Investigation of Mechanochromic and Solvatochromic Luminescence of Cyclometalated Heteroleptic Platinum(II) Complexes with Benzoylthiourea Derivatives
Abstract
1. Introduction
2. Results and Discussion
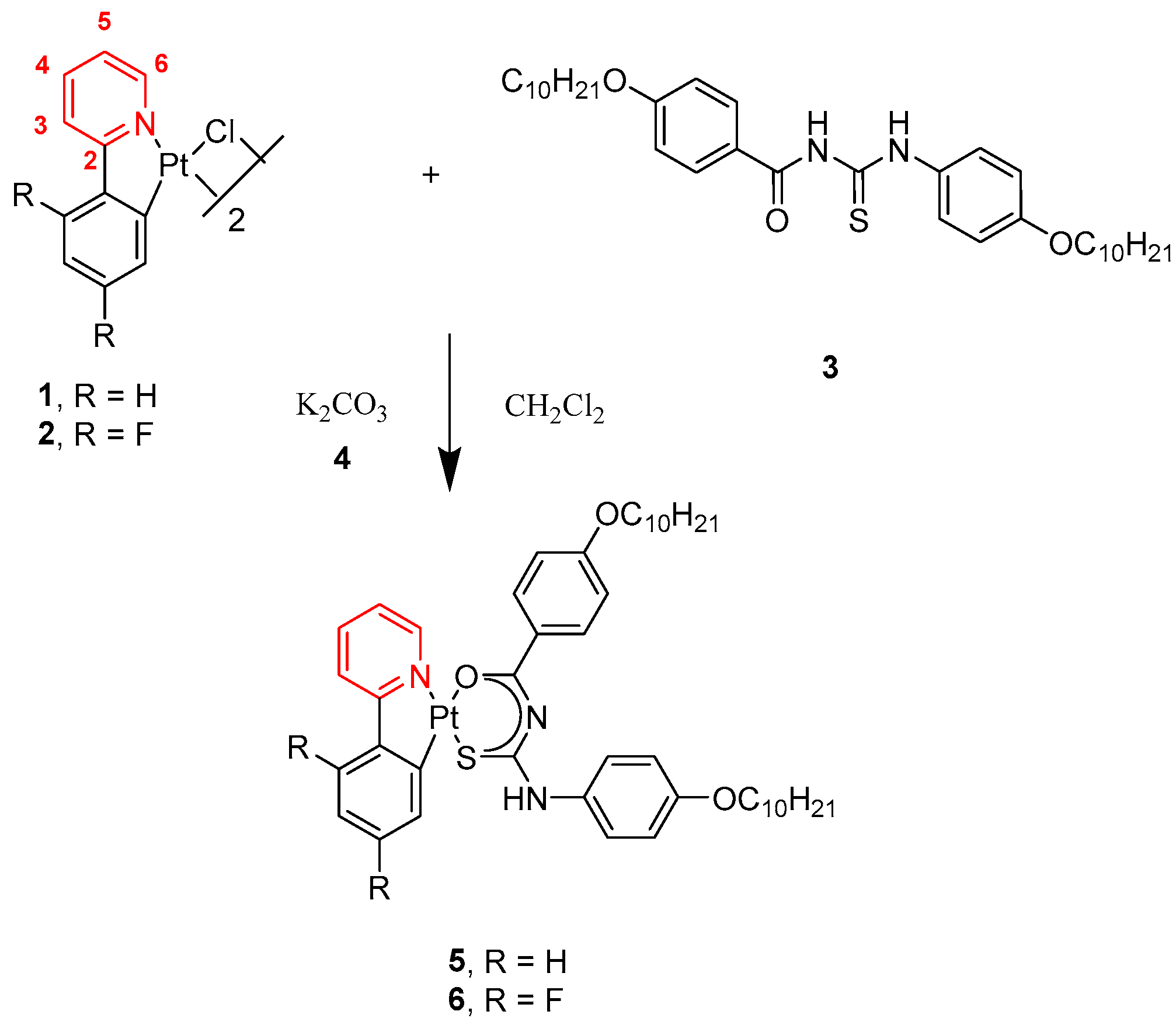
2.1. Synthesis and Structure of Pt Complexes
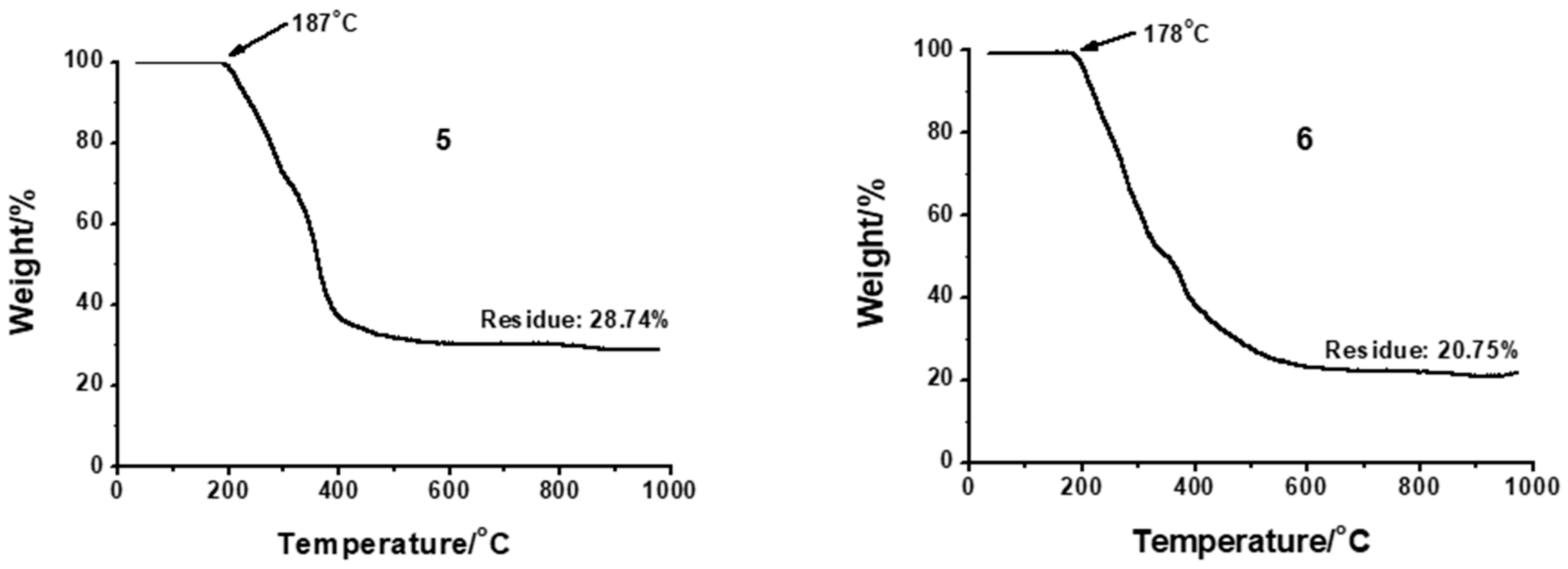
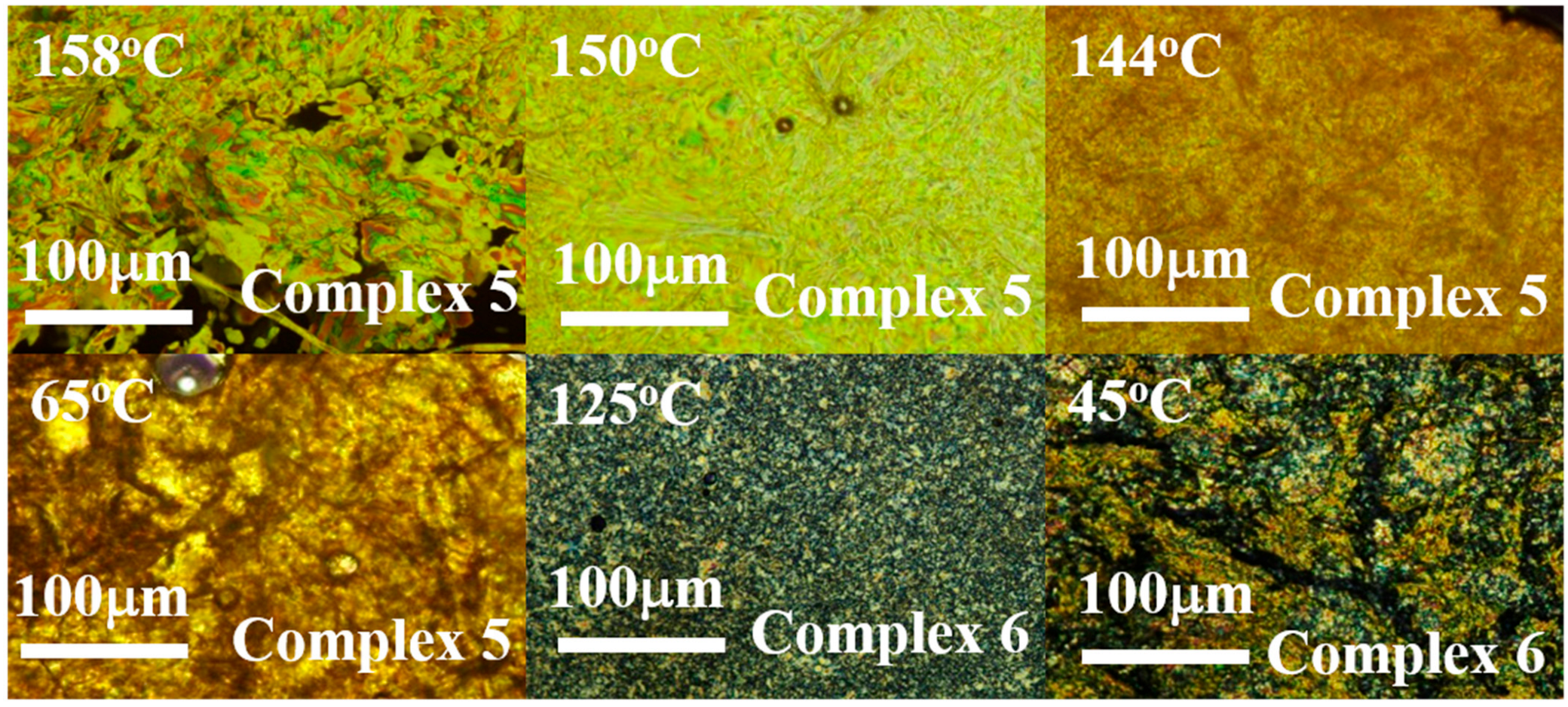
2.2. Thermal Characterization of Pt Complexes
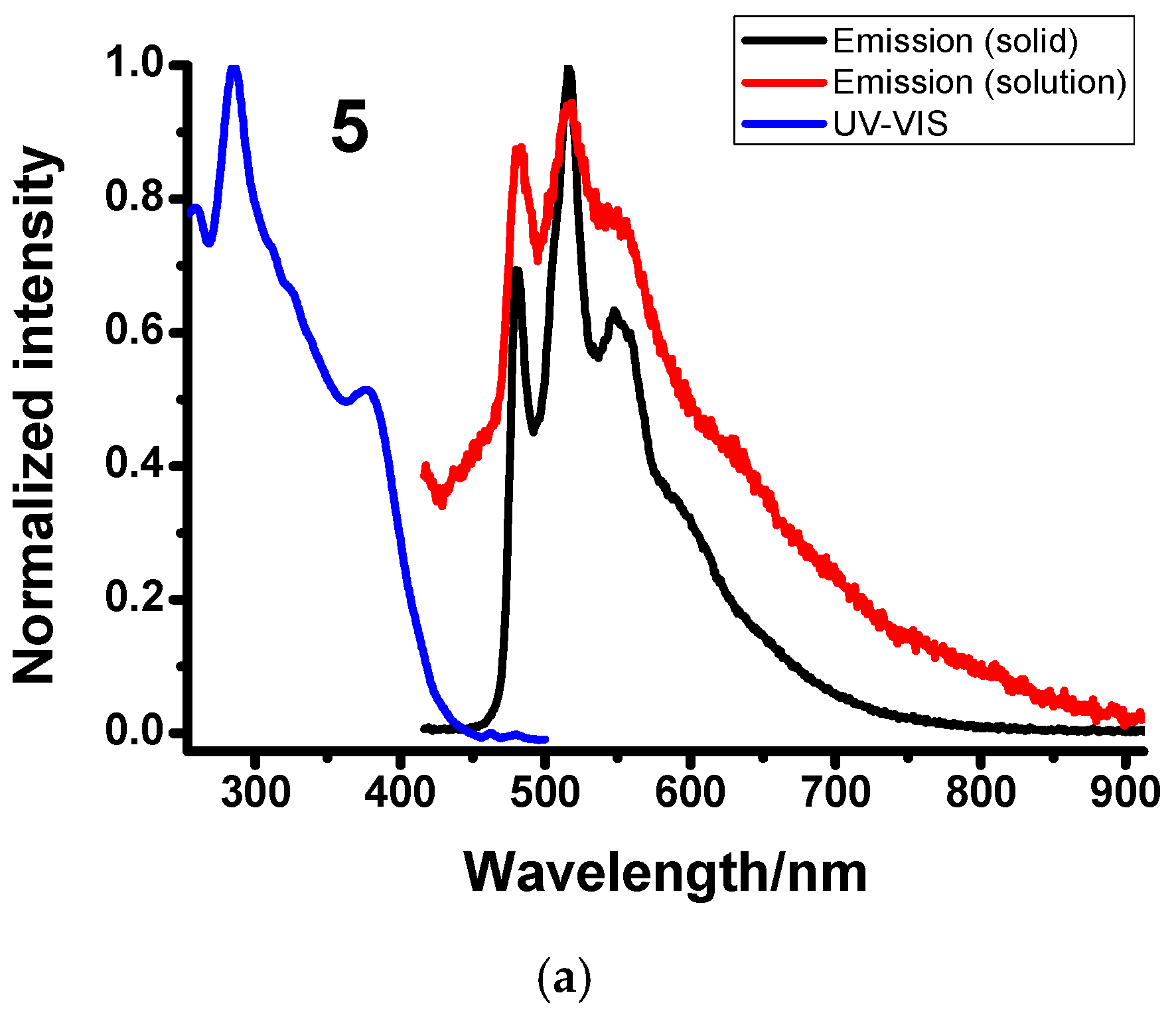
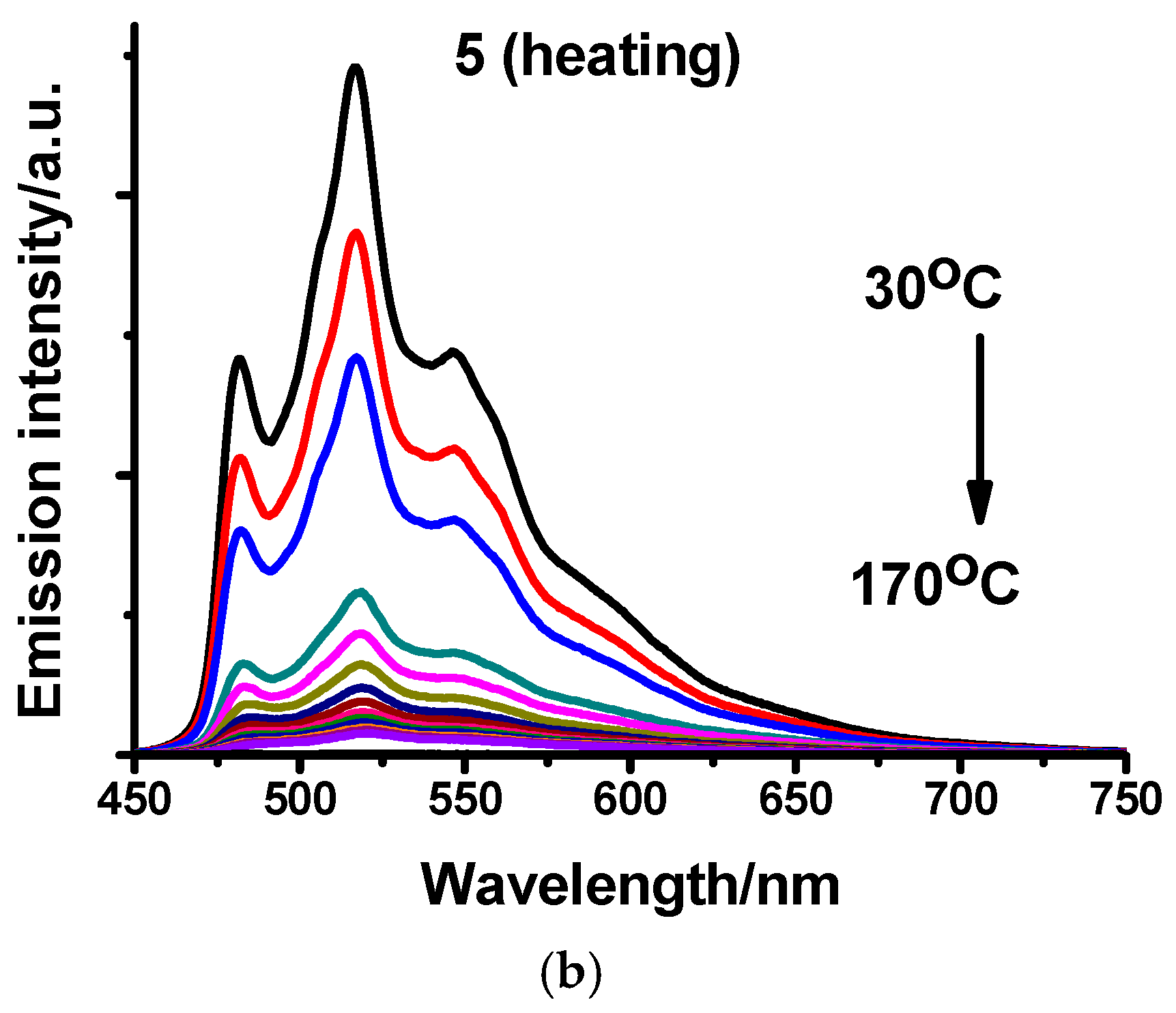
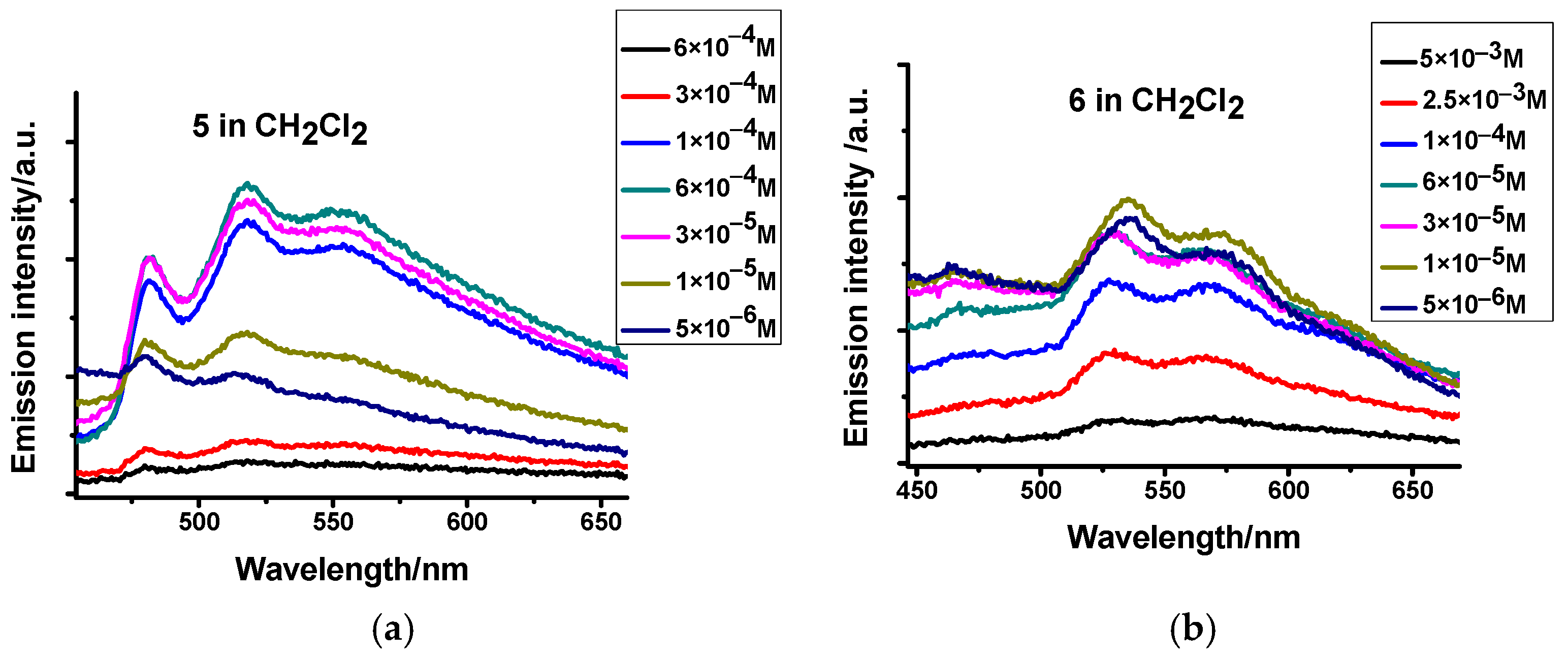
2.3. Photophysical Characterization
3. Materials and Methods
3.1. Materials and Characterization
3.2. Mechanochromic and Solvatochromic Tests
3.3. Chemical Synthesis of the Pt(II) Complexes 5 and 6
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.Y.; Lv, L.; Sun, L.; Hou, Y.; Hou, Z.; Chen, Z. Recent advances in mechanochromism of metal-organic compounds. Front. Chem. 2022, 10, 865198. [Google Scholar] [CrossRef]
- Xue, P.; Ding, J.; Wang, P.; Lu, R. Recent progress in the mechanochromism of phosphorescent organic molecules and metal complexes. J. Mater. Chem. C 2016, 4, 6688–6706. [Google Scholar] [CrossRef]
- Soto, M.A.; Kandel, R.; MacLachlan, M.J. Chromic platinum complexes containing multidentate ligands. Eur. J. Inorg. Chem. 2021, 2021, 894–906. [Google Scholar] [CrossRef]
- Kato, M. Chromic soft crystals based on luminescent platinum (II) complexes. IUCrJ 2024, 11, 442–452. [Google Scholar] [CrossRef]
- Bryant, M.J.; Skelton, J.M.; Hatcher, L.E.; Stubbs, C.; Madrid, E.; Pallipurath, A.R.; Thomas, L.H.; Woodall, C.H.; Christensen, J.; Fuertes, S. A rapidly-reversible absorptive and emissive vapochromic Pt(II) pincer-based chemical sensor. Nat. Commun. 2017, 8, 1800. [Google Scholar] [CrossRef] [PubMed]
- Wenger, O.S. Vapochromism in organometallic and coordination complexes: Chemical sensors for volatile organic compounds. Chem. Rev. 2013, 113, 3686–3733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, B.; Chen, Z.-H.; Chen, Z.-N. Luminescence vapochromism in solid materials based on metal complexes for detection of volatile organic compounds (VOCs). J. Mater. Chem. 2012, 22, 11427–11441. [Google Scholar] [CrossRef]
- Zhang, X.; Chi, Z.; Zhang, Y.; Liu, S.; Xu, J. Recent advances in mechanochromic luminescent metal complexes. J. Mater. Chem. C 2013, 1, 3376–3390. [Google Scholar] [CrossRef]
- Zhang, H.-H.; Wu, S.-X.; Wang, Y.-Q.; Xie, T.-G.; Sun, S.-S.; Liu, Y.-L.; Han, L.-Z.; Zhang, X.-P.; Shi, Z.-F. Mechanochromic luminescent property and anti-counterfeiting application of AIE-active cyclometalated platinum(II) complexes featuring a fused five-six-membered metallacycle. Dyes Pigments 2022, 197, 109857. [Google Scholar] [CrossRef]
- Haque, A.; Xu, L.; Al-Balushi, R.A.; Al-Suti, M.K.; Ilmi, R.; Guo, Z.; Khan, M.S.; Wong, W.-Y.; Raithby, P.R. Cyclometallated tridentate platinum(II) arylacetylide complexes: Old wine in new bottles. Chem. Soc. Rev. 2019, 48, 5547–5563. [Google Scholar] [CrossRef]
- Kalinowski, J.; Fattori, V.; Cocchi, M.; Williams, J.A.G. Light-emitting devices based on organometallic platinum complexes as emitters. Coord. Chem. Rev. 2011, 255, 2401–2425. [Google Scholar] [CrossRef]
- Kaswan, P.; Yadav, M.; Dhotre, S.; Minakshi, M.; Mehta, S. Applications of luminescent cyclometalated metal complexes as sensors and switches. Synlett 2025, 36, 921–943. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Au, V.K.-M.; Leung, S.Y.-L. Light-Emitting Self-Assembled Materials Based on d8 and d10 Transition Metal Complexes. Chem. Rev. 2015, 115, 7589–7728. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, B.-C.; Liao, C.-C.; Jung, P.-Y.; Chen, S.-Y.; Sun, B.-J.; Cheng, W.-C.; Chang, A.H.H.; Lee, G.-H. Luminescent Pt(II) Complexes Containing (1-Aza-15-crown-5)dithiocarbamate and (1-Aza-18-crown-6)dithiocarbamate: Mechanochromic and Solvent-Induced Luminescence. Inorg. Chem. 2023, 62, 916–929. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Carroll, J.; Vezzu, D.A. Design, synthesis, and applications of highly phosphorescent cyclometalated platinum complexes. Asian J. Org. Chem. 2015, 4, 1210–1245. [Google Scholar] [CrossRef]
- Swaminathan, S.; Jerome, P.; Deepak, R.J.; Karvembu, R.; Oh, T.H. Platinum group metal (PGM) complexes having acylthiourea ligand system as catalysts or anticancer agents. Coord. Chem. Rev. 2024, 503, 215620. [Google Scholar] [CrossRef]
- Molter, A.; Kathrein, S.; Kircher, B.; Mohr, F. Anti-tumour active gold (I), palladium (II) and ruthenium (II) complexes with thio-and selenoureato ligands: A comparative study. Dalton Trans. 2018, 47, 5055–5064. [Google Scholar] [CrossRef]
- Selcuk, O.; Azizi, N.; Aminzai, M.T.; Seferoglu, Z.; Erben, M.F.; Nural, Y. Acyl thiourea derivatives: Versatile tools for chemosensing and heavy metal remediation. J. Environ. Chem. Eng. 2024, 12, 114279. [Google Scholar] [CrossRef]
- Nkabyo, H.A.; Barnard, I.; Koch, K.R.; Luckay, R.C. Recent advances in the coordination and supramolecular chemistry of monopodal and bipodal acylthiourea-based ligands. Coord. Chem. Rev. 2021, 427, 213588. [Google Scholar] [CrossRef]
- Iliş, M.; Bucos, M.; Dumitraşcu, F.; Cîrcu, V. Mesomorphic behaviour of N-benzoyl-N′-aryl thioureas liquid crystalline compounds. J. Mol. Struct. 2011, 987, 1–6. [Google Scholar] [CrossRef]
- Iliş, M.; Micutz, M.; Pasuk, I.; Staicu, T.; Cîrcu, V. Synthesis and liquid crystalline properties of novel fluorinated N-benzoyl thiourea compounds. Effect of perfluoroalkyl chains on the thermal behavior and smectic phases stability. J. Fluor.Chem. 2017, 204, 84–89. [Google Scholar] [CrossRef]
- Ilis, M.; Batalu, D.; Pasuk, I.; Cîrcu, V. Cyclometalated palladium(II) metallomesogens with Schiff bases and N-benzoyl thiourea derivatives as co-ligands. J. Mol. Liq. 2017, 233, 45–51. [Google Scholar] [CrossRef]
- Cîrcu, V.; Ilie, M.; Iliş, M.; Dumitraşcu, F.; Neagoe, I.; Păsculescu, S. Luminescent cyclometallated platinum (II) complexes with N-benzoyl thiourea derivatives as ancillary ligands. Polyhedron 2009, 17, 3739–3746. [Google Scholar] [CrossRef]
- Cîrcu, V.; Micutz, M. X-Ray Structural Characterization of Cyclometalated Luminescent Pt(II) Complexes. In Current Trends in X-Ray Crystallography; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Micutz, M.; Iliş, M.; Staicu, T.; Dumitraşcu, F.; Pasuk, I.; Molard, Y.; Roisnel, T.; Cîrcu, V. Luminescent liquid crystalline materials based on palladium (II) imine derivatives containing the 2-phenylpyridine core. Dalton Trans. 2014, 43, 1151–1161. [Google Scholar] [CrossRef]
- Iliş, M.; Micutz, M.; Dumitraşcu, F.; Pasuk, I.; Molard, Y.; Roisnel, T.; Cîrcu, V. Enhancement of smectic C mesophase stability by using branched alkyl chains in the auxiliary ligands of luminescent Pt (II) and Pd (II) complexes. Polyhedron 2014, 69, 31–39. [Google Scholar] [CrossRef]
- Brooks, J.; Babayan, Y.; Lamansky, S.; Djurovich, P.I.; Tsyba, I.; Bau, R.; Thompson, M.E. Synthesis and Characterization of Phosphorescent Cyclometalated Platinum Complexes. Inorg. Chem. 2002, 41, 3055–3066. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-T.; Chen, H.-H.; Hsu, H.-F.; Poloek, A.; Yeh, H.-H.; Chi, Y.; Wang, K.-W.; Lai, C.-H.; Lee, G.-H.; Shih, C.-W.; et al. Mesomorphism and Luminescence Properties of Platinum(II) Complexes with Tris(alkoxy)phenyl-Functionalized Pyridyl Pyrazolate Chelates. Chem. Eur. J. 2011, 17, 546–556. [Google Scholar] [CrossRef]
- Ohno, K.; Yamaguchi, S.; Nagasawa, A.; Fujihara, T. Mechanochromism in the luminescence of novel cyclometalated platinum(II) complexes with α-aminocarboxylates. Dalton Trans. 2016, 45, 5492–5503. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Brédas, J.-L. Triplet Excimer Formation in Platinum-Based Phosphors: A Theoretical Study of the Roles of Pt–Pt Bimetallic Interactions and Interligand π–π Interactions. J. Am. Chem. Soc. 2009, 131, 11371–11380. [Google Scholar] [CrossRef]
- Martínez-Junquera, M.; Lara, R.; Lalinde, E.; Teresa Moreno, M. Isomerism, aggregation induced emission and mechanochromism of isocyanide cycloplatinated(II) complexes. J. Mater. Chem. C 2020, 8, 7221–7233. [Google Scholar] [CrossRef]
- Liu, S.; Sun, H.; Ma, Y.; Ye, S.; Liu, X.; Zhou, X.; Mou, X.; Wang, L.; Zhao, Q.; Huang, W. Rational design of metallophosphors with tunable aggregation-induced phosphorescent emission and their promising applications in time-resolved luminescence assay and targeted luminescence imaging of cancer cells. J. Mater. Chem. 2012, 22, 22167. [Google Scholar] [CrossRef]
- Kumar, P.; Escudero, D. Computational Protocol to Calculate the Phosphorescence Energy of Pt(II) Complexes: Is the Lowest Triplet Excited State Always Involved in Emission? A Comprehensive Benchmark Study. Inorg. Chem. 2021, 60, 17230–17240. [Google Scholar] [CrossRef] [PubMed]
- Begantsova, Y.E.; Bochkarev, L.N.; Ketkov, S.Y.; Baranov, E.V.; Bochkarev, M.N.; Yakhvarov, D.G. Synthesis, characterization and photophysical properties of new cyclometallated platinum(II) complexes with pyrazolonate ancillary ligand. J. Organomet. Chem. 2013, 733, 1–8. [Google Scholar] [CrossRef]
- Ni, J.; Zheng, W.; Qi, W.-J.; Guo, Z.-C.; Liu, S.-Q.; Zhang, J.-J. Synthesis, structure and luminescent switching properties of cycloplatinated(II) complexes bearing phenyl β-diketone ligands. J. Organomet. Chem. 2021, 952, 122048. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. C 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Adamo, C.; Barone, V.J. Toward reliable density functional methods without adjustable parameters: The PBE0 model. Chem. Phys. 1999, 110, 6158–6169. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Cho, J.-Y.; Suponitsky, K.Y.; Li, J.; Timofeeva, T.V.; Barlow, S.; Marder, S.R. Cyclometalated platinum complexes: High-yield synthesis, characterization, and a crystal structure. J. Organomet. Chem. 2005, 690, 4090. [Google Scholar] [CrossRef]
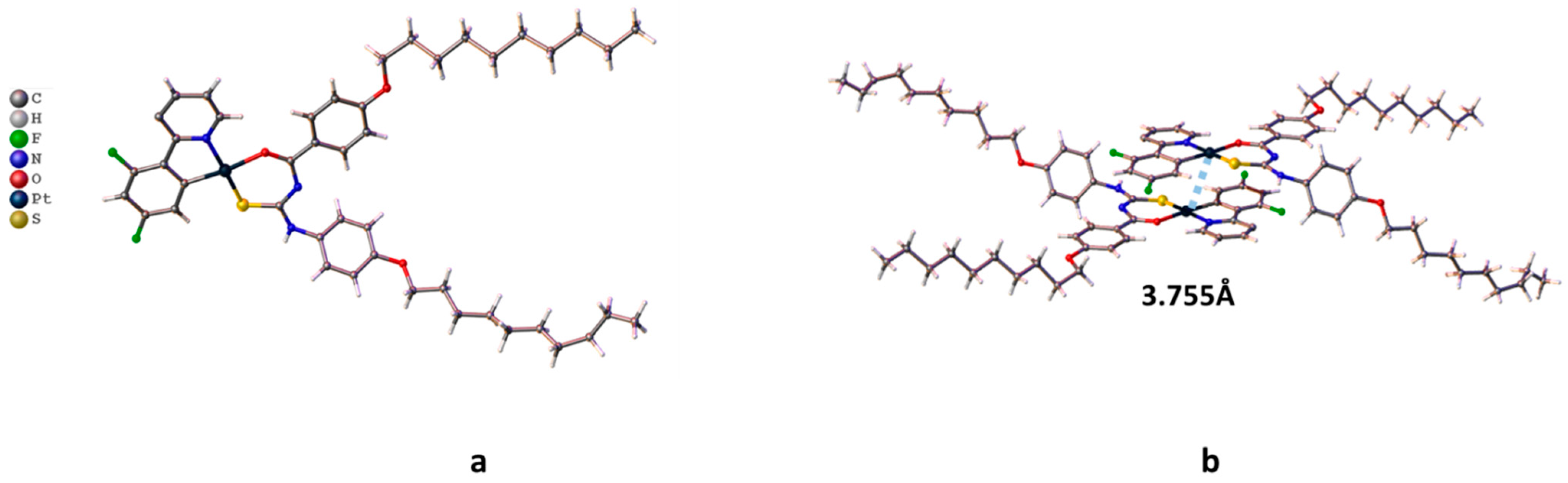
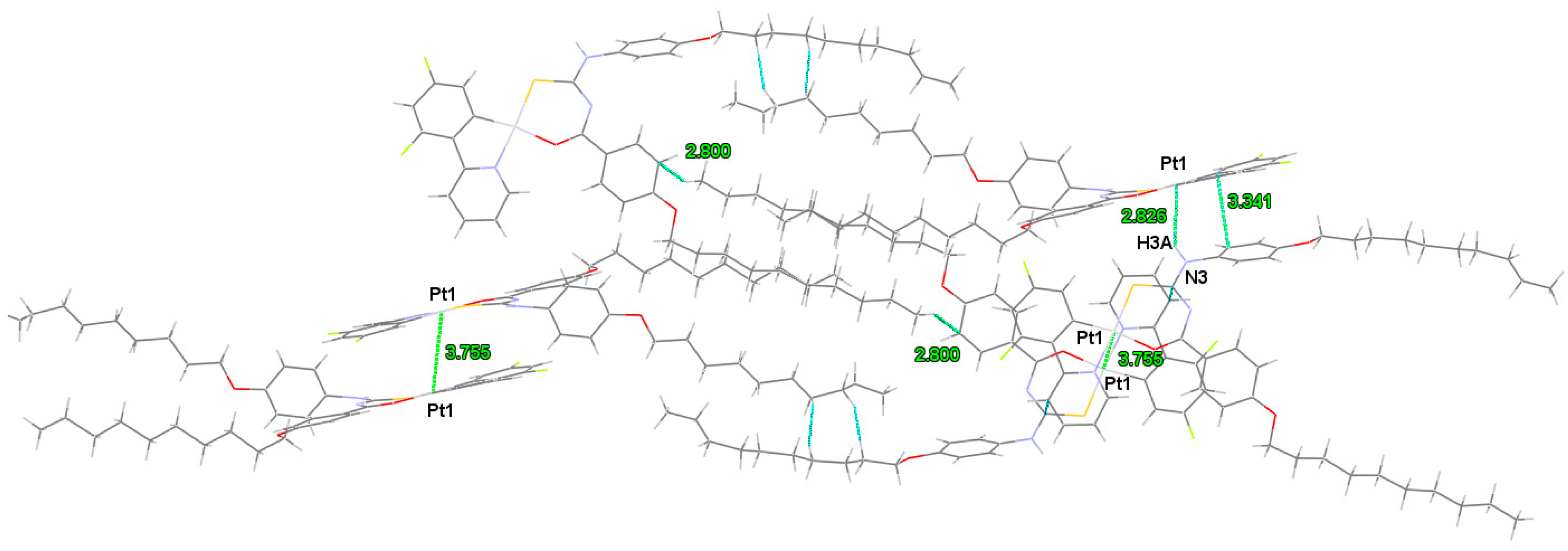



| Identification Code | Cvlcpt |
|---|---|
| Empirical formula | C45H57F2N3O3PtS |
| Formula weight | 953.08 |
| Temperature/K | 293.00 |
| Crystal system | monoclinic |
| Space group | P21/c |
| a/Å | 26.6340(18) |
| b/Å | 10.0262(6) |
| c/Å | 16.7109(10) |
| α/° | 90 |
| β/° | 104.840(7) |
| γ/° | 90 |
| Volume/Å3 | 4313.6(5) |
| Z | 4 |
| ρcalc(g/cm3) | 1.468 |
| μ/mm−1 | 3.352 |
| F(000) | 1936.0 |
| Crystal size/mm3 | 0.4 × 0.3 × 0.05 |
| Radiation | MoKα (λ = 0.71075) |
| 2Θ range for data collection/° | 6.082 to 50 |
| Index ranges | −31 ≤ h ≤ 31, −11 ≤ k ≤ 11, −19 ≤ l ≤ 17 |
| Reflections collected | 28,151 |
| Independent reflections | 6660 [Rint = 0.1162, Rsigma = 0.0950] |
| Data/restraints/parameters | 6660/387/560 |
| Goodness-of-fit on F2 | 1.030 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0757, wR2 = 0.2075 |
| Final R indexes [all data] | R1 = 0.1128, wR2 = 0.2408 |
| Largest diff. peak/hole/e Å−3 | 0.87/−1.46 |
| Bond Lengths, (Å) | Bond Angles, (o) | ||
|---|---|---|---|
| Pt1-S1 Pt1-N1 Pt1-O1 Pt1-C11 N2-C12 N2-C13 O1-C12 S1-C13 | 2.243(5) 2.013(9) 2.045(8) 1.974(12) 1.404(18) 1.371(16) 1.262(15) 1.732(13) | S1-Pt1-O1 S1-Pt1-C11 O1-Pt1-N1 N1-Pt1-C11 | 93.2(3) 96.4(4) 89.7(4) 80.7(5) |
| Complex | Transitions (T/°C, ∆H/kJ∙mol−1) |
|---|---|
| 5 | Cr1 57 (2.5) Cr2 73 (15.1) Cr3 164 (24.5) c Iso Iso 121 (−11.3) Cr3 89 (−0.2) Cr2 62 (−8.5) Cr1 |
| 6 | Cr1 106 (26.0) Cr2 151 (20.4) Iso Iso 139 (−3.9) Cr3 84 (−0.5) Cr2 56 (−2.6) Cr1 |
| Compound | Media | UV-VIS (Solution, 10−4 M, CH2Cl2) λabsmax (ε × 10−3/M−1cm−1) | Emission λemmax (nm) | Φ (%) | τ (μs) |
|---|---|---|---|---|---|
| 5 | CH2Cl2 | 285 (26.8), 310 (19.0), 378 (13.4) | 480, 519, 557 | 0.02 | <1 |
| Solid | / | 482, 518, 548 | 13 | 4 | |
| PMMA (1%) | / | 480, 514, 550 | 28.3 | 5 | |
| Ground | / | 625 | 7 | 2 | |
| 6 | CH2Cl2 | 277 (13.0), 315 (13.4), 369(sh) (6.9) | 467, 535, 572, 627(sh) | 0.17 | <1 |
| Solid | / | 468, 502, 530, 570 | 2 | 5 | |
| PMMA (1%) | / | 465, 500, 532, 570 | 5 | 6 | |
| Ground | / | 647 | 1 | 3 |
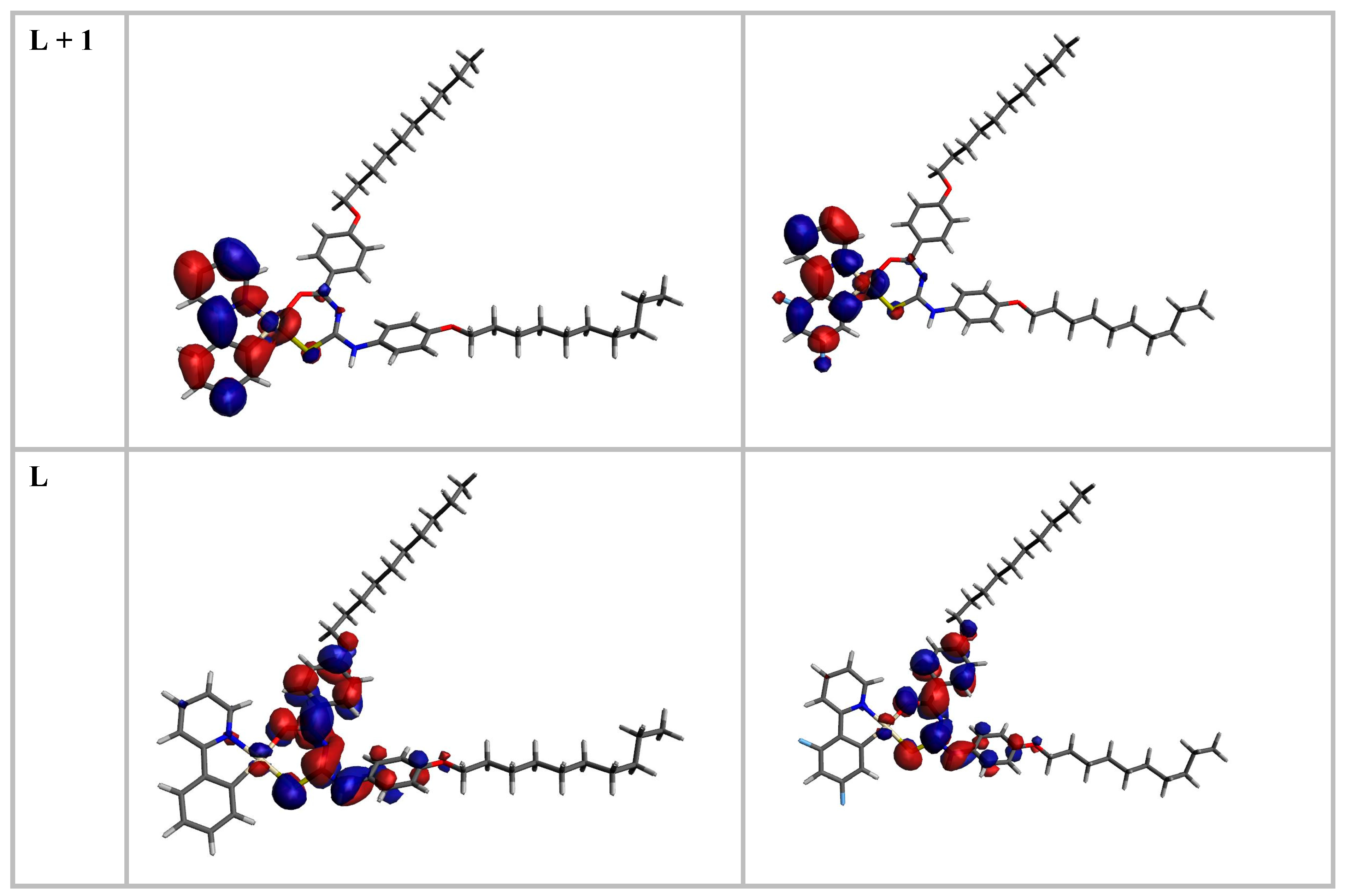
| Complex | λexp/nm | λcalcd/nm | E/eV fcalcd | Major Contribution | Assignment |
|---|---|---|---|---|---|
| 5 | 378 | 366 | 3.39 0.2874 | H → L (69%) H → L + 1 (10%) | 1MLCT, 1ILCT |
| 6 | 369 | 369 | 3.36 0.4704 | H → L (91%) | 1MLCT, 1ILCT |
| Fragment | H-4 | H-3 | H-2 | H-1 | H | L | L + 1 |
|---|---|---|---|---|---|---|---|
| 5 | |||||||
| 1 (Pt) | 8.12 | 88.11 | 16.06 | 33.83 | 15.48 | 2.43 | 6.38 |
| 2 (Ph) | 17.71 | 2.99 | 1.69 | 47.15 | 0.95 | 0.59 | 27.01 |
| 3 (PhN) | 10.18 | 1.03 | 3.01 | 7.83 | 2.43 | 1.86 | 63.72 |
| 4 (PhSN) | 10.07 | 5.69 | 30.68 | 3.37 | 69.44 | 36.93 | 1.09 |
| 5 (PhNO) | 38.11 | 2.17 | 36.10 | 7.10 | 1.78 | 55.72 | 1.74 |
| 6 (OC10H21) | 2.93 | 0.00 | 4.56 | 0.00 | 9.92 | 0.42 | 0.00 |
| 7 (OC10H21) | 12.88 | 0.00 | 7.91 | 0.72 | 0.00 | 2.04 | 0.06 |
| 6 | |||||||
| 1 (Pt) | 86.94 | 5.01 | 24.13 | 25.57 | 11.70 | 2.14 | 6.85 |
| 2 (PhFF) | 2.69 | 34.18 | 12.91 | 31.26 | 0.79 | 0.53 | 26.58 |
| 3 (PhN) | 0.91 | 15.67 | 4.16 | 8.47 | 1.85 | 1.43 | 64.19 |
| 4 (PhSN) | 6.37 | 6.91 | 28.35 | 2.23 | 72.39 | 38.63 | 0.97 |
| 5 (PhNO) | 3.05 | 27.02 | 24.16 | 25.16 | 2.01 | 54.76 | 1.36 |
| 6 (OC10H21) | 0.04 | 2.12 | 2.51 | 0.56 | 11.24 | 0.50 | 0.00 |
| 7 (OC10H21) | 0.00 | 9.08 | 3.78 | 6.74 | 0.02 | 2.00 | 0.04 |
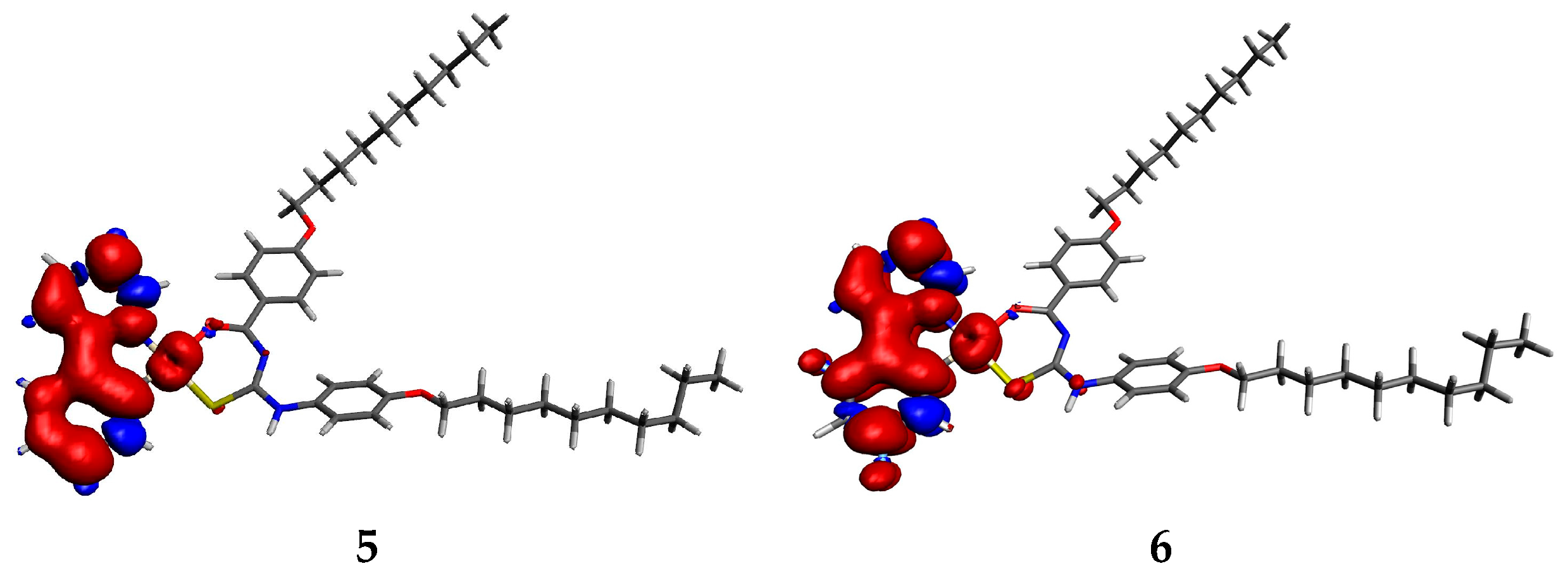
| Complex | λem (nm) | Pt Spin Density |
|---|---|---|
| 5 | 591.81 | 0.206238 |
| 6 | 569.33 | 0.172162 |
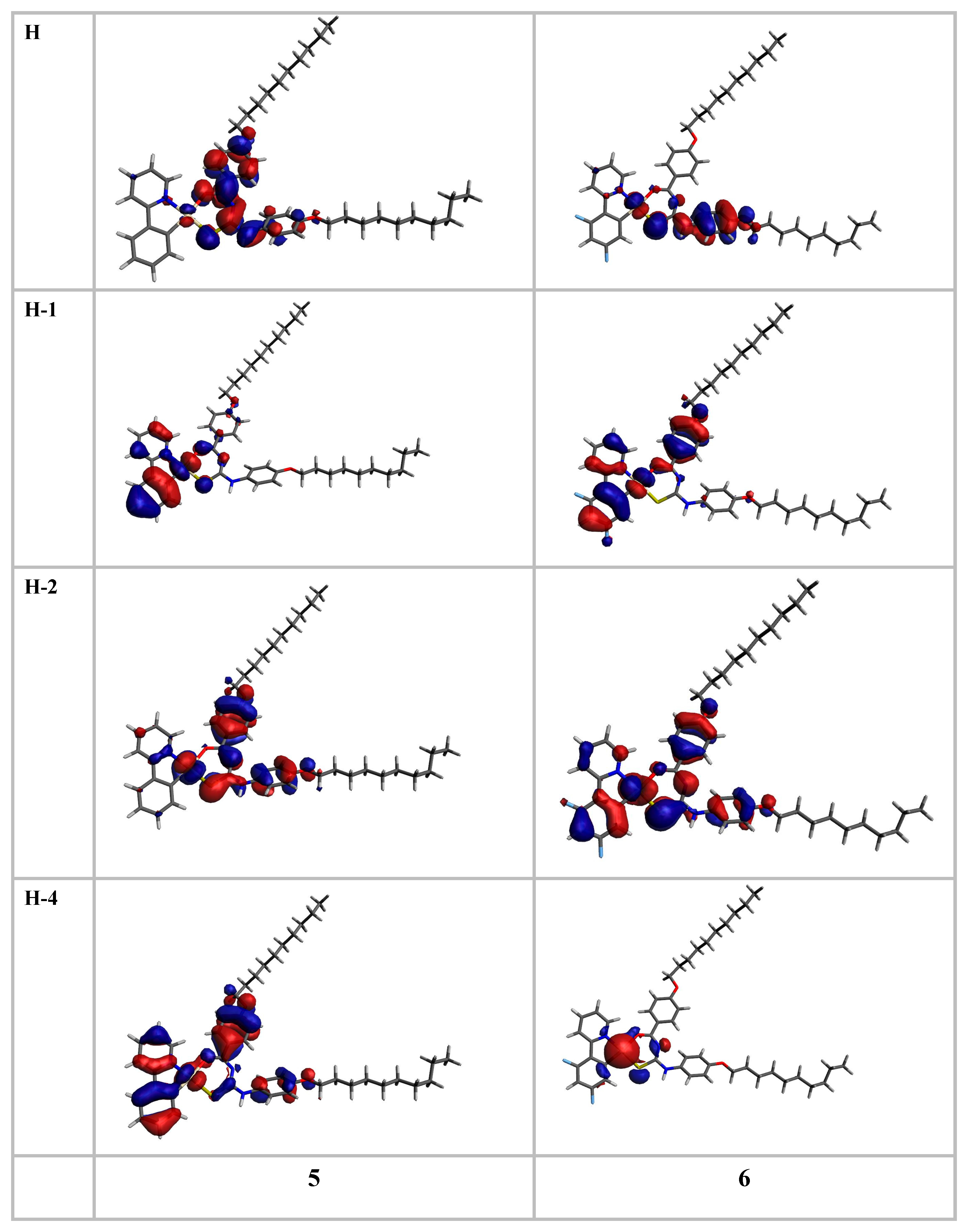
| Complex | λexp (nm) | λcalc (nm) | f | Main Contribution | Assignment | ΔE (eV) | f | Main Contribution | Assignment |
|---|---|---|---|---|---|---|---|---|---|
| S0 → S1 | S0 → Tn | ||||||||
| 5 | 378 | 366 | 0.2874 | H → L (69%) H → L + 1 (10%) | 1MLCT, 1ILCT | 2.7294 | 0.00 | H-1 → L + 1 (43%) H-4 → L + 1 (16%) | 3MLCT, 3ILCT |
| 2.8904 | 0.00 | H → L (83%) | 3ILCT, 3MLCT | ||||||
| 3.0382 | 0.00 | H-2 → L (58%) H-4 → L (12%) | 3ILCT, 3MLCT | ||||||
| 6 | 369 | 369 | 0.4704 | H → L (91%) | 1MLCT, 1ILCT | 2.8022 | 0.00 | H → L (76%) | 3MLCT, 3ILCT |
| 2.8193 | 0.00 | H-1 → L + 1 (34%) H-3 → L + 1 (24%) H → L + 1 (10%) H → L (10%) | 3MLCT, 3ILCT | ||||||
| 3.0219 | 0.00 | H-1 → L (37%) H-2 → L (32%) | 3MLCT, 3ILCT | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iliş, M.; Ferbinteanu, M.; Tablet, C.; Cîrcu, V. Investigation of Mechanochromic and Solvatochromic Luminescence of Cyclometalated Heteroleptic Platinum(II) Complexes with Benzoylthiourea Derivatives. Molecules 2025, 30, 2415. https://doi.org/10.3390/molecules30112415
Iliş M, Ferbinteanu M, Tablet C, Cîrcu V. Investigation of Mechanochromic and Solvatochromic Luminescence of Cyclometalated Heteroleptic Platinum(II) Complexes with Benzoylthiourea Derivatives. Molecules. 2025; 30(11):2415. https://doi.org/10.3390/molecules30112415
Chicago/Turabian StyleIliş, Monica, Marilena Ferbinteanu, Cristina Tablet, and Viorel Cîrcu. 2025. "Investigation of Mechanochromic and Solvatochromic Luminescence of Cyclometalated Heteroleptic Platinum(II) Complexes with Benzoylthiourea Derivatives" Molecules 30, no. 11: 2415. https://doi.org/10.3390/molecules30112415
APA StyleIliş, M., Ferbinteanu, M., Tablet, C., & Cîrcu, V. (2025). Investigation of Mechanochromic and Solvatochromic Luminescence of Cyclometalated Heteroleptic Platinum(II) Complexes with Benzoylthiourea Derivatives. Molecules, 30(11), 2415. https://doi.org/10.3390/molecules30112415







