Dependency of Catalytic Reactivity on the Characteristics of Expanded Graphites as Representatives of Carbonaceous Materials
Abstract
1. Introduction
2. Results and Discussion
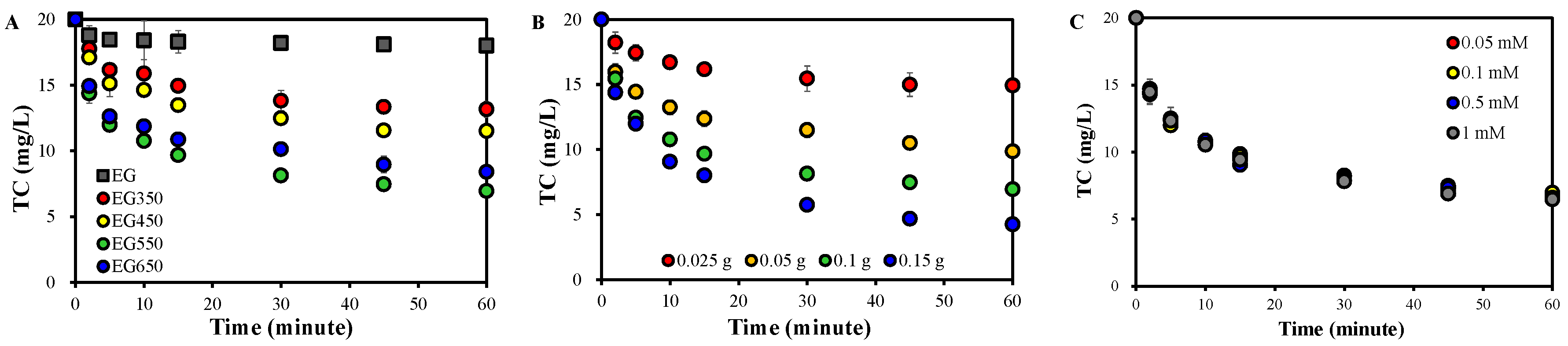
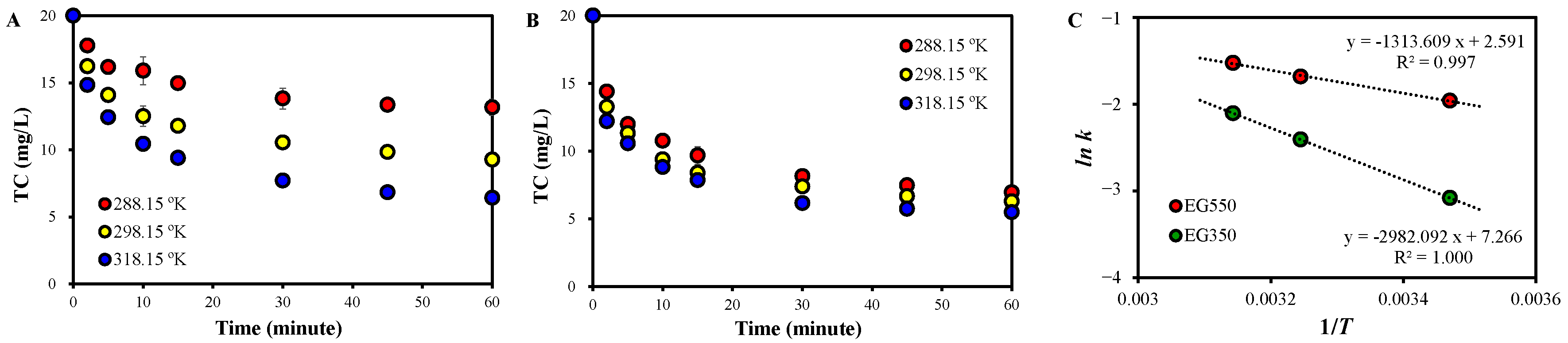
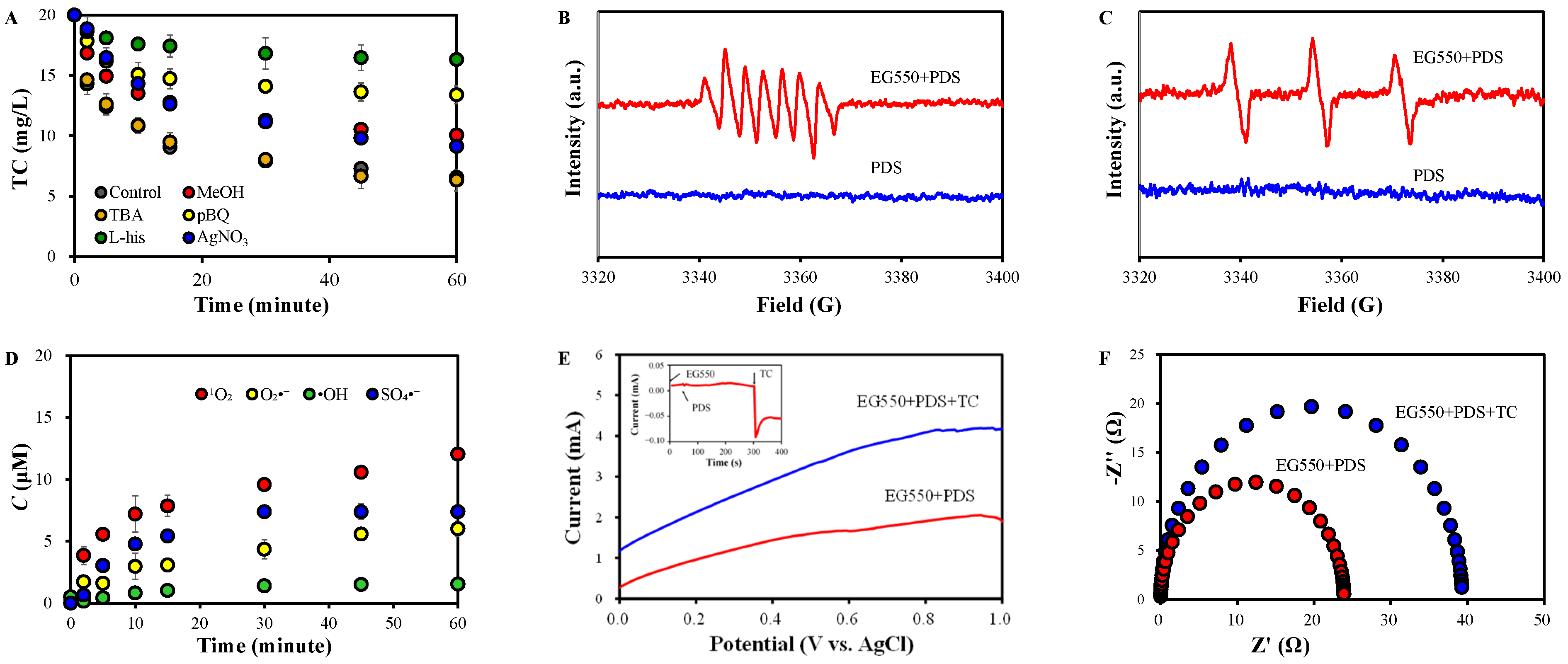
2.1. TC Removal
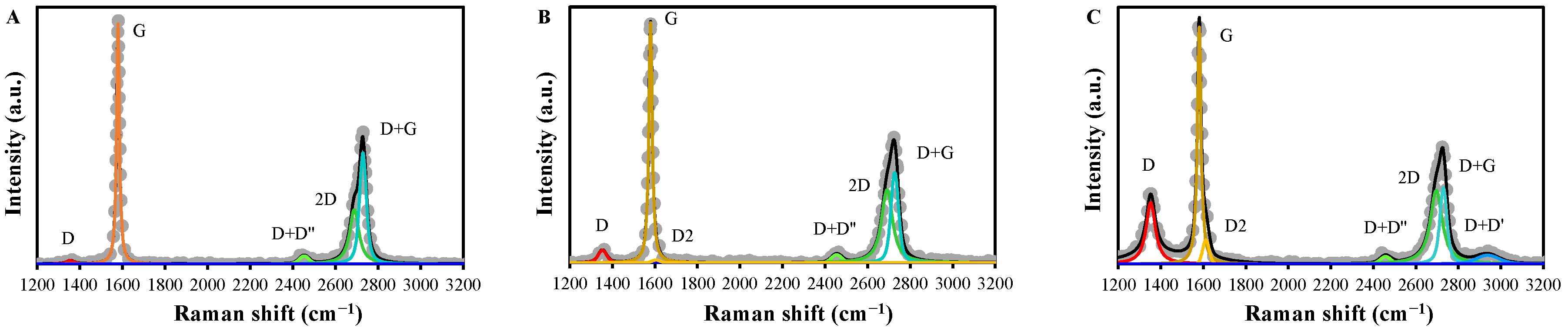
2.2. Characteristics of EGs
2.3. Identification of Reactive Species in EG550+PDS
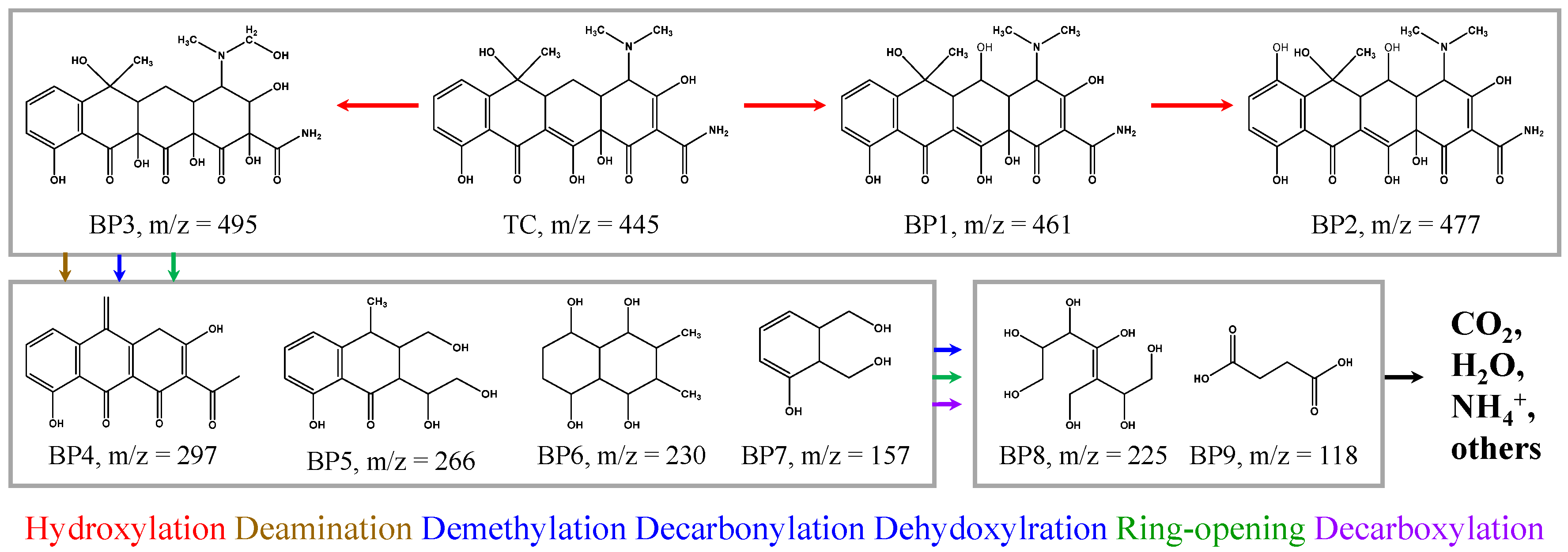
2.4. TC Degradation Intermediates and Pathways
2.5. Effects of Co-Existing Ions
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. TC Removal
3.4. Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | activated carbon |
| CM | carbonaceous material |
| CNT | carbon nanotube |
| DMPO | 5,5-dimethyl-1-pyrroline n-oxide |
| DPBF | 1,3-Diphenylisobenzofuran |
| EG | expanded graphite |
| ESR | electron spin resonance |
| FTIR | Fourier transform infrared |
| FWHM | full width at half maximum |
| gCN | graphitic carbon nitride |
| HBA | p-hydroxybenzoic acid |
| HPLC | high-performance liquid chromatography |
| L-his | L-histidine |
| MeOH | methyl alcohol |
| NBT | nitrotetrazolium blue chloride |
| Oads | adsorbed oxygen |
| pBQ | p-benzoquinone |
| PDS | peroxydisulfate |
| PMS | peroxymonosulfate |
| PVDF | polyvinylidene fluoride |
| SA | salicylic acid |
| SEM | scanning electron microscopy |
| SSA | specific surface |
| TBA | t-butanol |
| TC | tetracycline |
| TEMP | 2,2,6,6-tetramethylpiperidine |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
References
- Lee, J.; von Gunten, U.; Kim, J.-H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cui, Y.; Zhao, K.; Sun, H.; Zhang, W.; Kuang, P.; Ma, X.; Zhu, K.; Ma, K. Unleashing the Potential of Biomass-Doped Sludge Biochar: Promotion of Persulfate Activation by Biochar-Derived Dissolved Organic Matter. Sep. Purif. Technol. 2025, 361, 131468. [Google Scholar] [CrossRef]
- Chen, X.; Oh, W.-D.; Lim, T.-T. Graphene- and CNTs-Based Carbocatalysts in Persulfates Activation: Material Design and Catalytic Mechanisms. Chem. Eng. J. 2018, 354, 941–976. [Google Scholar] [CrossRef]
- Liu, S.; Lai, C.; Zhou, X.; Zhang, C.; Chen, L.; Yan, H.; Qin, L.; Huang, D.; Ye, H.; Chen, W.; et al. Peroxydisulfate Activation by Sulfur-Doped Ordered Mesoporous Carbon: Insight into the Intrinsic Relationship between Defects and 1O2 Generation. Water Res. 2022, 221, 118797. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, D.; Zhang, R.; Ding, Y.; Ren, Z.; Fu, M.; Cao, X.; Zeng, G. Singlet Oxygen-Dominated Activation of Peroxymonosulfate by Passion Fruit Shell Derived Biochar for Catalytic Degradation of Tetracycline through a Non-Radical Oxidation Pathway. J. Hazard. Mater. 2021, 419, 126495. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Kim, D.G.; Ko, S.O. Catalytic Degradation of Acetaminophen by C and O Co-Doped Graphitic Carbon Nitride: Peroxymonosulfate vs. Peroxydisulfate. Chem. Eng. J. 2024, 480, 148348. [Google Scholar] [CrossRef]
- Reza, M.S.; Afroze, S.; Kuterbekov, K.; Kabyshev, A.; Bekmyrza, K.Z.; Haque, M.N.; Islam, S.N.; Hossain, M.A.; Hassan, M.; Roy, H.; et al. Advanced Applications of Carbonaceous Materials in Sustainable Water Treatment, Energy Storage, and CO2 Capture: A Comprehensive Review. Sustainability 2023, 15, 8815. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Ali, S.; Mohyuddin, A.; Haider, A.; Riaz, M.; Khan, S.; Othman, M.H.D.; Goh, H.H.; Anouzla, A.; Aziz, F.; et al. Cultivating Sustainability: Harnessing Biochar-Derived Composites for Carbon-Neutral Wastewater Treatment. Process Saf. Environ. Prot. 2024, 187, 665–697. [Google Scholar] [CrossRef]
- Zhang, H.-C.; Han, J.-J.; Zhang, X.; Guo, P.-C.; Xie, D.-H.; Sheng, G.-P. Undiscovered Multiple Roles of Multivalent Cations in the Pollutant Removal from Actual Water by Persulfate Activated by Carbon Materials. ACS EST Eng. 2021, 1, 1227–1235. [Google Scholar] [CrossRef]
- Zhong, Q.; Lin, Q.; Huang, R.; Fu, H.; Zhang, X.; Luo, H.; Xiao, R. Oxidative Degradation of Tetracycline Using Persulfate Activated by N and Cu Codoped Biochar. Chem. Eng. J. 2020, 380, 122608. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Z.; Chen, Y.; Zhou, X.; Kong, W.; Lv, L.; Xu, Q.; Gao, B.; Li, Q. In-Situ Synthesis of Manganese Oxide-carbon Nanocomposite and Its Application in Activating Persulfate for Bisphenol F Degradation. Sci. Total Environ. 2021, 772, 144953. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhao, Z.; Ren, Y.; Chen, B.; Rheinlander, C.; Moore, A.X.; Li, L. Excellent Cycle Stability of Fe Loaded on N-Doped Activated Carbon for Microwave Hydrogenolysis of Lignin. Chem. Eng. J. 2024, 502, 158001. [Google Scholar] [CrossRef]
- Zhu, K.; Shen, Y.; Hou, J.; Gao, J.; He, D.; Huang, J.; He, H.; Lei, L.; Chen, W. One-Step Synthesis of Nitrogen and Sulfur Co-Doped Mesoporous Graphite-like Carbon Nanosheets as a Bifunctional Material for Tetracycline Removal via Adsorption and Catalytic Degradation Processes: Performance and Mechanism. Chem. Eng. J. 2021, 412, 128521. [Google Scholar] [CrossRef]
- Liang, Y.; Cui, J.; Ning, C.; Zhang, F.; Liang, F.; Gao, J. Construction of Adsorption-Oxidation Bifunction-Oriented Sludge Biochar for Non-Radical Ofloxacin Degradation via Persulfate: Emphasizing the Important Role of N-Species and Graphitized Structure. Sep. Purif. Technol. 2025, 354, 129239. [Google Scholar] [CrossRef]
- Kim, D.-G.; Ko, S.-O. Effects of Thermal Modification of a Biochar on Persulfate Activation and Mechanisms of Catalytic Degradation of a Pharmaceutical. Chem. Eng. J. 2020, 399, 125377. [Google Scholar] [CrossRef]
- Elbidi, M.; Resul, M.F.M.G.; Rashid, S.A.; Salleh, M.A.M. Preparation of Eco-Friendly Mesoporous Expanded Graphite for Oil Sorption. J. Porous Mater. 2023, 30, 1359–1368. [Google Scholar] [CrossRef]
- Elbidi, M.; Salleh, M.A.M.; Rashid, S.A.; Resul, M.F.M.G. The Potential of Thermally Expanded Graphite in Oil Sorption Applications. RSC Adv. 2024, 14, 16466–16485. [Google Scholar] [CrossRef]
- Fang, X.; Feng, Y.; Li, X.; Ding, D.; Wang, X.; Zhang, D. Efficient Fenton-like Catalysis Enabled by Single Cobalt Atoms Anchored on Expanded Graphite: Remarkable Intrinsic Activity of Co-N4 Sites and the Enhanced Mass Transfer Facilitated by Gradient Mesopore Structure. Chem. Eng. J. 2024, 479, 147840. [Google Scholar] [CrossRef]
- Xu, M.; Zhou, H.; Wu, Z.; Li, N.; Xiong, Z.; Yao, G.; Lai, B. Efficient Degradation of Sulfamethoxazole by NiCo2O4 Modified Expanded Graphite Activated Peroxymonosulfate: Characterization, Mechanism and Degradation Intermediates. J. Hazard. Mater. 2020, 399, 123103. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Shang, J.; Shen, C.; Joseph, S.D. Chemical Aging Changed Aggregation Kinetics and Transport of Biochar Colloids. Environ. Sci. Technol. 2019, 53, 8136–8146. [Google Scholar] [CrossRef]
- Azam, K.; Shezad, N.; Shafiq, I.; Akhter, P.; Akhtar, F.; Jamil, F.; Shafique, S.; Park, Y.-K.; Hussain, M. A Review on Activated Carbon Modifications for the Treatment of Wastewater Containing Anionic Dyes. Chemosphere 2022, 306, 135566. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-G.; Kim, T.-H.; Ko, S.-O. Enhanced Catalytic Activity of a Coal-Based Powdered Activated Carbon by Thermal Treatment. Water 2022, 14, 3308. [Google Scholar] [CrossRef]
- Suh, S.I.; Woo, H.; Song, S.-Y.; Park, D.; Ahn, Y.-Y.; Kim, E.; Lee, H.; Kim, D.-W.; Lee, C.; Ok, Y.S.; et al. Comparative Assessment of Biochars from Multiple Sources Based on Persulfate Activation Capability: Role of Iron Component in Enhancing Thermal Treatment Effect on Carbocatalytic Performance. Appl. Catal. B Environ. 2023, 330, 122647. [Google Scholar] [CrossRef]
- Crincoli, K.R.; Jones, P.K.; Huling, S.G. Fenton-Driven Oxidation of Contaminant-Spent Granular Activated Carbon (GAC): GAC Selection and Implications. Sci. Total Environ. 2020, 734, 139435. [Google Scholar] [CrossRef]
- Scaria, J.; Anupama, K.V.; Nidheesh, P.V. Tetracyclines in the Environment: An Overview on the Occurrence, Fate, Toxicity, Detection, Removal Methods, and Sludge Management. Sci. Total Environ. 2021, 771, 145291. [Google Scholar] [CrossRef]
- Lundström, S.V.; Östman, M.; Bengtsson-Palme, J.; Rutgersson, C.; Thoudal, M.; Sircar, T.; Blanck, H.; Eriksson, K.M.; Tysklind, M.; Flach, C.-F.; et al. Minimal Selective Concentrations of Tetracycline in Complex Aquatic Bacterial Biofilms. Sci. Total Environ. 2016, 553, 587–595. [Google Scholar] [CrossRef]
- Huang, A.; Yan, M.; Lin, J.; Xu, L.; Gong, H.; Gong, H. A Review of Processes for Removing Antibiotics from Breeding Wastewater. Int. J. Environ. Res. Public Health 2021, 18, 4909. [Google Scholar] [CrossRef]
- Menz, J.; Olsson, O.; Kümmerer, K. Antibiotic Residues in Livestock Manure: Does the EU Risk Assessment Sufficiently Protect against Microbial Toxicity and Selection of Resistant Bacteria in the Environment? J. Hazard. Mater. 2019, 379, 120807. [Google Scholar] [CrossRef]
- Yin, G.; Sun, Z.; Gao, Y.; Xu, S. Preparation of Expanded Graphite for Malachite Green Dye Removal from Aqueous Solution. Microchem. J. 2021, 166, 106190. [Google Scholar] [CrossRef]
- Chen, Q.; Lua, A.C. Kinetic Reaction and Deactivation Studies on Thermocatalytic Decomposition of Methane by Electroless Nickel Plating Catalyst. Chem. Eng. J. 2020, 389, 124366. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, Y.; Lyu, L.; Zeng, Q.; Xing, X.; Hu, C. Electronic Structure Modulation of Graphitic Carbon Nitride by Oxygen Doping for Enhanced Catalytic Degradation of Organic Pollutants through Peroxymonosulfate Activation. Environ. Sci. Technol. 2018, 52, 14371–14380. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, P. Effect of Ozone Treatment on Properties of Expanded Graphite. Chem. Eng. J. 2011, 172, 1096–1102. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Thommes, M. Progress in the Physisorption Characterization of Nanoporous Gas Storage Materials. Engineering 2018, 4, 559–566. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Kim, D.G.; Ko, S.O. Changes in the Catalytic Activity of Oxygen-Doped Graphitic Carbon Nitride for the Repeated Degradation of Oxytetracycline. Chemosphere 2022, 307, 135870. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Xu, J.; Mei, Y.; Fan, S.; Xu, H. Effect of Carbonization Methods on the Properties of Tea Waste Biochars and Their Application in Tetracycline Removal from Aqueous Solutions. Chemosphere 2021, 267, 129283. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Shamiri, A. A Review on Surface Modification of Activated Carbon for Carbon Dioxide Adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, J.; Wang, J. Pyrolytic Characteristics of Pine Wood in a Slowly Heating and Gas Sweeping Fixed-Bed Reactor. J. Anal. Appl. Pyrolysis 2009, 84, 179–184. [Google Scholar] [CrossRef]
- Lee, A.Y.; Yang, K.; Anh, N.D.; Park, C.; Lee, S.M.; Lee, T.G.; Jeong, M.S. Raman Study of D* Band in Graphene Oxide and Its Correlation with Reduction. Appl. Surf. Sci. 2021, 536, 147990. [Google Scholar] [CrossRef]
- Jaworski, S.; Wierzbicki, M.; Sawosz, E.; Jung, A.; Gielerak, G.; Biernat, J.; Jaremek, H.; Łojkowski, W.; Woźniak, B.; Wojnarowicz, J.; et al. Graphene Oxide-Based Nanocomposites Decorated with Silver Nanoparticles as an Antibacterial Agent. Nanoscale Res. Lett. 2018, 13, 116. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, M.; Wang, S.; Li, H.; Kunsági-Máté, S.; Yin, S. Raman Spectroscopy of Strained Monolayer Graphene Modulated by Monodispersed Au Nanoparticles. Appl. Surf. Sci. 2023, 610, 155531. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Gu, P.; Ma, R.; Wen, T.; Zhao, G.; Li, L.; Ai, Y.; Hu, C.; Wang, X. Enhanced Photodegradation of Toxic Organic Pollutants Using Dual-Oxygen-Doped Porous g-C3N4: Mechanism Exploration from Both Experimental and DFT Studies. Appl. Catal. B Environ. 2019, 248, 1–10. [Google Scholar] [CrossRef]
- Ghosh, S.; Ganesan, K.; Polaki, S.R.; Ravindran, T.R.; Krishna, N.G.; Kamruddin, M.; Tyagi, A.K. Evolution and Defect Analysis of Vertical Graphene Nanosheets. J. Raman Spectrosc. 2014, 45, 642–649. [Google Scholar] [CrossRef]
- Saha, A.; Basiruddin, S.K.; Ray, S.C.; Roy, S.S.; Jana, N.R. Functionalized Graphene and Graphene Oxide Solution via Polyacrylate Coating. Nanoscale 2010, 2, 2777–2782. [Google Scholar] [CrossRef]
- Stevanović, G.; Mojović, Z.; Bogdanović, D.B.; Barudžija, T.; Jović-Jovičić, N.; Banković, P.; Ajduković, M. The Influence of Preparation Method on the Efficiency of Clay-Carbon Composites for Detection of 4-Aminoantipyrine. Mater. Chem. Phys. 2025, 340, 130881. [Google Scholar] [CrossRef]
- Theodosiou, A.; Spencer, B.F.; Counsell, J.; Jones, A.N. An XPS/UPS Study of the Surface/near-Surface Bonding in Nuclear Grade Graphites: A Comparison of Monatomic and Cluster Depth-Profiling Techniques. Appl. Surf. Sci. 2020, 508, 144764. [Google Scholar] [CrossRef]
- Pinder, J.W.; Major, G.H.; Baer, D.R.; Terry, J.; Whitten, J.E.; Čechal, J.; Crossman, J.D.; Lizarbe, A.J.; Jafari, S.; Easton, C.D.; et al. Avoiding Common Errors in X-Ray Photoelectron Spectroscopy Data Collection and Analysis, and Properly Reporting Instrument Parameters. Appl. Surf. Sci. Adv. 2024, 19, 100534. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, H.; Liu, C.; Ge, Y.; Wang, L.; Zhang, B.; He, H.; Liu, S. Influence of Functional Groups on Toxicity of Carbon Nanomaterials. Atmos. Chem. Phys. 2019, 19, 8175–8187. [Google Scholar] [CrossRef]
- Fan, C.; Wei, J.; Huang, H.; Pan, M.; Fu, Z. Chemical Feature of the Soot Emissions from a Diesel Engine Fueled with Methanol-Diesel Blends. Fuel 2021, 297, 120739. [Google Scholar] [CrossRef]
- Abd, A.A.; Othman, M.R.; Kim, J. A Review on Application of Activated Carbons for Carbon Dioxide Capture: Present Performance, Preparation, and Surface Modification for Further Improvement. Environ. Sci. Pollut. Res. 2021, 28, 43329–43364. [Google Scholar] [CrossRef]
- Nwamba, O.C.; Echeverria, E.; McIlroy, D.N.; Austin, A.; Shreeve, J.M.; Aston, D.E. Thermal Modification of Graphite for Fast Electron Transport and Increased Capacitance. ACS Appl. Nano Mater. 2019, 2, 228–240. [Google Scholar] [CrossRef]
- Yang, B.; Kang, H.; Ko, Y.-J.; Woo, H.; Gim, G.; Choi, J.; Kim, J.; Cho, K.; Kim, E.-J.; Lee, S.-G.; et al. Persulfate Activation by Nanodiamond-Derived Carbon Onions: Effect of Phase Transformation of the Inner Diamond Core on Reaction Kinetics and Mechanisms. Appl. Catal. B Environ. 2021, 293, 120205. [Google Scholar] [CrossRef]
- Komeily-Nia, Z.; Chen, J.-Y.; Nasri-Nasrabadi, B.; Lei, W.-W.; Yuan, B.; Zhang, J.; Qu, L.-T.; Gupta, A.; Li, J.-L. The Key Structural Features Governing the Free Radicals and Catalytic Activity of Graphite/Graphene Oxide. Phys. Chem. Chem. Phys. 2020, 22, 3112–3121. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Wang, B.; Gao, B.; Jiang, T.; Feng, Q.; Chen, M.; Zhang, J.; Zhang, X. Roles and Mechanisms of Carbonaceous Materials in Advanced Oxidation Coupling Processes for Degradation Organic Pollutants in Wastewater: A Review. Biochar 2023, 5, 86. [Google Scholar] [CrossRef]
- Bellunato, A.; Arjmandi Tash, H.; Cesa, Y.; Schneider, G.F. Chemistry at the Edge of Graphene. ChemPhysChem 2016, 17, 785–801. [Google Scholar] [CrossRef]
- Sharma, R.; Baik, J.H.; Perera, C.J.; Strano, M.S. Anomalously Large Reactivity of Single Graphene Layers and Edges toward Electron Transfer Chemistries. Nano Lett. 2010, 10, 398–405. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, Y.; Fan, Z.; Liu, F.; Li, A. Carbonate-Enhanced Catalytic Activity and Stability of Co3O4 Nanowires for 1O2-Driven Bisphenol A Degradation via Peroxymonosulfate Activation: Critical Roles of Electron and Proton Acceptors. J. Hazard. Mater. 2020, 393, 122395. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, Z.; Zhu, Y.; Li, T.; Hu, C. New Insights into the Generation of Singlet Oxygen in the Metal-Free Peroxymonosulfate Activation Process: Important Role of Electron-Deficient Carbon Atoms. Environ. Sci. Technol. 2020, 54, 1232–1241. [Google Scholar] [CrossRef]
- Liu, J.; Yao, Z.; Qiu, G.; Wan, Y.; Song, W.; Zeng, H.; Yang, F.; Zhao, D.; Yuan, W.; Ju, P.; et al. Generation of Reactive Oxygen Species through Dissolved Oxygen Activation on Defected Porous Carbon for Efficient Degradation of Antibiotics. Chem. Eng. J. 2023, 455, 140602. [Google Scholar] [CrossRef]
- Yin, R.; Guo, W.; Wang, H.; Du, J.; Wu, Q.; Chang, J.-S.; Ren, N. Singlet Oxygen-Dominated Peroxydisulfate Activation by Sludge-Derived Biochar for Sulfamethoxazole Degradation through a Nonradical Oxidation Pathway: Performance and Mechanism. Chem. Eng. J. 2019, 357, 589–599. [Google Scholar] [CrossRef]
- Tian, L.; Chen, P.; Jiang, X.-H.; Chen, L.-S.; Tong, L.-L.; Yang, H.-Y.; Fan, J.-P.; Wu, D.-S.; Zou, J.-P.; Luo, S.-L. Mineralization of Cyanides via a Novel Electro-Fenton System Generating −OH and O2•−. Water Res. 2022, 209, 117890. [Google Scholar] [CrossRef]
- Huang, B.-C.; Jiang, J.; Huang, G.-X.; Yu, H.-Q. Sludge Biochar-Based Catalysts for Improved Pollutant Degradation by Activating Peroxymonosulfate. J. Mater. Chem. A 2018, 6, 8978–8985. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, Y.; Chen, Z.; Zeng, Q.; Hu, C. Insights into the Difference in Metal-Free Activation of Peroxymonosulfate and Peroxydisulfate. Chem. Eng. J. 2020, 394, 123936. [Google Scholar] [CrossRef]
- Murugan, P.; Nagarajan, R.D.; Shetty, B.H.; Govindasamy, M.; Sundramoorthy, A.K. Recent Trends in the Applications of Thermally Expanded Graphite for Energy Storage and Sensors—A Review. Nanoscale Adv. 2021, 3, 6294–6309. [Google Scholar] [CrossRef]
- Origins of Electron-Transfer Regime in Persulfate-Based Nonradical Oxidation Processes|Environmental Science & Technology. Available online: https://pubs.acs.org/doi/10.1021/acs.est.1c05374 (accessed on 17 May 2025).
- Jiang, Y.; Gao, K.; Chen, T.; Xiong, Y.; Li, Y.; Addisu, A.; Pillai, S.C.; Dionysiou, D.D.; Wang, D. Regulating the Generation of Singlet Oxygen (1O2) in Advanced Oxidation Processes by Catalyst Design for Water Treatment. Chem. Eng. J. 2024, 500, 156532. [Google Scholar] [CrossRef]
- Liang, J.; Fu, L.; Gao, K.; Duan, X. Accelerating Radical Generation from Peroxymonosulfate by Confined Variable Co Species toward Ciprofloxacin Mineralization: ROS Quantification and Mechanisms Elucidation. Appl. Catal. B Environ. 2022, 315, 121542. [Google Scholar] [CrossRef]
- Yu, Q.; Ye, J.; Liu, G.; Liu, M.; Tang, M.; Li, L. Exploring Electron-Transfer Pathways in Co-Pyrolyzed Waste-Derived Carbocatalyst for Enhanced Peroxydisulfate Activation. Sep. Purif. Technol. 2025, 353, 128313. [Google Scholar] [CrossRef]
- Li, L.; Chen, N.; An, N.; Feng, C.; Zheng, Y.; Zhao, L.; Li, J.; Zhang, Z.; Wang, D.; Cai, Y.; et al. Persulfate Activation by Iron Complex: A Novel Non-Radical Strategy for Enhanced Tetracycline Degradation. Sep. Purif. Technol. 2025, 361, 131364. [Google Scholar] [CrossRef]
- Wu, C.; Zuo, H.; Du, H.; Zhang, S.; Wang, L.; Yan, Q. Construction of Layered Embedding Dual Z-Scheme Bi2O2CO3/g-C3N4/Bi2O3: Tetracycline Degradation Pathway, Toxicity Analysis and Mechanism Insight. Sep. Purif. Technol. 2022, 282, 120096. [Google Scholar] [CrossRef]
- Ao, X.; Sun, W.; Li, S.; Yang, C.; Li, C.; Lu, Z. Degradation of Tetracycline by Medium Pressure UV-Activated Peroxymonosulfate Process: Influencing Factors, Degradation Pathways, and Toxicity Evaluation. Chem. Eng. J. 2019, 361, 1053–1062. [Google Scholar] [CrossRef]
- Xu, A.; Wei, Y.; Zou, Q.; Zhang, W.; Jin, Y.; Wang, Z.; Yang, L.; Li, X. The Effects of Nonredox Metal Ions on the Activation of Peroxymonosulfate for Organic Pollutants Degradation in Aqueous Solution with Cobalt Based Catalysts: A New Mechanism Investigation. J. Hazard. Mater. 2020, 382, 121081. [Google Scholar] [CrossRef]
- Zhu, J.-P.; Lin, Y.-L.; Zhang, T.-Y.; Cao, T.-C.; Xu, B.; Pan, Y.; Zhang, X.-T.; Gao, N.-Y. Modelling of Iohexol Degradation in a Fe(II)-Activated Persulfate System. Chem. Eng. J. 2019, 367, 86–93. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Effect of Inorganic Anions on the Performance of Advanced Oxidation Processes for Degradation of Organic Contaminants. Chem. Eng. J. 2021, 411, 128392. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, H. Mn-Based Catalysts for Sulfate Radical-Based Advanced Oxidation Processes: A Review. Environ. Int. 2019, 133, 105141. [Google Scholar] [CrossRef]
- Jiang, M.; Lu, J.; Ji, Y.; Kong, D. Bicarbonate-Activated Persulfate Oxidation of Acetaminophen. Water Res. 2017, 116, 324–331. [Google Scholar] [CrossRef]
- Liang, C.; Wang, Z.-S.; Bruell, C.J. Influence of pH on Persulfate Oxidation of TCE at Ambient Temperatures. Chemosphere 2007, 66, 106–113. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Z.; Ye, C.; Deng, J.; Ma, X.; Xu, Y.; Wang, L.; Tang, Z.; Luo, H.; Li, X. Magnetic Co/Fe Nanocomposites Derived from Ferric Sludge as an Efficient Peroxymonosulfate Catalyst for Ciprofloxacin Degradation. Chem. Eng. J. 2022, 432, 134180. [Google Scholar] [CrossRef]
- Ma, J.; Li, H.; Chi, L.; Chen, H.; Chen, C. Changes in Activation Energy and Kinetics of Heat-Activated Persulfate Oxidation of Phenol in Response to Changes in pH and Temperature. Chemosphere 2017, 189, 86–93. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y. A Comprehensive Review on Persulfate Activation Treatment of Wastewater. Sci. Total Environ. 2022, 831, 154906. [Google Scholar] [CrossRef]
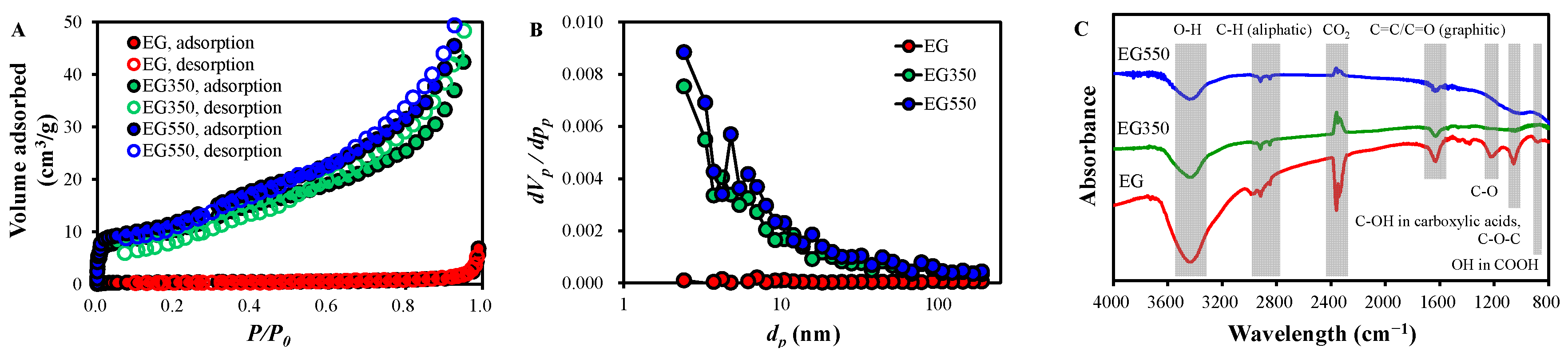
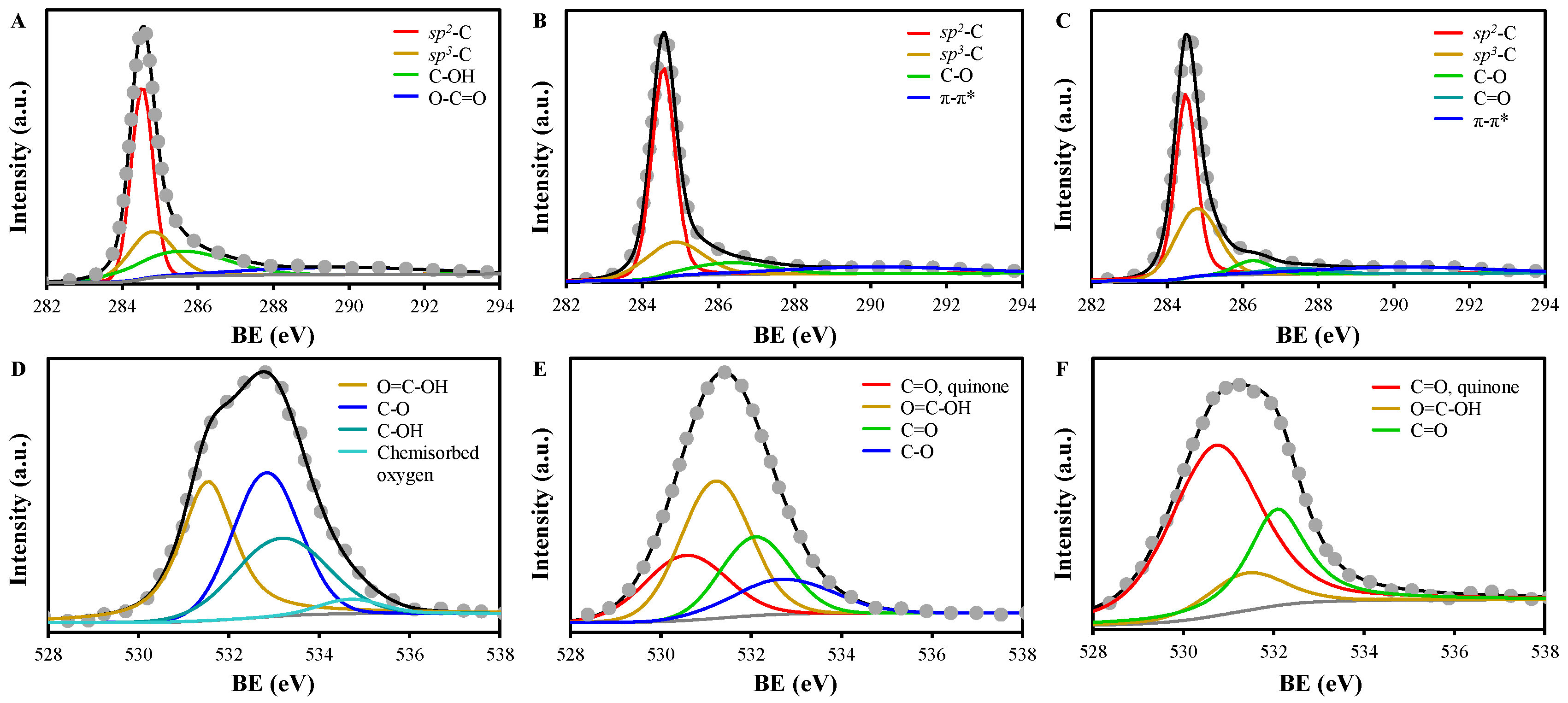

| SSA (m2/g) | Vt (cm3/g) | da (nm) | |
|---|---|---|---|
| EG | 1.209 | 0.0092 | 30.5 |
| EG350 | 34.942 | 0.1315 | 15.1 |
| EG550 | 40.219 | 0.1556 | 15.5 |
| D | G | D2 | D+D’’ | 2D | D+G | D+D’ | ID/IG | I2D/IG | ||
|---|---|---|---|---|---|---|---|---|---|---|
| EG | Center (cm−1) | 1357 | 1580 | 2453 | 2689 | 2729 | 0.04 | 0.75 | ||
| Area (%) | 1.4 | 35.4 | 3.1 | 26.4 | 33.8 | |||||
| FWHM (cm−1) | 43.3 | 15.9 | 53.9 | 52.1 | 38.5 | |||||
| EG | Center (cm−1) | 1353 | 1580 | 1606 | 2455 | 2692 | 2728 | 0.11 | 0.98 | |
| 350 | Area (%) | 3.8 | 34.9 | 0.6 | 2.4 | 34.1 | 24.2 | |||
| FWHM (cm−1) | 47.5 | 1.0 | 41.3 | 57.0 | 62.4 | 43.0 | ||||
| EG | Center (cm−1) | 1353 | 1581 | 1613 | 2458 | 2696 | 2730 | 2938 | 0.66 | 1.01 |
| 550 | Area (%) | 18.4 | 28.0 | 3.4 | 1.9 | 28.3 | 14.8 | 5.2 | ||
| FWHM (cm−1) | 53.0 | 20.9 | 36.4 | 59.3 | 67.7 | 39.6 | 125.8 | |||
| EG | Center (cm−1) | 1353 | 1579 | 1612 | 2462 | 2679 | 2719 | |||
| 650 | Area (%) | 1.3 | 34.8 | 0.8 | 1.4 | 16.0 | 45.7 | 0.04 | 0.46 | |
| FWHM (cm−1) | 23.9 | 23.6 | 17.4 | 46.7 | 100.1 | 41.7 |
| C1s | O1s | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sp2-C | sp3-C | C-OH | C-O | C=O | O-C=O | π-π* shake-up | C=O (quinone) | O=C-OH | C=O | C-O | C-OH | Oads a | ||
| EG | BE (eV) | 284.5 | 284.8 | 285.5 | 289.7 | 531.5 | 532.8 | 533.2 | 534.7 | |||||
| Fraction (%) | 42.6 | 21.5 | 22.9 | 13.0 | 36.1 | 35.0 | 25.7 | 3.2 | ||||||
| FWHM (eV) | 0.69 | 1.42 | 2.86 | 5.68 | 1.42 | 1.75 | 2.40 | 1.60 | ||||||
| EG | BE (eV) | 284.5 | 284.8 | 286.3 | 290.1 | 530.6 | 531.2 | 532.1 | 532.7 | |||||
| 350 | Fraction (%) | 56.4 | 21.0 | 10.2 | 12.4 | 22.4 | 40.6 | 22.5 | 14.5 | |||||
| FWHM (eV) | 0.73 | 1.82 | 2.55 | 5.50 | 2.13 | 1.82 | 1.80 | 2.59 | ||||||
| EG | BE (eV) | 284.5 | 284.8 | 286.3 | 287.3 | 290.2 | 530.7 | 531.4 | 532.1 | |||||
| 550 | Fraction (%) | 48.0 | 31.2 | 5.2 | 3.9 | 11.8 | 62.6 | 10.8 | 26.6 | |||||
| FWHM (eV) | 0.67 | 1.34 | 1.10 | 1.94 | 5.35 | 2.37 | 1.98 | 1.54 | ||||||
| EG | BE (eV) | 284.3 | 284.8 | 285.3 | 287.5 | 290.7 | 530.1 | 531.5 | 533.6 | |||||
| 650 | Fraction (%) | 1.8 | 61.6 | 25.5 | 4.5 | 6.7 | 31.8 | 37.7 | 30.5 | |||||
| FWHM (eV) | 0.60 | 0.74 | 2.26 | 2.23 | 3.56 | 1.94 | 2.09 | 4.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.G.; Im, S.W.; Ryu, K.H.; Jo, S.H.; Choe, M.G.; Ko, S.O. Dependency of Catalytic Reactivity on the Characteristics of Expanded Graphites as Representatives of Carbonaceous Materials. Molecules 2025, 30, 2275. https://doi.org/10.3390/molecules30112275
Kim DG, Im SW, Ryu KH, Jo SH, Choe MG, Ko SO. Dependency of Catalytic Reactivity on the Characteristics of Expanded Graphites as Representatives of Carbonaceous Materials. Molecules. 2025; 30(11):2275. https://doi.org/10.3390/molecules30112275
Chicago/Turabian StyleKim, Do Gun, Seong Won Im, Kyung Hwan Ryu, Seoung Ho Jo, Min Gyeong Choe, and Seok Oh Ko. 2025. "Dependency of Catalytic Reactivity on the Characteristics of Expanded Graphites as Representatives of Carbonaceous Materials" Molecules 30, no. 11: 2275. https://doi.org/10.3390/molecules30112275
APA StyleKim, D. G., Im, S. W., Ryu, K. H., Jo, S. H., Choe, M. G., & Ko, S. O. (2025). Dependency of Catalytic Reactivity on the Characteristics of Expanded Graphites as Representatives of Carbonaceous Materials. Molecules, 30(11), 2275. https://doi.org/10.3390/molecules30112275







