The Role of Lactoferrin in Combating Candida spp. Infections Through Regulation of Oxidative Stress, Immune Response, and Nutritional Support in Women and Newborns
Abstract
1. Introduction
2. Biological Activity of Lactoferrin and Its Mechanisms of Action in the Human Body
| Body Fluid | LF Concentration | Ref. | |
|---|---|---|---|
| Vaginal mucus | Before menstruation | 3.8–11.4 µg/mg | [17] |
| After menstruation | 62.9–218 µg/mg | [17] | |
| During contraception use | 19.8 µg/mg | [17] | |
| Amniotic fluid | Early pregnancy | 1–2 μg/ml | [22] |
| From the 32nd week of pregnancy until delivery | 5–15 μg/ml | [22] | |
| Cervical mucus plug | 10–1000 µg/ml | [22] | |
| Human colostrum | 6–7 g/L | [5,28] | |
| Mature human milk | 1–3 g/L | [5,28] | |
| Saliva | 10–100 µg/mL | [22] | |
| Blood | 10−3–200 µg/mL | [17] |
3. The Importance of Lactoferrin in Nutrition
4. Antifungal Functions of Lactoferrin: From Molecular Mechanisms to the Modulation of the Host Immune Response
5. Lactoferrin in Clinical Practice and the Treatment of Fungal Infections
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| 5-FC | Flucytosine |
| AMB | Amphotericin B |
| AMP | Antimicrobial peptide |
| Apo-LF | Iron-free form of lactoferrin |
| bCF | Bovine colostrum fortifier |
| bLf | Bovine lactoferrin |
| CAS | Caspofungin |
| CTZ | Clotrimazole |
| ELFIN trial | Enteral lactoferrin in neonates trial |
| FLC | Fluconazole |
| GM-CSF | Granulocyte–macrophage colony-stimulating factor |
| hLf | Human lactoferrin |
| Holo-LF | Iron-saturated form of lactoferrin |
| IL-6 | Interleukin 6 |
| ITZ | Itraconazole |
| LF | Lactoferrin |
| LOS | Late-onset sepsis |
| LPO | Lactoperoxidase |
| NEC | Necrotizing enterocolitis |
| NYS | Nystatin |
| rhLF | Recombinant human lactoferrin |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutases |
| TLR | Toll-like receptors |
| VRZ | Voriconazole |
References
- Benedict, K.; Singleton, A.L.; Jackson, B.R.; Molinari, N.A.M. Survey of Incidence, Lifetime Prevalence, and Treatment of Self-Reported Vulvovaginal Candidiasis, United States, 2020. BMC Women’s Health 2022, 22, 147. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.; Ferreras-Antolin, L.; Adhisivam, B.; Ballot, D.; Berkley, J.A.; Bernaschi, P.; Carvalheiro, C.G.; Chaikittisuk, N.; Chen, Y.; Chibabhai, V.; et al. Neonatal Invasive Candidiasis in Low-and Middle-Income Countries: Data from the NeoOBS Study. Med. Mycol. 2023, 61, myad010. [Google Scholar] [CrossRef] [PubMed]
- Willems, H.M.E.; Ahmed, S.S.; Liu, J.; Xu, Z.; Peters, B.M. Vulvovaginal Candidiasis: A Current Understanding and Burning Questions. J. Fungi 2020, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Liu, S.; Wang, H.; Su, H.; Liu, Z. Enhanced Antifungal Activity of Bovine Lactoferrin-Producing Probiotic Lactobacillus casei in the Murine Model of Vulvovaginal Candidiasis. BMC Microbiol. 2019, 19, 7. [Google Scholar] [CrossRef]
- Gawel, P.; Krolak-Olejnik, B. Lactoferrin Supplementation during Pregnancy—A Review of the Literature and Current Recommendations. Ginekol. Pol. 2023, 94, 570–580. [Google Scholar] [CrossRef]
- Dong, Z.; Fan, C.; Hou, W.; Rui, C.; Wang, X.; Fan, Y.; Zhao, L.; Wang, Q.; Wang, Z.; Zeng, X.; et al. Vaginal Exposure to Candida albicans During Early Gestation Results in Adverse Pregnancy Outcomes via Inhibiting Placental Development. Front. Microbiol. 2022, 12, 816161. [Google Scholar] [CrossRef]
- Maftei, N.-M.; Arbune, M.; Georgescu, C.V.; Elisei, A.M.; Iancu, A.V.; Tatu, A.L. Vulvovaginal Candidiasis in Pregnancy—Between Sensitivity and Resistance to Antimycotics. J. Xenobiotics 2023, 13, 312–322. [Google Scholar] [CrossRef]
- Payne, M.S.; Ireland, D.J.; Watts, R.; Nathan, E.A.; Furfaro, L.L.; Kemp, M.W.; Keelan, J.A.; Newnham, J.P. Ureaplasma parvum Genotype, Combined Vaginal Colonisation with Candida albicans, and Spontaneous Preterm Birth in an Australian Cohort of Pregnant Women. BMC Pregnancy Childbirth 2016, 16, 312. [Google Scholar] [CrossRef]
- Hemedez, C.; Trail-Burns, E.; Mao, Q.; Chu, S.; Shaw, S.K.; Bliss, J.M.; De Paepe, M.E. Pathology of Neonatal Non-Albicans Candidiasis: Autopsy Study and Literature Review. Pediatr. Dev. Pathol. 2019, 22, 98–105. [Google Scholar] [CrossRef]
- Barton, M.; Shen, A.; O’Brien, K.; Robinson, J.L.; Davies, H.D.; Simpson, K.; Asztalos, E.; Langley, J.; Le Saux, N.; Sauve, R.; et al. Early-Onset Invasive Candidiasis in Extremely Low Birth Weight Infants: Perinatal Acquisition Predicts Poor Outcome. Clin. Infect. Dis. 2017, 64, 921–927. [Google Scholar] [CrossRef]
- Talapko, J.; Juzbašić, M.; Matijević, T.; Pustijanac, E.; Bekić, S.; Kotris, I.; Škrlec, I. Candida albicans—The Virulence Factors and Clinical Manifestations of Infection. J. Fungi 2021, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Neuman-Łaniece, M. Management of Oral Candidiasis in Children—The General Practitioner’s Approach. Terapia 2016, 3, 65–67. [Google Scholar]
- Vainionpää, A.; Tuomi, J.; Kantola, S.; Anttonen, V. Neonatal Thrush of Newborns: Oral Candidiasis? Clin. Exp. Dent. Res. 2019, 5, 580–582. [Google Scholar] [CrossRef]
- Plachouri, K.-M.; Mulita, F.; Oikonomou, C.; Papadopoulou, M.; Akrida, I.; Vryzaki, E.; Verras, G.-I.; Georgiou, S. Nipple Candidiasis and Painful Lactation: An Updated Overview. Adv. Dermatol. Allergol. 2022, 39, 651–655. [Google Scholar] [CrossRef]
- Artym, J. Lactoferrin—A Sensor and Regulator of Iron Absorption. Postępy Biol. Komórki 2015, 2, 283–308. [Google Scholar]
- Fernandes, K.E.; Weeks, K.; Carter, D.A. Lactoferrin Is Broadly Active against Yeasts and Highly Synergistic with Amphotericin B. Antimicrob. Agents Chemother. 2020, 64, 10–1128. [Google Scholar] [CrossRef]
- De Felipe, L.O.; da Silva Júnior, W.F.; de Araújo, K.C.; Fabrino, D.L. Lactoferrin, Chitosan and Melaleuca alternifolia—Natural Products That Show Promise in Candidiasis Treatment. Braz. J. Microbiol. 2018, 49, 212–219. [Google Scholar] [CrossRef]
- Rascón-Cruz, Q.; Siqueiros-Cendón, T.S.; Siañez-Estrada, L.I.; Villaseñor-Rivera, C.M.; Ángel-Lerma, L.E.; Olivas-Espino, J.A.; León-Flores, D.B.; Espinoza-Sánchez, E.A.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F. Antioxidant Potential of Lactoferrin and Its Protective Effect on Health: An Overview. Int. J. Mol. Sci. 2024, 26, 125. [Google Scholar] [CrossRef]
- Alexander, D.B.; Iigo, M.; Yamauchi, K.; Suzui, M.; Tsuda, H. Lactoferrin: An Alternative View of Its Role in Human Biological Fluids. Biochem. Cell Biol. 2012, 90, 279–306. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Park, G.-T.; Cho, I.H.; Sim, S.-M.; Yang, J.-M.; Lee, D.-Y. An Antimicrobial Protein, Lactoferrin Exists in the Sweat: Proteomic Analysis of Sweat. Exp. Dermatol. 2011, 20, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.P.; Paz, E.; Conneely, O.M. Multifunctional Roles of Lactoferrin: A Critical Overview. Cell. Mol. Life Sci. 2005, 62, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- Artym, J.; Zimecki, M. Antimicrobial and Prebiotic Activity of Lactoferrin in the Female Reproductive Tract: A Comprehensive Review. Biomedicines 2021, 9, 1940. [Google Scholar] [CrossRef] [PubMed]
- Ohradanova-Repic, A.; Praženicová, R.; Gebetsberger, L.; Moskalets, T.; Skrabana, R.; Cehlar, O.; Tajti, G.; Stockinger, H.; Leksa, V. Time to Kill and Time to Heal: The Multifaceted Role of Lactoferrin and Lactoferricin in Host Defense. Pharmaceutics 2023, 15, 1056. [Google Scholar] [CrossRef] [PubMed]
- Rascón-Cruz, Q.; Espinoza-Sánchez, E.A.; Siqueiros-Cendón, T.S.; Nakamura-Bencomo, S.I.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F. Lactoferrin: A Glycoprotein Involved in Immunomodulation, Anticancer, and Antimicrobial Processes. Molecules 2021, 26, 205. [Google Scholar] [CrossRef]
- Curran, C.S.; Demick, K.P.; Mansfield, J.M. Lactoferrin Activates Macrophages via TLR4-Dependent and -Independent Signaling Pathways. Cell. Immunol. 2006, 242, 23–30. [Google Scholar] [CrossRef]
- de la Rosa, G.; Yang, D.; Tewary, P.; Varadhachary, A.; Oppenheim, J.J. Lactoferrin Acts as an Alarmin to Promote the Recruitment and Activation of APCs and Antigen-Specific Immune Responses. J. Immunol. 2008, 180, 6868–6876. [Google Scholar] [CrossRef]
- Otsuki, K.; Nishi, T.; Kondo, T.; Okubo, K. Review, Role of Lactoferrin in Preventing Preterm Delivery. BioMetals 2023, 36, 521–530. [Google Scholar] [CrossRef]
- Chen, K.; Jin, S.; Chen, H.; Cao, Y.; Dong, X.; Li, H.; Zhou, Z.; Liu, C. Dose Effect of Bovine Lactoferrin Fortification on Diarrhea and Respiratory Tract Infections in Weaned Infants with Anemia: A Randomized, Controlled Trial. Nutrition 2021, 90, 111288. [Google Scholar] [CrossRef]
- Andersson, Y.; Lindquist, S.; Lagerqvist, C.; Hernell, O. Lactoferrin Is Responsible for the Fungistatic Effect of Human Milk. Early Hum. Dev. 2000, 59, 95–105. [Google Scholar] [CrossRef]
- Perrin, M.T.; Fogleman, A.D.; Newburg, D.S.; Allen, J.C. A Longitudinal Study of Human Milk Composition in the Second Year Postpartum: Implications for Human Milk Banking. Matern. Child Nutr. 2017, 13, e12239. [Google Scholar] [CrossRef]
- Dombrowska-Pali, A.; Wiktorczyk-Kapischke, N.; Chrustek, A.; Olszewska-Słonina, D.; Gospodarek-Komkowska, E.; Socha, M.W. Human Milk Microbiome—A Review of Scientific Reports. Nutrients 2024, 16, 1420. [Google Scholar] [CrossRef] [PubMed]
- Miliku, K.; Azad, M. Breastfeeding and the Developmental Origins of Asthma: Current Evidence, Possible Mechanisms, and Future Research Priorities. Nutrients 2018, 10, 995. [Google Scholar] [CrossRef] [PubMed]
- Amitay, E.L.; Raz, G.D.; Keinan-Boker, L. Breastfeeding, Other Early Life Exposures and Childhood Leukemia and Lymphoma. Nutr. Cancer 2016, 68, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.L.; Gamborg, M.; Heitmann, B.L.; Lissner, L.; Sørensen, T.I.A.; Rasmussen, K.M. Breastfeeding Reduces Postpartum Weight Retention. Am. J. Clin. Nutr. 2008, 88, 1543–1551. [Google Scholar] [CrossRef]
- Calik-Ksepka, A.; Stradczuk, M.; Czarnecka, K.; Grymowicz, M.; Smolarczyk, R. Lactational Amenorrhea: Neuroendocrine Pathways Controlling Fertility and Bone Turnover. Int. J. Mol. Sci. 2022, 23, 1633. [Google Scholar] [CrossRef]
- Nagel, E.M.; Howland, M.A.; Pando, C.; Stang, J.; Mason, S.M.; Fields, D.A.; Demerath, E.W. Maternal Psychological Distress and Lactation and Breastfeeding Outcomes: A Narrative Review. Clin. Ther. 2022, 44, 215–227. [Google Scholar] [CrossRef]
- Amelia, R.; Masrul, M.; Sriyanti, R. The Effect of Breastfeeding on The Uterine Involution Post Partum Mothers. World J. Res. Rev. 2019, 8, 1–3. [Google Scholar] [CrossRef]
- Ip, S.; Chung, M.; Raman, G.; Trikalinos, T.A.; Lau, J. A Summary of the Agency for Healthcare Research and Quality’s Evidence Report on Breastfeeding in Developed Countries. Breastfeed. Med. 2009, 4, S-17–S-30. [Google Scholar] [CrossRef]
- Bjørnerem, Å.; Ahmed, L.A.; Jørgensen, L.; Størmer, J.; Joakimsen, R.M. Breastfeeding Protects against Hip Fracture in Postmenopausal Women: The Tromsø Study. J. Bone Miner. Res. 2011, 26, 2843–2850. [Google Scholar] [CrossRef]
- Stuebe, A. Associations Among Lactation, Maternal Carbohydrate Metabolism, and Cardiovascular Health. Clin. Obstet. Gynecol. 2015, 58, 827–839. [Google Scholar] [CrossRef]
- Zinaliyeva, A.N.; Tulegenova, G.A.; Zhelisbayeva, K.R.; Akhmetzhanova, M.B. The Role of Breastfeeding and Formula Feeding in Infant Health and Development: A Mini Review. J. Environ. Treat. Tech. 2024, 12, 30–38. [Google Scholar] [CrossRef]
- Telang, S. Lactoferrin: A Critical Player in Neonatal Host Defense. Nutrients 2018, 10, 1228. [Google Scholar] [CrossRef]
- Bialic, K.; Bialic, A.; Bakalarczyk, R.; Majewski, P.; Bednarz, L.; Szala-Czerwonka, K.; Woś, N.; Rejmer, A.; Rojek, K.; Wijas, K. Exploring the Potential of Lactoferrin in Neonatology and Obstetrics—Promising Advancements for Maternal and Infant Health. J. Educ. Health Sport 2023, 34, 34–50. [Google Scholar] [CrossRef]
- Baker, E.N.; Baker, H.M. A Structural Framework for Understanding the Multifunctional Character of Lactoferrin. Biochimie 2009, 91, 3–10. [Google Scholar] [CrossRef]
- King, J.C.; Cummings, G.E.; Guo, N.; Trivedi, L.; Readmond, B.X.; Keane, V.; Feigelman, S.; de Waard, R. A Double-Blind, Placebo-Controlled, Pilot Study of Bovine Lactoferrin Supplementation in Bottle-fed Infants. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 245–251. [Google Scholar] [CrossRef]
- Akin, I.; Atasay, B.; Dogu, F.; Okulu, E.; Arsan, S.; Karatas, H.; Ikinciogullari, A.; Turmen, T. Oral Lactoferrin to Prevent Nosocomial Sepsis and Necrotizing Enterocolitis of Premature Neonates and Effect on T-Regulatory Cells. Am. J. Perinatol. 2014, 31, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P. Clinical Benefits of Lactoferrin for Infants and Children. J. Pediatr. 2016, 173, S43–S52. [Google Scholar] [CrossRef]
- He, Y.; Cao, L.; Yu, J. Prophylactic Lactoferrin for Preventing Late-Onset Sepsis and Necrotizing Enterocolitis in Preterm Infants. Medicine 2018, 97, e11976. [Google Scholar] [CrossRef]
- Griffiths, J.; Jenkins, P.; Vargova, M.; Bowler, U.; Juszczak, E.; King, A.; Linsell, L.; Murray, D.; Partlett, C.; Patel, M.; et al. Enteral Lactoferrin Supplementation for Very Preterm Infants: A Randomised Placebo-Controlled Trial. Lancet 2019, 393, 423–433. [Google Scholar] [CrossRef]
- Supranoto, Y.T.N.; Tanuwijaya, D.C.D. Meta-Analysis of Enteral Lactoferrin Supplementation for Reducing the Risk of Preterm Infants Sepsis. Smart Med. J. 2022, 5, 78. [Google Scholar] [CrossRef]
- Gao, X.; Li, Y.; Olin, A.B.; Nguyen, D.N. Fortification with Bovine Colostrum Enhances Antibacterial Activity of Human Milk. J. Parenter. Enter. Nutr. 2021, 45, 1417–1424. [Google Scholar] [CrossRef]
- Zarzosa-Moreno, D.; Avalos-Gómez, C.; Ramírez-Texcalco, L.S.; Torres-López, E.; Ramírez-Mondragón, R.; Hernández-Ramírez, J.O.; Serrano-Luna, J.; de la Garza, M. Lactoferrin and Its Derived Peptides: An Alternative for Combating Virulence Mechanisms Developed by Pathogens. Molecules 2020, 25, 5763. [Google Scholar] [CrossRef]
- Gajda-Morszewski, P.; Śpiewak, K. Lactoferrin—A Multipotential Protein. Sci. J. Dr. Stud. Assoc. Jagiellonian Univ. Nat. Sci. 2015, 10, 177–188. [Google Scholar]
- Vogel, H.J.; Schibli, D.J.; Jing, W.; Lohmeier-Vogel, E.M.; Epand, R.F.; Epand, R.M. Towards a Structure-Function Analysis of Bovine Lactoferricin and Related Tryptophan- and Arginine-Containing Peptides. Biochem. Cell Biol. 2002, 80, 49–63. [Google Scholar] [CrossRef]
- Krupińska, A.M.; Bogucki, Z. Lactoferrin as a Potential Therapeutic for the Treatment of Candida-Associated Denture Stomatitis. J. Oral Biosci. 2024, 66, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, M.; Teixeira, M.C. Candida Biofilms: Threats, Challenges, and Promising Strategies. Front. Med. 2018, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Morici, P.; Fais, R.; Rizzato, C.; Tavanti, A.; Lupetti, A. Inhibition of Candida albicans Biofilm Formation by the Synthetic Lactoferricin Derived Peptide HLF1-11. PLoS ONE 2016, 11, e0167470. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Rosa, L.; Capobianco, D.; Lepanto, M.S.; Schiavi, E.; Cutone, A.; Paesano, R.; Mastromarino, P. Role of Lactobacilli and Lactoferrin in the Mucosal Cervicovaginal Defense. Front. Immunol. 2018, 9, 376. [Google Scholar] [CrossRef]
- Sherrington, S.L.; Sorsby, E.; Mahtey, N.; Kumwenda, P.; Lenardon, M.D.; Brown, I.; Ballou, E.R.; MacCallum, D.M.; Hall, R.A. Adaptation of Candida Albicans to Environmental PH Induces Cell Wall Remodelling and Enhances Innate Immune Recognition. PLoS Pathog. 2017, 13, e1006403. [Google Scholar] [CrossRef]
- Soukka, T. Fungicidal Effect of Human Lactoferrin against Candida albicans. FEMS Microbiol. Lett. 1992, 90, 223–228. [Google Scholar] [CrossRef][Green Version]
- Fais, R.; Rizzato, C.; Franconi, I.; Tavanti, A.; Lupetti, A. Synergistic Activity of the Human Lactoferricin-Derived Peptide HLF1-11 in Combination with Caspofungin against Candida Species. Microbiol. Spectr. 2022, 10, e01240-22. [Google Scholar] [CrossRef] [PubMed]
- Dantas, A.; Day, A.; Ikeh, M.; Kos, I.; Achan, B.; Quinn, J. Oxidative Stress Responses in the Human Fungal Pathogen, Candida albicans. Biomolecules 2015, 5, 142–165. [Google Scholar] [CrossRef] [PubMed]
- Tanida, T.; Rao, F.; Hamada, T.; Ueta, E.; Osaki, T. Lactoferrin Peptide Increases the Survival of Candida albicans—Inoculated Mice by Upregulating Neutrophil and Macrophage Functions, Especially in Combination with Amphotericin B and Granulocyte-Macrophage Colony-Stimulating Factor. Infect. Immun. 2001, 69, 3883–3890. [Google Scholar] [CrossRef]
- Nakano, M.; Suzuki, M.; Wakabayashi, H.; Hayama, K.; Yamauchi, K.; Abe, F.; Abe, S. Synergistic Anti-Candida Activities of Lactoferrin and the Lactoperoxidase System. Drug Discov. Ther. 2019, 13, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Y.; Samaramayake, Y.H.; Samaramayake, L.P.; Nikawa, H. In Vitro Susceptibility of Candida Species to Lactoferrin. Med. Mycol. 1999, 37, 35–41. [Google Scholar] [CrossRef]
- Samaranayake, Y.H.; Samaranayake, L.P.; Wu, P.C.; So, M. The Antifungal Effect of Lactoferrin and Lysozyme on Candida krusei and Candida albicans. APMIS 1997, 105, 875–883. [Google Scholar] [CrossRef]
- Cianci, A. Influence of Lactoferrin in Preventing Preterm Delivery: A Pilot Study. Mol. Med. Rep. 2011, 5, 162–166. [Google Scholar] [CrossRef]
- Manzoni, P.; Stolfi, I.; Messner, H.; Cattani, S.; Laforgia, N.; Romeo, M.G.; Bollani, L.; Rinaldi, M.; Gallo, E.; Quercia, M.; et al. Bovine Lactoferrin Prevents Invasive Fungal Infections in Very Low Birth Weight Infants: A Randomized Controlled Trial. Pediatrics 2012, 129, 116–123. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Li, Q.; Zhong, W.; Zou, H. The Increasing Trend of Triazole-Resistant Candida from Vulvovaginal Candidiasis. Infect. Drug Resist. 2024, 17, 4301–4310. [Google Scholar] [CrossRef]
- Patel, M.A.; Aliporewala, V.M.; Patel, D.A. Common Antifungal Drugs in Pregnancy: Risks and Precautions. J. Obstet. Gynecol. India 2021, 71, 577–582. [Google Scholar] [CrossRef]
- Farr, A.; Effendy, I.; Frey Tirri, B.; Hof, H.; Mayser, P.; Petricevic, L.; Ruhnke, M.; Schaller, M.; Schaefer, A.P.A.; Sustr, V.; et al. Guideline: Vulvovaginal Candidosis (AWMF 015/072, Level S2k). Mycoses 2021, 64, 583–602. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, M.; McMullan, P.; Truong, T.M.; Rothe, M.; Murase, J.; Grant-Kels, J.M. Safety of Dermatologic Medications in Pregnancy and Lactation: An Update—Part II: Lactation. J. Am. Acad. Dermatol. 2024, 91, 651–668. [Google Scholar] [CrossRef]
- Dermitzaki, N.; Balomenou, F.; Gialamprinou, D.; Giapros, V.; Rallis, D.; Baltogianni, M. Perspectives on the Use of Echinocandins in the Neonatal Intensive Care Unit. Antibiotics 2024, 13, 1209. [Google Scholar] [CrossRef] [PubMed]
- Danesi, R.; Senesi, S.; Wout, J.W.V.; van Dissel, J.T.; Lupetti, A.; Nibbering, P.H. Antimicrobial Peptides: Therapeutic Potential for the Treatment of Candida Infections. Expert Opin. Investig. Drugs 2002, 11, 309–318. [Google Scholar] [CrossRef]
- Haney, E.F.; Nazmi, K.; Bolscher, J.G.M.; Vogel, H.J. Structural and Biophysical Characterization of an Antimicrobial Peptide Chimera Comprised of Lactoferricin and Lactoferrampin. Biochim. Biophys. Acta-Biomembr. 2012, 1818, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Abe, S.; Okutomi, T.; Tansho, S.; Kawase, K.; Yamaguchi, H. Cooperative Anti-Candida Effects of Lactoferrin or Its Peptides in Combination with Azole Antifungal Agents. Microbiol. Immunol. 1996, 40, 821–825. [Google Scholar] [CrossRef]
- Kuipers, M.E.; de Vries, H.G.; Eikelboom, M.C.; Meijer, D.K.F.; Swart, P.J. Synergistic Fungistatic Effects of Lactoferrin in Combination with Antifungal Drugs against Clinical Candida Isolates. Antimicrob. Agents Chemother. 1999, 43, 2635–2641. [Google Scholar] [CrossRef]
- Lupetti, A.; Paulusma-Annema, A.; Welling, M.M.; Dogterom-Ballering, H.; Brouwer, C.P.J.M.; Senesi, S.; van Dissel, J.T.; Nibbering, P.H. Synergistic Activity of the N-Terminal Peptide of Human Lactoferrin and Fluconazole against Candida Species. Antimicrob. Agents Chemother. 2003, 47, 262–267. [Google Scholar] [CrossRef]
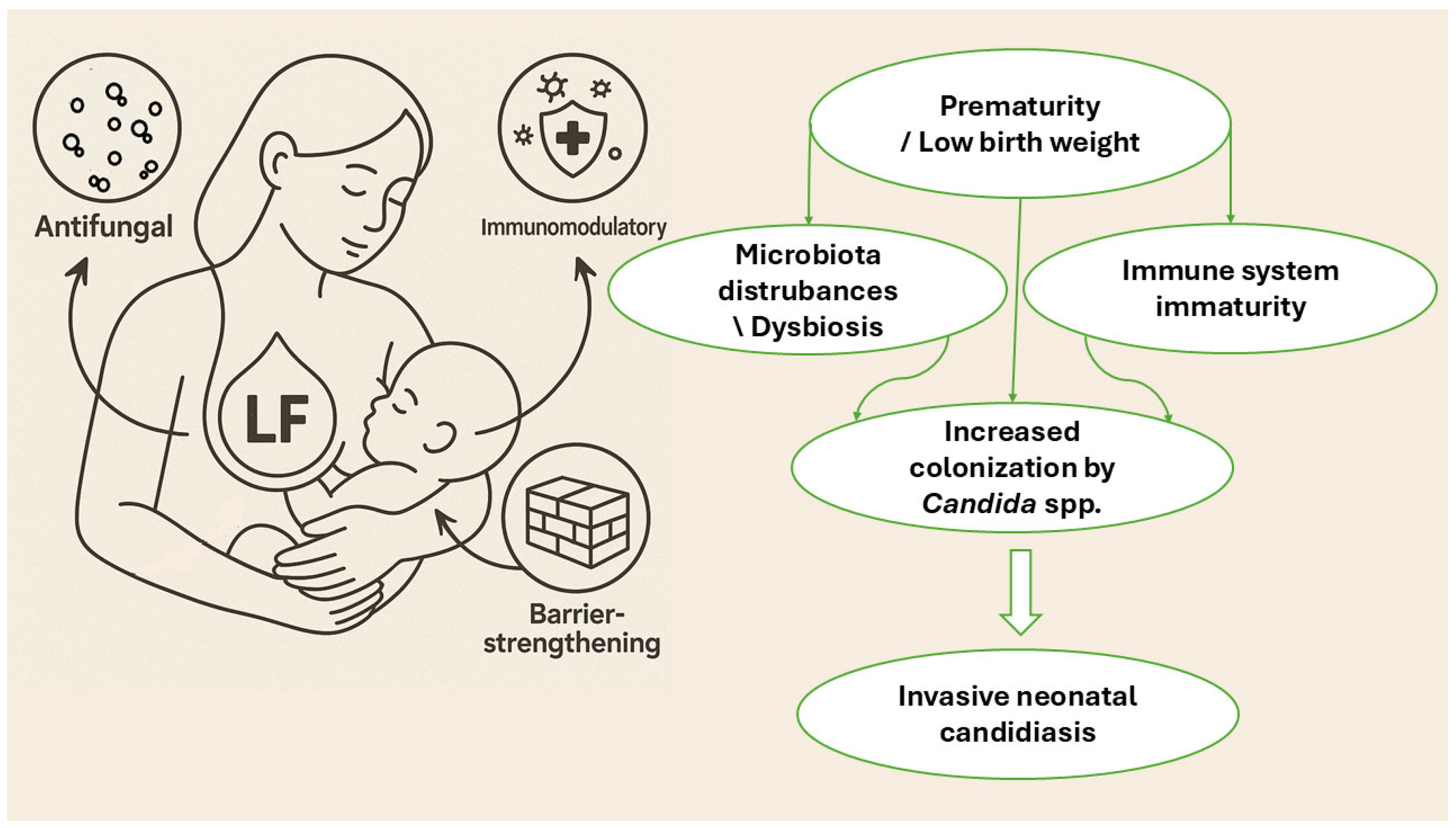
| Compound | Form and Origin | Target Pathogens | Synergistic Effect | Planktonic and Biofilm Forms | Ref. |
|---|---|---|---|---|---|
| LF | Intact bLf (whole protein) | C. albicans (including azole-sensitive and -resistant strains) | Synergistic with CTZ; also effective with FLC and ITZ | Planktonic only | [76] |
| LF-B | Isolated peptide from the N-terminal region of LF | C. albicans (including azole-sensitive and -resistant strains) | Synergistic with CTZ | Planktonic only | [76] |
| LFhyd | Enzymatically digested lactoferrin (mixture of peptides) | C. albicans (including azole-sensitive and -resistant strains) | Synergy with azole | Planktonic only | [76] |
| LF | Dairy-derived bLf (partially digested, low iron saturation) | C. albicans and C. glabrata | Strong synergy with AMB | Reduces hyphal growth; prevents biofilm formation; less effective against mature biofilm | [16] |
| hLF1-11 | Synthetic peptide from human LF (N-terminal region) | C. albicans, C. glabrata, C. tropicalis, and C. parapsilosis | Strong synergy with CAS | Active against planktonic cells; prevents biofilm formation; reduces metabolic activity of mature biofilm (fungistatic) | [61] |
| LF | Apo-lactoferrin | C. albicans, C. glabrata, and C. tropicalis | Synergistic with FLC; synergistic with 5-FC | Planktonic only | [77] |
| LF | Native bLf | C. albicans, C. glabrata, and C. tropicalis | Synergistic with FLC | Planktonic only | [77] |
| hLF(1-11) | Synthetic N-terminal peptide from hLF | C. albicans (FLC-sensitive and FLC- resistant), C. glabrata, C. krusei, C. tropicalis, and C. parapsilosis | Synergistic with FLC | Planktonic only | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Długosz, A.; Wróblewska, J.; Kołaczyk, P.; Wróblewska, W. The Role of Lactoferrin in Combating Candida spp. Infections Through Regulation of Oxidative Stress, Immune Response, and Nutritional Support in Women and Newborns. Molecules 2025, 30, 2416. https://doi.org/10.3390/molecules30112416
Długosz A, Wróblewska J, Kołaczyk P, Wróblewska W. The Role of Lactoferrin in Combating Candida spp. Infections Through Regulation of Oxidative Stress, Immune Response, and Nutritional Support in Women and Newborns. Molecules. 2025; 30(11):2416. https://doi.org/10.3390/molecules30112416
Chicago/Turabian StyleDługosz, Anna, Joanna Wróblewska, Paweł Kołaczyk, and Weronika Wróblewska. 2025. "The Role of Lactoferrin in Combating Candida spp. Infections Through Regulation of Oxidative Stress, Immune Response, and Nutritional Support in Women and Newborns" Molecules 30, no. 11: 2416. https://doi.org/10.3390/molecules30112416
APA StyleDługosz, A., Wróblewska, J., Kołaczyk, P., & Wróblewska, W. (2025). The Role of Lactoferrin in Combating Candida spp. Infections Through Regulation of Oxidative Stress, Immune Response, and Nutritional Support in Women and Newborns. Molecules, 30(11), 2416. https://doi.org/10.3390/molecules30112416






