A Pathway for Sugar Production from Agricultural Waste Catalyzed by Sulfonated Magnetic Carbon Microspheres
Abstract
1. Introduction
2. Results and Discussion
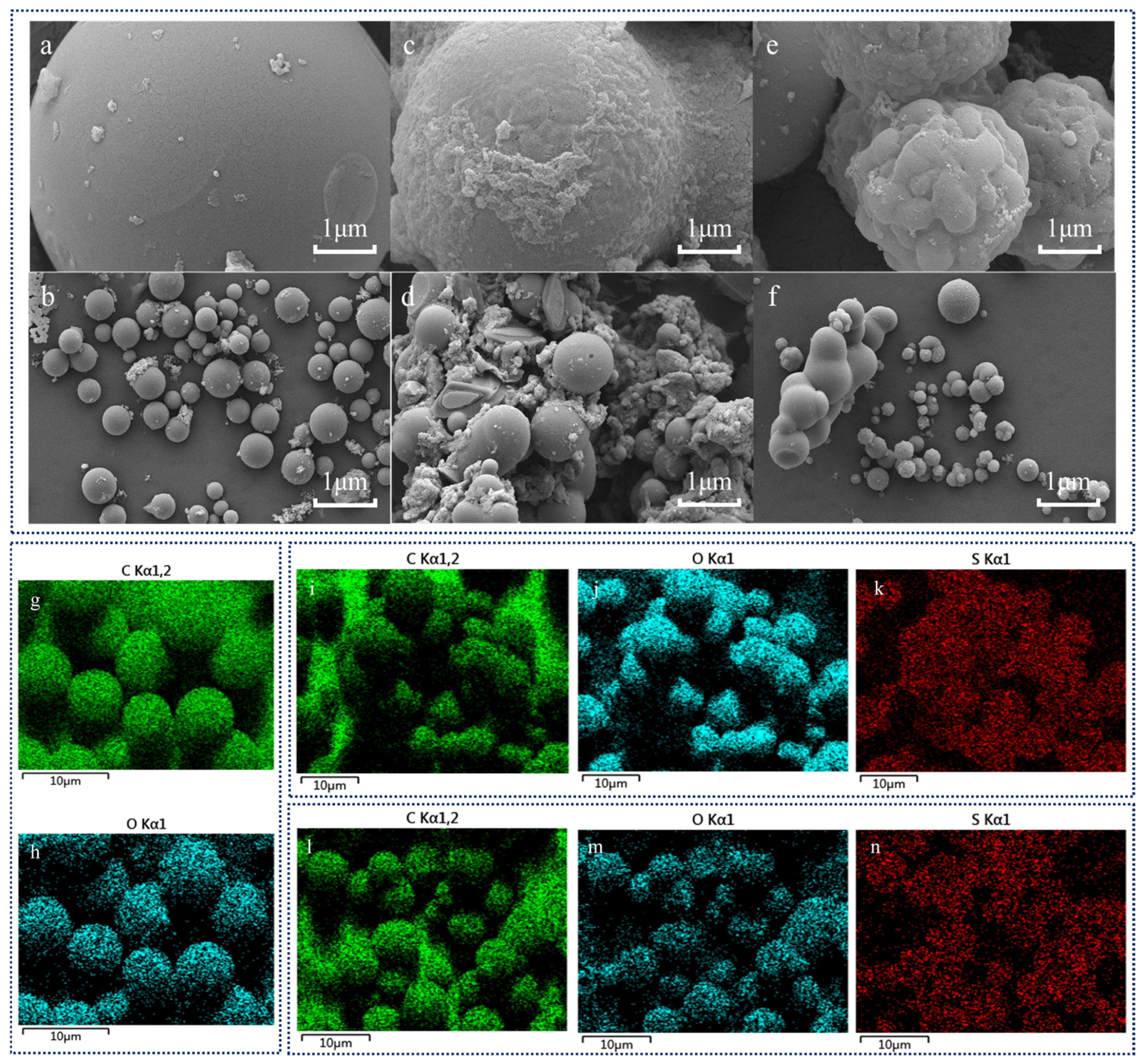
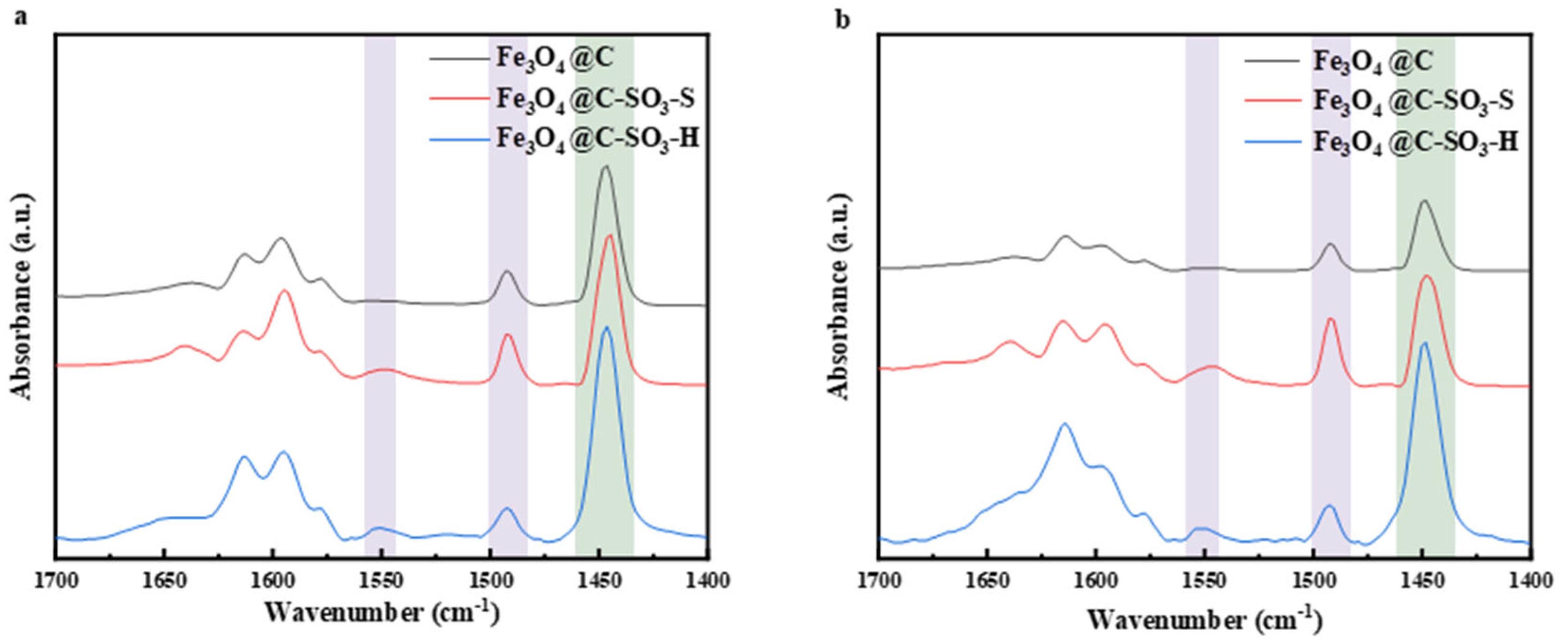
2.1. Characterization of a Carbon-Based Solid Acid Catalyst
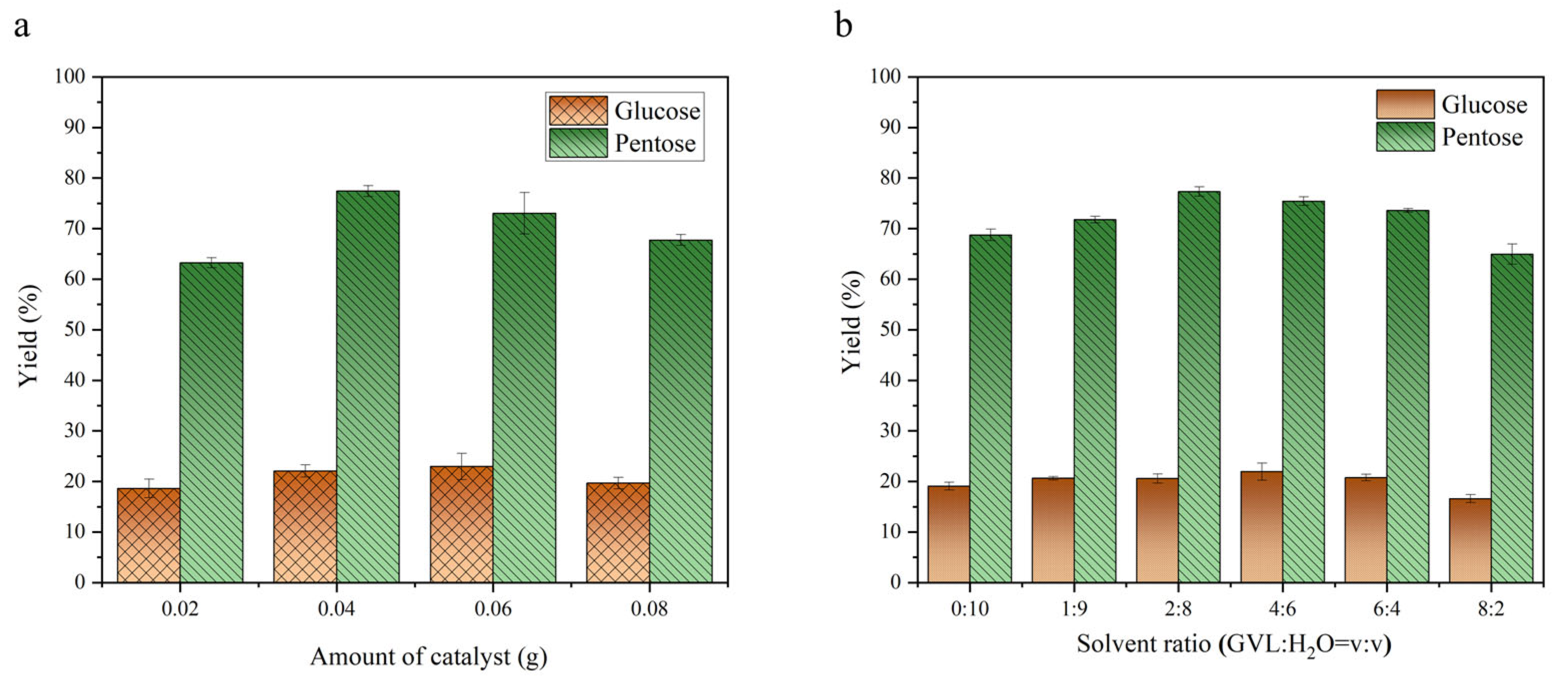
2.2. Catalytic Performance of a Carbon-Based Solid Acid Catalyst
3. Experimental Methods
3.1. Materials
3.2. The Preparation of the Catalyst
3.2.1. Preparation of Magnetic Nanoparticles Fe3O4
3.2.2. Preparation of Fe3O4@C Composite
3.2.3. Sulfonation of Fe3O4@C Composite
3.3. Characterization and Analysis of Catalysts
3.4. Test of Catalytic Performance of Catalyst for Production of Reducing Sugar
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mayer, A. Fossil fuel dependence and energy insecurity. Energy Sustain. Soc. 2022, 12, 1–13. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, J.; Zhang, L.; Chen, K.; Shen, N.; Yang, M.; Wang, X.; Wu, Z.; He, A.; Xu, J.; et al. High-Efficiency Production of Biomass-Derived 5-Alkoxymethylfurfurals over a Lewis-Brønsted Bifunctional Carbonaceous Acid Catalyst. ACS Sustain. Chem. Eng. 2023, 11, 16627–16640. [Google Scholar] [CrossRef]
- Xu, H.; Xin, G.; Hu, W.; Zhang, Z.; Si, C.; Chen, J.; Lu, L.; Peng, Y.; Li, X. Single-atoms Ru/NiFe layered double hydroxide electrocatalyst: Efficient for oxidation of selective oxidation of 5-hydroxymethylfurfural and oxygen evolution reaction. Appl. Catal. B Environ. 2023, 339, 123157. [Google Scholar] [CrossRef]
- Uzakov, G.; Beksultanova, A.; Aygumov, T.; Valeev, S.; Abdullozoda, R.; Bovtrikova, E.; Gibadullin, A.; Toshmamatov, B.; Morkovkin, D.; Sadullozoda, S. Achieving renewable energy goals through the utilization of renewable resources. E3S Web Conf. 2023, 411, 01008. [Google Scholar] [CrossRef]
- Li, X.; Yang, J.; Xu, R.; Lu, L.; Kong, F.; Liang, M.; Jiang, L.; Nie, S.; Si, C. Kinetic study of furfural production from Eucalyptus sawdust using H-SAPO-34 as solid Brønsted acid and Lewis acid catalysts in biomass-derived solvents. Ind. Crops Prod. 2019, 135, 196–205. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.; Hu, W.; Yu, Z.; Zhou, H.; Zhu, Y.; Lu, L.; Si, C. Research Progress of Highly Efficient Noble Metal Catalysts for the Oxidation of 5-Hydroxymethylfurfural. ChemSusChem 2022, 15, e202200352. [Google Scholar] [CrossRef]
- Kulikova, M.V.; Krylova, A.Y.; Zhagfarov, F.G.; Krysanova, K.O.; Lapidus, A.L. Plant Biomass as a Raw Material for Producing Basic Organic Sysnthesis Products. Chem. Technol. Fuels Oils 2022, 58, 320–326. [Google Scholar] [CrossRef]
- Terrer, R.P.P.C.; Hungate, B.A.; Rosende, J.; Pett-Ridge, J.; Craig, M.; van Groenigen, K.J.; Keenan, T.F.; Sulman, B.N.; Stocker, B.D.; Reich, P.B.; et al. A trade-off between plant and soil carbon storage under elevated CO2. Nature 2021, 591, 599–603. [Google Scholar] [CrossRef]
- Alper, K.; Tekin, K.; Karagöz, S.; Ragauskas, A.J. Sustainable energy and fuels from biomass: A review focusing on hydrothermal biomass processing. Sustain. Energy Fuels 2020, 4, 4390–4414. [Google Scholar] [CrossRef]
- Xu, R.; Liu, K.; Du, H.; Liu, H.; Cao, X.; Zhao, X.; Qu, G.; Li, X.; Li, B.; Si, C. Falling Leaves Return to Their Roots: A Review on the Preparation of γ-Valerolactone from Lignocellulose and Its Application in the Conversion of Lignocellulose. ChemSusChem 2020, 13, 6461–6476. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.; Hu, W.; Lu, L.; Chen, J.; Zhu, Y.; Zhou, H.; Zhou, H.; Si, C. Recent advances on solid acid catalyic systems for production of 5-Hydroxymethylfurfural from biomass derivatives. Fuel Process. Technol. 2022, 234, 107338. [Google Scholar] [CrossRef]
- Izydorczyk, G.; Skrzypczak, D.; Mironiuk, M.; Mikula, K.; Samoraj, M.; Gil, F.; Taf, R.; Moustakas, K.; Chojnacka, K. Lignocellulosic biomass fertilizers: Production; characterization; agri-applications. Sci. Total Environ. 2024, 923, 171343. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Fraceto, L.F.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; de Medeiros, G.A.; Pereira, A.D.E.S.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic biomass from agricultural waste to the circular economy: A review with focus on biofuels, biocomposites and bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Qiu, B.; Shi, J.; Hu, W.; Gao, J.; Li, S.; Chu, H. Construction of hydrothermal liquefaction system for efficient production of biomass-derived furfural: Solvents, catalysts and mechanisms. Fuel 2023, 354, 129278. [Google Scholar] [CrossRef]
- Cheenkachorn, K.; Mensah, R.Q.; Dharmalingam, B.; Gundupalli, M.P.; Rattanaporn, K.; Tantayotai, P.; Show, P.L.; Sriariyanun, M. The Versatility of Mixed Lignocellulose Feedstocks for Bioethanol Production: An Experimental Study and Empirical Prediction. Bioenerg. Res. 2023, 17, 1004–1014. [Google Scholar] [CrossRef]
- Li, X.; Xu, R.; Liu, Q.; Liang, M.; Yang, J.; Lu, S.; Li, G.; Lu, L.; Si, C. Valorization of corn stover into furfural and levulinic acid over SAPO-18 zeolites: Effect of Brønsted to Lewis acid sites ratios. Ind. Crops Prod. 2019, 141, 111759. [Google Scholar] [CrossRef]
- Li, X.; Xu, R.; Yang, J.; Nie, S.; Liu, D.; Liu, Y.; Si, C. Production of 5-hydroxymethylfurfural and levulinic acid from lignocellulosic biomass and catalytic upgradation. Ind. Crops Prod. 2019, 130, 184–197. [Google Scholar] [CrossRef]
- Yao, B.; Kang, Q.; Fu, J.; Liu, Y.; Ao, W.; Wang, L.; Jiang, Z.; Zhang, T.; Song, Y.; Deng, Z.; et al. Catalytic hydrolysis of corncob for production of furfural and cellulose-rich solids: Product characterization and analysis. Biomass Bioenergy 2023, 168, 106658. [Google Scholar] [CrossRef]
- Zhou, L.; Han, X.; Ma, Y.; Yang, X.; Lu, T. A mild oxidation strategy for oxidation-hydrolysis of cellulose without additional catalyst. Biomass Convers. Biorefinery 2022, 14, 7725–7733. [Google Scholar] [CrossRef]
- Ouyang, J.; He, W.-Q.; Li, Q.-M.; Chen, L.; Wu, X.-F.; Su, X.-J. Separation of Lignocellulose and Preparation of Xylose from Miscanthus lutarioriparius with a Formic Acid Method. Appl. Sci. 2022, 12, 1432. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Pan, X. Efficient sugar production from plant biomass: Current status, challenges, and future directions. Renew. Sustain. Energy Rev. 2022, 164, 112583. [Google Scholar] [CrossRef]
- Hu, W.; Xu, H.; Zhang, Z.; Duan, Y.; Lu, X.; Lu, L.; Si, C.; Peng, Y.; Li, X. Optimizing acidic site control for selective conversion of biomass-based sugar to furfural and levulinic acid through HSiW/MCM-41 catalyst. Biomass Bioenergy 2024, 186, 107275. [Google Scholar] [CrossRef]
- Li, X.; Lu, X.; Nie, S.; Liang, M.; Yu, Z.; Duan, B.; Yang, J.; Xu, R.; Lu, L.; Si, C. Efficient catalytic production of biomass-derived levulinic acid over phosphotungstic acid in deep eutectic solvent. Ind. Crops Prod. 2020, 145, 112154. [Google Scholar] [CrossRef]
- Li, X.; Lu, X.; Liang, M.; Xu, R.; Yu, Z.; Duan, B.; Lu, L.; Si, C. Conversion of waste lignocellulose to furfural using sulfonated carbon microspheres as catalyst. Waste Manag. 2020, 108, 119–126. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, G.; Wang, K. Efficient conversion of biomass derivatives to furfural with a novel carbon-based solid acid catalyst. Catal. Commun. 2023, 175, 106608. [Google Scholar] [CrossRef]
- Li, W.; Zhu, Y.; Lu, Y.; Liu, Q.; Guan, S.; Chang, H.-M.; Jameel, H.; Ma, L. Enhanced furfural production from raw corn stover employing a novel heterogeneous acid catalyst. Bioresour. Technol. 2017, 245, 258–265. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, J.; Shan, R.; Yuan, H.; Chen, Y. Advances in thermochemical valorization of biomass towards carbon neutrality. Resour. Conserv. Recycl. 2025, 212, 107905. [Google Scholar] [CrossRef]
- Bai, Y.-Y.; Xiao, L.-P.; Sun, R.-C. Efficient hydrolyzation of cellulose in ionic liquid by novel sulfonated biomass-based catalysts. Cellulose 2014, 21, 2327–2336. [Google Scholar] [CrossRef]
- Weerasai, K.; Champreda, V.; Sakdaronnarong, C.; Shotipruk, A.; Laosiripojana, N. Hydrolysis of eucalyptus wood chips under hot compressed water in the presence of sulfonated carbon-based catalysts. Food Bioprod. Process. 2018, 110, 136–144. [Google Scholar] [CrossRef]
- Qi, W.; He, C.; Wang, Q.; Liu, S.; Yu, Q.; Wang, W.; Leksawasdi, N.; Wang, C.; Yuan, Z. Carbon-Based Solid Acid Pretreatment in Corncob Saccharification: Specific Xylose Production and Efficient Enzymatic Hydrolysis. ACS Sustain. Chem. Eng. 2018, 6, 3640–3648. [Google Scholar] [CrossRef]
- Xu, X.L.Y.; Zhang, X.; Wang, W.; Liu, S.; Wei, Q.; Zhuang, X.; Luo, Y.; Yuan, Z. Hydrolysis of Corncob Using a Modified Carbon-based Solid Acid Catalyst. BioResources 2016, 11, 10469–10482. [Google Scholar] [CrossRef]
- Li, X.; Lu, X.; Hu, W.; Xu, H.; Chen, J.; Xiong, J.; Lu, L.; Yu, Z.; Si, C. Phosphotungstic acid functionalized biochar for furfural production from corncob. Fuel Process. Technol. 2022, 229, 107178. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Hu, W.; Zhou, H.; Zhu, Y.; Lu, L.; Si, C. One step synthesis of Mo-doped carbon microspheres for valorization corncob to levulinic acid. Ind. Crops Prod. 2022, 184, 115019. [Google Scholar] [CrossRef]
- Peymanfar, R.; Fazlalizadeh, F. Fabrication of expanded carbon microspheres/ZnAl2O4 nanocomposite and investigation of its microwave, magnetic, and optical performance. J. Alloys Compd. 2021, 854, 157273. [Google Scholar] [CrossRef]
- Khatun, R.; Mamun, M.S.A.; Islam, S.; Khatun, N.; Hakim, M.; Hossain, M.S.; Dhar, P.K.; Barai, H.R. Phytochemical-Assisted Synthesis of Fe3O4 Nanoparticles and Evaluation of Their Catalytic Activity. Micromachines 2022, 13, 2077. [Google Scholar] [CrossRef]
- Mahajan, V.P.; Kolekar, Y.A.; Bhanage, B.M. Magnetically separable Ni/Fe3O4: An efficient catalyst for phenoxy carbonylation of aryl iodides using bifunctional o-chlorophenyl formate as a CO source. Appl. Organomet. Chem. 2023, 37, e7032. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, Q.; Sun, X.; Zhang, Y.; Cai, Q.; Deng, W.; Rao, S.; Wu, X.; Ye, Q. Conversion of wet microalgae to biodiesel with microalgae carbon based magnetic solid acid catalyst. Energy Convers. Manag. 2023, 286, 117022. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, K.; Liu, Y.; Wu, S. Esterification of levulinic acid into ethyl levulinate catalyzed by sulfonated bagasse-carbonized solid acid. BioResources 2019, 14, 2186–2196. [Google Scholar] [CrossRef]
- Flores, K.P.; Omega, J.L.O.; Cabatingan, L.K.; Go, A.W.; Agapay, R.C.; Ju, Y.-H. Simultaneously carbonized and sulfonated sugarcane bagasse as solid acid catalyst for the esterification of oleic acid with methanol. Renew. Energy 2019, 130, 510–523. [Google Scholar] [CrossRef]
- Ribeiro, F.C.P.; Santos, J.L.; Araujo, R.O.; Santos, V.O.; Chaar, J.S.; Tenório, J.A.S.; de Souza, L.K.C. Sustainable catalysts for esterification: Sulfonated carbon spheres from biomass waste using hydrothermal carbonization. Renew. Energy 2024, 220, 119653. [Google Scholar] [CrossRef]
- Xu, H.; Xiong, S.; Zhao, Y.; Zhu, L.; Wang, S. Conversion of Xylose to Furfural Catalyzed by Carbon-Based Solid Acid Prepared from Pectin. Energ. Fuel 2021, 35, 9961–9969. [Google Scholar] [CrossRef]
- He, Q.; Lu, Y.; Peng, Q.; Chen, W.; Fan, G.; Chai, B.; Song, G. Synthesis of 5-hydroxymethylfurfural from fructose catalyzed by sulfonated carbon-based solid acid. Biomass Convers. Biorefinery 2021, 13, 9195–9203. [Google Scholar] [CrossRef]
- Mohan, V.B.; Jayaraman, K.; Bhattacharyya, D. Brunauer–Emmett–Teller (BET) specific surface area analysis of different graphene materials: A comparison to their structural regularity and electrical properties. Solid State Commun. 2020, 320, 114004. [Google Scholar] [CrossRef]
- Hu, X.; Jia, L.; Cheng, J.; Sun, Z. Magnetic ordered mesoporous carbon materials for adsorption of minocycline from aqueous solution: Preparation, characterization and adsorption mechanism. J. Hazard. Mater. 2019, 362, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kevin, M.W.; Liang, X. Synthesis of a Novel Porous Carbon Based Solid Acid and Its Catalytic Activities for Biodiesel Synthesis from Waste Oils. Kinet. Catal. 2020, 61, 486–493. [Google Scholar] [CrossRef]
- Chen, M.; Li, L.; Wang, Y.; Liang, D.; Zhou, Z.; Xin, H.; Li, C.; Yuan, G.; Wang, J. Sulfonated P-W modified nitrogen-containing carbon-based solid acid catalysts for one-pot conversion of cellulose to ethyl levulinate under water-ethanol medium. Int. J. Biol. Macromol. 2024, 260 Pt 1, 129472. [Google Scholar] [CrossRef]
- Bullo, T.A.; Bayisa, Y.M.; Bultum, M.S. Biosynthesis of sulfonated carbon catalyst from carbohydrate polymer derivatives for epoxidation of Croton macrostachyus seed oil. Carbohydr. Polym. Technol. Appl. 2022, 3, 100221. [Google Scholar] [CrossRef]
- Su, T.; Zeng, J.; Gao, H.; Jiang, L.; Bai, X.; Zhou, H.; Xu, F. One-pot synthesis of a chemically functional magnetic carbonaceous acid catalyst for fermentable sugars production from sugarcane bagasse. Fuel 2020, 262, 116512. [Google Scholar] [CrossRef]
- Ma, C.Y.; Cai, B.; Zhang, L.; Feng, J.F.; Pan, H. Acid-Catalyzed Conversion of Cellulose Into Levulinic Acid with Biphasic Solvent System. Front. Plant Sci. 2021, 12, 630807. [Google Scholar] [CrossRef]
- Sabry, T.M.; El-Korashy, S.A.E.-H.; Jahin, H.E.S.; Khairy, G.M.; Aal, N.F.A. Hydrothermal carbonization of Calotropis procera leaves as a biomass: Preparation and characterization. J. Mol. Struct. 2024, 1302, 137397. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, H.; Wu, K.; Liu, Y.; Zhu, Y.; Liang, B. Hierarchical porous carbon-based solid acid as a high-performance catalyst for conversion of fructose to 5-hydroxymethylfurfural. Fuel 2024, 363, 130835. [Google Scholar] [CrossRef]
- Panchal, B.; Sun, Y.; Zhao, C.; Wang, J.; Bian, K.; Zhao, Q.; Liu, B. Waste to value addition: Utilization of waste corn cob from corn plant derived novel green acidic catalyst for effective synthesis of esters. Energy Rep. 2024, 11, 4277–4289. [Google Scholar] [CrossRef]
- Jiang, H.; Dong, X.; Shou, J. Synthesis of Novel Magnetic Carbon Microtube-Based Solid Acid and Its Catalytic Activities for Biodiesel Synthesis. Catalysts 2022, 12, 305. [Google Scholar] [CrossRef]
- Deshavath, N.N.; Goud, V.V.; Veeranki, V.D. Commercialization of 2G bioethanol as a transportation fuel for the sustainable energy, environment, and economic growth of India: Theoretical and empirical assessment of bioethanol potential from agriculture crop residues. Biomass Convers. Biorefinery 2022, 14, 3551–3563. [Google Scholar] [CrossRef]
- Lin, Q.-X.; Zhang, C.-H.; Wang, X.-H.; Cheng, B.-G.; Mai, N.; Ren, J.-L. Impact of activation on properties of carbon-based solid acid catalysts for the hydrothermal conversion of xylose and hemicelluloses. Catal. Today 2019, 319, 31–40. [Google Scholar] [CrossRef]



| Sample | Temperature (°C) | Number of Acid Sites (μmol/g) | B/L | ||
|---|---|---|---|---|---|
| B Acid | L Acid | Total Acid | |||
| Fe3O4@C | 150 | 2.374 | 113.175 | 115.550 | 0.021 |
| 200 | 1.770 | 29.117 | 30.887 | 0.061 | |
| Fe3O4@C-S | 150 | 15.164 | 122.268 | 137.432 | 0.124 |
| 200 | 13.210 | 48.156 | 61.366 | 0.274 | |
| Fe3O4@C-H | 150 | 10.906 | 188.157 | 199.063 | 0.058 |
| 200 | 6.397 | 93.819 | 100.217 | 0.068 | |
| Time (min) | Glucose Yield (%) | Pentose Yield (%) | ||
|---|---|---|---|---|
| Fe3O4@C-S | Fe3O4@C-H | Fe3O4@C-S | Fe3O4@C-H | |
| 50 | 29.35 | 21.76 | 73.15 | 69.49 |
| 70 | 28.58 | 22.12 | 74.56 | 73.55 |
| 90 | 28.91 | 22.11 | 76.51 | 73.25 |
| 110 | 28.36 | 22.23 | 74.25 | 76.67 |
| 130 | 26.61 | 21.91 | 71.79 | 74.54 |
| 150 | 26.26 | 21.27 | 68.24 | 71.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Duan, Y.; Li, H.; He, S.; Zi, X.; Zhao, Y.; Jiao, C.; Li, X. A Pathway for Sugar Production from Agricultural Waste Catalyzed by Sulfonated Magnetic Carbon Microspheres. Molecules 2025, 30, 2675. https://doi.org/10.3390/molecules30132675
Xu M, Duan Y, Li H, He S, Zi X, Zhao Y, Jiao C, Li X. A Pathway for Sugar Production from Agricultural Waste Catalyzed by Sulfonated Magnetic Carbon Microspheres. Molecules. 2025; 30(13):2675. https://doi.org/10.3390/molecules30132675
Chicago/Turabian StyleXu, Maoru, Yanfeng Duan, Hongfu Li, Shoulin He, Xingyu Zi, Yanting Zhao, Cheng Jiao, and Xiaoyun Li. 2025. "A Pathway for Sugar Production from Agricultural Waste Catalyzed by Sulfonated Magnetic Carbon Microspheres" Molecules 30, no. 13: 2675. https://doi.org/10.3390/molecules30132675
APA StyleXu, M., Duan, Y., Li, H., He, S., Zi, X., Zhao, Y., Jiao, C., & Li, X. (2025). A Pathway for Sugar Production from Agricultural Waste Catalyzed by Sulfonated Magnetic Carbon Microspheres. Molecules, 30(13), 2675. https://doi.org/10.3390/molecules30132675






