Hyaluronic Acid Interactions with Pork Myofibrillar Proteins in Emulsion Gel-Type Systems
Abstract
:1. Introduction
2. Results and Discussion
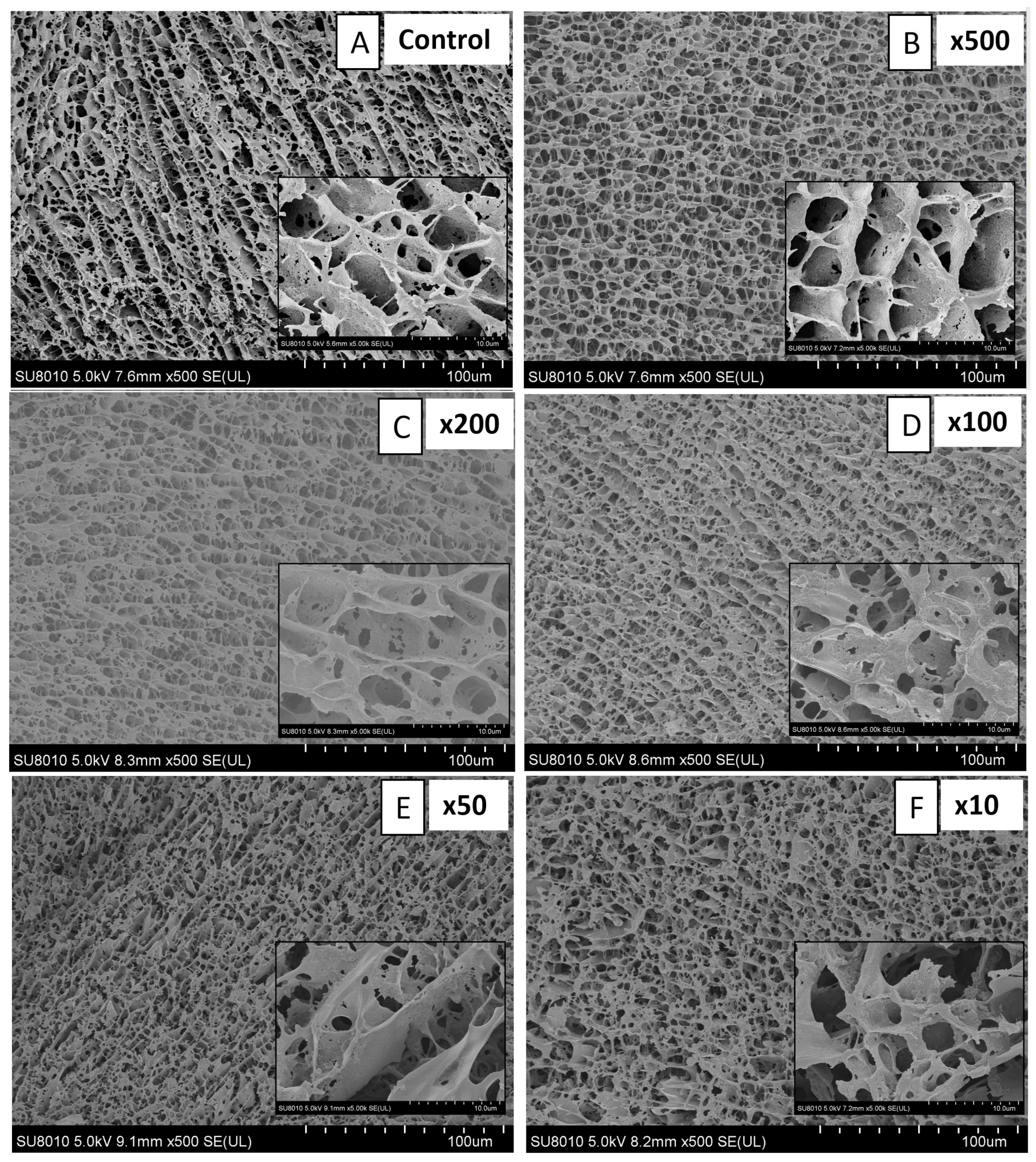
2.1. Properties of MP and HA Gels
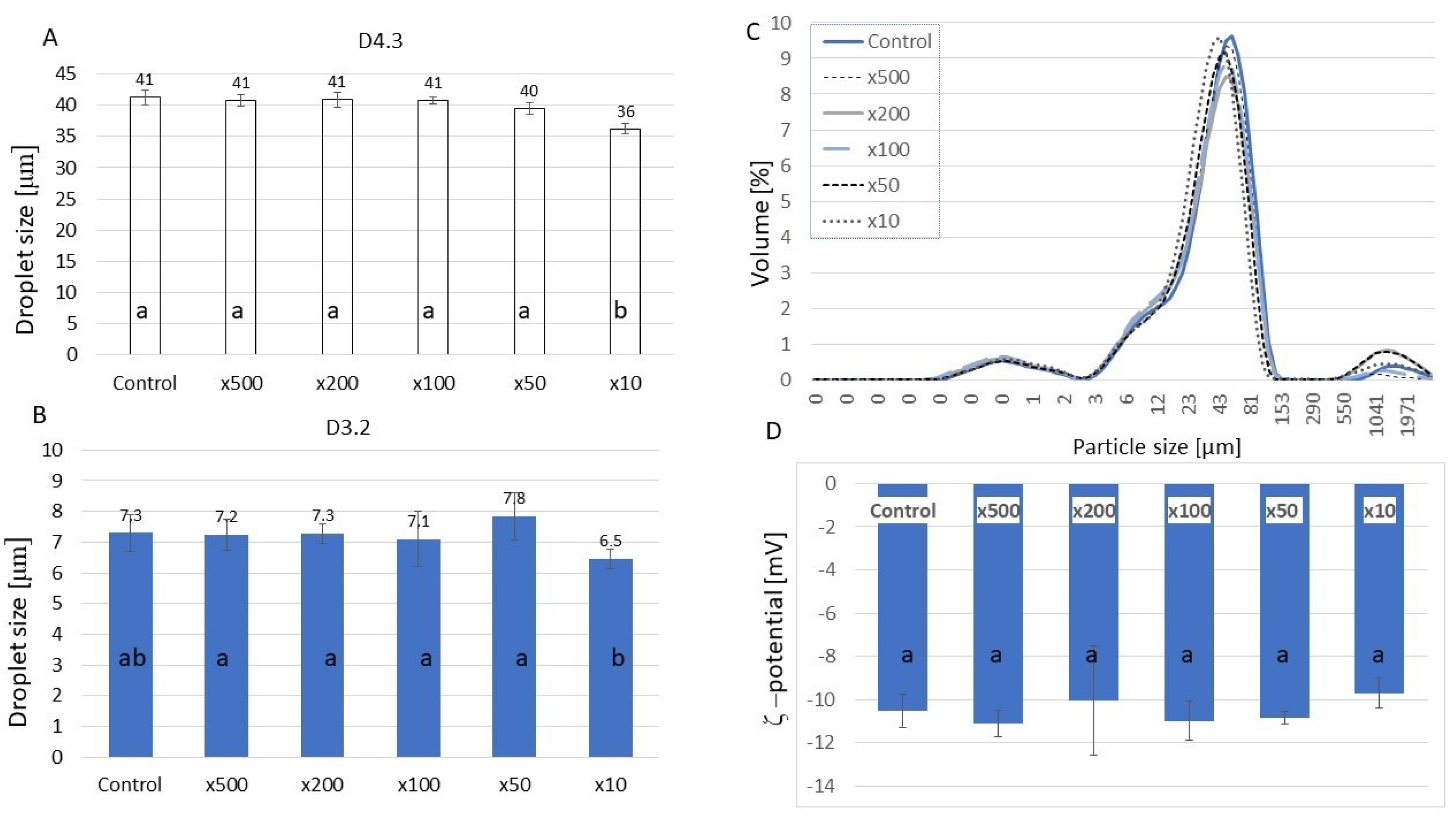
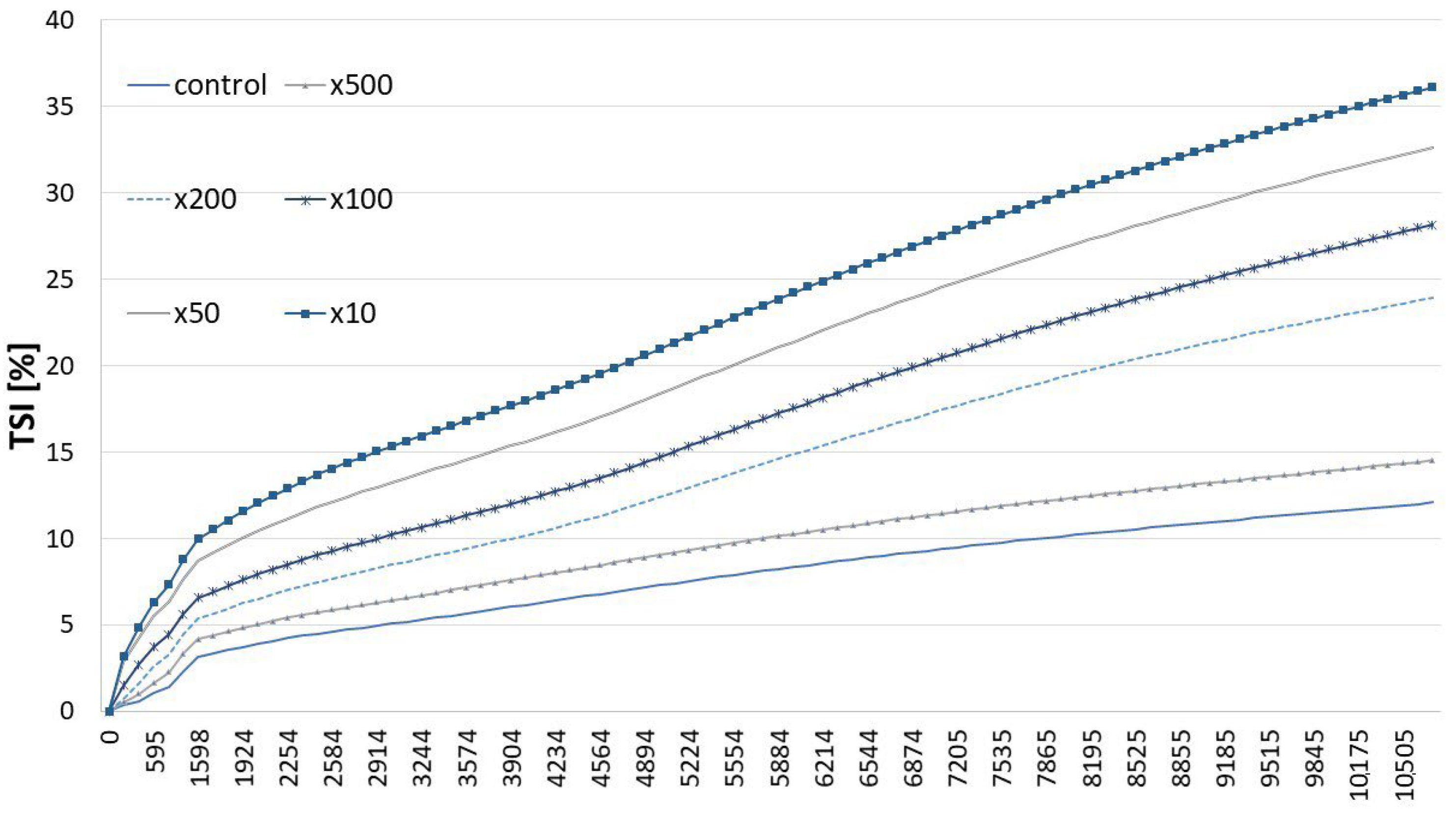
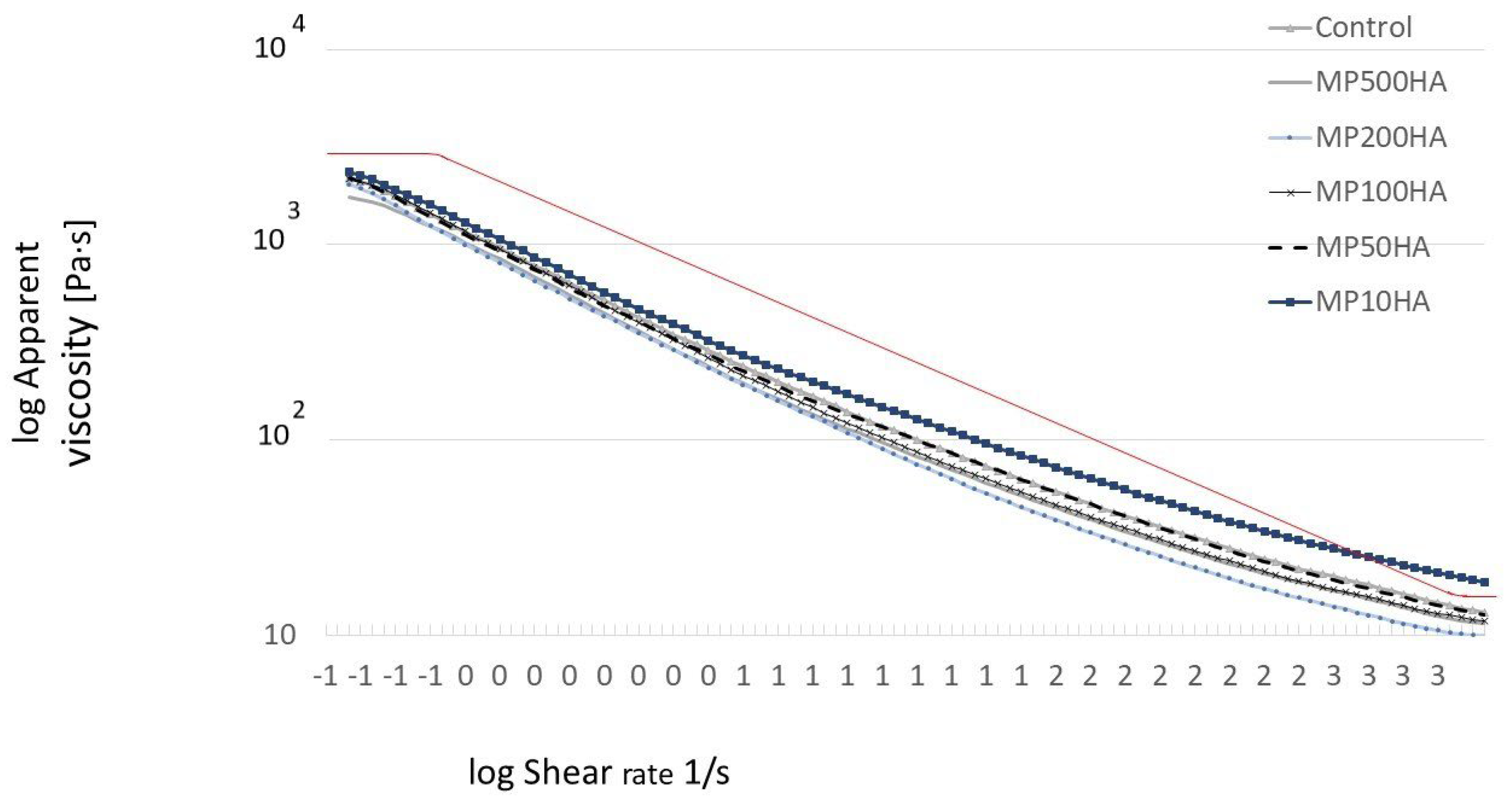
2.2. Properties of HA-Added Meat Emulsions
3. Materials and Methods
3.1. Chemical Reagents and Materials
3.2. Extraction of Meat Protein
3.3. The Analysis of HA Molecular Weight (MW)
3.4. Preparation of MP and HA Gels
3.4.1. Determination of Cooking Loss
3.4.2. Water-Holding Capacity
3.4.3. Texture Analysis
3.4.4. Cryo-Scanning Electron Microscope (Cryo-SEM)
3.5. Preparation of HA-Added Meat Emulsion
3.5.1. Emulsifying Activity and Emulsion Stability Indexes
3.5.2. Multiple Light-Scattering Measurement
3.5.3. Rheological Properties
3.5.4. Droplet Size Measurement
3.5.5. Zeta Potential Measurement
3.5.6. Protein and Oil Distribution Determination Using Confocal Laser Scanning Microscope (CLSM)
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MP | Myofibrillar Proteins |
| WHC | Water-Holding Capacity |
| EAI | Emulsion Activity Index |
| ESI | Emulsion Stability Index |
| WPI | Whey Protein Isolate |
| TSI | Turbiscan Stability Index |
| CLSM | Confocal Laser Scanning Microscope |
| Cryo-SEM | Cryo-Scanning Electron Microscopy |
References
- Kakehi, K.; Kinoshita, M.; Yasueda, S.-I. Hyaluronic acid: Separation and biological implications. J. Chromatogr. B 2003, 797, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Lierova, A.; Kasparova, J.; Filipova, A.; Cizkova, J.; Pekarova, L.; Korecka, L.; Mannova, N.; Bilkova, Z.; Sinkorova, Z. Hyaluronic acid: Known for almost a century, but still in vogue. Pharmaceutics 2022, 14, 838. [Google Scholar] [CrossRef] [PubMed]
- Zając, M.; Kulawik, P.; Tkaczewska, J.; Migdał, W.; Filipczak-Fiutak, M.; Fiutak, G. The effect of hyaluronic acid addition on the properties of smoked homogenised sausages. J. Sci. Food Agric. 2017, 97, 2316–2326. [Google Scholar] [CrossRef]
- Sionkowska, A.; Gadomska, M.; Musiał, K.; Piątek, J. Hyaluronic acid as a component of natural polymer blends for biomedical applications: A review. Molecules 2020, 25, 4035. [Google Scholar] [CrossRef]
- Pereira, H.; Sousa, D.A.; Cunha, A.; Andrade, R.; Espregueira-Mendes, J.; Oliveira, J.M.; Reis, R.L. Hyaluronic Acid. In Osteochondral Tissue Engineering: Challenges, Current Strategies, and Technological Advances; Oliveira, J.M., Pina, S., Reis, R.L., San Roman, J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 137–153. [Google Scholar]
- Cheng, Q.; Liu, C.; Zhao, J.; Li, W.; Guo, F.; Qin, J.; Wang, Y. Unlocking the potential of hyaluronic acid: Exploring its physicochemical properties, modification, and role in food applications. Trends Food Sci. Technol. 2023, 142, 104218. [Google Scholar] [CrossRef]
- Romanò, C.; Vecchi, E.D.; Bortolin, M.; Morelli, I.; Drago, L. Hyaluronic Acid and Its Composites as a Local Antimicrobial/Antiadhesive Barrier. J. Bone Jt. Infect. 2017, 2, 63–72. [Google Scholar] [CrossRef]
- Zamboni, F.; Okoroafor, C.; Ryan, M.P.; Pembroke, J.T.; Strozyk, M.; Culebras, M.; Collins, M.N. On the bacteriostatic activity of hyaluronic acid composite films. Carbohydr. Polym. 2021, 260, 117803. [Google Scholar] [CrossRef]
- Kaufmann, J.; Möhle, K.; Hofmann, H.-J.; Arnold, K. Molecular dynamics study of hyaluronic acid in water. J. Mol. Struct. THEOCHEM 1998, 422, 109–121. [Google Scholar] [CrossRef]
- Hu, H.; Pereira, J.; Xing, L.; Zhou, G.; Zhang, W. Thermal gelation and microstructural properties of myofibrillar protein gel with the incorporation of regenerated cellulose. LWT 2017, 86, 14–19. [Google Scholar] [CrossRef]
- Hu, L.; Shi, L.; Liu, S.; Xiao, Z.; Sun, J.; Shao, J.-H. Regulation mechanism of curcumin-loaded oil on the emulsification and gelation properties of myofibrillar protein: Emphasizing the dose–response of curcumin. Food Chem. 2023, 428, 136687. [Google Scholar] [CrossRef]
- Han, Z.; Liu, S.; Cao, J.; Yue, X.; Shao, J.-H. A review of oil and water retention in emulsified meat products: The mechanisms of gelation and emulsification, the application of multi-layer hydrogels. Crit. Rev. Food Sci. Nutr. 2024, 64, 8308–8324. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.; Barbut, S.; Schmidt, G. Mechanisms of meat batter stabilization: A review. Crit. Rev. Food Sci. Nutr. 1992, 32, 299–332. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Li, S.; Yang, Y.; Feng, X.; Tang, X.; Chen, Y. Mechanisms of inulin addition affecting the properties of chicken myofibrillar protein gel. Food Hydrocoll. 2022, 131, 107843. [Google Scholar] [CrossRef]
- Tarté, R. Ingredients in Meat Products: Properties, Functionality and Applications; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Wang, S.; Yang, J.; Shao, G.; Liu, J.; Wang, J.; Yang, L.; Li, J.; Liu, H.; Zhu, D.; Li, Y.; et al. pH-induced conformational changes and interfacial dilatational rheology of soy protein isolated/soy hull polysaccharide complex and its effects on emulsion stabilization. Food Hydrocoll. 2020, 109, 106075. [Google Scholar] [CrossRef]
- Tiwari, S.; Bahadur, P. Modified hyaluronic acid based materials for biomedical applications. Int. J. Biol. Macromol. 2019, 121, 556–571. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Raval, H.; Bhirud, D. Hyaluronic acid-functionalized carboxymethyl dextran-coated melatonin nanoconjugates for targeted etoposide delivery in metastatic colon cancer: Extensive in-vitro investigation in HCT116 cell lines, antimicrobial efficacy, and anti-angiogenic potential in chick chorioallantoic membrane (CAM) assay. Int. J. Biol. Macromol. 2024, 281, 136373. [Google Scholar]
- Wu, X.; Luan, M.; Yan, X.; Zhang, J.; Wu, X.; Zhang, Q. The impact of different concentrations of hyaluronic acid on the pasting and microstructural properties of corn starch. Int. J. Biol. Macromol. 2024, 254, 127555. [Google Scholar] [CrossRef]
- Wang, N.; Hu, J.; Zhang, K.; Zhang, Y.; Jiang, Y.; Wang, X.; Ban, Q. Development and characterization of a casein-hyaluronic acid emulsion gel with high water-holding capacity and excellent rheological properties for 3D printing. Food Hydrocoll. 2023, 140, 108632. [Google Scholar] [CrossRef]
- Joshi, R.; Sutariya, S.G.; Salunke, P. Effect of Different Molecular Weight Hyaluronic Acids on Skim Milk Functional Properties. Foods 2024, 13, 690. [Google Scholar] [CrossRef]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic Acid: The Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, F.; Deng, Y.; Tang, X.; Li, P.; Zhao, Z.; Zhang, M.; Liu, G. Texture characterization of 3D printed fibrous whey protein-starch composite emulsion gels as dysphagia food: A comparative study on starch type. Food Chem. 2024, 458, 140302. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, K.; Chen, Y.; Hu, J.; Jiang, Y.; Wang, X.; Ban, Q. Tuning whey protein isolate/hyaluronic acid emulsion gel structure to enhance quercetin bioaccessibility and in vitro digestive characteristics. Food Chem. 2023, 429, 136910. [Google Scholar] [CrossRef]
- Fan, X.; Gao, X.; Li, R.; Pan, D.; Zhou, C. Myofibrillar proteins’ intermolecular interaction weakening and degradation: Are they mainly responsible for the tenderization of meat containing l–arginine, l–lysine, or/and NaCl? Food Chem. 2024, 441, 138318. [Google Scholar] [CrossRef]
- Shen, R.; Tian, X.; Yang, Q.; Zhang, K.; Zhang, H.; Wang, X.; Bai, L.; Wang, W. Using nanocellulose to improve heat-induced cull cow meat myofibrillar protein gels: Effects of particle morphology and content. J. Sci. Food Agric. 2023, 103, 7550–7559. [Google Scholar] [CrossRef]
- Li, Q.; Wang, P.; Miao, S.; Zhang, L.; Zheng, B. Curdlan enhances the structure of myosin gel model. Food Sci. Nutr. 2019, 7, 2123–2130. [Google Scholar] [CrossRef]
- Gamini, A.; Paoletti, S.; Toffanin, R.; Micali, F.; Michielin, L.; Bevilacqua, C. Structural investigations of cross-linked hyaluronan. Biomaterials 2002, 23, 1161–1167. [Google Scholar] [CrossRef]
- Gravelle, A.J.; Marangoni, A.G.; Barbut, S. Insight into the mechanism of myofibrillar protein gel stability: Influencing texture and microstructure using a model hydrophilic filler. Food Hydrocoll. 2016, 60, 415–424. [Google Scholar] [CrossRef]
- Bourne, M.C. Chapter 1—Texture, Viscosity, and Food. In Food Texture and Viscosity, 2nd ed.; Bourne, M.C., Ed.; Academic Press: London, UK, 2002; pp. 1–32. [Google Scholar]
- He, S.; Li, M.; Sun, Y.; Pan, D.; Zhou, C.; Lan, H. Effects of limited enzymatic hydrolysis and polysaccharide addition on the physicochemical properties of emulsions stabilized with duck myofibrillar protein under low-salt conditions. Food Chem. 2024, 430, 137053. [Google Scholar] [CrossRef]
- McClements, D.J. Comments on viscosity enhancement and depletion flocculation by polysaccharides. Food Hydrocoll. 2000, 14, 173–177. [Google Scholar] [CrossRef]
- McClements, D.J. Critical Review of Techniques and Methodologies for Characterization of Emulsion Stability. Crit. Rev. Food Sci. Nutr. 2007, 47, 611–649. [Google Scholar] [CrossRef]
- Huang, A.; McClements, D.J.; Luo, S.; Chen, T.; Ye, J.; Liu, C. Fabrication of rutin-protein complexes to form and stabilize bilayer emulsions: Impact of concentration and pretreatment. Food Hydrocoll. 2022, 122, 107056. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, P.; Xu, X.; Chen, J.; Huang, M.; Zhang, W. Effects of ultrasound treatment on the morphological characteristics, structures and emulsifying properties of genipin cross-linked myofibrillar protein. Ultrason. Sonochemistry 2023, 97, 106467. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Shukla, P.; Misra, A.; Mishra, P.R. Chapter 8—Interfacial and colloidal properties of emulsified systems: Pharmaceutical and biological perspective. In Colloid and Interface Science in Pharmaceutical Research and Development; Ohshima, H., Makino, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 149–172. [Google Scholar]
- Sun, W.; Zhou, F.; Sun, D.-W.; Zhao, M. Effect of oxidation on the emulsifying properties of myofibrillar proteins. Food Bioprocess Technol. 2013, 6, 1703–1712. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, X.; Jiang, Y.; Ban, Q.; Wang, X. Enhancing the stability of oil-in-water emulsions by non-covalent interaction between whey protein isolate and hyaluronic acid. Int. J. Biol. Macromol. 2023, 225, 1085–1095. [Google Scholar] [CrossRef]
- Zhao, N.; Zou, H.; Sun, S.; Yu, C. The interaction between sodium alginate and myofibrillar proteins: The rheological and emulsifying properties of their mixture. Int. J. Biol. Macromol. 2020, 161, 1545–1551. [Google Scholar] [CrossRef]
- Wang, K.; Li, G.; Zhang, B. Opposite results of emulsion stability evaluated by the TSI and the phase separation proportion. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 402–409. [Google Scholar] [CrossRef]
- Mengual, O.; Meunier, G.; Cayré, I.; Puech, K.; Snabre, P. TURBISCAN MA 2000: Multiple light scattering measurement for concentrated emulsion and suspension instability analysis. Talanta 1999, 50, 445–456. [Google Scholar] [CrossRef]
- Xie, J.; Jin, Y.-C. Parameter determination for the Cross rheology equation and its application to modeling non-Newtonian flows using the WC-MPS method. Eng. Appl. Comput. Fluid Mech. 2016, 10, 111–129. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansal, N.; Bhandari, B. Rheology of emulsion-filled alginate microgel suspensions. Food Res. Int. 2016, 80, 50–60. [Google Scholar] [CrossRef]
- Kwiatkowski, A.L.; Makarova, A.L.; Ospennikov, A.S.; Shibaev, A.V.; Boronin, S.A.; Strizhnev, G.K.; Osiptsov, A.A.; Shvets, P.V.; Shel, E.V.; Paderin, G.V.; et al. Rheology of polyacrylamide-based fluids and its impact on proppant transport in hydraulic fractures. Phys. Fluids 2024, 36, 123101. [Google Scholar] [CrossRef]
- Michaud, T. Rheology of hyaluronic acid and dynamic facial rejuvenation: Topographical specificities. J. Cosmet. Dermatol. 2018, 17, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, J.; Lorenzo, J.M.; Zhang, W. Effects of ultrasound emulsification on the properties of pork myofibrillar protein-fat mixed gel. Food Chem. 2021, 345, 128751. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Hernández, L.A.; Camacho-Ruíz, R.M.; Arriola-Guevara, E.; Padilla-Camberos, E.; Kirchmayr, M.R.; Corona-González, R.I.; Guatemala-Morales, G.M. Validation of an Analytical Method for the Simultaneous Determination of Hyaluronic Acid Concentration and Molecular Weight by Size-Exclusion Chromatography. Molecules 2021, 26, 5360. [Google Scholar] [CrossRef]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Hu, H.-Y.; Xing, L.-J.; Hu, Y.-Y.; Qiao, C.-L.; Wu, T.; Zhou, G.-H.; Zhang, W.-G. Effects of regenerated cellulose on oil-in-water emulsions stabilized by sodium caseinate. Food Hydrocoll. 2016, 52, 38–46. [Google Scholar] [CrossRef]

| Cooking Loss [%] | WHC [%] [ns] | |
|---|---|---|
| Control | 6.72 b ± 0.38 | 67.14 ± 1.56 |
| ×500 | 6.14 b ± 0.44 | 71.12 ± 2.26 |
| ×200 | 5.96 b ± 0.37 | 70.27 ± 2.56 |
| ×100 | 9.66 b ± 1.26 | 70.40 ± 2.30 |
| ×50 | 22.45 a ± 1.96 | 74.71 ± 3.84 |
| ×10 | 35.91 a ± 2.92 | 69.05 ± 1.38 |
| Sample ID | Hardness [N] | Adhesiveness | Springiness | Chewiness [N] |
|---|---|---|---|---|
| Control | 1.37 b ± 0.09 | −14.02 b ± 1.86 | 0.42 b ± 0.01 | 0.21 b ± 0.03 |
| ×500 | 1.69 ab ± 0.25 | −11.02 ab ± 0.66 | 0.50 ab ± 0.05 | 0.31 bc ± 0.09 |
| ×200 | 1.81 ab ± 0.30 | −10.67 ab ± 0.54 | 0.52 ab ± 0.04 | 0.38 ab ± 0.11 |
| ×100 | 1.67 ab ± 0.24 | −11.08 ab ± 0.18 | 0.52 ab ± 0.04 | 0.33 abc ± 0.08 |
| ×50 | 2.32 a ± 0.35 | −8.24 a ± 0.51 | 0.57 a ± 0.03 | 0.56 a ± 0.13 |
| ×10 | 1.75 ab ± 0.09 | −10.63 ab ± 0.71 | 0.46 ab ± 0.02 | 0.31 bc ± 0.04 |
| Sample ID | EAI [g/m2] | ESI [%] |
|---|---|---|
| Control | 1.8 c ± 0.1 | 106.8 b ± 1.5 |
| ×500 | 1.8 c ± 0.1 | 105.5 b ± 2.2 |
| ×200 | 2.0 bc ± 0.1 | 103.9 b ± 2.8 |
| ×100 | 2.1 ab ± 0.1 | 111.7 b ± 3.5 |
| ×50 | 2.2 a ± 0.1 | 109.9 b ± 2.8 |
| ×10 | 2.1 ab ± 0.0 | 94.6 a ± 4.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zając, M.; Zhou, L.; Mika, M.; Yang, Z.; Wang, J.; Tao, Y.; Zhang, W. Hyaluronic Acid Interactions with Pork Myofibrillar Proteins in Emulsion Gel-Type Systems. Molecules 2025, 30, 2230. https://doi.org/10.3390/molecules30102230
Zając M, Zhou L, Mika M, Yang Z, Wang J, Tao Y, Zhang W. Hyaluronic Acid Interactions with Pork Myofibrillar Proteins in Emulsion Gel-Type Systems. Molecules. 2025; 30(10):2230. https://doi.org/10.3390/molecules30102230
Chicago/Turabian StyleZając, Marzena, Lei Zhou, Magdalena Mika, Ziyi Yang, Jingyu Wang, Ye Tao, and Wangang Zhang. 2025. "Hyaluronic Acid Interactions with Pork Myofibrillar Proteins in Emulsion Gel-Type Systems" Molecules 30, no. 10: 2230. https://doi.org/10.3390/molecules30102230
APA StyleZając, M., Zhou, L., Mika, M., Yang, Z., Wang, J., Tao, Y., & Zhang, W. (2025). Hyaluronic Acid Interactions with Pork Myofibrillar Proteins in Emulsion Gel-Type Systems. Molecules, 30(10), 2230. https://doi.org/10.3390/molecules30102230








