Chemical Composition, Anti-Tyrosinase and Antioxidant Potential of Essential Oils from Acorus calamus (L.) and Juniperus communis (L.)
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of the Essential Oils Extracted from A. calamus and J. communis Plants
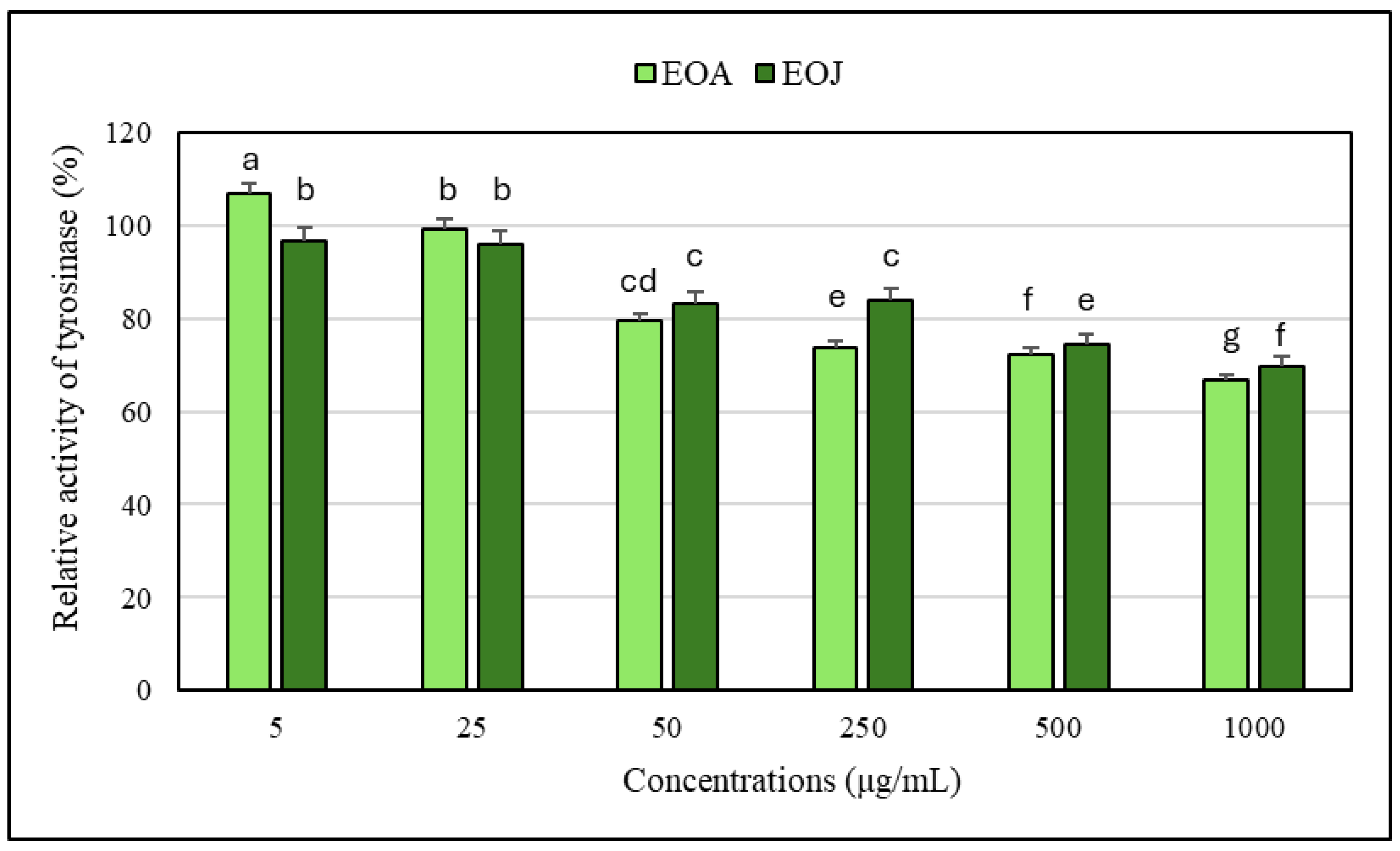
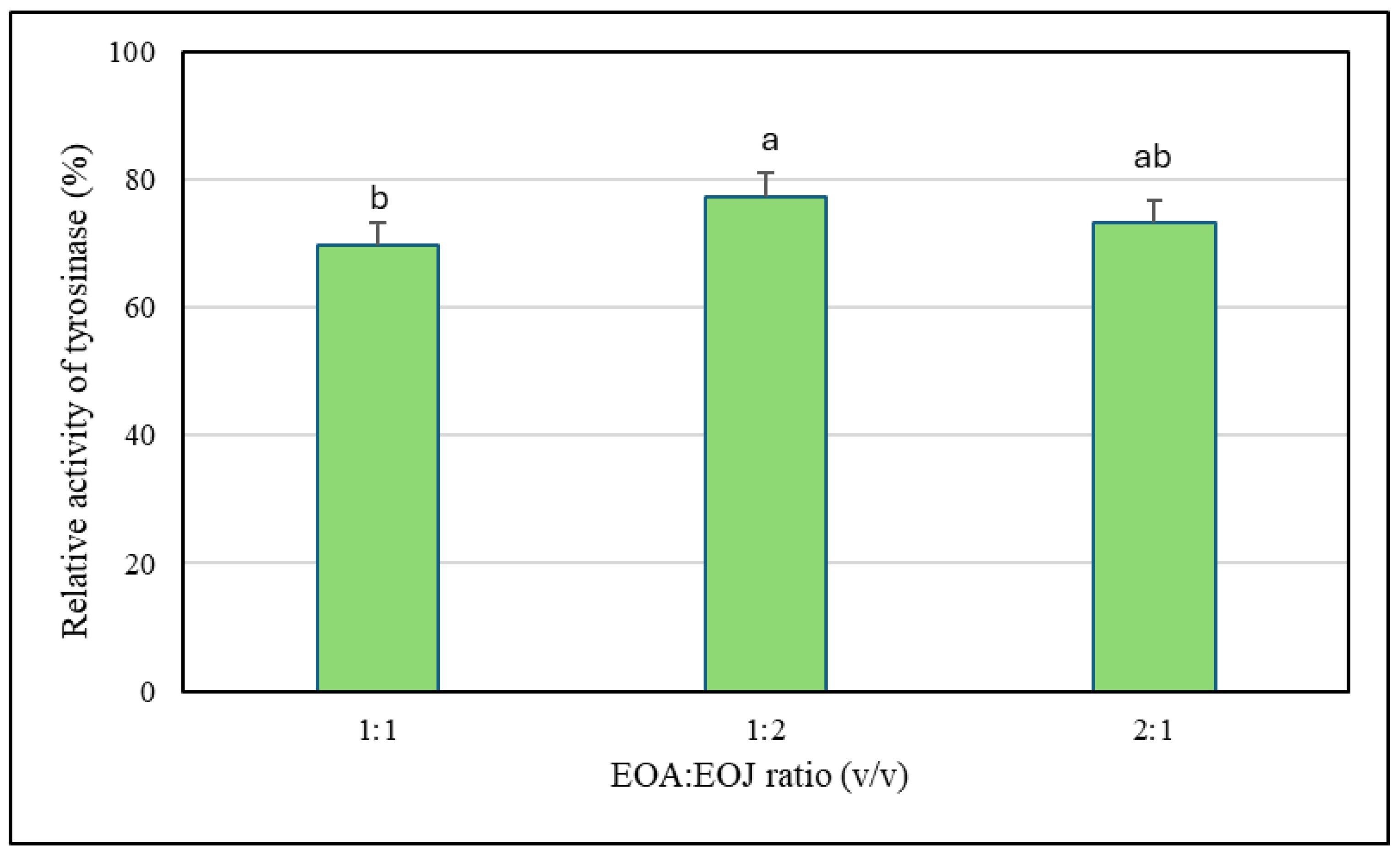
2.2. Assessment of Anti-Tyrosinase Potential of the Tested Essential Oils
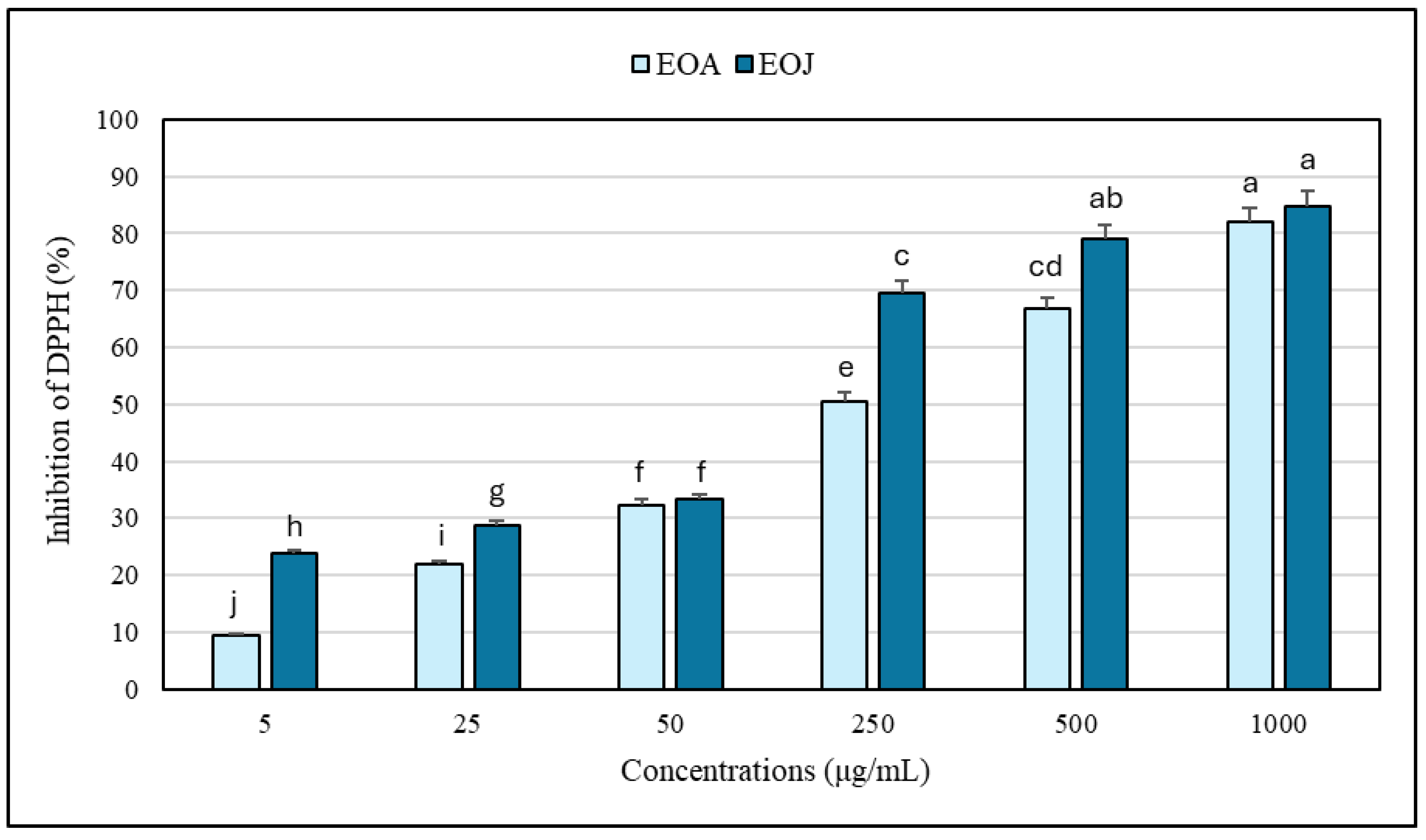
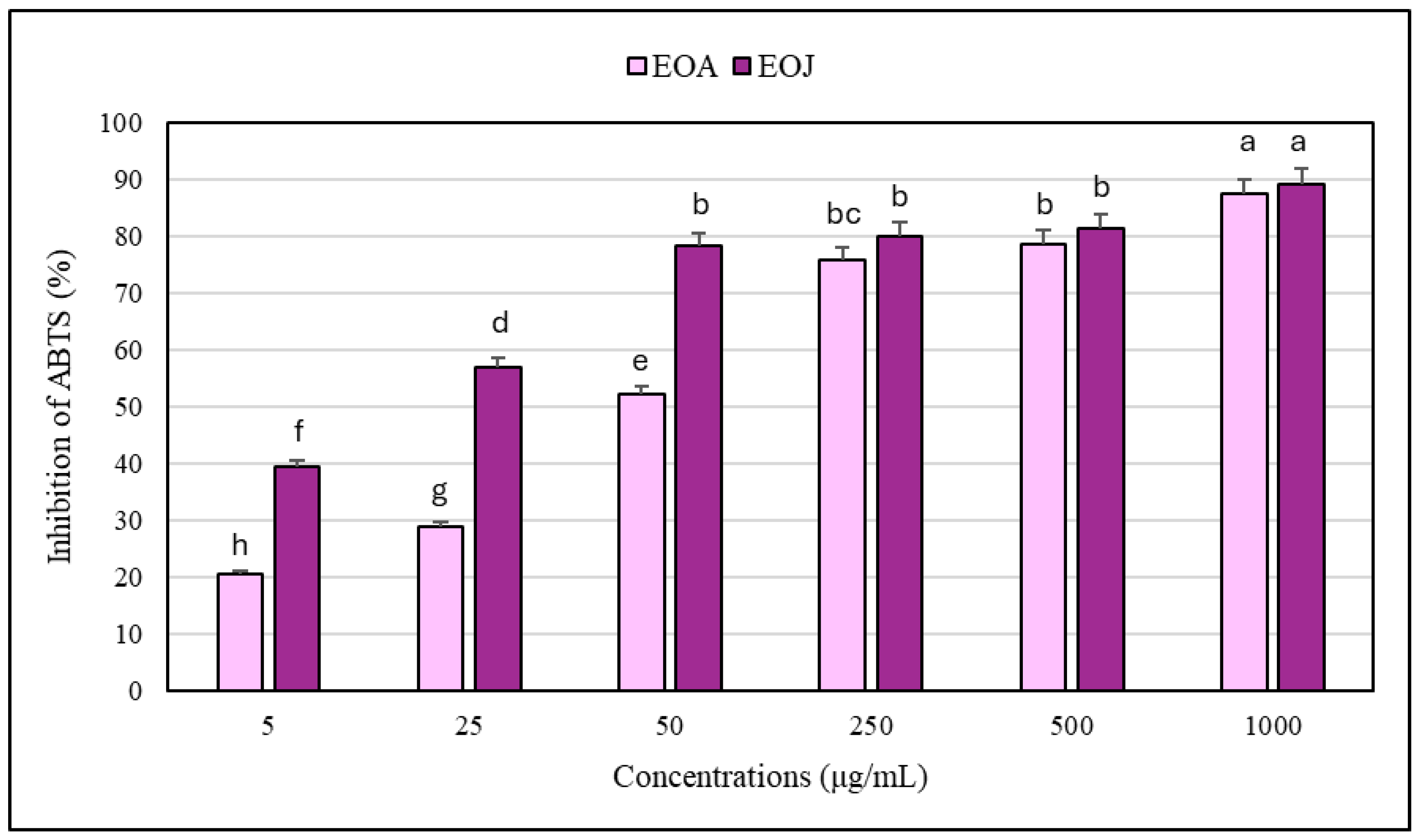
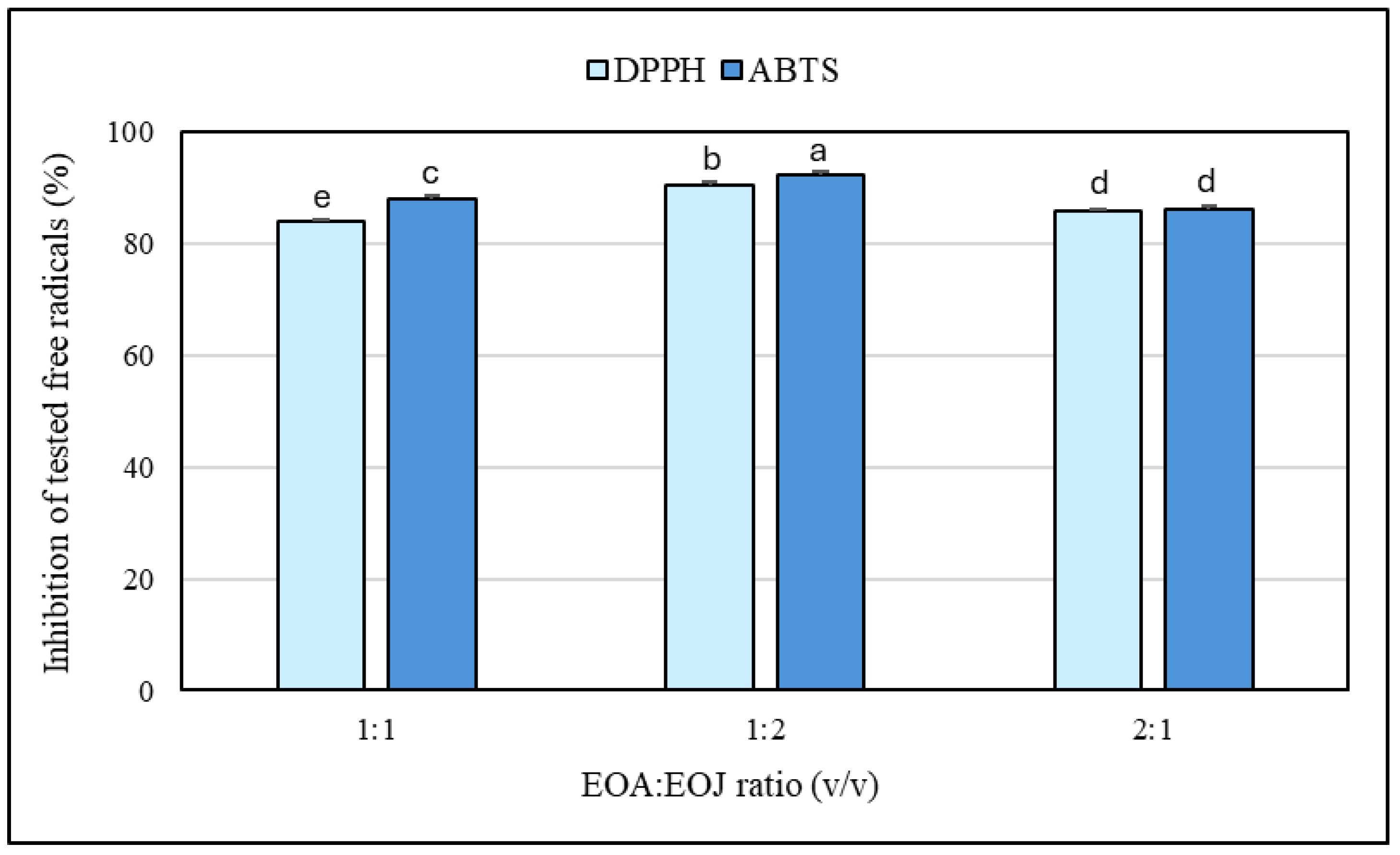
2.3. Antioxidant Capacity of the Examined Essential Oils
3. Materials and Methods
3.1. Plant Materials and Extraction of the Examined Essential Oils
3.2. Analysis of Chemical Compounds in the Tested Essential Oils Using Gas Chromatography–Mass Spectrometry (GC-MS)
3.3. Determination of Anti-Tyrosinase Potential
3.4. Measurement of Antioxidant Capacity
3.4.1. DPPH• Scavenging Assay
3.4.2. ABTS•+ Scavenging Assay
3.5. Determination of Combination Index of Essential Oil Mixtures
3.6. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yihan, W.; Jinjin, D.; Yingqi, W.; Guanai, M.; Xiwu, Z. Advances in Plant Essential Oils and Drug Delivery Systems for Skincare. Front. Pharmacol. 2025, 16, 1578280. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Aslam, M.; Alsagaby, S.A.; Saeed, F.; Ahmad, I.; Afzaal, M.; Arshad, M.U.; Abdelgawad, M.A.; El-Ghorab, A.H.; Khames, A.; et al. Therapeutic Application of Carvacrol: A Comprehensive Review. Food Sci. Nutr. 2022, 10, 3544–3561. [Google Scholar] [CrossRef] [PubMed]
- Altyar, A.E.; Ashour, M.L.; Youssef, F.S. Premna odorata: Seasonal Metabolic Variation in the Essential Oil Composition of Its Leaf and Verification of Its Anti-Ageing Potential via In Vitro Assays and Molecular Modelling. Biomolecules 2020, 10, 879. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, J.; Cao, G.; Zhao, D.; Li, G.; Zhang, H.; Yan, M. Ethnic, Botanic, Phytochemistry and Pharmacology of the Acorus L. Genus: A Review. Molecules 2023, 28, 7117. [Google Scholar] [CrossRef]
- Sunandini; Dutt, B.; Kumar, R. An Ethnopharmacological Review on the Antibacterial, Antifungal and Therapeutic Potentials of Acorus calamus Linn. Euro. J. Med. Plants 2025, 36, 98–105. [Google Scholar] [CrossRef]
- Yue, C.; Li, H.; Shi, X. Geographical Distribution Dynamics of Acorus calamus in China Under Climate Change. Plants 2024, 13, 3352. [Google Scholar] [CrossRef]
- Hrytsyna, M.; Salamon, I.; Peleno, R.; Vargova, V. Identification and Analysis of the Content of Biologically Active Substances of Juniper Cone Berries and Their Antioxidant Activity. Horticulturae 2024, 10, 1237. [Google Scholar] [CrossRef]
- Höferl, M.; Stoilova, I.; Schmidt, E.; Wanner, J.; Jirovetz, L.; Trifonova, D.; Krastev, L.; Krastanov, A. Chemical Composition and Antioxidant Properties of Juniper Berry (Juniperus communis L.) Essential Oil. Action of the Essential Oil on the Antioxidant Protection of Saccharomyces cerevisiae Model Organism. Antioxidants 2014, 3, 81–98. [Google Scholar] [CrossRef]
- Salamon, I.; Otepka, P.; Kryvtsova, M.; Kolesnyk, O.; Hrytsyna, M. Selected Biotopes of Juniperus communis L. in Slovakia and Their Chemotype Determination. Horticulturae 2023, 9, 686. [Google Scholar] [CrossRef]
- Khwairakpam, A.D.; Damayenti, Y.D.; Deka, A.; Monisha, J.; Roy, N.K.; Padmavathi, G.; Kunnumakkara, A.B. Acorus calamus: A Bio-Reserve of Medicinal Values. J. Basic Clin. Physiol. Pharmacol. 2018, 29, 107–122. [Google Scholar] [CrossRef]
- He, X.; Chen, X.; Yang, Y.; Liu, Y.; Xie, Y. Acorus calamus var. angustatus Besser: Insight into Current Research on Ethnopharmacological Use, Phytochemistry, Pharmacology, Toxicology, and Pharmacokinetics. Phytochemistry 2023, 210, 113626. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Sharma, R.; Gautam, D.S.; Kuca, K.; Nepovimova, E.; Martins, N. Role of Vacha (Acorus calamus Linn.) in Neurological and Metabolic Disorders: Evidence from Ethnopharmacology, Phytochemistry, Pharmacology and Clinical Study. J. Clin. Med. 2020, 9, 1176. [Google Scholar] [CrossRef]
- Bai, D.; Li, X.; Wang, S.; Zhang, T.; Wei, Y.; Wang, Q.; Dong, W.; Song, J.; Gao, P.; Li, Y.; et al. Advances in Extraction Methods, Chemical Constituents, Pharmacological Activities, Molecular Targets and Toxicology of Volatile Oil from Acorus calamus var. angustatus Besser. Front. Pharmacol. 2022, 13, 1004529. [Google Scholar] [CrossRef]
- Wu, Q.; Fang, W.; Liu, H.; Liu, Z.; Xu, X. Rosa × damascena Herrm. Essential Oil: Anti-Tyrosinase Activity and Phytochemical Composition. Front. Pharmacol. 2024, 15, 1451452. [Google Scholar] [CrossRef]
- Zolghadri, S.; Beygi, M.; Mohammad, T.F.; Alijanianzadeh, M.; Pillaiyar, T.; Garcia-Molina, P.; Garcia-Canovas, F.; Munoz-Munoz, J.; Saboury, A.A. Targeting Tyrosinase in Hyperpigmentation: Current Status, Limitations and Future Promises. Biochem. Pharmacol. 2023, 212, 115574. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Shahzadi, S.; Kloczkowski, A. Tyrosinase Inhibitors Naturally Present in Plants and Synthetic Modifications of These Natural Products as Anti-Melanogenic Agents: A Review. Molecules 2023, 28, 378. [Google Scholar] [CrossRef]
- Doğan, A.; Akocak, S. Chapter Four—Natural Products as Tyrosinase Inhibitors. Enzymes 2024, 56, 85–109. [Google Scholar] [CrossRef] [PubMed]
- Nasir, O. Protective Effect of Acorus calamus on Kidney and Liver Functions in Healthy Mice. Saud. J. Biol. Sci. 2021, 28, 2701–2708. [Google Scholar] [CrossRef]
- Hajlaoui, H.; Arraouadi, S.; Noumi, E.; Aouadi, K.; Adnan, M.; Khan, M.A.; Kadri, A.; Snoussi, M. Antimicrobial, Antioxidant, Anti-Acetylcholinesterase, Antidiabetic, and Pharmacokinetic Properties of Carum carvi L. and Coriandrum sativum L. Essential Oils Alone and in Combination. Molecules 2021, 26, 3625. [Google Scholar] [CrossRef]
- Falasca, A.; Caprari, C.; Felice, V.D.; Fortini, P.; Saviano, G.; Zollo, F.; Iorizzi, M. GC-MS Analysis of the Essential Oils of Juniperus communis L. Berries Growing Wild in the Molise Region: Seasonal Variability and in vitro Antifungal Activity. Biochem. Syst. Ecol. 2016, 69, 166–175. [Google Scholar] [CrossRef]
- Majewska, E.; Kozłowska, M.; Kowalska, D.; Gruczynska, E. Characterization of the Essential Oil from Cone-Berries of Juniperus communis L. (Cupressaceae). Herba Pol. 2017, 63, 48–55. [Google Scholar] [CrossRef]
- Jovanović, D.Z.; Nešić, M.S.; Dekić, M.S.; Ristić, N.R.; Radulović, N.S. Resolving Unresolvable Multiplets: 1H NMR Spectral Simulation of Selected Sesquiterpenoids from the Acorenone-Rich Rhizome Essential Oil of Sweet Flag, Acorus calamus L. J. Mol. Struct. 2025, 1321, 140205. [Google Scholar] [CrossRef]
- Süzgeç-Selçuk, S.; Özek, G.; Meriçli, A.H.; Baser, K.H.C.; Haliloglu, Y.; Özek, T. Chemical and Biological Diversity of the Leaf and Rhizome Volatiles of Acorus calamus L. from Turkey. J. Essent. Oil-Bear. Plants 2017, 20, 646–661. [Google Scholar] [CrossRef]
- Loying, R.; Gogoi, R.; Sarma, N.; Borah, A.; Munda, S.; Pandey, S.K.; Lal, M. Chemical Compositions, In-vitro Antioxidant, Anti-microbial, Anti-inflammatory and Cytotoxic Activities of Essential Oil of Acorus calamus L. Rhizome from North-East India. J. Essent. Oil-Bear. Plants 2019, 22, 1299–1312. [Google Scholar] [CrossRef]
- Ahlawat, A.; Katoch, M.; Ram, G.; Ahuja, A. Genetic Diversity in Acorus Calamus L. as Revealed by RAPD Markers and its Relationship with β-Asarone Content and Ploidy Level. Sci. Hortic. 2010, 124, 294–297. [Google Scholar] [CrossRef]
- Uebel, T.; Hermes, L.; Haupenthal, S.; Müller, L.; Esselen, M. α-Asarone, β-asarone, and γ-asarone: Current Status of Toxicological Evaluation. J. Appl. Toxicol. 2021, 41, 1166–1179. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Off. J. Eur. Union 2009, L342, 59–209. [Google Scholar]
- Szczeblewski, P.; Wróblewski, M.; Borzyszkowska-Bukowska, J.; Bairamova, T.; Górska, J.; Laskowski, T.; Samulewicz, A.; Kosno, M.; Sobiech, Ł.; Polit, J.T.; et al. The Role of Centrifugal Partition Chromatography in the Removal of β-Asarone from Acorus calamus Essential Oil. Sci. Rep. 2022, 12, 22217. [Google Scholar] [CrossRef]
- Raal, A.; Orav, A.; Gretchushnikova, T. β-Asarone Content and Essential Oil Composition of Acorus calamus L. Rhizomes from Estonia. J. Essent. Oil-Bear. Plants 2016, 28, 299–304. [Google Scholar] [CrossRef]
- Chou, T.-C.; Talalay, P. Quantitative Analysis of Dose-Effect Relationships: The Combined Effects of Multiple Drugs or Enzyme Inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Jegal, J.; Park, S.A.; Chung, K.; Chung, H.Y.; Lee, J.; Jeong, E.J.; Yang, M.H. Tyrosinase Inhibitory Flavonoid from Juniperus communis Fruits. Biosci. Biotechnol. Biochem. 2016, 80, 2311–2317. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.J.; Jegal, J.; Chung, K.W.; Noh, S.G.; Chung, H.Y.; Nam, Y.H.; Kang, T.H.; Kim, S.-N.; Yang, M.H. Hypolaetin-7-O-β-D-xyloside from Juniperus communis Fruits Inhibits Melanogenesis on Zebrafish Pigmentation. Nat. Prod. Comm. 2017, 12, 1687–1690. [Google Scholar] [CrossRef]
- Ha, S.Y.; Jung, J.Y.; Yang, J.K. Camellia japonica Essential Oil Inhibits α-MSH-Induced Melanin Production and Tyrosinase Activity in B16F10 Melanoma Cells. Evid. Based Complement Alternat. Med. 2021, 2021, 6328767. [Google Scholar] [CrossRef] [PubMed]
- Salihu, A.S.; Salleh, W.M.N.H.W.; Setzer, W.N. Essential Oil Composition, Anti-Tyrosinase Activity, and Molecular Docking Studies of Knema intermedia Warb. (Myristicaceae). Z. Naturforsch C. J. Biosci. 2023, 78, 293–298. [Google Scholar] [CrossRef]
- Michalak, M. Plant Extracts as Skin Care and Therapeutic Agents. Int. J. Mol. Sci. 2023, 24, 15444. [Google Scholar] [CrossRef]
- Murray, A.F.; Satooka, H.; Shimizu, K.; Chavasiri, W.; Kubo, I. Polygonum odoratum Essential Oil Inhibits the Activity of Mushroom Derived Tyrosinase. Heliyon 2019, 5, e02817. [Google Scholar] [CrossRef]
- Chang, C.T.; Chang, W.L.; Hsu, J.C.; Shih, Y.; Chou, S.T. Chemical Composition and Tyrosinase Inhibitory Activity of Cinnamomum cassia Essential Oil. Bot. Stud. 2013, 54, 10. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and Terpenoids as Main Bioactive Compounds of Essential Oils, Their Roles in Human Health and Potential Application as Natural Food Preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Ashraf, Z.; Rafiq, M.; Nadeem, H.; Hassan, M.; Afzal, S.; Waseem, M.; Afzal, K.; Latip, J. Carvacrol Derivatives as Mushroom Tyrosinase Inhibitors; Synthesis, Kinetics Mechanism and Molecular Docking Studies. PLoS ONE 2017, 12, e0178069. [Google Scholar] [CrossRef]
- Matsuura, R.; Ukeda, H.; Sawamura, M. Tyrosinase Inhibitory Activity of Citrus Essential Oils. J. Agric. Food Chem. 2006, 54, 2309–2313. [Google Scholar] [CrossRef]
- Capetti, F.; Tacchini, M.; Marengo, A.; Cagliero, C.; Bicchi, C.; Rubiolo, P.; Sgorbini, B. Citral-Containing Essential Oils as Potential Tyrosinase Inhibitors: A Bio-Guided Fractionation Approach. Plants 2021, 10, 969. [Google Scholar] [CrossRef] [PubMed]
- Capetti, F.; Cagliero, C.; Argenziano, M.; Cavalli, R.; Dianzani, C.; Pavarino, M.; Bicchi, C.; Rubiolo, P.; Sgorbini, B. A New Blend of Litsea cubeba, Pinus mugo, and Cymbopogon winterianus Essential Oil Active as an Anti-tyrosinase Ingredient in Topical Formulations. Planta Med. 2024, 90, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-D.; Choi, H.; Abekura, F.; Park, J.-Y.; Yang, W.-S.; Yang, S.-H.; Kim, C.-H. Naturally-Occurring Tyrosinase Inhibitors Classified by Enzyme Kinetics and Copper Chelation. Int. J. Mol. Sci. 2023, 24, 8226. [Google Scholar] [CrossRef]
- da Silva, A.P.; Silva, N.F.; Andrade, E.H.A.; Gratieri, T.; Setzer, W.N.; Maia, J.G.S.; da Silva, J.K.R. Tyrosinase Inhibitory Activity, Molecular Docking Studies and Antioxidant Potential of Chemotypes of Lippia Origanoides (Verbenaceae) Essential Oils. PLoS ONE 2017, 12, e0175598. [Google Scholar] [CrossRef]
- Di Martile, M.; Garzoli, S.; Ragno, R.; Del Bufalo, D. Essential Oils and Their Main Chemical Components: The Past 20 Years of Preclinical Studies in Melanoma. Cancers 2020, 12, 2650. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.-T.; Chang, W.-L.; Chang, C.-T.; Hsu, S.-L.; Lin, Y.-C.; Shih, Y. Cinnamomum cassia Essential Oil Inhibits α-MSH-Induced Melanin Production and Oxidative Stress in Murine B16 Melanoma Cells. Int. J. Mol. Sci. 2013, 14, 19186–19201. [Google Scholar] [CrossRef]
- Wang, R.; Wang, G.; Sui, W.; Zhou, C.; Li, S.; Ji, Y.; Si, C. Tyrosinase Inhibitory Performance of Hydrolysate from Post-Washing Liquor of Steam Exploded Corn Stalk and Its Fractionation Enhancement. Ind. Crops Prod. 2020, 154, 112652. [Google Scholar] [CrossRef]
- Khanal, S.; Bhandari, D.P.; Bhandari, L.; Dangol, S.; Adhikari, A. Chemical Profiling and Antioxidant Activities of Essential Oil from the Rhizomes of Acorus calamus L. Bibechana 2020, 17, 89–95. [Google Scholar] [CrossRef]
- Emami, S.A.; Javadi, B.; Hassanzadeh, M.K. Antioxidant Activity of the Essential Oils of Different Parts of Juniperus communis. Subsp. Hemisphaerica and Juniperus oblonga. Pharm. Biol. 2007, 45, 769–776. [Google Scholar] [CrossRef]
- Assaggaf, H.; Jeddi, M.; Mrabti, H.N.; Ez-Zoubi, A.; Qasem, A.; Attar, A.; Goh, B.H.; Tan, S.L.; Bouyahya, A.; Goh, K.W.; et al. Design of Three-Component Essential Oil Extract Mixture from Cymbopogon flexuosus, Carum carvi, and Acorus calamus with Enhanced Antioxidant Activity. Sci. Rep. 2024, 14, 9195. [Google Scholar] [CrossRef]
- Czerniewicz, P.; Sytykiewicz, H.; Chrzanowski, G. The Effect of Essential Oils from Asteraceae Plants on Behavior and Selected Physiological Parameters of the Bird Cherry-Oat Aphid. Molecules 2024, 29, 1673. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 5th ed.; Texensis Publishing: Gruver, TX, USA, 2017; ISBN 978-0-9981557-2-2. [Google Scholar]
- National Institute of Standards and Technology (NIST). In NIST Mass Spectral Library, 2023rd ed.; U.S. Department of Commerce: Washington, DC, USA, 2023. Available online: https://www.nist.gov/srd/nist-standard-reference-database-1a (accessed on 2 January 2025).
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of Free Radical Method to Evaluate Antioxidant Activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
| Chemical Constituents | RT | RI | Peak Area (%) |
|---|---|---|---|
| α-Pinene | 11.00 | 931 | 0.4 |
| Camphene | 11.56 | 946 | 2.1 |
| Sabinene | 12.55 | 971 | 0.1 |
| β-Pinene | 12.64 | 974 | 0.1 |
| α-Phellandrene | 13.75 | 1003 | <0.1 |
| p-Cymene | 14.53 | 1023 | 0.1 |
| Limonene | 14.69 | 1027 | 0.1 |
| 1,8-Cineole | 14.77 | 1029 | 0.1 |
| Linalool | 17.46 | 1099 | 0.3 |
| Camphor | 19.09 | 1143 | 3.5 |
| α-Copaene | 27.27 | 1377 | 0.2 |
| β-Elemene | 27.80 | 1393 | 0.2 |
| β-Cedrene | 28.53 | 1416 | 2.2 |
| Aristolene | 28.68 | 1421 | 1.6 |
| β-Copaene | 28.74 | 1423 | 1.1 |
| cis-Thujopsene | 28.98 | 1431 | 0.2 |
| β-Gurjunene | 29.11 | 1435 | 4.0 |
| (E)-α-Bergamotene | 29.18 | 1437 | 0.8 |
| Prezizaene | 29.57 | 1450 | 0.9 |
| Zizaene | 29.73 | 1455 | 2.6 |
| α-Acoradiene | 30.19 | 1469 | 0.2 |
| α-Neocallitropsene | 30.38 | 1475 | 0.5 |
| Ledene | 30.84 | 1490 | 1.0 |
| 6-Epishyobunone | 30.99 | 1495 | 2.0 |
| α-Selinene | 31.08 | 1498 | 4.0 |
| Shyobunone | 31.63 | 1516 | 7.5 |
| δ-Cadinene | 31.91 | 1526 | 1.0 |
| Isoshyobunone | 32.10 | 1532 | 5.7 |
| α-Calacorene | 32.51 | 1546 | 2.5 |
| β-Calacorene | 33.13 | 1567 | 0.6 |
| Spathulenol | 33.54 | 1581 | 1.3 |
| Caryophyllene oxide | 33.71 | 1586 | 0.8 |
| Cedrol | 34.27 | 1606 | 0.8 |
| Preisocalamendiol | 34.38 | 1610 | 12.0 |
| 1,2-Humulenepoxide | 34.48 | 1613 | 1.6 |
| β-Asarone | 34.81 | 1625 | 4.2 |
| Dehydroisocalamendiol | 35.08 | 1634 | 2.7 |
| Epi-α-muurolol | 35.41 | 1646 | 0.3 |
| 1-Isopropyl-4,8-dimethylspiro[4.5]decan-7-one | 35.50 | 1649 | 2.4 |
| α-Cadinol | 35.76 | 1658 | 0.9 |
| 10β-Hydroxy-cis-calamenene | 35.87 | 1662 | 0.9 |
| 10α-Hydroxy-cis-calamenene | 36.12 | 1671 | 0.4 |
| α-Asarone | 36.43 | 1682 | 0.6 |
| Acorenone | 36.74 | 1693 | 18.1 |
| Acora-7(11),9-dien-2-one | 37.69 | 1728 | 0.2 |
| Isocalamendiol | 38.24 | 1749 | 3.0 |
| Acorone isomer 1 | 39.92 | 1812 | 0.5 |
| Acorone isomer 2 | 40.45 | 1832 | 0.3 |
| Total | - | - | 96.6 |
| Chemical Constituents | RT | RI | Peak Area (%) |
|---|---|---|---|
| Tricyclene | 10.55 | 919 | 0.1 |
| α-Thujene | 10.79 | 926 | 0.5 |
| α-Pinene | 11.00 | 931 | 22.1 |
| Camphene | 11.56 | 946 | 0.3 |
| 2,4(10)-Thujadiene | 11.79 | 952 | <0.1 |
| Sabinene | 12.55 | 971 | 2.3 |
| β-Pinene | 12.64 | 974 | 1.8 |
| β-Myrcene | 13.30 | 991 | 3.2 |
| Δ3-Carene | 13.98 | 1009 | <0.1 |
| p-Cymene | 14.53 | 1023 | 3.6 |
| Limonene | 14.69 | 1027 | 2.3 |
| 1,8-Cineole | 14.77 | 1029 | 0.1 |
| (Z)-Sabinene hydrate | 16.19 | 1066 | 0.1 |
| α-Pinene oxide | 17.35 | 1096 | 1.1 |
| Perillene | 17.50 | 1100 | 0.2 |
| α-Fenchol | 17.94 | 1112 | <0.1 |
| β-Thujone | 18.04 | 1115 | 0.2 |
| Dehydrosabinaketone | 18.15 | 1118 | 0.1 |
| (Z)-p-Menth-2-en-1-ol | 18.24 | 1120 | 0.2 |
| α-Campholenal | 18.42 | 1125 | 0.4 |
| cis-Limonene-oxide | 18.69 | 1132 | <0.1 |
| trans-Pinocarveol | 18.89 | 1138 | 0.9 |
| cis-Verbenol | 19.13 | 1144 | 1.3 |
| (E)-p-Menth-2-en-1-ol | 19.24 | 1147 | 0.1 |
| Pinocarvone | 19.77 | 1162 | 0.3 |
| Borneol | 19.90 | 1165 | 0.4 |
| Terpinen-4-ol | 20.33 | 1177 | 3.3 |
| p-Cymen-8-ol | 20.62 | 1185 | 1.0 |
| α-Terpineol | 20.82 | 1190 | 0.7 |
| Myrtenal | 21.01 | 1195 | 0.5 |
| Methyl chavicol | 21.10 | 1198 | 0.5 |
| Verbenone | 21.48 | 1208 | 2.0 |
| trans-Carveol | 21.84 | 1219 | 0.3 |
| Cuminyl aldehyde | 22.57 | 1239 | 0.1 |
| Carvone | 22.72 | 1244 | 0.2 |
| p-Anisaldehyde | 23.07 | 1254 | 1.6 |
| p-Menth-2-en-1,4-diol | 23.60 | 1269 | 0.1 |
| trans-Anethol | 24.17 | 1285 | 0.6 |
| Bornyl acetate | 24.21 | 1286 | 0.7 |
| 2-Undecanone | 24.45 | 1293 | 0.2 |
| α-Cubebene | 26.38 | 1351 | 1.1 |
| Eugenol | 26.62 | 1358 | 0.1 |
| α-Ylangene | 27.12 | 1373 | 0.1 |
| α-Copaene | 27.27 | 1377 | 1.5 |
| β-Elemene | 27.80 | 1393 | 2.1 |
| Bicyclo-4(15)-oppositene | 28.24 | 1407 | 0.1 |
| (E)-β-caryophyllene | 28.69 | 1421 | 0.4 |
| β-Copaene | 28.99 | 1431 | 0.1 |
| γ-Elemene | 29.12 | 1435 | 0.1 |
| (E)-α-Bergamotene | 29.18 | 1437 | 0.1 |
| Guaia-6,9-diene | 29.39 | 1444 | 0.1 |
| α-Caryophyllene | 29.77 | 1456 | 0.7 |
| γ-Muurolene | 30.48 | 1479 | 1.5 |
| β-Selinene | 30.81 | 1489 | 1.3 |
| epi-Cubebol | 31.06 | 1497 | 0.7 |
| γ-Cadinene | 31.64 | 1517 | 1.9 |
| trans-Calamenene | 31.90 | 1525 | 0.5 |
| Selina-3,7(11)-diene | 32.29 | 1539 | 0.1 |
| Nootkatene | 32.34 | 1540 | 0.2 |
| α-Calacorene | 32.47 | 1545 | 0.2 |
| Elemol | 32.68 | 1552 | 0.3 |
| β-Calacorene | 33.06 | 1565 | 0.2 |
| Mintoxide | 33.22 | 1570 | 0.2 |
| Spathulenol | 33.54 | 1581 | 3.1 |
| Caryophyllene oxide | 33,71 | 1586 | 3.7 |
| 4(14)-Salvialene-1-one | 34.02 | 1597 | 0.4 |
| trans-Caryophyllene oxide | 34.18 | 1602 | 0.2 |
| 1,2-Humulenepoxide | 34.48 | 1613 | 3.4 |
| 1-epi-Cubenol | 35.00 | 1631 | 1.0 |
| epi-α-Cadinol | 35.39 | 1645 | 1.8 |
| α-Muurolol | 35.51 | 1649 | 0.5 |
| β-Eudesmol | 35.64 | 1654 | 0.2 |
| α-Cadinol | 35.76 | 1658 | 2.3 |
| 10β-Hydroxy-cis-calamenene | 35.87 | 1662 | 0.4 |
| ar-Turmerone | 35.99 | 1666 | 0.2 |
| 10α-Hydroxy-cis-calamenene | 36.12 | 1671 | 0.7 |
| Cadalene | 36.32 | 1678 | 0.4 |
| Oplopanone | 38.04 | 1741 | 0.3 |
| Benzyl benzoate | 38.70 | 1766 | 0.1 |
| Manoyl oxide | 44.55 | 1997 | 0.1 |
| Abietatriene | 46.04 | 2060 | 0.4 |
| Total | - | - | 85.7 |
| Mixture Ratio (EOA:EOJ) | CI | Effect of Interaction |
|---|---|---|
| 1:1 | 0.805 | Synergism |
| 1:2 | 0.920 | Slight Synergism/Near-Additive |
| 2:1 | 0.904 | Slight Synergism/Near-Additive |
| Mixture Ratio (EOA:EOJ) | DPPH• | ABTS•+ | ||
|---|---|---|---|---|
| CI | Effect of Interaction | CI | Effect of Interaction | |
| 1:1 | 0.910 | Slight Synergism/Near-Additive | 0.815 | Synergism |
| 1:2 | 0.847 | Synergism | 0.718 | Synergism |
| 2:1 | 0.944 | Slight Synergism/Near-Additive | 0.850 | Synergism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sytykiewicz, H.; Łukasik, I.; Goławska, S. Chemical Composition, Anti-Tyrosinase and Antioxidant Potential of Essential Oils from Acorus calamus (L.) and Juniperus communis (L.). Molecules 2025, 30, 2417. https://doi.org/10.3390/molecules30112417
Sytykiewicz H, Łukasik I, Goławska S. Chemical Composition, Anti-Tyrosinase and Antioxidant Potential of Essential Oils from Acorus calamus (L.) and Juniperus communis (L.). Molecules. 2025; 30(11):2417. https://doi.org/10.3390/molecules30112417
Chicago/Turabian StyleSytykiewicz, Hubert, Iwona Łukasik, and Sylwia Goławska. 2025. "Chemical Composition, Anti-Tyrosinase and Antioxidant Potential of Essential Oils from Acorus calamus (L.) and Juniperus communis (L.)" Molecules 30, no. 11: 2417. https://doi.org/10.3390/molecules30112417
APA StyleSytykiewicz, H., Łukasik, I., & Goławska, S. (2025). Chemical Composition, Anti-Tyrosinase and Antioxidant Potential of Essential Oils from Acorus calamus (L.) and Juniperus communis (L.). Molecules, 30(11), 2417. https://doi.org/10.3390/molecules30112417







