.-N.; Zhao, B.-B.; Lou, H.-X.; Ren, D.-M.; Shen, T. Withanolides from the Genus Physalis: A Review on Their Phytochemical and Pharmacological Aspects. J. Pharm. Pharmacol. 2020, 72, 649–669. [Google Scholar] [CrossRef] [PubMed]
- Samadi, A.K. Potential Anticancer Properties and Mechanisms of Action of Withanolides. Enzymes 2015, 37, 73–94. [Google Scholar] [CrossRef]
- Chirumamilla, C.S.; Pérez-Novo, C.; Van Ostade, X.; Vanden Berghe, W. Molecular Insights into Cancer Therapeutic Effects of the Dietary Medicinal Phytochemical Withaferin A. Proc. Nutr. Soc. 2017, 76, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Atteeq, M. Evaluating Anticancer Properties of Withaferin A-a Potent Phytochemical. Front. Pharmacol. 2022, 13, 975320. [Google Scholar] [CrossRef]
- White, P.T.; Subramanian, C.; Motiwala, H.F.; Cohen, M.S. Natural Withanolides in the Treatment of Chronic Diseases. Adv. Exp. Med. Biol. 2016, 928, 329–373. [Google Scholar] [CrossRef] [PubMed]
- De Souza E Silva, G.W.; Marques, A.M.; Fontão, A.P.G.A.; DE Moura Lima, S.C.; Kaplan, M.A.C.; Figueiredo, M.R.; Sampaio, A.L.F. Aurelianolides from Aureliana Fasciculata Var. Fasciculata Trigger Apoptosis With Caspase Activation in Human Leukemia Cells. Anticancer Res. 2023, 43, 1245–1253. [Google Scholar] [CrossRef]
- Lem, F.F.; Yong, Y.S.; Goh, S.; Chin, S.N.; Chee, F.T. Withanolides, the Hidden Gem in Physalis Minima: A Mini Review on Their Anti-Inflammatory, Anti-Neuroinflammatory and Anti-Cancer Effects. Food Chem. 2022, 377, 132002. [Google Scholar] [CrossRef]
- Singh, A.; Raza, A.; Amin, S.; Damodaran, C.; Sharma, A.K. Recent Advances in the Chemistry and Therapeutic Evaluation of Naturally Occurring and Synthetic Withanolides. Molecules 2022, 27, 886. [Google Scholar] [CrossRef]
- Kashyap, V.K.; Peasah-Darkwah, G.; Dhasmana, A.; Jaggi, M.; Yallapu, M.M.; Chauhan, S.C. Withania Somnifera: Progress towards a Pharmaceutical Agent for Immunomodulation and Cancer Therapeutics. Pharmaceutics 2022, 14, 611. [Google Scholar] [CrossRef]
- Paul, S.; Chakraborty, S.; Anand, U.; Dey, S.; Nandy, S.; Ghorai, M.; Saha, S.C.; Patil, M.T.; Kandimalla, R.; Proćków, J.; et al. Withania somnifera (L.) Dunal (Ashwagandha): A Comprehensive Review on Ethnopharmacology, Pharmacotherapeutics, Biomedicinal and Toxicological Aspects. Biomed. Pharmacother. 2021, 143, 112175. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Xu, W.; Liu, Y.; Zhang, J.-H.; Yang, Y.-Y.; Wang, Z.-W.; Sun, D.-J.; Li, H.; Liu, B.; Chen, L.-X. Anomanolide C Suppresses Tumor Progression and Metastasis by Ubiquitinating GPX4-Driven Autophagy-Dependent Ferroptosis in Triple Negative Breast Cancer. Int. J. Biol. Sci. 2023, 19, 2531–2550. [Google Scholar] [CrossRef] [PubMed]
- Hahm, E.-R.; Kim, S.-H.; Singh, S.V. Withaferin A Inhibits Breast Cancer-Induced Osteoclast Differentiation. Mol. Carcinog. 2023, 62, 1051–1061. [Google Scholar] [CrossRef]
- Almeida, A.A.; Lima, G.D.A.; Eiterer, M.; Rodrigues, L.A.; do Vale, J.A.A.; Zanatta, A.C.; Bressan, G.C.; de Oliveira, L.L.; Leite, J.P.V. A Withanolide-Rich Fraction of Athenaea Velutina Induces Apoptosis and Cell Cycle Arrest in Melanoma B16F10 Cells. Planta Med. 2022, 88, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, Y.; Okuzaki, D.; Fukushima, K.; Mukai, S.; Ohno, S.; Ozaki, Y.; Yabuta, N.; Nojima, H. Withaferin A Induces Cell Death Selectively in Androgen-Independent Prostate Cancer Cells but Not in Normal Fibroblast Cells. PLoS ONE 2015, 10, e0134137. [Google Scholar] [CrossRef]
- Agamah, F.E.; Mazandu, G.K.; Hassan, R.; Bope, C.D.; Thomford, N.E.; Ghansah, A.; Chimusa, E.R. Computational/in Silico Methods in Drug Target and Lead Prediction. Brief. Bioinform. 2020, 21, 1663–1675. [Google Scholar] [CrossRef]
- Sadybekov, A.V.; Katritch, V. Computational Approaches Streamlining Drug Discovery. Nature 2023, 616, 673–685. [Google Scholar] [CrossRef]
- Rayan, A.; Raiyn, J.; Falah, M. Nature Is the Best Source of Anticancer Drugs: Indexing Natural Products for Their Anticancer Bioactivity. PLoS ONE 2017, 12, e0187925. [Google Scholar] [CrossRef]
- Ferreira, L.L.G.; Andricopulo, A.D. ADMET Modeling Approaches in Drug Discovery. Drug Discov. Today 2019, 24, 1157–1165. [Google Scholar] [CrossRef]
- Lombardo, F.; Desai, P.V.; Arimoto, R.; Desino, K.E.; Fischer, H.; Keefer, C.E.; Petersson, C.; Winiwarter, S.; Broccatelli, F. In Silico Absorption, Distribution, Metabolism, Excretion, and Pharmacokinetics (ADME-PK): Utility and Best Practices. An Industry Perspective from the International Consortium for Innovation through Quality in Pharmaceutical Development. J. Med. Chem. 2017, 60, 9097–9113. [Google Scholar] [CrossRef]
- Alqahtani, S. In Silico ADME-Tox Modeling: Progress and Prospects. Expert. Opin. Drug Metab. Toxicol. 2017, 13, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Shaker, B.; Ahmad, S.; Lee, J.; Jung, C.; Na, D. In Silico Methods and Tools for Drug Discovery. Comput. Biol. Med. 2021, 137, 104851. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Abraham, M.H.; Le, J.; Hersey, A.; Luscombe, C.N.; Beck, G.; Sherborne, B.; Cooper, I. Rate-Limited Steps of Human Oral Absorption and QSAR Studies. Pharm. Res. 2002, 19, 1446–1457. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef]
- Lea, T. Caco-2 Cell Line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Switzerland, 2015; ISBN 978-3-319-15791-7. [Google Scholar]
- Kajiwara, M.; Ban, T.; Matsubara, K.; Nakanishi, Y.; Masuda, S. Urinary Dopamine as a Potential Index of the Transport Activity of Multidrug and Toxin Extrusion in the Kidney. Int. J. Mol. Sci. 2016, 17, 1228. [Google Scholar] [CrossRef] [PubMed]
- Fasinu, P.; Pillay, V.; Ndesendo, V.M.K.; du Toit, L.C.; Choonara, Y.E. Diverse Approaches for the Enhancement of Oral Drug Bioavailability. Biopharm. Drug Dispos. 2011, 32, 185–209. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The Blood-Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Emmerová, R.; Engelová, J.; Vinakurau, S.; Ondrová, B. CNS Tumors—Clinical and Radiological Aspects. Cesk Patol. 2022, 58, 150–160. [Google Scholar]
- Cascella, M.; Di Napoli, R.; Carbone, D.; Cuomo, G.F.; Bimonte, S.; Muzio, M.R. Chemotherapy-Related Cognitive Impairment: Mechanisms, Clinical Features and Research Perspectives. Recenti Prog. Med. 2018, 109, 523–530. [Google Scholar] [CrossRef]
- Shaker, B.; Lee, J.; Lee, Y.; Yu, M.-S.; Lee, H.-M.; Lee, E.; Kang, H.-C.; Oh, K.-S.; Kim, H.W.; Na, D. A machine learning-based quantitative model (LogBB_Pred) to predict the blood-brain barrier permeability (logBB value) of drug compounds. Bioinformatics 2023, 39, btad577. [Google Scholar] [CrossRef]
- Modi, S.J.; Tiwari, A.; Ghule, C.; Pawar, S.; Saste, G.; Jagtap, S.; Singh, R.; Deshmukh, A.; Girme, A.; Hingorani, L. Pharmacokinetic Study of Withanosides and Withanolides from Withania Somnifera Using Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry (UHPLC-MS/MS). Molecules 2022, 27, 1476. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Jiang, W.; Guo, Z.; Wang, Z.; Huang, M.; Zhong, G.; Liang, C.; Pei, X.; Dai, R. Studies on Oral Bioavailability and First-Pass Metabolism of Withaferin A in Rats Using LC-MS/MS and Q-TRAP. Biomed. Chromatogr. 2019, 33, e4573. [Google Scholar] [CrossRef]
- Floyd, J.; Mirza, I.; Sachs, B.; Perry, M.C. Hepatotoxicity of Chemotherapy. Semin. Oncol. 2006, 33, 50–67. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Yang, H.; Lou, C.; Sun, L.; Li, J.; Cai, Y.; Wang, Z.; Li, W.; Liu, G.; Tang, Y. admetSAR 2.0: Web-Service for Prediction and Optimization of Chemical ADMET Properties. Bioinformatics 2019, 35, 1067–1069. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Dar, N.J.; Hamid, A.; Ahmad, M. Pharmacologic Overview of Withania Somnifera, the Indian Ginseng. Cell Mol. Life Sci. 2015, 72, 4445–4460. [Google Scholar] [CrossRef]
- Xia, Y.; Yan, M.; Wang, P.; Hamada, K.; Yan, N.; Hao, H.; Gonzalez, F.J.; Yan, T. Withaferin A in the Treatment of Liver Diseases: Progress and Pharmacokinetic Insights. Drug Metab. Dispos. 2022, 50, 685–693. [Google Scholar] [CrossRef]
- Siddiqui, S.; Ahmed, N.; Goswami, M.; Chakrabarty, A.; Chowdhury, G. DNA Damage by Withanone as a Potential Cause of Liver Toxicity Observed for Herbal Products of Withania Somnifera (Ashwagandha). Curr. Res. Toxicol. 2021, 2, 72–81. [Google Scholar] [CrossRef]
- Ferri, N.; Siegl, P.; Corsini, A.; Herrmann, J.; Lerman, A.; Benghozi, R. Drug Attrition during Pre-Clinical and Clinical Development: Understanding and Managing Drug-Induced Cardiotoxicity. Pharmacol. Ther. 2013, 138, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, J.I.; Perry, M.D.; Perrin, M.J.; Mann, S.A.; Ke, Y.; Hill, A.P. hERG K(+) Channels: Structure, Function, and Clinical Significance. Physiol. Rev. 2012, 92, 1393–1478. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Chen, H. Cardiotoxicity of Anticancer Therapeutics. Front. Cardiovasc. Med. 2018, 5, 9. [Google Scholar] [CrossRef]
- Ducroq, J.; Moha ou Maati, H.; Guilbot, S.; Dilly, S.; Laemmel, E.; Pons-Himbert, C.; Faivre, J.F.; Bois, P.; Stücker, O.; Le Grand, M. Dexrazoxane Protects the Heart from Acute Doxorubicin-Induced QT Prolongation: A Key Role for I(Ks). Br. J. Pharmacol. 2010, 159, 93–101. [Google Scholar] [CrossRef]
- Tiribelli, M.; Medeot, M. Cardiotoxicity of Imatinib: At the Heart of the Problem. Leuk. Res. 2011, 35, 36–37. [Google Scholar] [CrossRef]
- Dong, Q.; Fu, X.-X.; Du, L.-L.; Zhao, N.; Xia, C.-K.; Yu, K.-W.; Cheng, L.-X.; Du, Y.-M. Blocking of the Human Ether-à-Go-Go-Related Gene Channel by Imatinib Mesylate. Biol. Pharm. Bull. 2013, 36, 268–275. [Google Scholar] [CrossRef][Green Version]
- Hamza, A.; Amin, A.; Daoud, S. The Protective Effect of a Purified Extract of Withania Somnifera against Doxorubicin-Induced Cardiac Toxicity in Rats. Cell Biol. Toxicol. 2008, 24, 63–73. [Google Scholar] [CrossRef]
- Poindexter, B.J.; Allison, A.W.; Bick, R.J.; Dasgupta, A. Ginseng: Cardiotonic in Adult Rat Cardiomyocytes, Cardiotoxic in Neonatal Rat Cardiomyocytes. Life Sci. 2006, 79, 2337–2344. [Google Scholar] [CrossRef]
- Zeiger, E. The Test That Changed the World: The Ames Test and the Regulation of Chemicals. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 841, 43–48. [Google Scholar] [CrossRef]
- Mortelmans, K.; Zeiger, E. The Ames Salmonella/Microsome Mutagenicity Assay. Mutat. Res. 2000, 455, 29–60. [Google Scholar] [CrossRef]
- Dhar, N.; Razdan, S.; Rana, S.; Bhat, W.W.; Vishwakarma, R.; Lattoo, S.K. A Decade of Molecular Understanding of Withanolide Biosynthesis and In Vitro Studies in Withania Somnifera (L.) Dunal: Prospects and Perspectives for Pathway Engineering. Front. Plant Sci. 2015, 6, 1031. [Google Scholar] [CrossRef] [PubMed]
- Werk, A.N.; Cascorbi, I. Functional Gene Variants of CYP3A4. Clin. Pharmacol. Ther. 2014, 96, 340–348. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Wong, Y.N. Enzyme Kinetics for Clinically Relevant CYP Inhibition. Curr. Drug Metab. 2005, 6, 241–257. [Google Scholar] [CrossRef][Green Version]
- Shilpashree, H.B.; Sudharshan, S.J.; Shasany, A.K.; Nagegowda, D.A. Molecular Characterization of Three CYP450 Genes Reveals Their Role in Withanolides Formation and Defense in Withania Somnifera, the Indian Ginseng. Sci. Rep. 2022, 12, 1602. [Google Scholar] [CrossRef] [PubMed]
- Mollazadeh, S.; Sahebkar, A.; Hadizadeh, F.; Behravan, J.; Arabzadeh, S. Structural and Functional Aspects of P-Glycoprotein and Its Inhibitors. Life Sci. 2018, 214, 118–123. [Google Scholar] [CrossRef]
- Czyzewski, K.; Styczynski, J. Imatinib Is a Substrate for Various Multidrug Resistance Proteins. Neoplasma 2009, 56, 202–207. [Google Scholar] [CrossRef] [PubMed]
- van Hoppe, S.; Rood, J.J.M.; Buil, L.; Wagenaar, E.; Sparidans, R.W.; Beijnen, J.H.; Schinkel, A.H. P-Glycoprotein (MDR1/ABCB1) Restricts Brain Penetration of the Bruton’s Tyrosine Kinase Inhibitor Ibrutinib, While Cytochrome P450-3A (CYP3A) Limits Its Oral Bioavailability. Mol. Pharm. 2018, 15, 5124–5134. [Google Scholar] [CrossRef]
- Maia, R.C.; Silva, E.A.; Harab, R.C.; Lucena, M.; Pires, V.; Rumjanek, V.M. Sensitivity of Vincristine-Sensitive K562 and Vincristine-Resistant K562-Lucena 1 Cells to Anthracyclines and Reversal of Multidrug Resistance. Braz. J. Med. Biol. Res. 1996, 29, 467–472. [Google Scholar]
- Issa, M.E.; Wijeratne, E.M.K.; Gunatilaka, A.a.L.; Cuendet, M. Withanolide D Exhibits Similar Cytostatic Effect in Drug-Resistant and Drug-Sensitive Multiple Myeloma Cells. Front. Pharmacol. 2017, 8, 610. [Google Scholar] [CrossRef]
- Arruda, A.C.; Perilhão, M.S.; Santos, W.A.; Gregnani, M.F.; Budu, A.; Neto, J.C.R.; Estrela, G.R.; Araujo, R.C. PPARα-Dependent Modulation by Metformin of the Expression of OCT-2 and MATE-1 in the Kidney of Mice. Molecules 2020, 25, 392. [Google Scholar] [CrossRef]
- Koepsell, H. Organic Cation Transporters in Health and Disease. Pharmacol. Rev. 2020, 72, 253–319. [Google Scholar] [CrossRef]
- Kis, E.; Ioja, E.; Rajnai, Z.; Jani, M.; Méhn, D.; Herédi-Szabó, K.; Krajcsi, P. BSEP Inhibition: In Vitro Screens to Assess Cholestatic Potential of Drugs. Toxicol. In Vitro 2012, 26, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Weikum, E.R.; Liu, X.; Ortlund, E.A. The Nuclear Receptor Superfamily: A Structural Perspective. Protein Sci. 2018, 27, 1876–1892. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.H.E.; Li, J.; Xu, H.E.; Melcher, K.; Yong, E. Androgen Receptor: Structure, Role in Prostate Cancer and Drug Discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [CrossRef]
- Saatci, O.; Huynh-Dam, K.-T.; Sahin, O. Endocrine Resistance in Breast Cancer: From Molecular Mechanisms to Therapeutic Strategies. J. Mol. Med. 2021, 99, 1691–1710. [Google Scholar] [CrossRef] [PubMed]
- Soh, S.; Ong, W.-Y. Effect of Withanolide A on 7-Ketocholesterol Induced Cytotoxicity in hCMEC/D3 Brain Endothelial Cells. Cells 2022, 11, 457. [Google Scholar] [CrossRef]
- Duan, W.; Meng, F.; Cui, H.; Lin, Y.; Wang, G.; Wu, J. Ecotoxicity of Phenol and Cresols to Aquatic Organisms: A Review. Ecotoxicol. Environ. Saf. 2018, 157, 441–456. [Google Scholar] [CrossRef]
- Duan, W.; Cui, H.; Jia, X.; Huang, X. Occurrence and Ecotoxicity of Sulfonamides in the Aquatic Environment: A Review. Sci. Total Environ. 2022, 820, 153178. [Google Scholar] [CrossRef]
- Barrick, A.; Châtel, A.; Bruneau, M.; Mouneyrac, C. The Role of High-Throughput Screening in Ecotoxicology and Engineered Nanomaterials. Environ. Toxicol. Chem. 2017, 36, 1704–1714. [Google Scholar] [CrossRef]
- Sripriya, J.; Dash, A. Analytical Study of Kharif Food Grain Production in Odisha. Int. J. Plant Soil Sci. 2021, 33, 78–85. [Google Scholar] [CrossRef]
- Rim, K.-T. In Silico Prediction of Toxicity and Its Applications for Chemicals at Work. Toxicol. Environ. Health Sci. 2020, 12, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-C.; Tsai, Y.-L.; Wu, Y.-C.; Chang, F.-R.; Liu, M.-H.; Chen, W.-Y.; Wu, C.-C. Withanolides-Induced Breast Cancer Cell Death Is Correlated with Their Ability to Inhibit Heat Protein 90. PLoS ONE 2012, 7, e37764. [Google Scholar] [CrossRef]
- Hassannia, B.; Logie, E.; Vandenabeele, P.; Vanden Berghe, T.; Vanden Berghe, W. Withaferin A: From Ayurvedic Folk Medicine to Preclinical Anti-Cancer Drug. Biochem. Pharmacol. 2020, 173, 113602. [Google Scholar] [CrossRef]
- Wang, T.; Subramanian, C.; Yu, M.; White, P.T.; Kuai, R.; Sanchez, J.; Moon, J.J.; Timmermann, B.N.; Schwendeman, A.; Cohen, M.S. Mimetic sHDL Nanoparticles: A Novel Drug-Delivery Strategy to Target Triple-Negative Breast Cancer. Surgery 2019, 166, 1168–1175. [Google Scholar] [CrossRef]
- Maldonado, E.; Pérez-Castorena, A.L.; Garcés, C.; Martínez, M. Philadelphicalactones C and D and Other Cytotoxic Compounds from Physalis Philadelphica. Steroids 2011, 76, 724–728. [Google Scholar] [CrossRef]
- Ma, T.; Fan, B.-Y.; Zhang, C.; Zhao, H.-J.; Han, C.; Gao, C.-Y.; Luo, J.-G.; Kong, L.-Y. Metabonomics Applied in Exploring the Antitumour Mechanism of Physapubenolide on Hepatocellular Carcinoma Cells by Targeting Glycolysis through the Akt-P53 Pathway. Sci. Rep. 2016, 6, 29926. [Google Scholar] [CrossRef]
- Wadegaonkar, V.P.; Wadegaonkar, P.A. Withanone as an Inhibitor of Survivin: A Potential Drug Candidate for Cancer Therapy. J. Biotechnol. 2013, 168, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.-J.; Tang, J.-Y.; Lin, L.-C.; Lien, W.-J.; Cheng, Y.-B.; Chang, F.-R.; Ou-Yang, F.; Chang, H.-W. Withanolide C Inhibits Proliferation of Breast Cancer Cells via Oxidative Stress-Mediated Apoptosis and DNA Damage. Antioxidants 2020, 9, 873. [Google Scholar] [CrossRef]
- Wang, S.-B.; Zhu, D.-R.; Nie, B.; Li, J.; Zhang, Y.-J.; Kong, L.-Y.; Luo, J.-G. Cytotoxic withanolides from the aerial parts of Tubocapsicum anomalum. Bioorg. Chem. 2018, 81, 396–404. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Liaw, C.-C.; Chen, B.-W.; Chen, P.-C.; Su, J.-H.; Sung, P.-J.; Dai, C.-F.; Chiang, M.Y.; Sheu, J.-H. Withanolide-Based Steroids from the Cultured Soft Coral Sinularia Brassica. J. Nat. Prod. 2013, 76, 1902–1908. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
| Molinspiration | SwissADME | pkCSM | admetSAR | |||||
|---|---|---|---|---|---|---|---|---|
| Compound Group | TPSA | % Absorption a | Lipinski’s Rule of Five (Violations) | P-gp Substrate | Human Intestinal Absorption | Caco-2 Permeability | Blood–Brain Barrier Permeability | P-gp Substrate |
| 5β,6β epoxides | 118.2 ± 3.96 | 68.23 ± 1.37 | 0 (7/16); 1 (9/16) | Yes (13/16); No (3/16) | 0.9062 ± 0.0115 | 0.2785 ± 0.0127 | 0.6125 ± 0.0306 | 0.5832 ± 0.0108 |
| 6α,7α epoxides | 103.1 ± 8.74 | 73.47 ± 2.33 | 0 (3/3) | Yes (3/3) | 0.9391 ± 0.0093 | 0.3424 ± 0.0128 | 0.6333 ± 0.0167 | 0.4871 ± 0.0029 |
| 16β,17β epoxide | 116.59 | 68.8 | 0 | Yes | 0.8657 | 0.265 | 0.5000 | 0.5123 |
| Intermediate Withanolides | 117.2 ± 7.08 | 68.55 ± 2.45 | 1 (2/2) | Yes (2/2) | 0.9489 ± 0.0204 | 0.2888 ± 0.0243 | 0.6250 ± 0.0500 | 0.5536 ± 0.0098 |
| Actinistins | 140.7 ± 24.08 | 60.50 | 0 (1/2); 2(1/2) | Yes (2/2) | 0.8943 ± 0.0528 | 0.2268 ± 0.0333 | 0.5125 ± 0.0876 | 0.4371 ± 0.0218 |
| Ixocarpalactones | 87.42 ± 25.38 | 78.83 ± 8.75 | 0 (1/3); 1 (2/3) | Yes (2/3); No (1/3) | 0.9471 ± 0.0423 | 0.3182 ± 0.0602 | 0.6833 ± 0.0417 | 0.4561 ± 0.0683 |
| PREDICTION TOOL | ||||||
|---|---|---|---|---|---|---|
| pkCSM | admetSAR | |||||
| Compound Group | Hepatotoxicity | hERG Inhibitor | Ames Mutagenicity | Hepatotoxicity | hERG Inhibition | Ames Mutagenicity |
| 5β,6β epoxides | Yes (1/16); No (15/16) | No (16/16) | Yes (1/16); No (15/16) | 0.5295 ± 0.0244 | 0.6403 ± 0.0250 | 0.4177 ± 0.0118 |
| 6α,7α epoxides | No (3/3) | No (3/3) | No (3/3) | 0.5884 ± 0.0252 | 0.6921 ± 0.0183 | 0.3131 ± 0.0060 |
| 16β,17β epoxide | No | No | No | 0.5717 | 0.5774 | 0.4100 |
| Intermediate Withanolides | No (2/2) | No (2/2) | No (2/2) | 0.6163 ± 0.0661 | 0.7452 ± 0.0801 | 0.3447 ± 0.0283 |
| Actinistins | No (2/2) | No (2/2) | No (2/2) | 0.5929 ± 0.0021 | 0.4366 ± 0.0246 | 0.3089 ± 0.0589 |
| Ixocarpalactones | No (3/3) | No (3/3) | No (3/3) | 0.5844 ± 0.0290 | 0.6722 ± 0.0525 | 0.2387 ± 0.0759 |
| admetSAR CYP Predictions | ||||||||
|---|---|---|---|---|---|---|---|---|
| Substrate | Inhibition | |||||||
| Compound Group | CYP3A4 Substrate | CYP2C9 Substrate | CYP2D6 Substrate | CYP3A4 Inhibition | CYP2C9 Inhibition | CYP2C19 Inhibition | CYP2D6 Inhibition | CYP1A2 Inhibition |
| 5β,6β epoxides | 0.7375 ± 0.0038 | 0 | 0.1026 ± 0.0010 | 0.1766 ± 0.0173 | 0.1323 ± 0.0062 | 0.1032 ± 0.0067 | 0.0486 ± 0.0012 | 0.2498 ± 0.0186 |
| 6α,7α epoxides | 0.7128 ± 0.0108 | 0 | 0.0921 ± 0.0005 | 0.2445 ± 0.0027 | 0.1190 ± 0.0112 | 0.1116 ± 0.0019 | 0.03907 ± 0.0025 | 0.2613 ± 0.0075 |
| 16β,17β epoxide | 0.7111 | 0 | 0.1016 | 0.2184 | 0.1072 | 0.0935 | 0.0495 | 0.1412 |
| Intermediate Withanolides | 0.7362 ± 0.0076 | 0 | 0.0902 ± 0.0070 | 0.1314 ± 0.0534 | 0.1188 ± 0.0145 | 0.1085 ± 0.0248 | 0.0661 ± 0.0174 | 0.3473 ± 0.1894 |
| Actinistins | 0.7196 ± 0.0032 | 0 | 0.1018 ± 0.0051 | 0.2853 ± 0.0287 | 0.1048 ± 0.0129 | 0.1254 ± 0.0111 | 0.0372 ± 0.0009 | 0.1902 ± 0.0195 |
| Ixocarpalactones | 0.7485 ± 0.0108 | 0.0607 ± 0.0607 | 0.1034 ± 0.0057 | 0.3099 ± 0.0438 | 0.0863 ± 0.0304 | 0.0867 ± 0.0324 | 0.0456 ± 0.0031 | 0.2653 ± 0.0607 |
| Prediction of Ecotoxicity | ||||||
|---|---|---|---|---|---|---|
| pkCSM | admetSAR | |||||
| Compound Group | Tetrahymena pyriformis Toxicity (log µg/L) | Minnow Toxicity (log mM) | Honey Bee Toxicity | Biodegradation | Crustacea Aquatic Toxicity | Fish Aquatic Toxicity |
| 5β,6β epoxides | 0.288 ± 0.002 | 2.350 ± 0.317 | 0.2830 ± 0.0150 | 0.1594 ± 0.0139 | 0.5281 ± 0.0195 | 0.9745 ± 0.0024 |
| 6α,7α epoxides | 0.290 ± 0.004 | 1.664 ± 0.3495 | 0.1777 ± 0.0082 | 0.1917 ± 0.0221 | 0.5967 ± 0.0433 | 0.9722 ± 0.0029 |
| 16β,17β epoxide | 0.285 | 1.663 | 0.2019 | 0.1000 | 0.4700 | 0.9730 |
| Intermediate Withanolides | 0.290 ± 0.004 | 1.956 ± 0.5645 | 0.2219 ± 0.0375 | 0.1500 ± 0.0250 | 0.5800 ± 0.0800 | 0.9906 ± 0.0013 |
| Actinistins | 0.285 ± 0.001 | 3.231 ± 0.3735 | 0.2507 ± 0.0328 | 0.2125 ± 0.0125 | 0.5900 ± 0.0100 | 0.9767 ± 0.0010 |
| Ixocarpalactones | 0.3193 ± 0.024 | 0.8740 ± 1.226 | 0.3357 ± 0.0204 | 0.2167 ± 0.0167 | 0.5800 ± 0.0577 | 0.9871 ± 0.0044 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, G.W.d.S.e.; Marques, A.M.; Sampaio, A.L.F. Anticancer Effects of Withanolides: In Silico Prediction of Pharmacological Properties. Molecules 2025, 30, 2457. https://doi.org/10.3390/molecules30112457
Silva GWdSe, Marques AM, Sampaio ALF. Anticancer Effects of Withanolides: In Silico Prediction of Pharmacological Properties. Molecules. 2025; 30(11):2457. https://doi.org/10.3390/molecules30112457
Chicago/Turabian StyleSilva, Gustavo Werneck de Souza e, André Mesquita Marques, and André Luiz Franco Sampaio. 2025. "Anticancer Effects of Withanolides: In Silico Prediction of Pharmacological Properties" Molecules 30, no. 11: 2457. https://doi.org/10.3390/molecules30112457
APA StyleSilva, G. W. d. S. e., Marques, A. M., & Sampaio, A. L. F. (2025). Anticancer Effects of Withanolides: In Silico Prediction of Pharmacological Properties. Molecules, 30(11), 2457. https://doi.org/10.3390/molecules30112457






