Functional Approaches to Discover New Compounds via Enzymatic Modification: Predicted Data Mining Approach and Biotransformation-Guided Purification
Abstract
1. Introduction
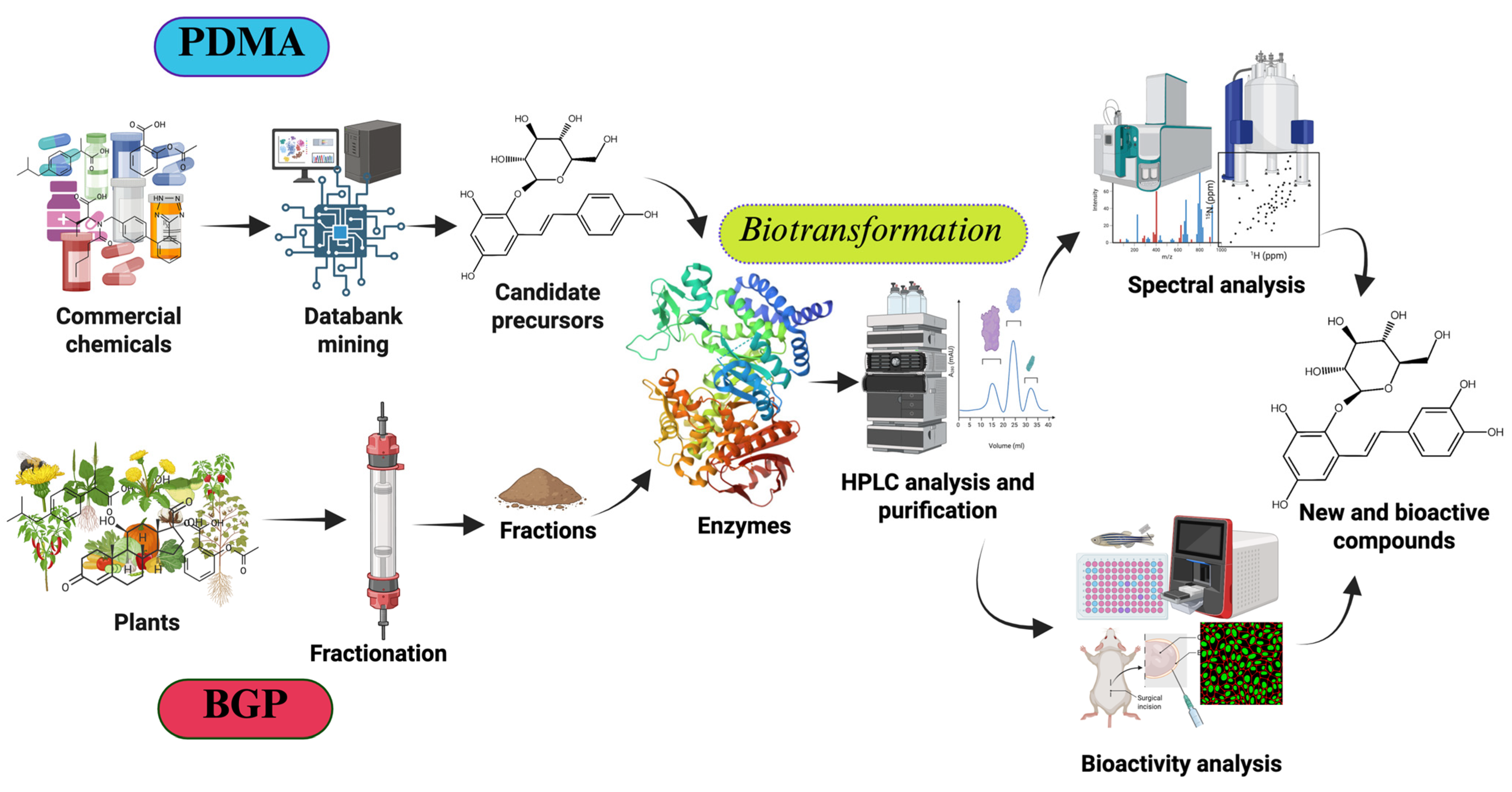
2. Finding New Compounds Using the Predicted Data Mining Approach
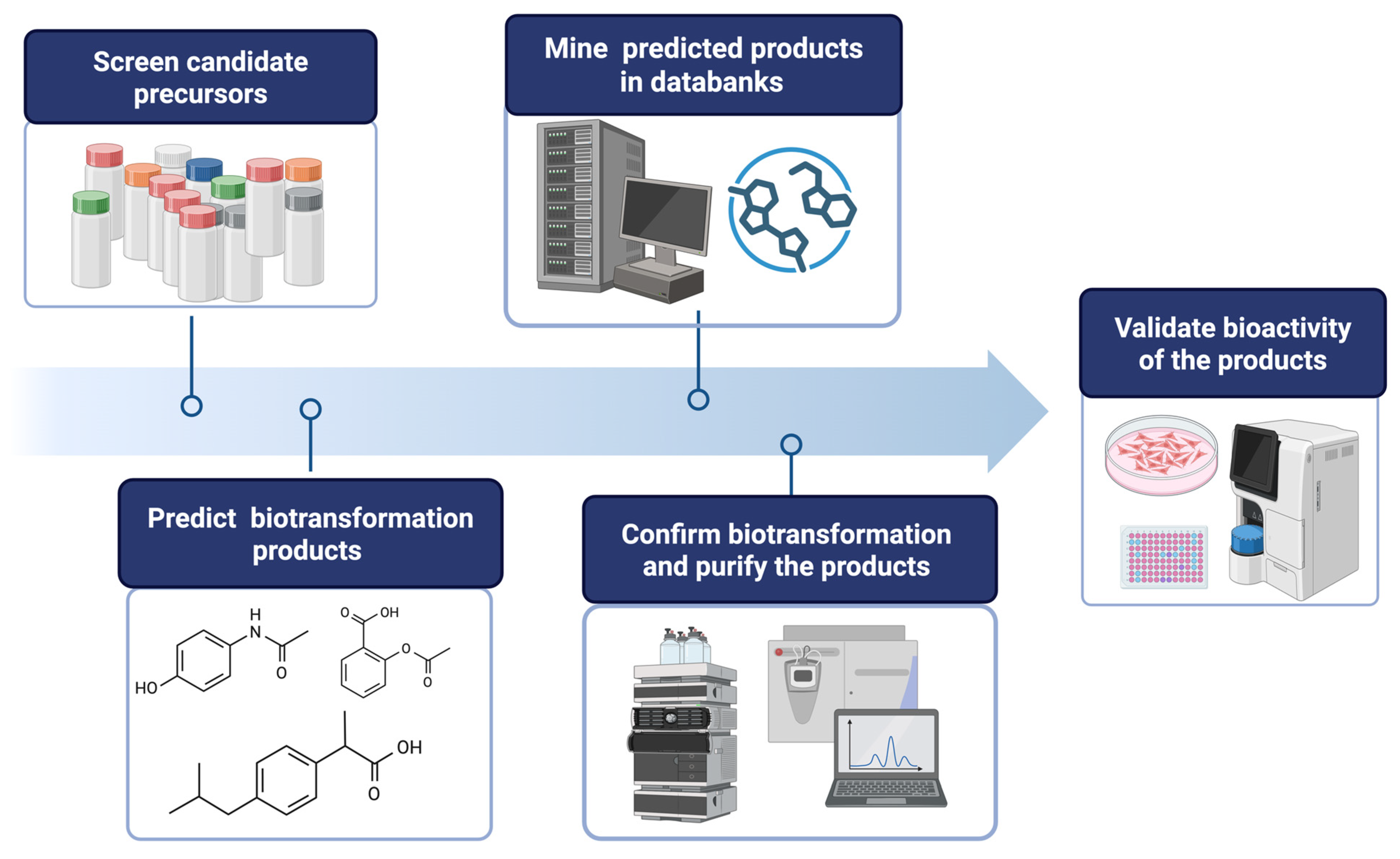
- Setting Screening Criteria (selecting a suitable enzymatic reaction): Define clear screening criteria based on the target enzyme’s known catalytic mechanism, substrate preference, and desired product characteristics. These criteria may include specific functional groups, structural features, physicochemical properties of precursor compounds, and their availability at an industrial scale. For example, hydroxylation by tyrosinase (BmTYR) requires precursors containing a phenyl group mimicking the structure of tyrosine; glycosylation by glycosyltransferases (GTs) requires precursors with at least one hydroxyl group that can be glycosylated; and O-methylation by O-methyltransferases (OMTs) requires precursors with a catechol structure.
- Screening Candidate Precursors: Based on the defined screening criteria, potential candidate precursors can be screened from commercial chemical or natural product databases. These databases usually contain a vast amount of compound structures and related information. In some cases, customized catalogs of commercially available compounds are used.
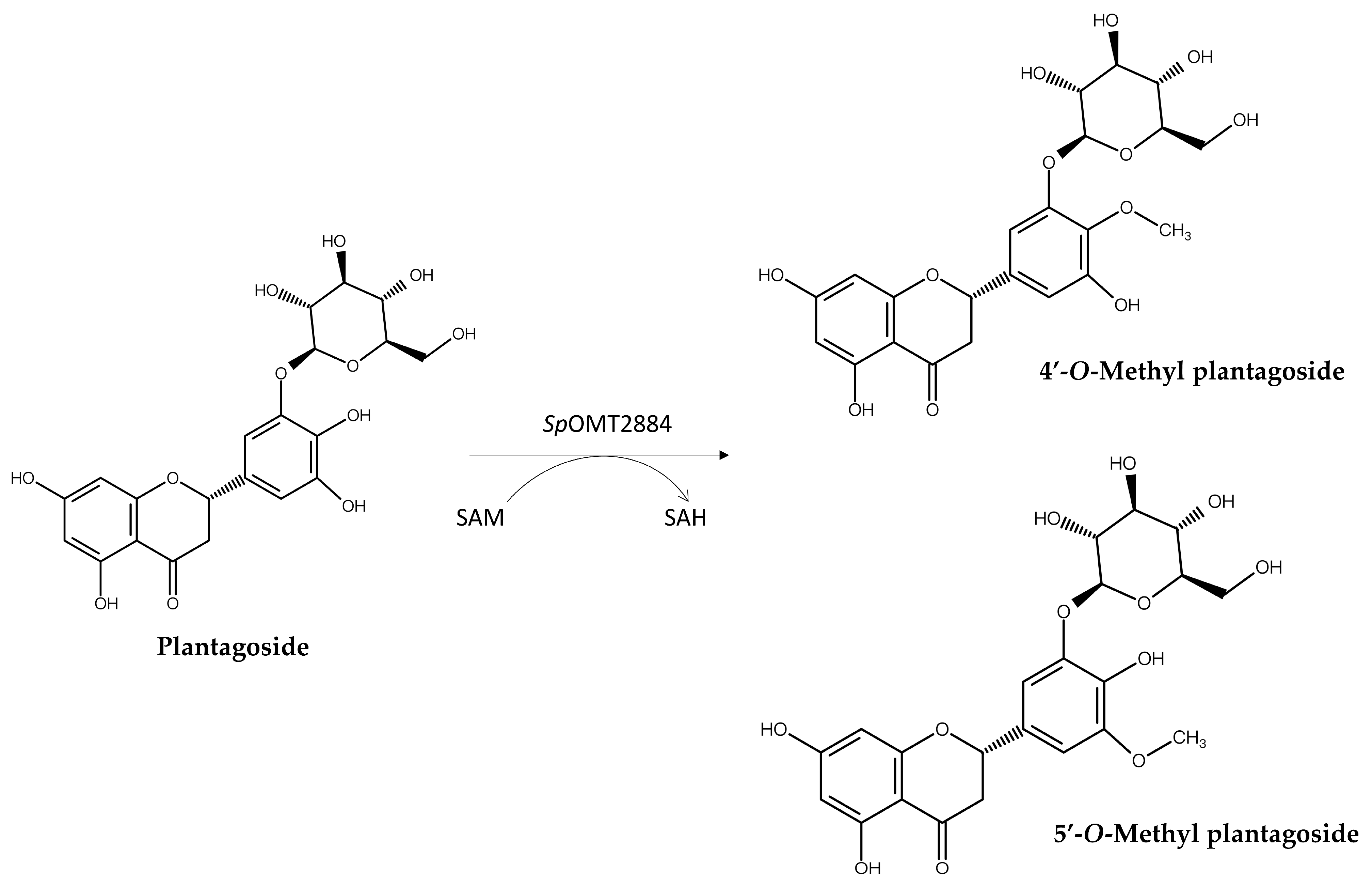
- Predicting Biotransformation Product Structure: For the selected candidate precursors, the structures of potential biotransformation products under the action of the target enzyme are determined using on-line chemical drawing software supplying by databases, such as Reaxys® or SciFinder®. This step requires researchers to have a certain knowledge of the enzyme’s catalytic mechanism; for instance, BmTYR primarily catalyzes ortho-hydroxylation, GTs catalyze the transfer of sugar moieties, and OMTs catalyze the transfer of methyl groups.
- Verifying Product Novelty: The predicted biotransformation product structures are uploaded to chemical databases (e.g., Reaxys®, PubChem®, or SciFinder®) in order to verify their novelty, confirming whether each product is a known compound. Only precursors that yield novel derivatives are further selected for subsequent experimental validation.
- In Vitro Biotransformation and Product Identification: The selected precursors are reacted with the target enzyme in vitro. The biotransformed products are analyzed using isolation methods, such as high-performance liquid chromatography (HPLC). Once the putative new compounds are purified, their chemical structures can be identified using techniques such as mass spectrometry (MS) and nuclear magnetic resonance (NMR).
- Bioactivity Evaluation: Alternatively, the identified compounds may undergo bioactivity testing to evaluate their potential application value. The tested activities may include antioxidant, anti-inflammatory, anticancer, and anti-diabetic properties, among others.
- High Efficiency: The PDMA enables rapid in silico screening of a large number of compounds, targeting potential precursors and thereby significantly reducing the time required to find suitable biotransformation substrates.
- Reduced Cost: Through minimizing the number of trial-and-error experiments, the PDMA helps to lower the consumption of experimental reagents, enzymes, and human resources, as well as reducing costs associated with clinical trials.
- Predicting Novelty: The PDMA predicts whether a given product is a novel compound before experimentation, avoiding the risk of redundant research on known compounds and increasing the likelihood of discovering new entities.
- Knowledge-Based Guidance: The PDMA can predict outcomes based on the enzyme’s characteristics, the precursor’s structure, and the experimental design, helping researchers better understand the potential results of biotransformation reactions.
- Applicable to Various Enzymes and Reactions: The PDMA concept is not limited to specific enzymes or reaction types. It can be adapted based on the catalytic properties of different enzymes and applied to various biotransformation processes, including hydroxylation, glycosylation, and methylation.
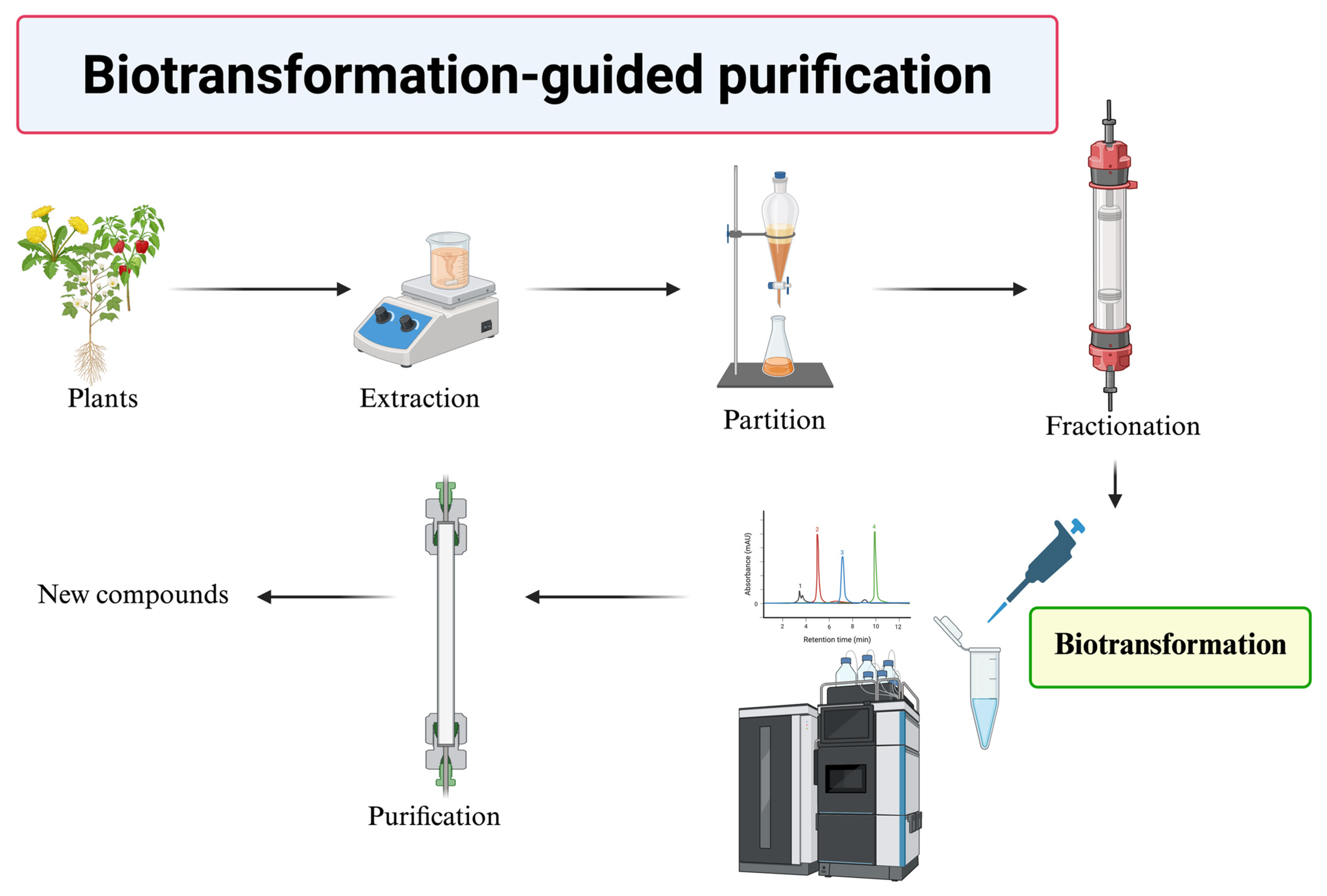
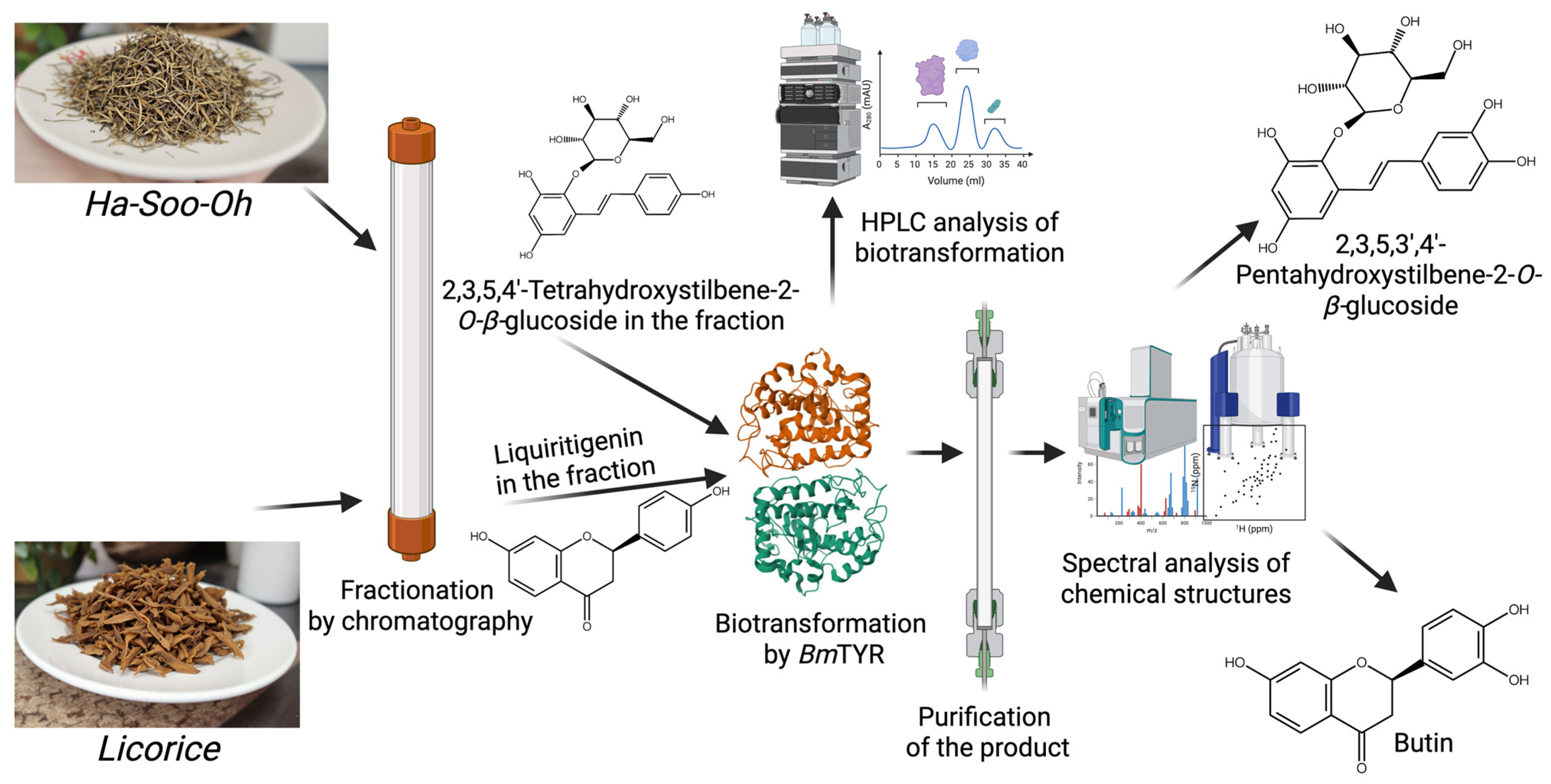
3. Finding New Compounds via Biotransformation-Guided Purification
- Discovery of Novel Bioactive Compounds: BGP facilitates the identification of new molecules that are structurally related to known bioactive precursors but possess altered or enhanced properties.
- Enhanced Bioactivity: Enzymatic biotransformation can modify the functional groups of precursor molecules, leading to derivatives with significantly improved bioactivity, as demonstrated by the enhanced antioxidant activities of butin and PSG.
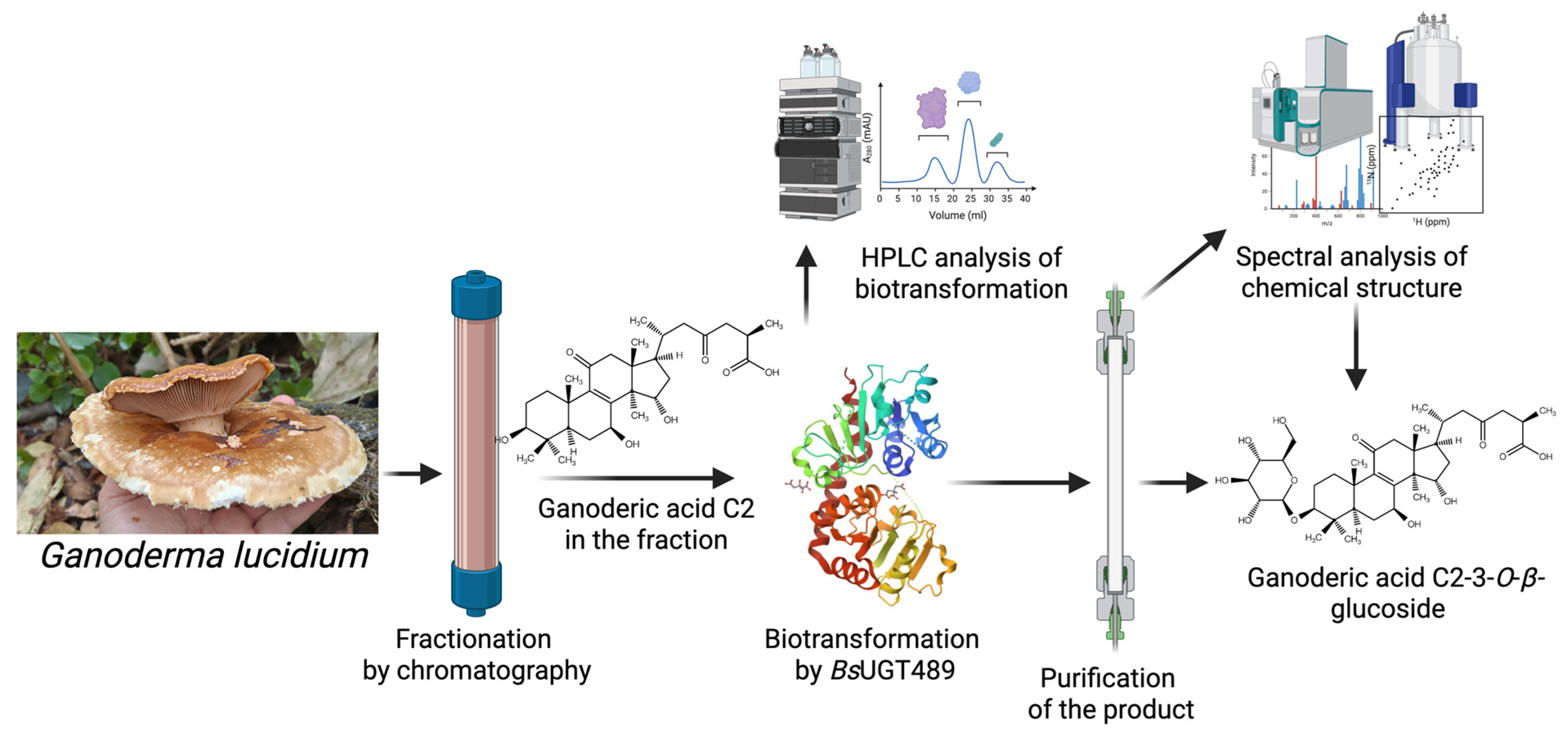
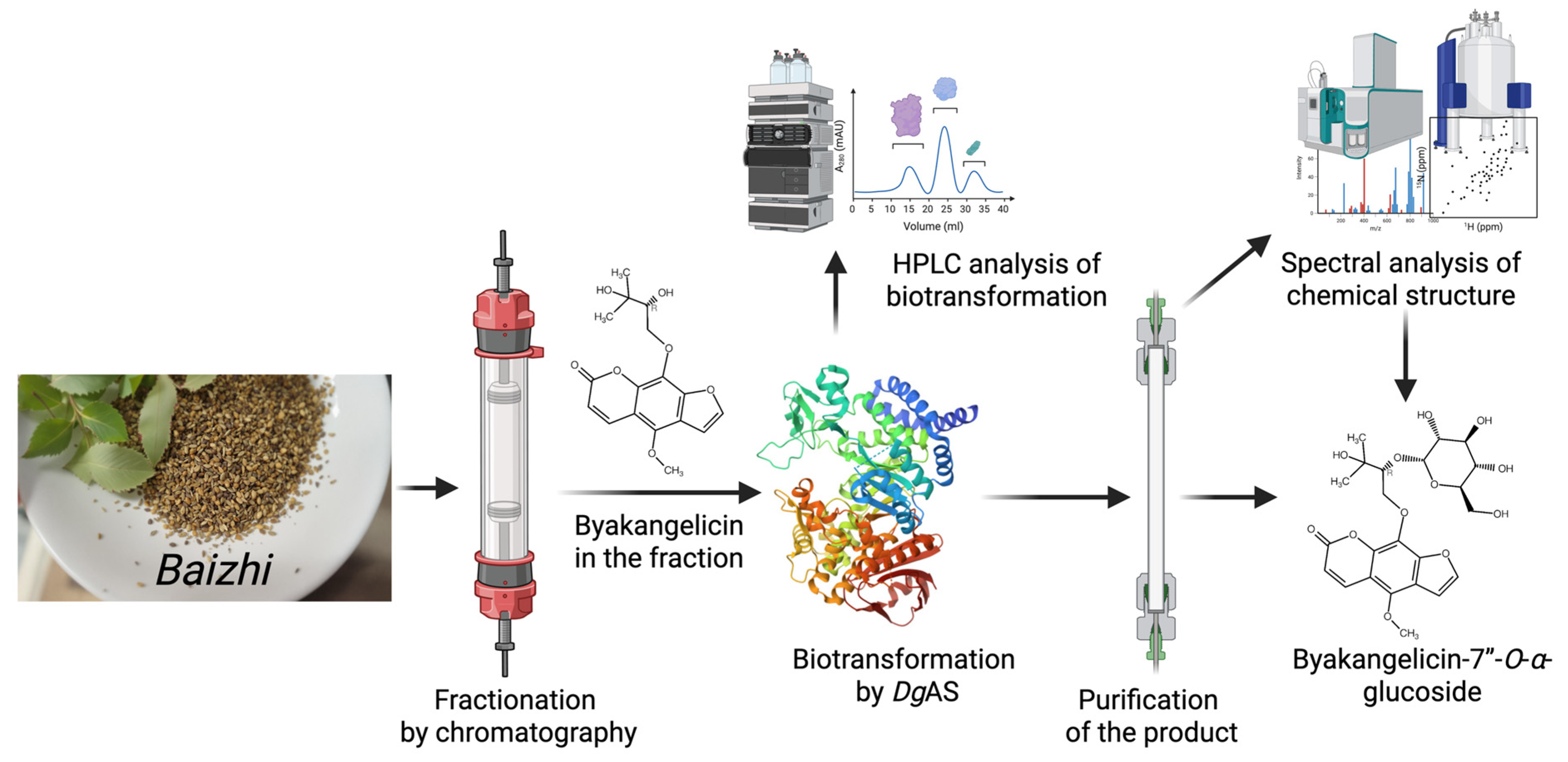
- Improved Physicochemical Properties: BGP can be used to generate derivatives with enhanced pharmaceutical properties, such as significantly increased aqueous solubility, as seen with the Ganoderma glucosides GAC2-3-O-β-glucoside and GAC2-3,15-O-β-diglucoside, as well as byakangelicin-7″-O-α-glucoside from Baizhi.
- Increased Yield of Active Ingredients: By selectively biotransforming a specific precursor within a complex mixture and then purifying the valuable product, BGP can sometimes lead to higher yields of the target compound when compared to direct isolation from the natural source, as observed in the production of butin.
- Cost-Effectiveness: BGP can utilize crude or partially purified extracts as starting materials, potentially reducing the need for extensive initial purification of precursors and leading to a more economical process.
- Efficiency in Screening Biotransformable Compounds: BGP offers an efficient method to screen complex natural extracts for compounds that can be biotransformed by specific enzymes, rather than testing expensive pure compounds individually.
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ekiert, H.M.; Szopa, A. Biological activities of natural products. Molecules 2020, 25, 5769. [Google Scholar] [CrossRef] [PubMed]
- Muffler, K.; Leipold, D.; Scheller, M.-C.; Haas, C.; Steingroewer, J.; Bley, T.; Neuhaus, H.E.; Mirata, M.A.; Schrader, J.; Ulber, R. Biotransformation of triterpenes. Process Biochem. 2011, 46, 1–15. [Google Scholar] [CrossRef]
- Cao, H.; Chen, X.; Jassbi, A.R.; Xiao, J. Microbial biotransformation of bioactive flavonoids. Biotechnol. Adv. 2015, 33, 214–223. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Wozniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Sultana, N.; Saify, Z.S. Enzymatic biotransformation of terpenes as bioactive agents. J. Enzym. Inhib. Med. Chem. 2013, 28, 1113–1128. [Google Scholar] [CrossRef] [PubMed]
- Andres, P.; Francisco, R.; Andres, G.-G.; Antonio, M. Microbial transformation of triterpenoids. Mini-Rev. Org. Chem. 2009, 6, 307–320. [Google Scholar] [CrossRef]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral activities of flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Ding, H.-Y.; Wang, T.-Y.; Cai, C.-Z.; Chang, T.-S. Antioxidant and anti-α-glucosidase activities of biotransformable dragon’s blood via predicted data mining approach. Process Biochem. 2023, 130, 166–173. [Google Scholar] [CrossRef]
- Wu, C.Y.; Ding, H.Y.; Wang, T.Y.; Liu, C.W.; Wu, J.Y.; Chang, T.S. Development of a new isoxsuprine hydrochloride-based hydroxylated compound with potent antioxidant and anti-inflammatory activities. J. Microbiol. Biotechnol. 2024, 34, 2693–2701. [Google Scholar] [CrossRef]
- Chang, T.S.; Wu, J.Y.; Ding, H.Y.; Tayo, L.L.; Suratos, K.S.; Tsai, P.W.; Wang, T.Y.; Fong, Y.N.; Ting, H.J. Predictive production of a new highly soluble glucoside, corylin-7-O-beta-glucoside with potent anti-inflammatory and anti-melanoma activities. Appl. Biochem. Biotechnol. 2024, 197, 1174–1191. [Google Scholar] [CrossRef]
- Chang, T.S.; Ding, H.Y.; Wang, T.Y.; Wu, J.Y.; Tsai, P.W.; Suratos, K.S.; Tayo, L.L.; Liu, G.C.; Ting, H.J. In silico-guided synthesis of a new, highly soluble, and anti-melanoma flavone glucoside: Skullcapflavone II-6′-O-beta-glucoside. Biotechnol. Appl. Biochem. 2024, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.Y.; Wang, T.Y.; Wu, J.Y.; Zhang, Y.R.; Chang, T.S. Novel Ganoderma triterpenoid saponins from the biotransformation-guided purification of a commercial Ganoderma extract. J. Biosci. Bioeng. 2023, 135, 402–410. [Google Scholar] [CrossRef]
- Chang, T.S.; Ding, H.Y.; Wu, J.Y.; Wang, M.L.; Ting, H.J. Biotransformation-guided purification of a novel glycoside derived from the extracts of Chinese berb Baizhi. J. Biosci. Bioeng. 2024, 137, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Ding, H.Y.; Wang, T.Y.; Hsu, M.H.; Chang, T.S. A new stilbene glucoside from biotransformation-guided purification of Chinese herb Ha-Soo-Oh. Plants 2022, 11, 2286. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Ding, H.-Y.; Wang, T.-Y.; Cai, C.-Z.; Chang, T.-S. Application of biotransformation-guided purification in Chinese medicine: An example to produce butin from licorice. Catalysts 2022, 12, 718. [Google Scholar] [CrossRef]
- Chang, T.-S.; Ding, H.-Y.; Wu, J.-Y.; Lee, C.-C.; Yang, Z.; Liu, Y.-C.; Wang, T.-Y. New methyl compounds using the predicted data mining approach (PDMA), coupled with the biotransformation of Streptomyces peucetius O-methyltransferase. Biocatal. Biotransform. 2025, 43, 84–96. [Google Scholar] [CrossRef]
- Lee, S.H.; Baek, K.; Lee, J.E.; Kim, B.G. Using tyrosinase as a monophenol monooxygenase: A combined strategy for effective inhibition of melanin formation. Biotechnol. Bioeng. 2016, 113, 735–743. [Google Scholar] [CrossRef]
- Slamova, K.; Kapesova, J.; Valentova, K. “Sweet flavonoids”: Glycosidase-catalyzed modifications. Int. J. Mol. Sci. 2018, 19, 2126. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, J.; Xie, Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. 2019, 570, 118642. [Google Scholar] [CrossRef]
- Wang, T.H.; Tseng, W.C.; Leu, Y.L.; Chen, C.Y.; Lee, W.C.; Chi, Y.C.; Cheng, S.F.; Lai, C.Y.; Kuo, C.H.; Yang, S.L.; et al. The flavonoid corylin exhibits lifespan extension properties in mouse. Nat. Commun. 2022, 13, 1238. [Google Scholar] [CrossRef]
- Kopycki, J.G.; Rauh, D.; Chumanevich, A.A.; Neumann, P.; Vogt, T.; Stubbs, M.T. Biochemical and structural analysis of substrate promiscuity in plant Mg2+-dependent O-methyltransferases. J. Mol. Biol. 2008, 378, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Kim, H.; Hur, H.G.; Lim, Y.; Ahn, J.H. Regioselectivity of 7-O-methyltransferase of poplar to flavones. J. Biotechnol. 2006, 126, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Solnier, J.; Martin, L.; Bhakta, S.; Bucar, F. Flavonoids as novel efflux pump inhibitors and antimicrobials against both environmental and pathogenic intracellular mycobacterial species. Molecules 2020, 25, 734. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, B.G.; Lee, Y.; Ryu, J.Y.; Lim, Y.; Hur, H.G.; Ahn, J.H. Regiospecific methylation of naringenin to ponciretin by soybean O-methyltransferase expressed in Escherichia coli. J. Biotechnol. 2005, 119, 155–162. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Z.; Zhang, L.; Wang, J.; Wu, C. Glycosyltransferase GT1 family: Phylogenetic distribution, substrates coverage, and representative structural features. Comput. Struct. Biotechnol. J. 2020, 18, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xu, W.; Guang, C.; Zhang, W.; Mu, W. Glycosylation of flavonoids by sucrose- and starch-utilizing glycoside hydrolases: A practical approach to enhance glycodiversification. Crit. Rev. Food Sci. Nutr. 2023, 63, 7408–7425. [Google Scholar] [CrossRef]
- Mestrom, L.; Przypis, M.; Kowalczykiewicz, D.; Pollender, A.; Kumpf, A.; Marsden, S.R.; Bento, I.; Jarzebski, A.B.; Szymanska, K.; Chrusciel, A.; et al. Leloir glycosyltransferases in applied biocatalysis: A multidisciplinary approach. Int. J. Mol. Sci. 2019, 20, 5263. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, W.; Zhang, W.; Zhang, T.; Guang, C.; Mu, W. Amylosucrase as a transglucosylation tool: From molecular features to bioengineering applications. Biotechnol. Adv. 2018, 36, 1540–1552. [Google Scholar] [CrossRef]
- Park, H.-S.; Choi, K.-H.; Park, Y.-D.; Park, C.-S.; Cha, J.-H. Enzymatic Synthesis of Polyphenol Glycosides by Amylosucrase. J. Life Sci. 2011, 21, 1631–1635. [Google Scholar] [CrossRef]
- Moulis, C.; Andre, I.; Remaud-Simeon, M. GH13 amylosucrases and GH70 branching sucrases, atypical enzymes in their respective families. Cell. Mol. Life Sci. 2016, 73, 2661–2679. [Google Scholar] [CrossRef]
- Rha, C.S.; Kim, H.G.; Baek, N.I.; Kim, D.O.; Park, C.S. Using Amylosucrase for the Controlled Synthesis of Novel Isoquercitrin Glycosides with Different Glycosidic Linkages. J. Agric. Food Chem. 2020, 68, 13798–13805. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.H.; Yoo, S.H.; Choi, S.J.; Kim, Y.R.; Park, C.S. Versatile biotechnological applications of amylosucrase, a novel glucosyltransferase. Food Sci. Biotechnol. 2020, 29, 1–16. [Google Scholar] [CrossRef]
- Seo, D.H.; Jung, J.H.; Ha, S.J.; Cho, H.K.; Jung, D.H.; Kim, T.J.; Baek, N.I.; Yoo, S.H.; Park, C.S. High-yield enzymatic bioconversion of hydroquinone to alpha-arbutin, a powerful skin lightening agent, by amylosucrase. Appl. Microbiol. Biotechnol. 2012, 94, 1189–1197. [Google Scholar] [CrossRef]
- Cho, H.K.; Kim, H.H.; Seo, D.H.; Jung, J.H.; Park, J.H.; Baek, N.I.; Kim, M.J.; Yoo, S.H.; Cha, J.; Kim, Y.R.; et al. Biosynthesis of (+)-catechin glycosides using recombinant amylosucrase from Deinococcus geothermalis DSM 11300. Enzym. Microbiol. Technol. 2011, 49, 246–253. [Google Scholar] [CrossRef]
- Kim, M.D.; Jung, D.H.; Seo, D.H.; Jung, J.H.; Seo, E.J.; Baek, N.I.; Yoo, S.H.; Park, C.S. Acceptor specificity of amylosucrase from Deinococcus radiopugnans and its application for synthesis of rutin derivatives. J. Microbiol. Biotechnol. 2016, 26, 1845–1854. [Google Scholar] [CrossRef]
- Kim, E.R.; Rha, C.S.; Jung, Y.S.; Choi, J.M.; Kim, G.T.; Jung, D.H.; Kim, T.J.; Seo, D.H.; Kim, D.O.; Park, C.S. Enzymatic modification of daidzin using heterologously expressed amylosucrase in Bacillus subtilis. Food Sci. Biotechnol. 2019, 28, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Rha, C.S.; Kim, E.R.; Kim, Y.J.; Jung, Y.S.; Kim, D.O.; Park, C.S. Simple and efficient production of highly soluble daidzin glycosides by amylosucrase from Deinococcus geothermalis. J. Agric. Food Chem. 2019, 67, 12824–12832. [Google Scholar] [CrossRef]
- Jung, Y.S.; Kim, Y.J.; Kim, A.T.; Jang, D.; Kim, M.S.; Seo, D.H.; Nam, T.G.; Rha, C.S.; Park, C.S.; Kim, D.O. Enrichment of polyglucosylated isoflavones from soybean isoflavone aglycones using optimized amylosucrase transglycosylation. Molecules 2020, 25, 181. [Google Scholar] [CrossRef]
- Wahab, S.; Annadurai, S.; Abullais, S.S.; Das, G.; Ahmad, W.; Ahmad, M.F.; Kandasamy, G.; Vasudevan, R.; Ali, M.S.; Amir, M. Glycyrrhiza glabra (Licorice): A comprehensive review on its phytochemistry, biological activities, clinical evidence and toxicology. Plants 2021, 10, 2751. [Google Scholar] [CrossRef] [PubMed]
| Strategy | Enzyme | Precursor | Novel Product | Properties of the New Compounds | Reference |
|---|---|---|---|---|---|
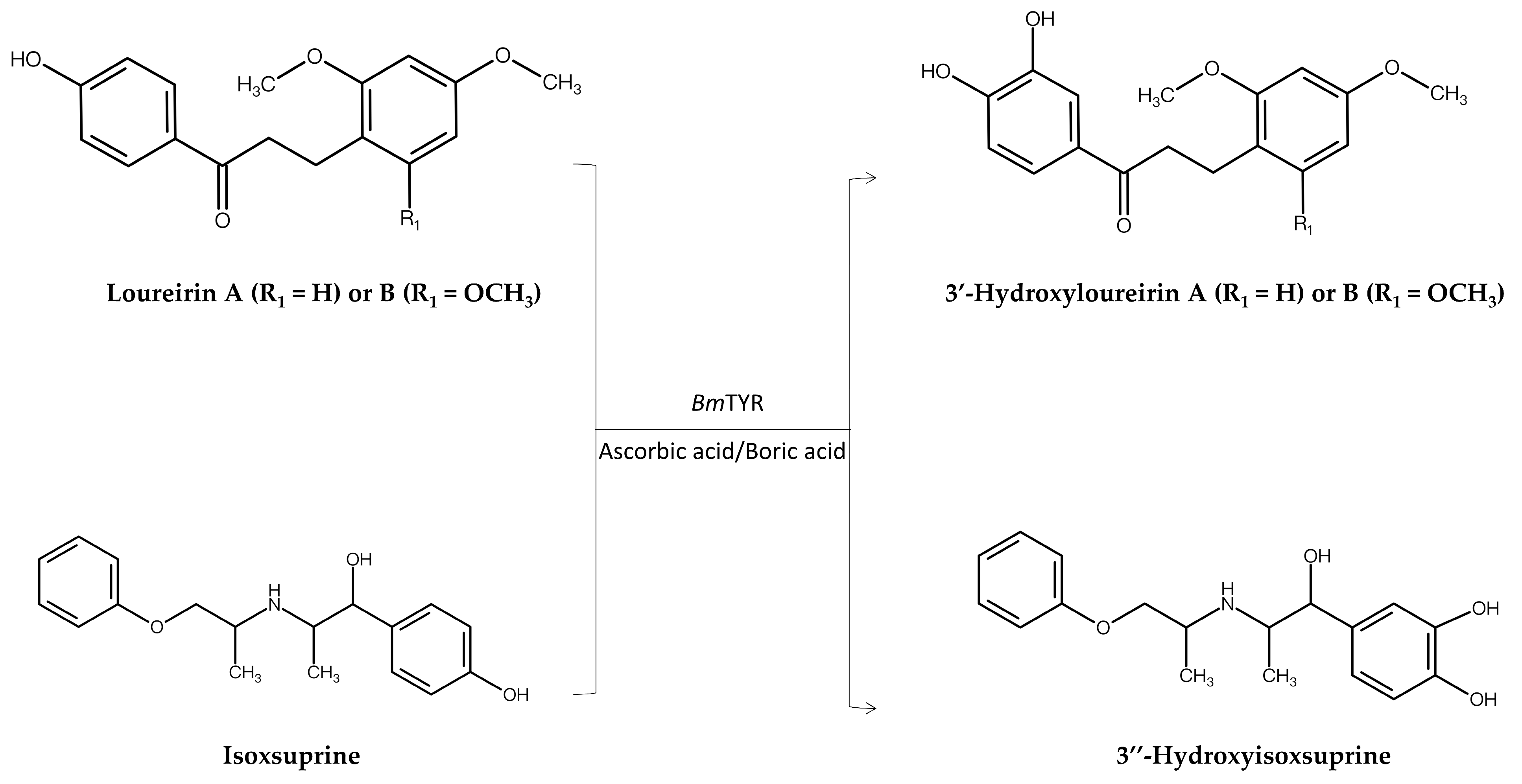
| Predicted data mining approach (PDMA) | BmTYR 1,2 | Loureirin A Loureirin B | 3′-Hydroxyloureirin A 3′-Hydroxyloureirin B | Improved both antioxidant and anti-α-glucosidase activity | [8] |
| BmTYR | Isoxsuprine | 3″-Hydroxyisoxsuprine | Improved both antioxidant and anti-inflammatory activity | [9] | |
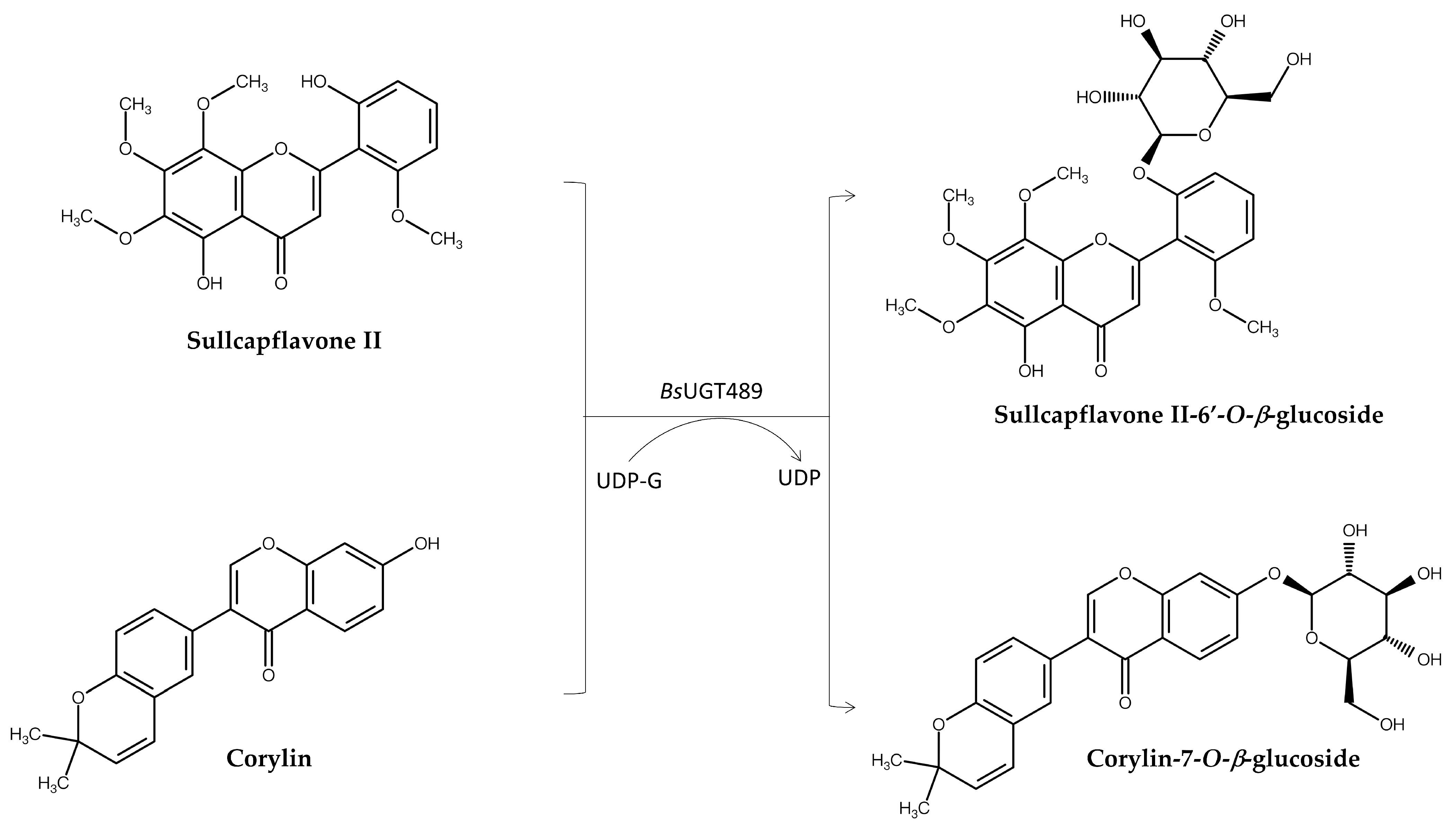
| BsUGT489 1,3 | Corylin | Corylin-7-O-β-glucoside | Improved both anti-inflammatory and anti-melanoma activity | [10] | |
| BsUGT489 | Skullcapflavone II | Sullcapflavone II-6′-O-β-glucoside | Improved both solubility and anti-melanoma activity | [11] | |
| SpOMT2884 1,4 | Plantagoside | 4′-O-Methyl plantagoside 5′-O-Methyl plantagoside | Not mentioned | [16] | |
| Biotransformation-guided purification (BGP) | BsUGT489 | Ganoderma extract | Ganoderic acid C2-3-O-β-glucoside | Improved solubility and maintains anti-α-glucosidase activity | [12] |
| DgAS 1,5 | Baizhi herb | Byakangelicin-7″-O-α-glucoside | Improved solubility | [13] | |
| BmTYR | Ha-Soo-Oh herb | 2,3,5,3′,4′-Pentahydroxystilbene-2-O-β-glucoside | Improved antioxidant activity | [14] | |
| BmTYR | Licorice herb | Butin | Improved antioxidant activity | [15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, T.-S. Functional Approaches to Discover New Compounds via Enzymatic Modification: Predicted Data Mining Approach and Biotransformation-Guided Purification. Molecules 2025, 30, 2228. https://doi.org/10.3390/molecules30102228
Chang T-S. Functional Approaches to Discover New Compounds via Enzymatic Modification: Predicted Data Mining Approach and Biotransformation-Guided Purification. Molecules. 2025; 30(10):2228. https://doi.org/10.3390/molecules30102228
Chicago/Turabian StyleChang, Te-Sheng. 2025. "Functional Approaches to Discover New Compounds via Enzymatic Modification: Predicted Data Mining Approach and Biotransformation-Guided Purification" Molecules 30, no. 10: 2228. https://doi.org/10.3390/molecules30102228
APA StyleChang, T.-S. (2025). Functional Approaches to Discover New Compounds via Enzymatic Modification: Predicted Data Mining Approach and Biotransformation-Guided Purification. Molecules, 30(10), 2228. https://doi.org/10.3390/molecules30102228








