Enhanced Yield of Methyl Ethyl Ketone through Levulinic Acid Decarboxylation in the AgNO3/K2S2O8 System: Mechanistic Insights and Characterization of Metallic Species
Abstract
1. Introduction
2. Results and Discussion
2.1. Influence of K2S2O8 and AgNO3
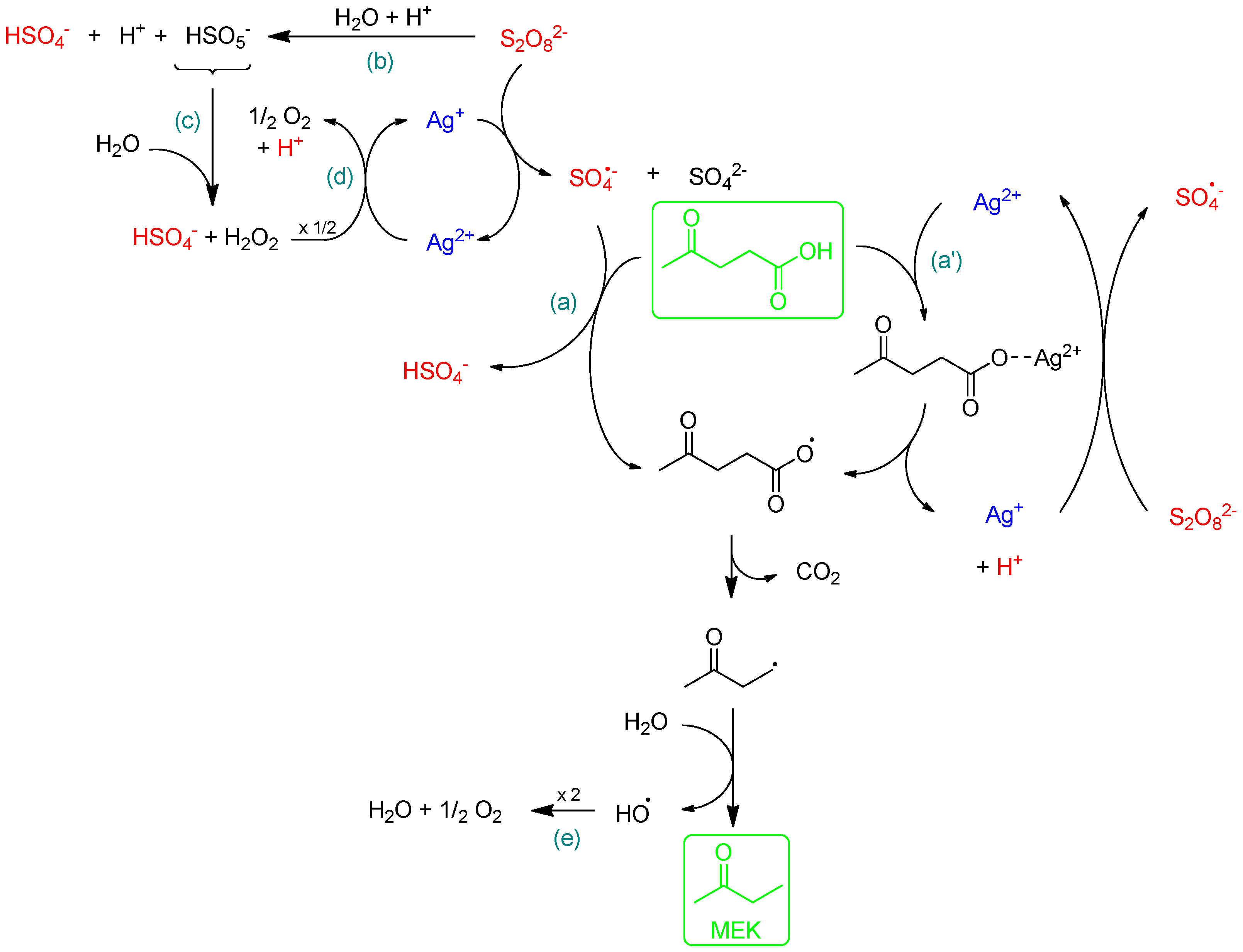
2.2. Role of Ag Salts
2.3. Role of K2S2O8 in the Presence of AgNO3
2.4. Influence of AgNO3/K2S2O8 Ratio
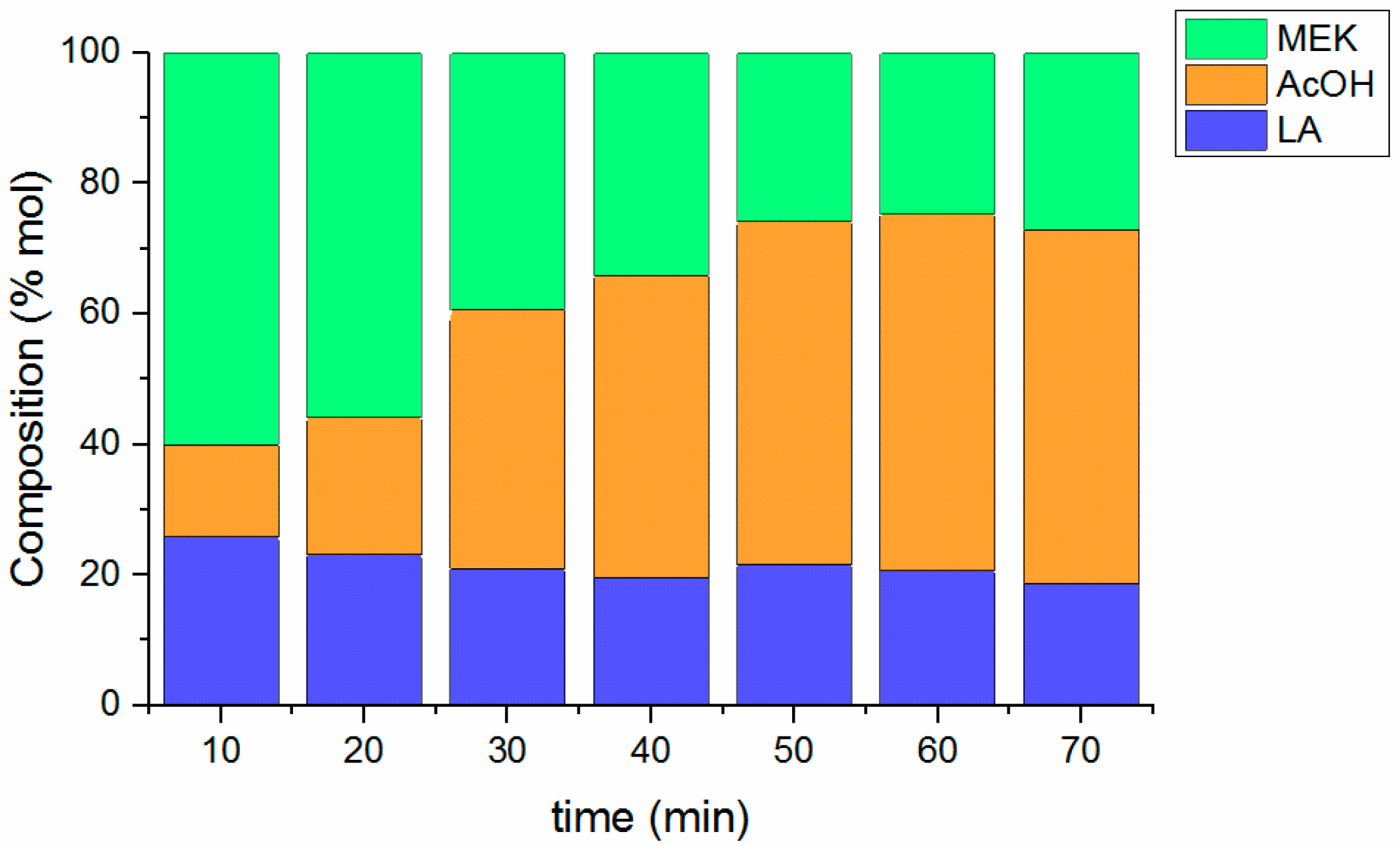
2.5. Influence of Temperature and Reaction Time
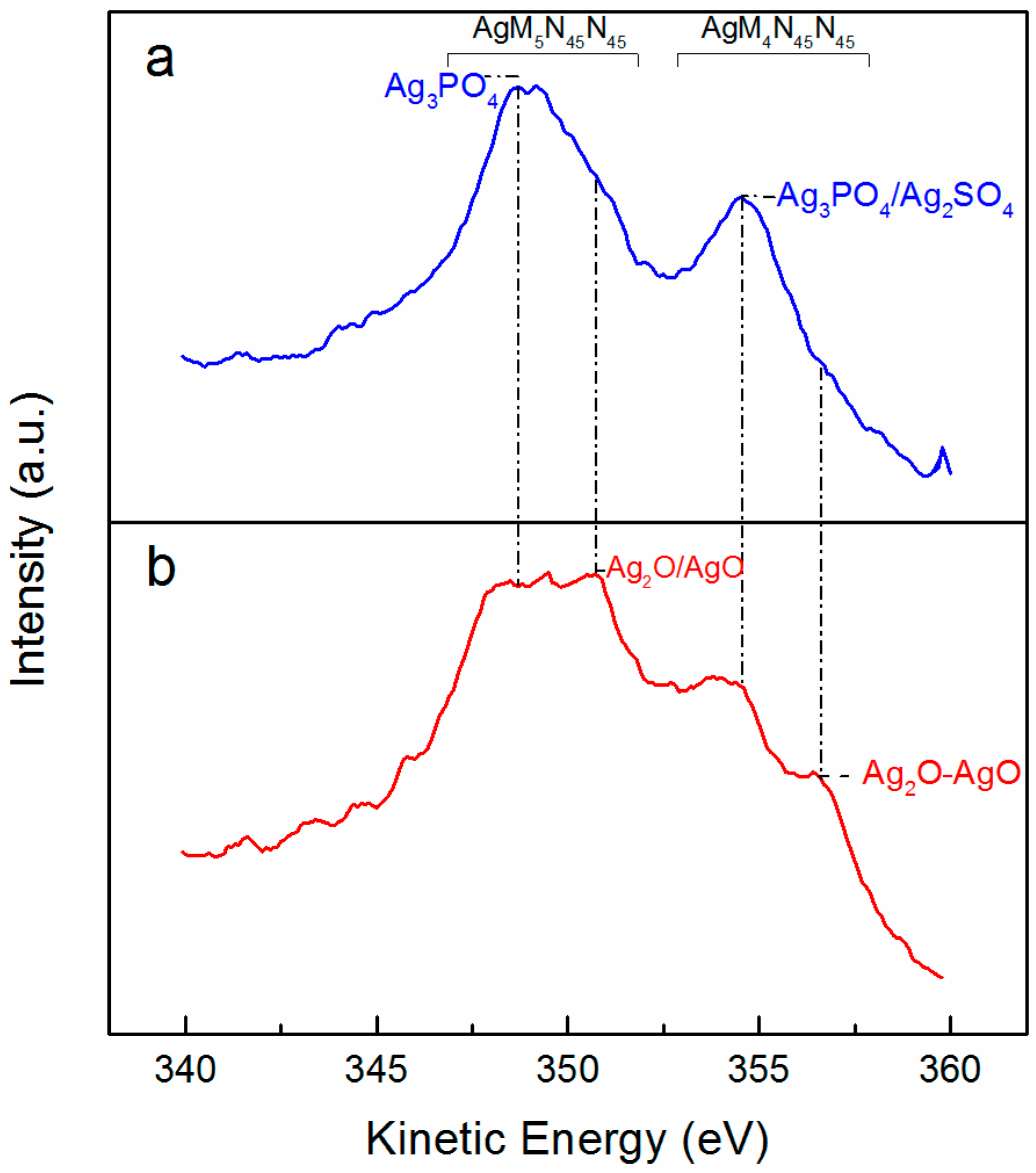
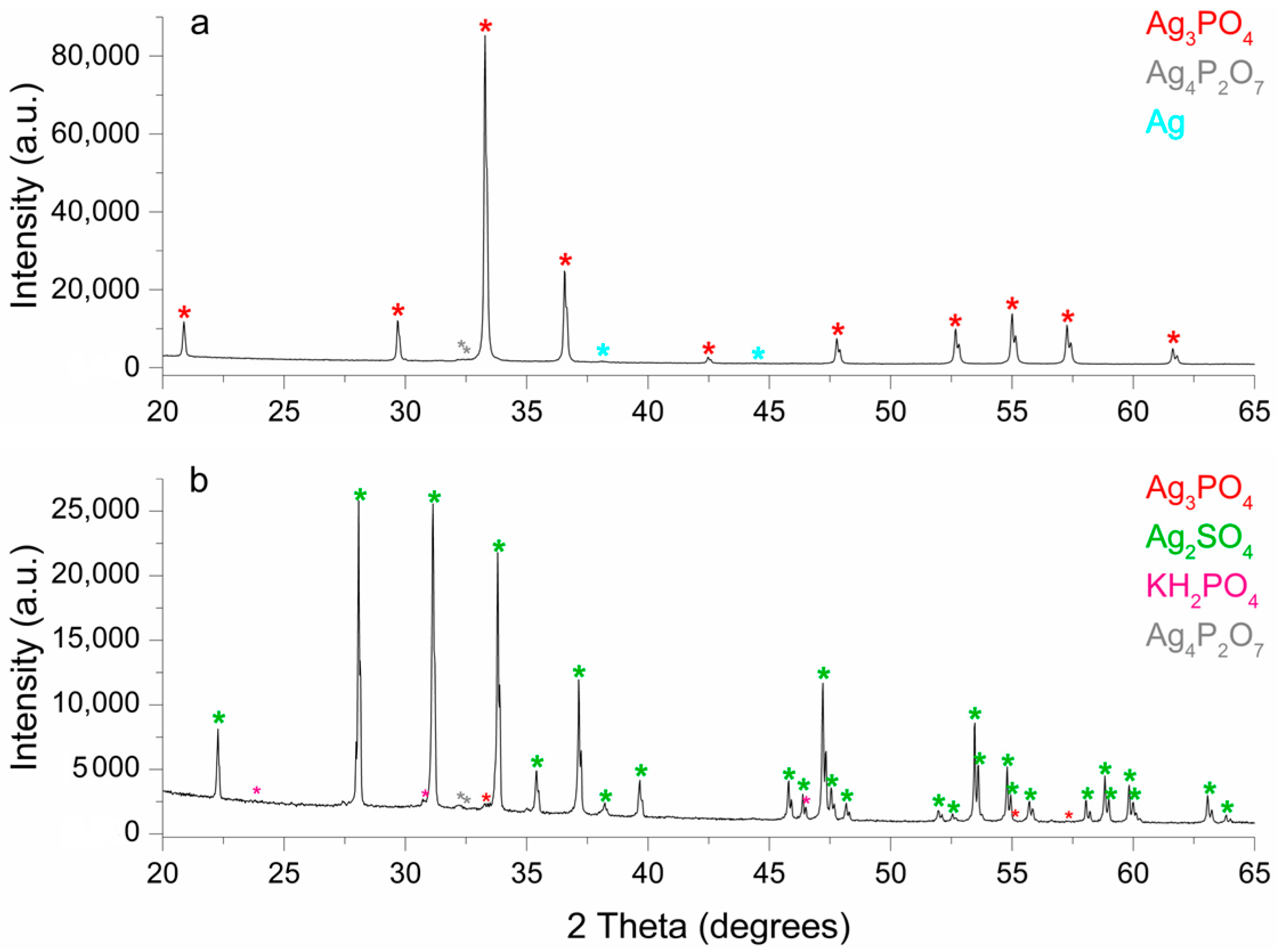
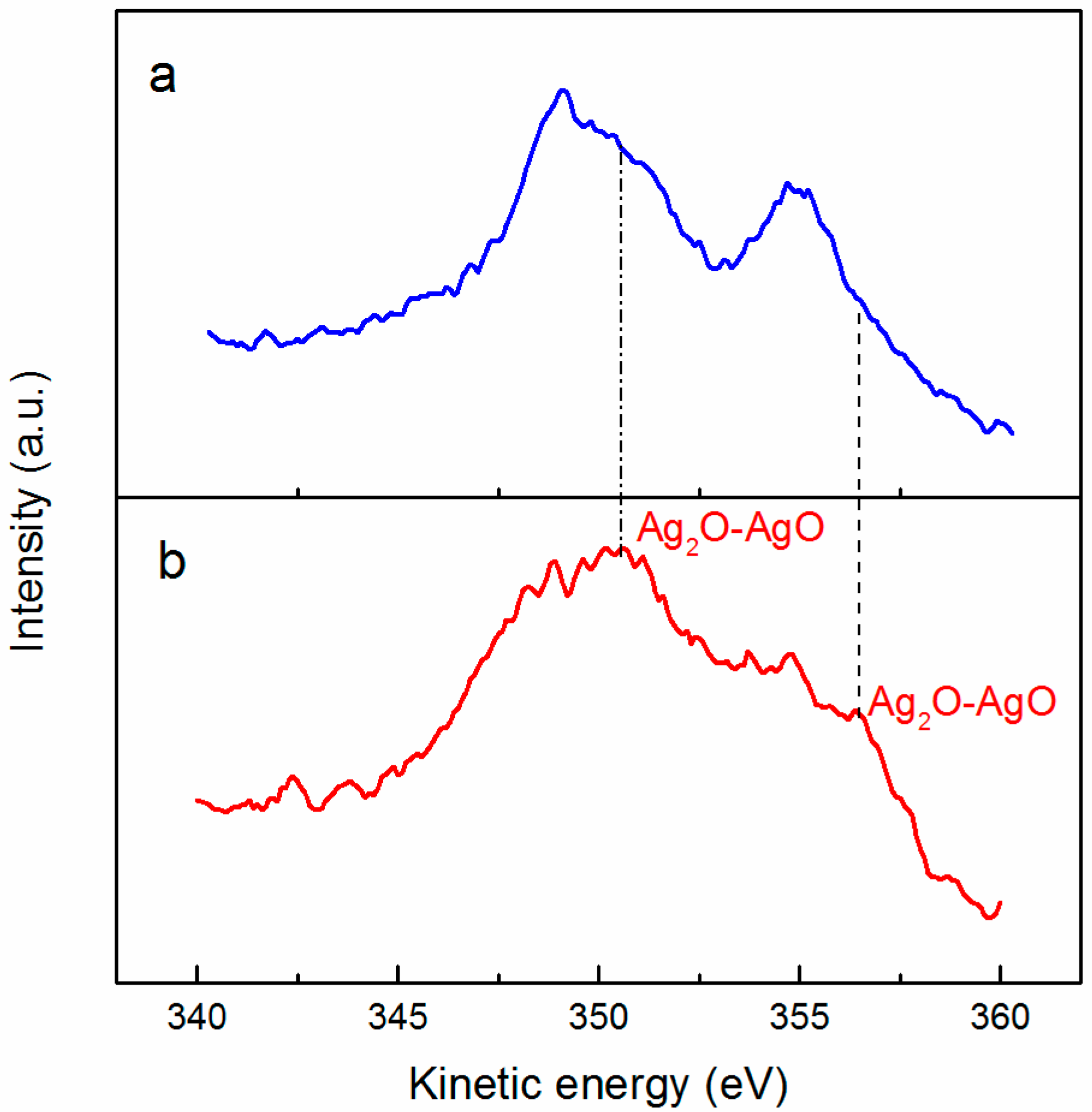
2.6. Characterization of Solid Phases
2.7. Influence of pH Variation
3. Materials and Methods
3.1. LA Decarboxylation
3.2. XPS Analyses
3.3. XRD Analyses
3.4. Thermodynamic Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sunnyside Corp. Available online: https://www.sunnysidecorp.com/product.php?p=t&b=s&n=847G1 (accessed on 3 October 2024).
- Indestructible Paint. Available online: https://indestructible.co.uk/wp-content/uploads/download-manager-files/MEK.pdf (accessed on 3 October 2024).
- Werpy, T.; Petersen, G.; Aden, A.; Bozell, J.; Holladay, J.; White, J.; Manheim, A.; Elliot, D.; Lasure, L.; Jones, S.; et al. Volumen I: Results of Screening for Potential Candidates from Sugars and Synthesis Gas. In Top Value Added Chemicals from Biomass; U.S. Department of Energy: Oak Ridge, TN, USA, 2004; p. 76. [Google Scholar]
- Grand View Research. Available online: https://www.grandviewresearch.com/press-release/global-levulinic-acid-market (accessed on 3 October 2017).
- Bozell, J.J.; Moens, L.; Elliott, D.C.; Wang, Y.; Neuenscwander, G.G.; Fitzpatrick, S.W.; Bilski, R.J.; Jarnefeld, J.L. Production of Levulinic Acid and Use as a Platform Chemical for Derived Products. Resour. Conserv. Recycl. 2000, 28, 227–239. [Google Scholar] [CrossRef]
- Stapley, J.A.; Bemiller, J.N. The Hofer-Moest Decarboxylation of D-Glucuronic Acid and D-Glucuronosides. Carbohydr. Res. 2007, 342, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Mundle, S.O.C.; Kluger, R. Decarboxylation via Addition of Water to a Carboxyl Group: Acid Catalysis of Pyrrole-2-Carboxylic Acid. J. Am. Chem. Soc. 2009, 131, 11674–11675. [Google Scholar] [CrossRef]
- Rodríguez, N.; Goossen, L.J. Decarboxylative Coupling Reactions: A Modern Strategy for C-C-Bond Formation. Chem. Soc. Rev. 2011, 40, 5030–5048. [Google Scholar] [CrossRef]
- Mirkhani, V.; Tangestaninejad, S.; Moghadam, M.; Moghbel, M. Rapid and Efficient Oxidative Decarboxylation of Carboxylic Acids with Sodium Periodate Catalyzed by Manganese (III) Schiff Base Complexes. Bioorg. Med. Chem. 2004, 12, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hakim, S.H.; Alonso, D.M.; Dumesic, J.A. A Highly Selective Route to Linear Alpha Olefins from Biomass-Derived Lactones and Unsaturated Acids. Chem. Commun. 2013, 49, 7040–7042. [Google Scholar] [CrossRef][Green Version]
- Monig, J.; Chapman, R.; Asmus, K.-D. Effect of the Protonation State of the Amino Group on the *OH Radical Induced Decarboxylation of Amino Acids in Aqueous Solution. J. Phys. Chem 1985, 89, 3139–3144. [Google Scholar] [CrossRef]
- Steffen, L.K.; Glass, R.S.; Sabahi, M.; Wilson, G.S.; Schoeneich, C.; Mahling, S.; Asmus, K.D. Hydroxyl Radical Induced Decarboxylation of Amino Acids. Decarboxylation vs. Bond Formation in Radical Intermediates. J. Am. Chem. Soc. 1991, 113, 2141–2145. [Google Scholar] [CrossRef]
- Goldstein, S.; Czapski, G.; Cohen, H.; Meyerstein, D. Hydroxyl Radical Induced Decarboxylation and Deamination of 2-Methylalanine Catalyzed by Copper Ions. Inorg. Chem. 1992, 31, 2439–2444. [Google Scholar] [CrossRef]
- Bobrowski, K.; Pogocki, D.; Schoneich, C. Mechanism of the Hydroxyl Radical-Induced Decarboxylation of 2-(Alkylthio)Ethanoic Acid Derivatives. J. Phys. Chem. 1993, 97, 13677–13684. [Google Scholar] [CrossRef]
- Guitton, J.; Tinardon, F.; Lamrini, R.; Lacan, P.; Desage, M.; Francina, A. Decarboxylation of [1-13C]Leucine by Hydroxyl Radicals. Free Radic. Biol. Med. 1998, 25, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, X.; Wang, X.; Huang, X.; Shen, T.; Zhang, Y.; Sun, X.; Zou, M.; Song, S.; Jiao, N. Silver-Catalyzed Decarboxylative Azidation of Aliphatic Carboxylic Acids. Org. Lett. 2015, 17, 4702–4705. [Google Scholar] [CrossRef] [PubMed]
- Fristad, W.E.; Fry, M.A.; Klang, J.A. Persulfate/Silver Ion Decarboxylation of Carboxylic Acids. Preparation of Alkanes, Alkenes, and Alcohols. J. Org. Chem. 1983, 48, 3575–3577. [Google Scholar] [CrossRef]
- Wan, W.; Li, J.; Ma, G.; Chen, Y.; Jiang, H.; Deng, H.; Hao, J. Ag(I)-Catalyzed Oxidative Decarboxylation of Difluoroacetates with Activated Alkenes to Form Difluorooxindoles. Org. Biomol. Chem. 2017, 15, 5308. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Hu, P.; Zhang, M.; Su, W. Metal-Catalyzed Decarboxylative C−H Functionalization. Chem. Rev. 2017, 117, 8864–8907. [Google Scholar] [CrossRef]
- Kiméné, A.; Wojcieszak, R.; Paul, S.; Dumeignil, F. Catalytic Decarboxylation of Fatty Acids to Hydrocarbons over Non-Noble Metal Catalysts: The State of the Art. J. Chem. Technol. Biotechnol. 2018, 94, 658–669. [Google Scholar] [CrossRef]
- Chumaidi, A.; Dewajani, H.; Sulaiman, M.A.; Angestine, F.; Putri, A.; Pravitasari, S.A. Effect of Temperature and Mg-Zn Catalyst Ratio on Decarboxylation Reaction to Produce Green Diesel from Kapok Oil with Saponification Pretreatment Using NaOH. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1073, 012002. [Google Scholar] [CrossRef]
- Chum, H.L.; Ratcliff, M.; Posey, F.L.; Turner, J.A.; Nozik, A.J. Photoelectrochemistry of Levulinic Acid on Undoped Platinized N-Titanium Dioxide Powders. J. Phys. Chem. 1983, 87, 3089–3093. [Google Scholar] [CrossRef]
- Gong, Y.; Lin, L.; Shi, J.; Liu, S. Oxidative Decarboxylation of Levulinic Acid by Cupric Oxides. Molecules 2010, 15, 7946–7960. [Google Scholar] [CrossRef]
- Gong, Y.; Lin, L. Oxidative Decarboxylation of Levulinic Acid by Silver(I)/Persulfate. Molecules 2011, 16, 2714–2725. [Google Scholar] [CrossRef]
- Mehrer, C.R.; Rand, J.M.; Incha, M.R.; Cook, T.B.; Demir, B.; Hussain Motagamwala, A.; Kim, D.; Dumesic, J.A.; Pfleger, B.F. Growth-Coupled Bioconversion of Levulinic Acid to Butanone. Metab. Eng. 2019, 55, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Grilc, M.; Likozar, B. Levulinic Acid Hydrodeoxygenation, Decarboxylation and Oligmerization over NiMo/Al2O3 Catalyst to Bio-Based Value-Added Chemicals: Modelling of Mass Transfer, Thermodynamics and Micro-Kinetics. Chem. Eng. J. 2017, 330, 383–397. [Google Scholar] [CrossRef]
- Novodárszki, G.; Valyon, J.; Illés, Á.; Dóbé, S.; Deka, D.; Hancsók, J.; Mihályi, M.R.; Hu, M.M.M. Heterogeneous Hydroconversion of Levulinic Acid over Silica-Supported Ni Catalyst. React. Kinet. Mech. Catal. 2019, 126, 795–810. [Google Scholar] [CrossRef]
- Walling, C.; Camaioni, D.M. Role of Silver(II) in Silver-Catalyzed Oxidations by Peroxydisulfate. J. Org. Chem. 1978, 43, 3266–3271. [Google Scholar] [CrossRef]
- Fristad, W.E.; Klang, J.A. Silver(I)/Persulfate Oxidative Decarboxylation of Carboxylic Acids. Arylacetic Acid Dimerization. Tetrahedron Lett. 1983, 24, 2219–2222. [Google Scholar] [CrossRef]
- Tanner, D.D.; Osman, S.A.A. Oxidative Decarboxylation. On the Mechanism of the Potassium Persulfate-Promoted Decarboxylation Reaction. J. Org. Chem. 1987, 52, 4689–4693. [Google Scholar] [CrossRef]
- Seiple, I.B.; Su, S.; Rodriguez, R.A.; Gianatassio, R.; Fujiwara, Y.; Sobel, A.L.; Baran, P.S. Direct C−H Arylation of Electron-Deficient Heterocycles with Arylboronic Acids. J. Am. Chem. Soc. 2010, 132, 13194–13196. [Google Scholar] [CrossRef]
- Patel, N.R.; Flowers, R.A. Uncovering the Mechanism of the Ag(I)/Persulfate-Catalyzed Cross-Coupling Reaction of Arylboronic Acids and Heteroarenes. J. Am. Chem. Soc. 2013, 135, 4672–4675. [Google Scholar] [CrossRef]
- Kan, J.; Huang, S.; Lin, J.; Zhang, M.; Su, W. Silver-Catalyzed Arylation of (Hetero)Arenes by Oxidative Decarboxylation of Aromatic Carboxylic Acids. Angew. Chem. Int. Ed. Engl. 2015, 54, 2199–2203. [Google Scholar] [CrossRef]
- Chang, S.; Jian, A.; Wang, F.; Dong, L.L.; Wang, D.; Feng, B.; Shi, Y.T. Ag(I)/Persulfate-Catalyzed Decarboxylative Coupling of a-Oxocarboxylates with Organotrifluoroborates in Water under Room Temperature. RSC Adv. 2017, 7, 51928–51934. [Google Scholar] [CrossRef]
- Anderson, J.M.; Kochi, J.K. Silver(I)-Catalyzed Oxidative Decarboxylation of Acids by Peroxydisulfate. Role of Silver(II). J. Am. Chem. Soc. 1970, 92, 1651–1659. [Google Scholar] [CrossRef]
- Bartlett, P.D.; Cotman, J.D. The Kinetics of the Decomposition of Potassium Persulfate in Aqueous Solutions of Methanol. J. Am. Chem. Soc. 1949, 71, 1419–1422. [Google Scholar] [CrossRef]
- Kolthoff, I.M.; Miller, I.K. The Chemistry of Persulfate. I. The Kinetics and Mechanism of the Decomposition of the Persulfate Ion in Aqueous Medium 1. J. Am. Chem. Soc. 1951, 73, 3055–3059. [Google Scholar] [CrossRef]
- Berlin, A.A. Kinetics of Radical-Chain Decomposition of Persulfate in Aqueous Solutions of Organic Compounds. Kinet. Catal. 1986, 27, 34–39. [Google Scholar]
- Berlin, A.A.; Kislenko, V.N.; Medvedevskikh, Y.G. Conditions for Occurrence of Long-Chain Oxidation Reaction of Organic Compounds by Persulfate in an Aqueous Medium. Theor. Exp. Chem. 1990, 26, 337–341. [Google Scholar] [CrossRef]
- Hussain, I.; Zhang, Y.; Huang, S.; Du, X. Degradation of P-Chloroaniline by Persulfate Activated with Zero-Valent Iron. Chem. Eng. J. 2012, 203, 269–276. [Google Scholar] [CrossRef]
- Couttenye, R.A.; Huang, K.C.; Hoag, G.E.; Suib, S.L. Evidence of Sulfate Free Radical (SO4−) Formation under Heat-Assisted Persulfate Oxidation of MTBE. In Proceedings of the 19th Petroleum Hydrocarbons and Organic Chemicals in Ground Water: Prevention, Assessment, and Remediation, Conference and Exposition, Atlanta, GA, USA; 2002; pp. 345–350. [Google Scholar]
- Dawes, G.J.S.; Scott, E.L.; Le Nôtre, J.; Sanders, J.P.M.; Bitter, J.H. Deoxygenation of Biobased Molecules by Decarboxylation and Decarbonylation—A Review on the Role of Heterogeneous, Homogeneous and Bio-Catalysis. Green Chem. 2015, 17, 3231–3250. [Google Scholar] [CrossRef]
- Herrera-Ordonez, J. The Role of Sulfate Radicals and PH in the Decomposition of Persulfate in Aqueous Medium: A Step towards Prediction. Chem. Eng. J. Adv. 2022, 11, 100331–100341. [Google Scholar] [CrossRef]
- Kimura, M.; Kawajiri, T.; Tanida, M. Kinetics of the Silver(I)-Catalyzed Decomposition of Peroxodisulphate in Aqueous Solution. J. Chem. Soc. Dalton Trans. 1980, 5, 726. [Google Scholar] [CrossRef]
- Xia, X.-F.; Zhu, S.-L.; Chen, C.; Wang, H.; Liang, Y.-M. Silver-Catalyzed Decarboxylative Addition/Cyclization of Activated Alkenes with Aliphatic Carboxylic Acids. J. Org. Chem. 2016, 81, 1277–1284. [Google Scholar] [CrossRef]
- Liu, J.; Fan, C.; Yin, H.; Qin, C.; Zhang, G.; Zhang, X.; Yi, H.; Lei, A. Synthesis of 6-Acyl Phenanthridines by Oxidative Radical Decarboxylation-Cyclization of α-Oxocarboxylates and Isocyanides. Chem. Commun. 2014, 50, 2145–2147. [Google Scholar] [CrossRef]
- Hobbs, C.C.; Van’t Hof, H. Liquid Phase Oxidation of Methyl Ethyl Ketone to Form Acetic Acid Therefrom. U.S. Patent No. US3947497A, 4 January 1974. [Google Scholar]
- Clark, L.W. The Decarboxylation of Methylmalonic Acid Andn-Octadecylmalonic Acid in Normal Alkanols. Int. J. Chem. Kinet. 1976, 8, 609–624. [Google Scholar] [CrossRef]
- Stockbridge, R.B.; Lewis, C.A.; Yuan, Y.; Wolfenden, R.; Wolfenden, R. Impact of Temperature on the Time Required for the Establishment of Primordial Biochemistry, and for the Evolution of Enzymes. Proc. Natl. Acad. Sci. USA 2010, 107, 22102–22105. [Google Scholar] [CrossRef]
- Wolfenden, R.; Lewis, C.A.; Yuan, Y.; Yuan, Y. Kinetic Challenges Facing Oxalate, Malonate, Acetoacetate, and Oxaloacetate Decarboxylases. J. Am. Chem. Soc. 2011, 133, 5683–5685. [Google Scholar] [CrossRef]
- Ng, H.N.; Calvo, C.; Faggiani, R.; IUCr. A New Investigation of the Structure of Silver Orthophosphate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1978, 34, 898–899. [Google Scholar] [CrossRef]
- Keen, R.C. The Crystal Structure of Potassium Persulfate K2S2O8. Z. Krist.—Cryst. Mater. 1935, 91, 129–135. [Google Scholar] [CrossRef]
- Morris, M.; McMurdie, H.; Evans, E.; Paretzkin, B.; Groot, J.; Hubbard, C.; Carmel, S. Standard X-ray Diffraction Powder Patterns; National Bureau of Standards Monograph 25; U.S. Department of Commerce: Washington, DC, USA, 1976; p. 37.
- Tibballs, J.E.; Nelmes, R.J.; McIntyre, G.J. The Crystal Structure of Tetragonal KH2PO4 and KD2PO4 as a Function of Temperature and Pressure. J. Phys. C Solid State Phys. 1982, 15, 37–58. [Google Scholar] [CrossRef]
- Morgan, J.L.R.; Crist, R.H. The Photochemical Decomposition of Potassium Persulfate. I. J. Am. Chem. Soc. 1927, 49, 16–25. [Google Scholar] [CrossRef]
- Morgan, J.L.R.; Crist, R.H. The Photochemical Decomposition of Potassium Persulfate. II. J. Am. Chem. Soc. 1927, 49, 338–346. [Google Scholar] [CrossRef]
- Heidt, L.J. The Photolysis of Persulfate. J. Chem. Phys. 1942, 10, 297–302. [Google Scholar] [CrossRef]
- House, D.A. Kinetics and Mechanism of Oxidations by Peroxydisulfate. Chem. Rev. 1962, 62, 185–203. [Google Scholar] [CrossRef]
- Jette, E.R.; Foote, F. Precision Determination of Lattice Constants. J. Chem. Phys. 1935, 3, 605–616. [Google Scholar] [CrossRef]
- Deb, A.; Manna, S.; Modak, A.; Patra, T.; Maity, S.; Maiti, D. Oxidative Trifluoromethylation of Unactivated Olefins: An Efficient and Practical Synthesis of α-Trifluoromethyl-Substituted Ketones. Angew. Chem. Int. Ed. 2013, 52, 9747–9750. [Google Scholar] [CrossRef]
- Hatamura, M.; Yamaguchi, S.; Takane, S.; Chen, Y.; Suganuma, K. Decarboxylation and Simultaneous Reduction of Silver(I) β-Ketocarboxylates with Three Types of Coordinations. Dalton Trans. 2015, 44, 8993–9003. [Google Scholar] [CrossRef]
- Standke, B.; Jansen, M. Ag3O4, the First Silver(II,III) Oxide. Angew. Chem. Int. Ed. Engl. 1986, 25, 77–78. [Google Scholar] [CrossRef]
- Waterhouse, G.I.N.; Metson, J.B.; Bowmaker, G.A. Synthesis, Vibrational Spectra and Thermal Stability of Ag3O4 and Related Ag7O8X Salts (X=NO3−,ClO4−,HSO4−). Polyhedron 2007, 26, 3310–3322. [Google Scholar] [CrossRef]
- Kaspar, T.C.; Droubay, T.; Chambers, S.A.; Bagus, P.S. Spectroscopic Evidence for Ag(III) in Highly Oxidized Silver Films by X-Ray Photoelectron Spectroscopy. J. Phys. Chem. C 2010, 114, 21562–21571. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, B.; Yu, J.; Liu, G.; Fan, W. Visible-Light Photocatalytic Activity and Deactivation Mechanism of Ag3PO4 Spherical Particles. Chem.—Asian J. 2012, 7, 1902–1908. [Google Scholar] [CrossRef]
- Turner, N.H.; Murday, J.S.; Ramaker, D.E. Quantitative Determination of Surface Composition of Sulfur Bearing Anion Mixtures by Auger Electron Spectroscopy. Anal. Chem. 1980, 52, 84–92. [Google Scholar] [CrossRef]
- Gaarenstroom, S.W.; Winograd, N. Initial and Final State Effects in the ESCA Spectra of Cadmium and Silver Oxides. J. Chem. Phys. 1977, 67, 3500–3506. [Google Scholar] [CrossRef]
- Tjeng, L.H.; Meinders, B.J.; Van Elp, J.; Ghinjsen, J.; Sawatzky, G.A.; Johnson, R.L. Electronic Structure of Ag2O. Phys. Rev. B 1990, 41, 3190–3199. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, V.K. XPS Core Level Spectra and Auger Parameters for Some Silver Compounds. J. Electron. Spectrosc. Relat. Phenom. 1991, 56, 273–277. [Google Scholar] [CrossRef]
- Simulis Thermodynamics, a Thermodynamic Properties and Phase Equilibria Calculator from ProSim® Software. Available online: https://www.prosim.net/en/product/simulis-thermodynamics-mixture-properties-and-fluid-phase-equilibria-calculations/ (accessed on 24 September 2024).
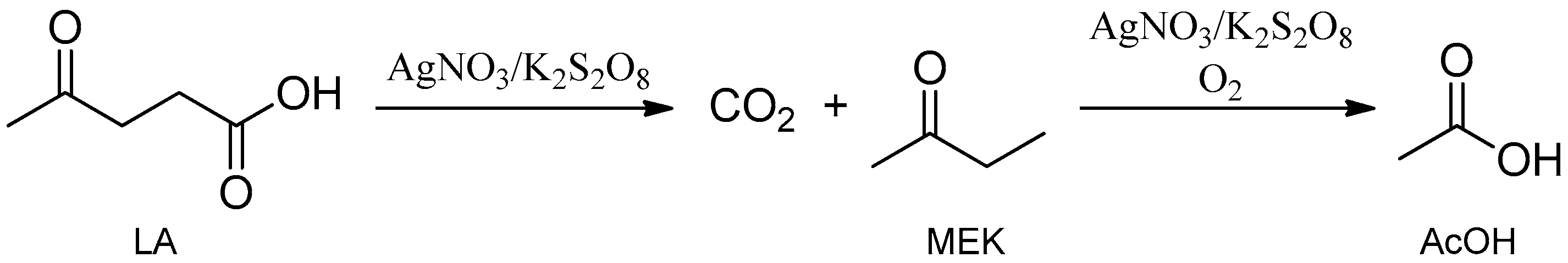


| # | neq * AgNO3/K2S2O8 | Initial pH | Final pH | LA Conversion (%) | AcOH Yield (%) | MEK Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 1/0 | 5 | 4 | 0.0 | 0.0 | 0.0 |
| 2 | 0/0.25 | 5 | 5 | 3.6 | 1.0 | 2.6 |
| 3 | 0/0.5 | 5 | 5 | 12.1 | 3.3 | 8.8 |
| 4 | 0/1 | 4 | 2 | 13.6 | 6.8 | 6.8 |
| 5 | 0/2 | 4 | 1 | 18.8 | 14.5 | 4.3 |
| # | Ag Salt | neq Ag Salt/K2S2O8 | Initial pH | Final pH | LA Conversion (%) | AcOH Yield (%) | MEK Yield (%) |
|---|---|---|---|---|---|---|---|
| 1 | AgNO3 | 1/1 | 5 | 2 | 46.9 | 14.4 | 32.5 |
| 2 | AgCl | 1/1 | 6 | 5 | 38.7 | 13.5 | 25.2 |
| 3 | Ag2O | 1/1 | 4 | 3 | 54.3 | 17.8 | 36.5 |
| 4 | AgO | 1/1 | 5 | 3 | 57.6 | 22.9 | 34.8 |
| 5 | AgO | 1/0 | 4 | 1 | 0.0 | 0.0 | 0.0 |
| # | neq AgNO3/K2S2O8 | Initial pH | Final pH | LA Conversion (%) | AcOH Yield (%) | MEK Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 0.5/0.5 | 5 | 3 | 22.8 | 13.6 | 9.2 |
| 2 | 1/0.5 | 5 | 2 | 34.2 | 15.8 | 18.4 |
| 3 | 1/1 | 5 | 2 | 46.9 | 14.4 | 32.5 |
| 4 | 1/2 | 4 | 1 | 49.2 | 21.3 | 27.9 |
| # | Aqueous Composition | Solution Concentration [M] | Solution pH * | Initial pH | Final pH | LA Conversion (%) | AcOH Yield (%) | MEK Yield (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | Water | - | 6 | 3 | 1 | 29.6 | 14.8 | 14.8 |
| 2 | KCl/NaOH | 0.2 | 12 | 4 | 1 | 48.8 | 12.3 | 36.5 |
| 3 | K2HPO4/KH2PO4 | 0.2 | 7 | 5 | 2 | 46.9 | 14.4 | 32.5 |
| 4 | K2HPO4/KH2PO4 | 0.2 | 8 | 5 | 2 | 47.6 | 15.7 | 31.0 |
| 5 | Na2HPO4/NaH2PO4 | 0.2 | 7 | 4 | 2 | 50.5 | 10.9 | 39.6 |
| 6 | KH2PO4 | 0.2 | 4 | 3 | 1 | 48.9 | 21.3 | 27.9 |
| 7 | K2HPO4 | 0.2 | 8 | 5 | 1 | 50.0 | 11.0 | 39.0 |
| 8 | NaOH | 9 × 10–4 | 8 | 5 | 1 | 30.5 | 9.7 | 20.8 |
| # | Description of Reaction | neq AgNO3/K2S2O8 | Initial pH | Final pH | LA Conversion (%) | AcOH Yield (%) | MEK Yield (%) |
|---|---|---|---|---|---|---|---|
| 1 | Initial reaction: medium 1 (M1) a | 1/1 | 5 | 2 | 46.9 | 14.4 | 32.5 |
| After adjustment at pH 5 | |||||||
| 2 | Addition of NaOH to M1 b | 0/0 | 5 | 2 | 43.8 | 11.8 | 32.0 |
| 3 | Addition of NaOH/K2S2O8 to M1 b | 0/1 | 5 | 1 | 97.9 | 11.3 | 86.6 |
| Without adjustment of pH | |||||||
| 4 | Addition of K2S2O8 to M1 b | 0/1 | 2 | 1 | 54.3 | 22.8 | 31.5 |
| 5 | Addition of AgNO3/K2S2O8 to M1 b | 1/1 | 2 | 1 | 67.0 | 28.7 | 38.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán Barrera, N.I.; Peydecastaing, J.; Esvan, J.; Albet, J.; Vaca-Garcia, C.; Behra, P.; Vedrenne, E.; Thiébaud-Roux, S. Enhanced Yield of Methyl Ethyl Ketone through Levulinic Acid Decarboxylation in the AgNO3/K2S2O8 System: Mechanistic Insights and Characterization of Metallic Species. Molecules 2024, 29, 4822. https://doi.org/10.3390/molecules29204822
Guzmán Barrera NI, Peydecastaing J, Esvan J, Albet J, Vaca-Garcia C, Behra P, Vedrenne E, Thiébaud-Roux S. Enhanced Yield of Methyl Ethyl Ketone through Levulinic Acid Decarboxylation in the AgNO3/K2S2O8 System: Mechanistic Insights and Characterization of Metallic Species. Molecules. 2024; 29(20):4822. https://doi.org/10.3390/molecules29204822
Chicago/Turabian StyleGuzmán Barrera, Nydia I., Jérôme Peydecastaing, Jérôme Esvan, Joël Albet, Carlos Vaca-Garcia, Philippe Behra, Emeline Vedrenne, and Sophie Thiébaud-Roux. 2024. "Enhanced Yield of Methyl Ethyl Ketone through Levulinic Acid Decarboxylation in the AgNO3/K2S2O8 System: Mechanistic Insights and Characterization of Metallic Species" Molecules 29, no. 20: 4822. https://doi.org/10.3390/molecules29204822
APA StyleGuzmán Barrera, N. I., Peydecastaing, J., Esvan, J., Albet, J., Vaca-Garcia, C., Behra, P., Vedrenne, E., & Thiébaud-Roux, S. (2024). Enhanced Yield of Methyl Ethyl Ketone through Levulinic Acid Decarboxylation in the AgNO3/K2S2O8 System: Mechanistic Insights and Characterization of Metallic Species. Molecules, 29(20), 4822. https://doi.org/10.3390/molecules29204822







