Abstract
Adsorbate-induced surface segregation significantly influences the catalytic and electrochemical performance of bimetallic alloys. Using density functional theory (DFT), we investigated Rh segregation in Au–Rh(111) alloys under the influence of adsorbed NO, CO, or O2. The computational results reveal that these adsorbates can markedly alter Rh segregation trends on the Au–Rh(111) surface. Under vacuum conditions, the Rh atom remains preferentially in the bulk of the alloy; whereas, in the presence of adsorption, it segregates to the topmost layer, where NO has the greatest influence, followed by CO and O2. Electronic structure analysis and adsorption energy evaluations further reveal that the strength of the surface–adsorbate interactions critically governs the Rh segregation behavior under reactive conditions. These findings establish a theoretical framework for designing Au–Rh alloys as efficient catalysts for CO oxidation.
1. Introduction
Bimetallic alloys are recognized for their potential to enhance the performance of catalysts through synergistic effects, making them a focal point of research in catalysis [1,2]. Consequently, they have attracted significant interest in both industrial applications and fundamental studies [3,4]. The catalytic efficacy of an alloy surface is strongly influenced by its composition and structural features. These surface characteristics frequently differ from the bulk material’s properties, primarily because of elemental segregation within the alloy [5,6]. Especially in a reactive environment, the composition and structure of an alloy surface may further change if one of the alloying elements interacts strongly with adsorbed molecules, causing adsorbate-induced segregation [7,8]. A wide range of experimental and computational research has revealed that the adsorbate-induced segregation could occur across a variety of bimetallic systems, exerting a profound influence on their catalytic behavior [9,10,11,12,13]. For instance, Wang et al. [11] reported that chemisorbed oxygen prompted Mo segregation in Ni-based hydrogen evolution electrodes, which in turn reduced the reaction rate. Gasteiger et al. [12] showed that the segregation of Co induced by O2 reduced the catalytic efficiency of Pt–Co catalysts in the oxygen reduction reaction. Similarly, McCrory et al. [13] observed that OH adsorption on the surface of Ni–Fe catalysts led to the segregation of Fe, significantly decreasing the oxygen evolution reaction activity. Therefore, it is critical to understand whether a bimetallic system with targeted properties remains stable under the environmental conditions of its intended application.
Among bimetallic systems, Au alloys are recognized as effective catalysts for reactions, such as hydrogen oxidation reaction [14], dehydrogenation reaction [15], hydrogen evolution reaction [16], hydrogenation reaction [17], oxygen reduction reaction [18], reforming reaction [19], water–gas shift reaction [20], and so forth. In the context of CO oxidation, many studies have demonstrated that Au alloys with Rh could enhance the catalytic activity for CO oxidation and further improve the stability of the catalytic reaction [21,22]. This is because Rh atoms could promote the formation of an Au-segregated surface structure with higher catalytic activity, which is conducive to the enhancement of catalytic stability for CO oxidation [22]. In addition, the results obtained by Mancilla et al. [21] also demonstrated that Au alloys with Rh tended to form Au-segregated surface structures, which not only exhibited superior catalytic activity, but prevented the oxidation of Rh during CO oxidation. These exceptional catalytic properties have also led to the use of Au–Rh alloys in automotive catalytic converters to improve efficiency and reduce emissions. However, in this practical application, the alloy catalysts are inevitably exposed to a variety of reactive gases, including NO, CO, and O2 [23]. The presence of these reaction gases may induce Rh segregation on the catalyst surface, which could significantly decrease the catalytic activity of the Au–Rh catalysts in the CO oxidation, thus affecting the efficiency of automotive catalytic converters [24]. However, there are few experimental and theoretical studies discussing which reactive gases lead to the experimentally observed segregation of Rh on alloy surfaces. Obviously, comprehensive theoretical calculations are essential for gaining a deeper understanding of the experimental observations.
In this study, we employ first-principles calculations to examine how chemisorbed NO, CO, or O2 influence the segregation tendencies of Rh atoms within the Au–Rh(111) alloy surfaces. The results reveal that these adsorbates significantly affect Rh segregation on the Au–Rh(111) surface. Under vacuum conditions, the Rh atom remains preferentially in the bulk of the alloy; whereas, in the presence of adsorption, it segregates to the topmost layer, where NO has the greatest influence, followed by CO and O2. Electronic structure analysis and adsorption energy evaluations further reveal that the strength of the surface–adsorbate interactions critically governs the Rh segregation behavior under reactive conditions. The remainder of this paper is structured as follows: Section 2 outlines the theoretical methods and computational details, Section 3 presents the results and discussion, and Section 4 provides a brief summary.
2. Materials and Methods
The spin-polarized DFT calculations were performed employing the Vienna Ab initio Simulation Program (VASP) [25,26,27] with the Perdew–Burke–Ernzerhof (PBE) functional [28] as exchange–correlation functional to obtain the energy. All calculations were conducted on the (111) crystallographic planes, which are recognized as the most thermodynamically stable configuration for face-centered cubic (fcc) metals. For core electron interactions, the projector augmented wave (PAW) approach was utilized [29,30]. A plane-wave cutoff energy of 400 eV was selected after optimization. To calculate electron occupancies, the Methfessel–Paxton method [31] with a width of the smearing in 0.2 eV was applied. The calculations are carried out using the 3 × 3 × 1 Monkhorst–Pack [32] mesh k-points for surface calculations, and the electric dipole was neglected. The converge criteria for force and electronic self-consistency were 0.02 eV/Å and 10−5 eV, respectively.
The DFT-calculated lattice constant for bulk Au is 4.16 Å, which is consistent with previously reported values [33,34,35]. The pure copper slab model included six atomic layers representing a 3 × 3 supercell, separated by 15 Å of vacuum space. This choice was based on prior studies of Au-based bimetallic systems [33,36,37], where the six–seven atomic layer was shown to balance computational cost and accuracy in surface energy and adsorption property calculations. In the Au–Rh alloy system, one Rh atom replaced one Au atom in each layer of the pure Au slab model, as illustrated in Figure 1. To simulate both bulk and surface environments, the bottom two layers of the slab were fixed, while the top four layers, along with the adsorbed reactive gases, were allowed to relax.
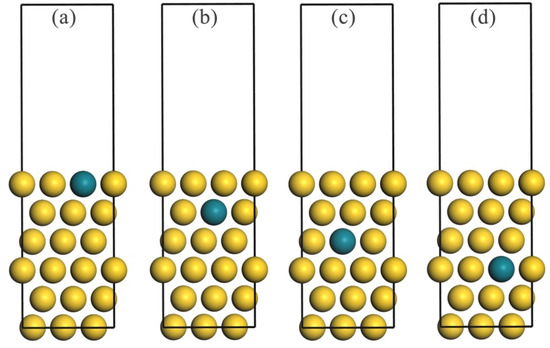
Figure 1.
The Au–Rh(111) alloy slab model showing substitution of an Au atom with an Rh atom in the (a) first, (b) second, (c) third, and (d) fourth atomic layer. Gold and green spheres correspond to Au and Rh atoms, respectively.
The surface segregation energy () is defined as the energy difference between configurations where the Rh atom occupies the upper surface layer versus the bulk [33,38]. Therefore, this parameter is computed using Equation (1) as follows:
In this equation, and denote the total energies of the Au–Rh alloy system. Here, Au–Rh(Rh, x-layer) corresponds to configurations where the Rh atom is positioned within the upper x-th Au atomic layer (x = 1, 2, or 3), while Au–Rh(Rh, 4th-layer) refers to the configuration where the Rh atom is positioned within the fourth Au layer, which is analogous to the “bulk” Au matrix. Equation (1) above indicates that the higher value of correspond to the increased difficulty in achieving surface segregation.
The adsorption energies () of molecular CO, O2, and NO adsorbed on the pure Au and Au–Rh(111) surfaces were computed using the following approach:
In this equation, refers to the total energy of the adsorbed system, denotes the bare surface energy, and represents the energy contribution from the reactive gas phase.
The d-band center, which quantifies the change in the d-states of the alloy component, is given by the following equation:
In this equation, E denotes the specified energy, while ρ(E) represents the electronic state density.
3. Results and Discussion
3.1. Adsorption Behavior
Given that both Au and Rh are metals with face-centered cubic structures, it is observed that CO, NO, and O2 molecules exhibit a preference for adsorption on the (111) surface of these metals [33,39]. For both the CO and NO molecules, four primary stable adsorption sites are identified on this surface: Top, Bridge, fcc, and hcp. For O2 molecules, the stable adsorption sites primarily comprise top–bridge–top (bridge), top–hcp–bridge (hcp), and top–fcc–bridge (fcc).
Table 1 summarizes the adsorption energies for the studied gas molecules on both Au(111) and Rh(111) surfaces. For the Au(111) surface, CO exhibits the strongest adsorption, surpassing NO and O2. Both CO and NO adopt a linear binding geometry, where the carbon atom of CO and the nitrogen atom of NO orient toward the surface. These molecules also share identical adsorption site preferences, specifically occupying the top sites on the (111) surface. Additionally, in accordance with the findings of the previous research [40,41], the Au(111) surface does not readily support the chemisorption of O2 unless external energy inputs, such as electron bombardment or UV irradiation, provide the necessary activation energy.

Table 1.
Adsorption energies (eV) for NO, CO, and O2 molecules on both Au(111) and Rh(111) surfaces. Configurations with the lowest energy are highlighted in bold.
For the Rh(111) surface, it was observed that the adsorption of CO is stronger than that of O2, but remains weaker than that of NO. Both CO and NO still adopt a linear binding geometry, and these molecules demonstrate a uniform adsorption site preference, exhibiting a marked predilection for occupying the fcc or hcp sites on the (111) surface. Additionally, in comparison with Au, Rh exhibits a higher affinity toward reactive gaseous molecules. For instance, the lowest adsorption energies for CO and NO on the Rh(111) surface were found to be −1.86 and −2.37 eV, respectively, whereas the relevant values for the same molecules on the Au(111) surface were −0.35 and −0.28 eV, respectively.
The adsorption configurations of gas molecules on the Au–Rh(111) surface have been systematically investigated, and the lowest adsorption energies of gas molecules on the alloy surface have been determined by calculations for different positions of the Rh atoms, as shown in Table 2. The calculated results reveal that, when the Rh atom is situated in the topmost layer of the Au–Rh(111) surface, the order of the adsorption strength is NO > CO > O2, and both NO and CO preferentially bind at the top site of Rh atom. Nevertheless, the most stable adsorption configuration of O2 on the alloy surface is top–fcc–bridge, where one oxygen atom occupies the top site of Rh and the other occupies the bridge site formed by two Au atoms. When the Rh atom migrates into the second layer of the Au–Rh(111) surface, the adsorption of O2 on the alloy surface is not stable, and both NO and CO preferentially occupy the top of the Au atom in close proximity to the Rh atom. Nevertheless, their adsorption strength is considerably diminished. Notably, when the Rh atom migrates into bulk regions (third and fourth layers), The adsorption behavior of the three gases on the alloy surface is very similar to that on the surface of Au(111).

Table 2.
The adsorption energies (eV) for NO, CO, and O2 molecules on the Au–Rh(111) surface for different positions of the Rh atom.
3.2. Segregation Behavior
The segregation energies of Rh in Au–Rh(111) alloys were computed with and without adsorbed NO, CO, and O2 molecules, as illustrated in Figure 2. For the clean surface, i.e., without adsorbed gas molecules, Rh exhibits a positive segregation energy of 0.53 eV in the first atomic layer, indicating a thermodynamic preference to remain within the bulk. Surface segregation in metallic alloys is fundamentally driven by the minimization of the system’s Gibbs free energy, where two dominant factors govern this process under vacuum conditions, including (1) the relative surface energies of constituent elements, and (2) the elastic strain energy relaxation [42,43]. While the entropy-driven randomization of the atomic distributions becomes significant at elevated temperatures, its contribution is inherently excluded in our T = 0 K DFT framework. For clean surfaces, elements with higher surface energies and smaller atomic radii tend to resist segregation to the surface [6,38]. These principles explain Rh’s bulk preference in Au–Rh alloys: Rh exhibits a surface energy nearly double that of Au (2.7 vs. 1.5 eV/atom [44]) and a smaller atomic radius (2.34 Å vs. 2.43 Å for Au [45]), creating both thermodynamic and steric barriers to surface migration. This interpretation aligns with prior computational studies showing limited Au segregation in Rh-rich matrices under vacuum [46,47], confirming that surface energy disparities and atomic size effects dominate segregation behavior in the absence of adsorbates. When Rh is situated in the second layer of the alloy, the segregation energy decreases significantly, with a value of only 0.12 eV. Notably, when Rh is situated in the third layer of the alloy, the value of the segregation energy is close to zero, suggesting this layer can be considered to be a bulk layer like the fourth layer. Analogous behavior is observed under gas adsorption conditions.
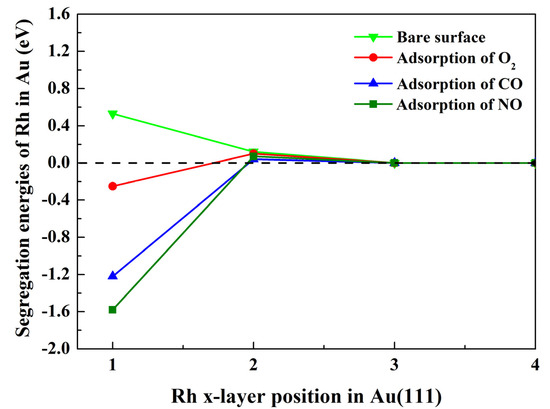
Figure 2.
Evolution of the segregation energies (eV) of Rh from the Au ‘bulk’ (4th layer) to the upper surface layers with and without adsorbed NO, CO, and O2 molecules.
In the case of the adsorption of gas molecules, when Rh is situated in the first atomic layer, the segregation energy is reversed to negative values. These were found to be −1.58 eV, −1.22 eV, and −0.25 eV, for NO, CO, and O2, respectively. Negative segregation energies indicate a thermodynamic preference for Rh to segregate to the Au–Rh(111) surface under adsorbate influence, and the order of influence on Rh segregation is NO > CO > O2. This phenomenon aligns with the Le Chatelier principle: adsorbate-induced surface chemical potential gradients drive segregation to stabilize the system [48]. In fact, some adsorbate-induced negative segregation has been validated in Au–Rh systems. For example, DFT calculations by Sansa et al. [37] predicted Rh segregation in the Au–Rh(111) surface under CO adsorption, closely matching our calculated results. The experimental studies by Mancilla et al. [21] and Wang et al. [22] demonstrated CO-induced Rh segregation in Au–Rh alloys, consistent with our DFT predictions for CO–Au–Rh systems. According to the findings of previous studies [8,49], adsorbate-induced segregation is determined by a combination of three factors. These factors comprise the surface energy of the alloying element, the atomic radius of the alloying element, and the adsorption strength between the adsorbed gas and the alloy surface. There are two offsetting factors that limit this segregation trend. One is that the surface energy of Rh is higher than that of Au [44], and the other is that the atomic radius of Rh is smaller than that of Au [45]. In addition, the standard DFT calculations are performed at 0 K and do not explicitly include temperature effects. However, even if the temperature effect is not taken into account, it does not affect the tendency of Rh to segregate. This is because, for strong adsorbates, the chemisorption effect dominates over thermal energy. Therefore, this change in the trend of segregation is attributed to the stronger binding of NO, CO, and O2 to the alloy surface.
3.3. Electronic Structure Analysis
To evaluate the local effect of Rh alloying on the electronic characteristics of the Au–Rh(111) surface, the d-band DOS for Rh and Au atoms in the outermost surface layer under vacuum conditions was calculated, as illustrated in Figure 3. Relative to pure Rh, Rh within the alloy displays a narrowed d-band DOS shifted toward higher energy levels near the Fermi energy (Figure 3a). This finding implies electron donation from Rh to adjacent Au atoms, which results in an increase in d-band electrons in Au. This will cause the d-band center for Au to shift down, away from the Fermi energy level. This phenomenon is consistent with the results shown in Figure 3b. According to the d-band center model developed by Hammer and Nørskov [50,51], these shifts of the d-band centers, as shown in Figure 3, are closely related to the gas adsorption strength, which will drive the segregation of Rh on the surface of the alloy.
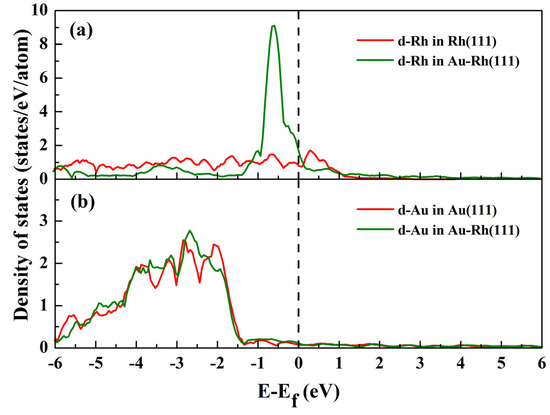
Figure 3.
Calculated density of states (DOS) for Rh and Au atoms in the alloy and pure metal surfaces. (a,b) present the d-band DOS for Rh and Au atoms in Au–Rh(111) surfaces, respectively. The d-band DOS for pure metal surfaces is presented for comparison.
To further explore the preferential location of the Rh atom within the Au ‘bulk’ without adsorption and the surface segregation with adsorption, we evaluated the d-band DOS for the Rh atom located in three upper layers of the Au–Rh(111) surface without adsorption (Figure 4a), and in the first layer of the Au–Rh(111) surface with adsorption (Figure 4b), respectively. As shown in Figure 4a, the shape and position of the Rh atom’s d-band DOS depend critically on its position within the surface layers. When Rh migrates from the third to the outermost atomic layer, its d-band DOS progressively shifts to higher energy levels and narrows in width. This demonstrates that electronic interactions under vacuum conditions govern Rh’s preferential occupancy within the Au ‘bulk’ structure [33]. As shown in Figure 4b, the d-band DOS for surface Rh atoms in the alloy exhibits significant broadening under adsorbate exposure, particularly with NO adsorption. This narrowing in DOS under non-adsorbed conditions arises from the reduced coordination of surface atoms relative to bulk atoms. In adsorbed systems, the presence of adsorbates enhances surface atom coordination, leading to a broadening of the DOS [52]. Additionally, the broadened DOS shifts toward lower energy levels under adsorption conditions. Such a redistribution likely lowers the system’s total energy, thereby enhancing its thermodynamic stability.
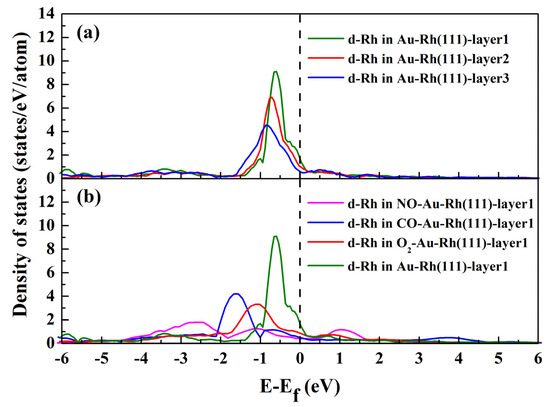
Figure 4.
Calculated d-band DOS for the Rh atom located in three upper layers of Au–Rh(111) surface without adsorption (a), and in the first layer of Au–Rh(111) surface with adsorption (b), respectively.
To probe how adsorbates modulate the electronic structure of the Au–Rh(111) surface, we performed Bader charge analysis on the top-layer Rh atoms under both clean and adsorbate-covered conditions. In the absence of adsorbates, the Au atoms gained 0.1 electrons from their neighboring Rh atom, demonstrating significant charge transfer that stabilizes Ni within the bulk through covalent Cu–Ni bonding. However, adsorbate interactions dramatically alter this charge balance: when NO, CO, and O2 adsorb onto the surface, they gained 0.14, 0.15, and 0.18 electrons from Rh atom, respectively. This electron depletion weakens the covalent Au–Rh bonds that originally anchored Rh in the bulk, creating a thermodynamic driving force for Rh segregation toward the surface to compensate for the charge loss.
4. Conclusions
First-principle computational methods were employed to investigate Rh segregation in Au–Rh(111) alloys under the influence of adsorbed NO, CO, or O2. Our findings reveal that these adsorbates can markedly alter Rh segregation trends on the Au–Rh(111) surface. Under vacuum conditions, Rh atom remains preferentially in the bulk of the alloy; whereas, in the presence of adsorption, it segregates to the topmost layer, where NO has the greatest influence, followed by CO and O2. Examining the adsorption behavior of these adsorbates on Au–Rh(111) demonstrates a binding strength order of NO > CO > O2, with all three adsorbates exhibiting stronger affinity for Rh than Au. An electronic structure analysis for the clean Au–Rh(111) surface shows that electron donation from Rh to the adjacent Au atoms results in an increase in d-band electrons in Au. This will cause the d-band center for Au to shift down, away from the Fermi energy level. The shape and position of Rh’s d-band DOS demonstrates that electronic interactions under vacuum conditions govern Rh’s preferential occupancy within the Au ‘bulk’ structure. Notably, in the presence of adsorption, the d-band DOS broadens and shifts to lower energies, potentially lowering the system’s total energy and enhancing stability. These insights offer a theoretical foundation for leveraging Au–Rh alloys in CO oxidation catalysis.
Author Contributions
Conceptualization, Y.Y.; methodology, Y.W.; software, G.Z.; validation, Y.K.; formal analysis, H.G.; investigation, Y.K.; resources, H.G.; data curation, Y.L.; writing—original draft preparation, Y.W.; writing—review and editing, Y.Y.; visualization, Y.L.; supervision, Y.Y.; project administration, Q.H.; funding acquisition, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Specialized Fund for the Doctoral of Kaili University, grant number: BS202502013.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Acknowledgments
The authors would like to acknowledge the support of the Specialized Fund for the Doctoral of Kaili University (BS202502013) and the National Natural Science Foundation of China (51661013 and 12064019).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, S.; Jelic, J.; Rein, D.; Najafishirtari, S.; Schmidt, F.P.; Girgsdies, F.; Kang, L.; Wandzilak, A.; Rabe, A.; Doronkin, D.E. Highly loaded bimetallic iron-cobalt catalysts for hydrogen release from ammonia. Nat. Commun. 2024, 15, 871. [Google Scholar] [CrossRef]
- Gholinejad, M.; Bashirimousavi, S.; Sansano, J.M. Novel magnetic bimetallic AuCu catalyst for reduction of nitroarenes and degradation of organic dyes. Sci. Rep. 2024, 14, 5852. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Yang, F.; Chen, S.; Wu, H.; Yang, J.; Shen, F. Highly Efficient Catalytic Oxidation of Glucose to Formic Acid over Mn-Mo Doped Carbon Nanotube. Molecules 2025, 30, 1639. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Zhu, S.; Hitch, M. Electronic–Oxygen Synergy at Ca-Fe Dual-Metal Interfaces for Selective Syngas Regulation in Biomass Chemical Looping Gasification. Molecules 2025, 30, 1471. [Google Scholar] [CrossRef]
- Yu, Y.; Hu, Q.; Xiao, W.; Wang, J.; Wang, L. Design of highly efficient Ni-based water-electrolysis catalysts by a third transition metal addition into Ni3Mo. Intermetallics 2018, 94, 99–105. [Google Scholar] [CrossRef]
- Yu, Y.; Xiao, W.; Wang, J.; Wang, L. Understanding the surface segregation behavior of transition metals on Ni (111): A first-principles study. Phys. Chem. Chem. Phys. 2016, 18, 26616–26622. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Z.; Huang, W.; Zhou, S.; Hu, Z.; Wang, L. Density functional theory study of Ni segregation in CuNi (111) alloy with chemisorbed CO, O, or H. J. Phys. Chem. Solids 2022, 171, 111021. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, W.; Liu, Z.; Hu, Z.; Wang, L. First-principles study of surface segregation in bimetallic Cu3M (1 1 1) (M= Au, Ag, and Zn) alloys in presence of adsorbed CO. Comput. Mater. Sci. 2022, 212, 111550. [Google Scholar] [CrossRef]
- Mashkovsky, I.; Bukhtiyarov, A.; Markov, P.; Bragina, G.; Baeva, G.; Smirnova, N.; Panafidin, M.; Chetyrin, I.; Gerasimov, E.Y.; Zubavichus, Y. Catalytic performance of a single atom Pd1Ag10/Al2O3 catalyst for the selective hydrogenation of acetylene: The role of CO-induced segregation. Appl. Surf. Sci. 2025, 681, 161516. [Google Scholar] [CrossRef]
- Lee, K.S.; Park, H.Y.; Ham, H.C.; Yoo, S.J.; Kim, H.J.; Cho, E.; Manthiram, A.; Jang, J.H. Reversible surface segregation of Pt in a Pt3Au/C catalyst and its effect on the oxygen reduction reaction. J. Phys. Chem. C 2013, 117, 9164–9170. [Google Scholar] [CrossRef]
- Wang, J.W.; Wang, Y.F.; Zhang, J.G.; Yu, Y.L.; Zhou, G.G. Optimization of electrocatalytic properties of NiMoCo foam electrode for water electrolysis by post-treatment processing. Rare Met. 2015, 34, 802–807. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Kocha, S.S.; Sompalli, B.; Wagner, F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B Environ. 2005, 56, 9–35. [Google Scholar] [CrossRef]
- McCrory, C.C.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J. Am. Chem. Soc. 2015, 137, 4347–4357. [Google Scholar] [CrossRef]
- Lu, L.; Peng, L.; Li, L.; Li, J.; Huang, X.; Wei, Z. Improved hydrogen oxidation reaction under alkaline conditions by Au–Pt alloy nanoparticles. J. Energy Chem. 2020, 40, 52–56. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, P.; Huang, X.; Hu, C.; Wen, Z.; Lu, Z.H. Anchoring PdAu nanoclusters inside aminated metal-organic framework for fast dehydrogenation of formic acid. Fuel 2024, 359, 130459. [Google Scholar] [CrossRef]
- Weng, X.; Liu, Y.; Wang, K.K.; Feng, J.J.; Yuan, J.; Wang, A.J.; Xu, Q.Q. Single-step aqueous synthesis of AuPt alloy nanodendrites with superior electrocatalytic activity for oxygen reduction and hydrogen evolution reaction. Int. J. Hydrogen Energy 2016, 41, 18193–18202. [Google Scholar] [CrossRef]
- Wei, H.; Wei, X.; Yang, X.; Yin, G.; Wang, A.; Liu, X.; Huang, Y.; Zhang, T. Supported Au-Ni nano-alloy catalysts for the chemoselective hydrogenation of nitroarenes. Chin. J. Catal. 2015, 36, 160–167. [Google Scholar] [CrossRef]
- Luo, J.; Njoki, P.N.; Lin, Y.; Wang, L.; Zhong, C.J. Activity-composition correlation of AuPt alloy nanoparticle catalysts in electrocatalytic reduction of oxygen. Electrochem. Commun. 2006, 8, 581–587. [Google Scholar] [CrossRef]
- Chin, Y.; King, D.L.; Roh, H.S.; Wang, Y.; Heald, S.M. Structure and reactivity investigations on supported bimetallic AuNi catalysts used for hydrocarbon steam reforming. J. Catal. 2006, 244, 153–162. [Google Scholar] [CrossRef]
- Barakat, T.; Rooke, J.C.; Genty, E.; Cousin, R.; Siffert, S.; Su, B.L. Gold catalysts in environmental remediation and water-gas shift technologies. Energy Environ. Sci. 2013, 6, 371–391. [Google Scholar] [CrossRef]
- Mancilla, A.; Mendoza-Cruz, R.; Portales, B.; Zanella, R. Catalytic oxidation of carbon monoxide at low temperature using AuRh/TiO2 bimetallic catalysts: Effect of the synthesis method. Mater. Today Chem. 2024, 38, 102092. [Google Scholar] [CrossRef]
- Wang, X.; Lu, G.; Guo, Y.; Zhang, Z.; Guo, Y. Role of Rh promoter on increasing stability of Au/Al2O3 catalyst for CO oxidation at low temperature. Environ. Chem. Lett. 2011, 9, 185–189. [Google Scholar] [CrossRef]
- Van Delft, F.; Siera, J.; Nieuwenhuys, B. The transient behaviour of Pt-Rh (410) alloy surfaces upon interaction with O2, NO and CO. Surf. Sci. 1989, 208, 365–382. [Google Scholar] [CrossRef]
- Piccolo, L.; Li, Z.; Demiroglu, I.; Moyon, F.; Konuspayeva, Z.; Berhault, G.; Afanasiev, P.; Lefebvre, W.; Yuan, J.; Johnston, R.L. Understanding and controlling the structure and segregation behaviour of AuRh nanocatalysts. Sci. Rep. 2016, 6, 35226. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B 1993, 49, 14251–14269. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B Condens. Matter 1994, 50, 2665–2668. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Methfessel, M.; Paxton, A.T. High-precision sampling for Brillouin-zone integration in metals. Phys. Rev. B Condens. Matter 1989, 40, 3616–3621. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Hendrik, J.; James, D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Dhifallah, M.; Dhouib, A.; Aldulaijan, S.; Di Renzo, F.; Guesmi, H. First-principles study of Au–Cu alloy surface changes induced by gas adsorption of CO, NO, or O2. J. Chem. Phys. 2016, 145, 024701. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Dai, C.; Fisher, A.; Shen, Y.; Cheng, D. A full understanding of oxygen reduction reaction mechanism on Au (1 1 1) surface. J. Phys.-Condens. Matter 2017, 29, 365201. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Triangular nanoplates of silver: Synthesis, characterization, and use as sacrificial templates for generating triangular nanorings of gold. Adv. Mater. 2003, 15, 695–699. [Google Scholar] [CrossRef]
- Dhouib, A.; Guesmi, H. DFT study of the M segregation on MAu alloys (M=Ni, Pd, Pt) in presence of adsorbed oxygen O and O2. Chem. Phys. Lett. 2012, 521, 98–103. [Google Scholar] [CrossRef]
- Sansa, M.; Dhouib, A.; Guesmi, H. Density functional theory study of CO-induced segregation in gold-based alloys. J. Chem. Phys. 2014, 141, 064709. [Google Scholar] [CrossRef]
- Yu, Y.; Xiao, W.; Wang, J.; Wang, L. First-Principles Study of Mo Segregation in MoNi(111): Effects of Chemisorbed Atomic Oxygen. Materials 2016, 9, 5. [Google Scholar] [CrossRef]
- Ishikawa, A.; Tateyama, Y. First-principles microkinetic analysis of NO+ CO reactions on Rh (111) surface toward understanding NOx reduction pathways. J. Phys. Chem. C 2018, 122, 17378–17388. [Google Scholar] [CrossRef]
- Mavrikakis, M.; Stoltze, P.; Nørskov, J.K. Making gold less noble. Catal. Lett. 2000, 64, 101–106. [Google Scholar] [CrossRef]
- Gottfried, J.; Schmidt, K.; Schroeder, S.; Christmann, K. Spontaneous and electron-induced adsorption of oxygen on Au (110)-(1×2). Surf. Sci. 2002, 511, 65–82. [Google Scholar] [CrossRef]
- Wang, G.; Hove, M.A.V.; Ross, P.N.; Baskes, M.I. Quantitative prediction of surface segregation in bimetallic Pt–M alloy nanoparticles (M=Ni, Re, Mo). Prog. Surf. Sci. 2005, 79, 28–45. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, Z.; Xiao, C.; Wang, G. Density functional theory calculation of platinum surface segregation energy in Pt3Ni (111) surface doped with a third transition metal. Surf. Sci. 2011, 605, 1577–1582. [Google Scholar] [CrossRef]
- DeBoer, F.R.; Boom, R.; Miedema, A.R. Cohesion in Metals, 2nd ed.; North-Holland Physics Publishing: Amsterdam, The Netherlands, 1989; pp. 657–660. [Google Scholar]
- Allinger, N.L.; Zhou, X.; Bergsma, J. Molecular mechanics parameters. J. Mol. Struct. THEOCHEM 1994, 312, 69–83. [Google Scholar] [CrossRef]
- Ruban, A.V.; Skriver, H.L. Calculated surface segregation in transition metal alloys. Comput. Mater. Sci. 1999, 15, 119–143. [Google Scholar] [CrossRef]
- Ruban, A.V.; Skriver, H.L.; Norskov, J.K. Surface segregation energies in transition-metal alloys. Phys. Rev. B 1999, 59, 15990–16000. [Google Scholar] [CrossRef]
- Krebs, E.; Silvi, B.; Raybaud, P. Mixed sites and promoter segregation: A DFT study of the manifestation of Le Chatelier’s principle for the Co (Ni) MoS active phase in reaction conditions. Catal. Today 2008, 130, 160–169. [Google Scholar] [CrossRef]
- Ma, Y.; Balbuena, P.B. Surface segregation in bimetallic Pt3M (M = Fe, Co, Ni) alloys with adsorbed oxygen. Surf. Sci. 2009, 603, 349–353. [Google Scholar] [CrossRef]
- Hammer, B.; Norskov, J.K. Why gold is the noblest of all the metals. Nature 1995, 376, 238–240. [Google Scholar] [CrossRef]
- Hammer, B.; Nørskov, J.K. Electronic factors determining the reactivity of metal surfaces. Surf. Sci. 1995, 343, 211–220. [Google Scholar] [CrossRef]
- Wang, L.G.; Tsymbal, E.Y.; Jaswal, S.S. Structural and magnetic properties of clean and methylthiolate-adsorbed Co(0001) surfaces: A first-principles study. J. Magn. Magn. Mater. 2005, 286, 119–123. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).