Abstract
Dy3+ single-doped and Dy3+/Eu3+ co-doped ZnO:B2O3:WO3:Nb2O5 glass was successfully synthesized using the melt quenching method. The amorphous character of the prepared samples was confirmed by X-ray diffraction (XRD). The glass transition and crystallization temperatures were examined by differential scanning calorimetry (DSC). Raman spectroscopy was applied to investigate the glass microstructure. Physical properties like the density, molar volume, oxygen molar volume and oxygen packing density of the glass were also determined. The photoluminescence excitation (PLE) and emission (PL) spectra of the resultant glass types were measured. The obtained Dy3+ single-doped glass was characterized by strong luminescence at 482 and 574 nm, corresponding to the 4F9/2 → 6H15/2 (blue) and 4F9/2 → 6H13/2 (yellow) transitions, respectively, and weak luminescence at 663 nm and 753 nm due to the 4F9/2 → 6H11/2 (red) and 4F9/2 → 6H9/2 + 6F11/2 (red) transitions. The luminescence results indicate that energy transfer from the Dy3+ to Eu3+ ions occurs in the proposed glass system. The emitted light from the Dy3+ single-doped glass was found to be yellow-orange. The Dy3+/Eu3+ co-doped samples emitted darker orange light. The obtained results show that the investigated types of glass have the potential to be used as orange light-emitting materials.
1. Introduction
For many years, luminescent materials doped with rare-earth (RE) elements have garnered significant attention because of their diverse applications [1,2]. These light-emitting materials are used in a variety of areas, including optoelectronic devices, displays, biomarkers, optical fibers and lasers [3,4,5,6,7]. A considerable part of the research in this domain is dedicated to rare-earth-ion-doped phosphors for solid-state lighting (SSL)-based devices, such as light-emitting diodes (LEDs) [8,9]. These materials offer several benefits compared to traditional incandescent or fluorescent lamps, including high brightness, efficiency, a long lifespan, reliability and reduced energy consumption [10]. This aspect is particularly vital considering that the electrical lighting industry accounts for nearly 19% of global electricity usage [11]. Consequently, the creation of innovative and environmentally friendly materials that are both efficient and durable is of utmost importance [2,12,13,14]. In this context, the significance of RE ions is considerable. These ions possess a distinctive capability to emit light due to their 4f-4f or 4d-4f transitions when excited at an appropriate wavelength [8,15]. Dy3+ single-doped materials can produce neutral white light emission. The luminescence spectrum of trivalent dysprosium ions reveals multiple characteristic lines, with two main bands appearing in the blue (470–500 nm) and yellow (570–600 nm) regions, corresponding to the transitions 4F9/2 → 6H15/2 and 4F9/2 → 6H13/2, respectively [16,17]. White light emission can be achieved through the proper adjustment of the blue and yellow luminescence intensity ratio [15,18,19]. Nonetheless, there is often a preference for producing warm white light rather than a cold, artificial white hue [20]. To generate this warm light, red emitters such as Eu3+ ions can be utilized, as their strongest band related to the 5D0 → 7F2 transition of Eu3+ occurs at approximately 615 nm, placing it within the red section of the visible light spectrum [21,22]. Furthermore, a suitable ratio of Eu3+/Dy3+ within the host matrix can facilitate color adjustment, resulting in the emission of warm white light. This phenomenon has been observed in various Dy3+/Eu3+-doped matrices, such as yttrium alumino bismuth borosilicate and CdO-GeO2-TeO2 glass [4,23], as well as phosphate glass [24]. Additionally, warm white light was achieved in lithium fluoride bismuth borate glass co-doped with Dy3+ and Eu3+ [25]. Research on Zn(PO3)2 glass doped with Eu3+ and Dy3+ ions shows the transition of white light emission from neutral white at 348 nm excitation to warm white at 445 nm [26]. On the other hand, when a pair of rare-earth (RE) ions have matched energy levels and are co-doped in a host, a process in which one RE is sensitized by the energy from the other ion may occur. This process is defined as energy transfer (ET), a common way to improve the luminescence properties of RE ion-doped materials [27]. Combining Dy3+ with Eu3+ tends to enforce the europium emission because when materials with Dy3+ and Eu3+ are excited with a UV light source, the Dy3+ ions absorb the incident light, and they non-radiatively transfer part of the absorbed energy to Eu3+ ions, causing emissions.
It is well known that binary ZnO-B2O3 glass has been attracting continuous scientific interest as a host for luminescence applications due to its high transparency from the visible to mid-infrared region of the spectrum, its relatively low melting temperature, and its good chemical and thermal stability. We have experience in the synthesis, structural analysis and luminescent properties of ZnO-B2O3 glass containing WO3 and Nb2O5 doped with Eu3+ ions [28]. Our results have shown the existence of a charge transfer from the matrix (WO3 and Nb2O5) to Eu3+, resulting in enhanced Eu3+ emission. We are also expecting the occurrence of such a charge transfer from the matrix to Dy3+ (which we established through spectral results). On the other hand, it is well known from the literature that in a Dy3+ and Eu3+ co-doped matrix, a non-radiative charge transfer from Dy3+ to Eu3+ active ions is observed, resulting in an increase in Eu3+ emission intensity. In this way, we are sensitizing Eu3+ in two ways—from the matrix and from Dy3+. The novelty and significance of this study is the composition of the matrix, which is suitable for hosting Eu3+ and Dy3+ active ions and can also serve as a host sensitizer of both ions. The aim of this work was to synthesize ZnO:B2O3:WO3:Nb2O5 glass doped with different concentrations of Dy3+ ions and co-doped with Eu3+ ions, which could be used as a phosphor in LEDs. This study provides a detailed analysis of the thermal and luminescent properties and structural features of these prepared types of glass. Physical parameters such as the density, molar volume, oxygen molar volume and oxygen packing density, which provide some structural information, were also evaluated.
2. Results
2.1. XRD Data and Thermal Analysis
Two series of glass were obtained. Their compositions and respective names are listed in Table 1 and Table 2, respectively.

Table 1.
Molar compositions of the investigated Dy3+ single-doped 50ZnO:40B2O3:5WO3:5Nb2O5 glass (mol%).

Table 2.
Molar compositions of the investigated Dy3+/Eu3+ co-doped 50ZnO:40B2O3:WO3:Nb2O5 glass (mol%).
The measured X-ray diffraction patterns are shown in Figure 1a,b. The absence of sharp diffraction peaks in the spectra showed that prepared samples are glassy in nature.
Figure 1.
XRD patterns of the investigated glasses: (a) Dy3+-doped 50ZnO:40B2O3:5WO3:5Nb2O5 glass; (b) Dy3+/Eu3+ co-doped 50ZnO:40B2O3:5WO3:5Nb2O5 glass.
The amorphous nature of the obtained materials was further confirmed by differential scanning calorimetry (DSC). The DSC curves for the two series of samples—Dy3+-doped and Dy3+/Eu3+ co-doped glasses—are shown in Figure 2a and Figure 2b, respectively.
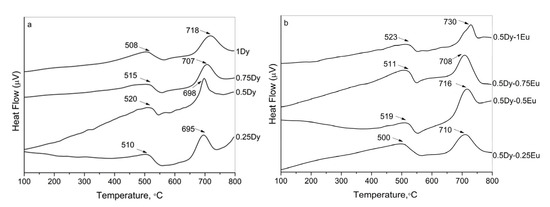
Figure 2.
DSC curves of the investigated glasses: (a) Dy3+-doped 50ZnO:40B2O3:5WO3:5Nb2O5 glass; (b) Dy3+/Eu3+ co-doped 50ZnO:40B2O3:5WO3:5Nb2O5 glass.
The endothermic dips corresponding to the glass transition temperature (Tg) and the exothermic peaks due to the crystallization temperature (Tc) are observed. The estimated values of Tg and Tc are pointed out in the figure. As can be seen from the figure, the glass transition temperatures of all glasses are above 500 °C and do not vary significantly with composition, indicating that the addition of Dy2O3 and Eu2O3 does not significantly affect the structure of the matrix glass. All glasses are characterized by a high thermal stability, i.e., ΔT = Tc − Tg between 178 and 210 °C.
2.2. Physical Properties
Structural information of the glasses was gained by density (ρg) measurements, which provided the base for the determination of several physical parameters, such as molar volume (Vm), oxygen molar volume (Vo) and oxygen packing density (OPD). These were evaluated using the following relations:
where xi is the molar fraction of each component i, Mi is the molecular weight, ρg is the glass density, ni is the number of oxygen atoms in each oxide, C is the number of oxygen atoms per formula units, and M is the total molecular weight of the glass compositions. The values obtained are listed in Table 3 and Table 4.

Table 3.
Values of physical parameters of 50ZnO:40B2O3:5WO3:5Nb2O5:xDy2O3 (0 ≤ x ≤ 1) glass: density, ρg, molar volume, Vm, oxygen molar volume, Vo, oxygen packing density, OPD.

Table 4.
Values of physical parameters of 50ZnO:40B2O3:5WO3:5Nb2O5:0.5Dy2O3:xEu2O3 (0 ≤ x ≤ 1) glass: density, ρg, molar volume, Vm, oxygen molar volume, Vo, oxygen packing density, OPD.
As seen from Table 3, the density of glass 50ZnO:40B2O3:5WO3:5Nb2O5 from the first series with increasing content of Dy2O3 increases with the Dy2O3 loading, which is mostly due to the increase in average molecular mass of the glasses [29]. Molar volume (Vm) is also an important physical property and normally follows a trend opposite to that of density. However, Vm also enhances with increasing Dy2O3 content, indicating an increasing inter-atomic spacing in the network due to the insertion of Dy3+ ions with high ionic radius (0.91 Å). Oxygen molar volume (Vo) and OPD are two parameters that provide information on the packing of the oxygen ions in the glass structure [30]. Lower Vo and higher OPD indicate a higher degree of network connectivity. In this series of glasses, the lowest Vo and the highest OPD are observed for the Dy0.5 glass, evidencing the highest cross-linking and bonding in its glass network. The highest value of Vo and the lowest OPD for Dy0.25, Dy0.75, Dy1 glasses indicate lower cross-linking density and the formation of less reticulated glass networks. For the second series of 50ZnO:40B2O3:5WO3:5Nb2O5 glasses, containing a constant Dy2O3 content of 0.5 mol% and increasing Eu2O3 concentration from 0.25 to 1 mol% (Table 4), both density and oxygen molar values increase with the Eu2O3 loading. Vo increases and OPD decreases for glasses having up to 0.75 mol% Eu2O3 (samples 0.5Dy-0.25Eu2O3, 0.5Dy-0.5Eu2O3 and 0.5Dy-0.75Eu2O3). With future increase in Eu2O3 content of 1 mol% (sample 0.5Dy-1Eu), the oxygen molar volume decreases and oxygen packing density enhances. The increasing glass density can be attributed to the increase in the average molecular mass of glasses [29]. As the molar volume (volume occupied by a mass of the glass equal to 1 mole) is strongly affected by the ionic radii of the incorporated ionic species in the glass, the increasing trend of Vm is due to the insertion of Eu3+ ions, which are known to have a high ionic radius (0.95 Å), resulting in the formation of an excess free volume, which increases the overall molar volume of these glasses [31]. The increasing Vo and decreasing OPD for glasses having up to 0.75 mol% Eu2O3 revealed insufficient cross-linking (high number of non-bridging oxygen atoms) of the borate network. Decreasing Vo and enhancing OPD for Dy0.5-1Eu glass suggest increased cross-linking in the glass network.
2.3. Raman Analysis
The structure of the obtained glasses was studied by Raman spectroscopy. Figure 3a shows the Raman spectra of 50ZnO:40B2O3:5WO3:5Nb2O5 glasses from the first series, containing increasing Dy2O3 content. As can be seen, all glass spectra contain a band at 960 cm−1, a high-intensity band at 905–885 cm−1, a shoulder at 805 cm−1, a broad shoulder at 710–740 cm−1, broad Raman activity in the range of 200–450 cm−1 and a band at 126–132 cm−1. Taking into account previous spectral investigations on glasses 50ZnO:40B2O3:5WO3:5Nb2O5:xEu2O3 (x = 0, 0.1, 0.5, 1, 2, 5, and 10 mol%) and (50-x)WO3:25La2O3:25B2O3:xNb2O5 reported in refs. [28,32], we attribute the band at 960 cm−1 in the Raman spectra of the first series glasses to the ν1 mode of the isolated tetrahedral species, [WO4]2−. This band becomes more intense with the addition of Dy2O3, indicating an increasing quantity of isolated [WO4]2− groups as a result of the WO6 → WO4 transformation. The intensive Raman peak at 905–885 cm−1 is assigned to overlapping contributions from the asymmetric stretching mode ν3 of [WO4]2− tetrahedra and the ν1 stretching vibration of short Nb–O bonds in strongly deformed and isolated NbO6 octahedra [28,32]. The broadening of this band with increasing Dy2O3 content indicates the formation of [WO4]2− tetrahedral and NbO6 octahedral units with different distortions upon the addition of Dy2O3. The shoulder at 805 cm−1 is a superposition of the symmetric stretching ν1 mode of tetrahedral [NbO4]3− groups, and vibrations of B–O–Nb bonds [28,32]. The broad Raman shoulder at ca. 710–740 cm−1 is attributed to the stretching of the bridging oxygen atoms linking the tungsten ion and niobium polyhedral units, via Nb–O–W bridges that overlap with the vibrations of chain-type metaborate units, [BØ2O]− [28,33]. In addition, the observed upshift to 740 cm−1 indicates that metaborate units, [BØ2O]−, are charge balanced by Dy3+ ions. The increasing intensity of this shoulder upon increasing Dy2O3 concentration indicates a growing number of Nb–O–W linkages and metaborate groups in the glass networks. The vibrational features in the lower-frequency region 200–450 cm−1 correspond mainly to bending modes of [WO4]2− and [NbO4]3− tetrahedra coupled with the Zn–O (at ca. 235 cm−1) and Dy–O vibrations (378 cm−1) [28,34,35]. The band observed at 126–132 cm−1 in all glass compositions was related to the out-of-plane W–O deformation mode [36].
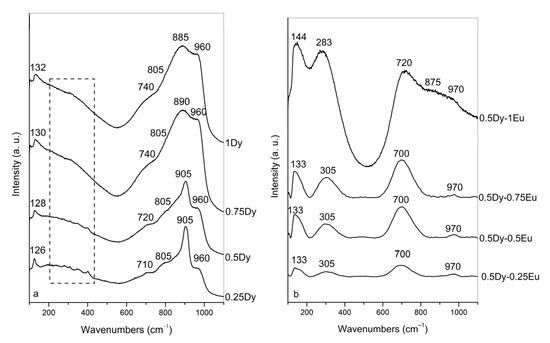
Figure 3.
Raman spectra of the investigated glasses: (a) Dy3+-doped 50ZnO:40B2O3:5WO3:5Nb2O5 glass; (b) Dy3+/Eu3+ co-doped 50ZnO:40B2O3:5WO3:5Nb2O5 glass.
Figure 3b presents the Raman spectra of the second series of 50ZnO:40B2O3:5WO3:5Nb2O5 glasses containing a constant Dy2O3 content of 0.5 mol% and increasing Eu2O3 concentration from 0.25 to 1 mol%. As can be seen from the figure, all glass spectra contain Raman bands at 133–144 cm−1, at 305–283 cm−1, at 700–720 cm−1 and at 970 cm−1. In the spectrum of glass 0.5Dy-1Eu, a band is also present at about 875 cm−1 that is not well resolved.
Having in mind our previous spectral investigation on glasses 50ZnO:40B2O3:5WO3:5Nb2O5:xEu2O3 (x = 0, 0.1, 0.5, 1, 2, 5, and 10 mol%) reported in ref. [28], we ascribe the weak band at 970 cm−1 to the ν1 mode of the isolated tetrahedra, [WO4]2−. The not-well-resolved band at 875 cm−1 is due to a coupled mode including the ν1 stretching vibration of short Nb–O bonds in strongly deformed and isolated NbO6 octahedra and the asymmetric stretching mode ν3 of [WO4]2− tetrahedra [28]. The band at 700–720 cm−1 is a superposition of the peaks associated with the vibrational modes of the Nb–O–W linkages and metaborate units [28,33]. The band at 305–283 cm−1 is connected with the overlapping Zn–O, Dy–O, and Eu–O stretching vibrations [28,35,37]. The low- frequency band at 133–144 cm−1 is assigned to the out-of-plane W–O deformation mode [36]. As seen from Figure 3b, with increasing Eu2O3 content, the overall intensity of the Raman spectra for all glasses increases, reflecting enhanced crosslinking in the glass network [38]. In addition, the observed upshift of the band at 700 to 720 (vibrations of Nb–O–W and [BØ2O]−) and the band at 133 to 144 (W–O deformation mode) in the Raman spectrum of the glass having the highest Eu2O3 content of 1 mol% (Figure 3b, spectrum 0.5Dy-1Eu2O3) indicates that the short-range order of niobate, tungstate and borate groups is influenced by the added Eu2O3. Most probably, the Eu3+ ions are situated in the local environment of these structural arrangements, charge balancing them via the formation of Nb/W–O–Eu and B–O–Eu bonding and thus increasing the reticulation of the glass network. This observation coincides well with the physical parameters obtained above and the data of the thermal analysis.
2.4. Photoluminescent Properties
The excitation spectra of Dy3+-doped 50ZnO:40B2O3:5WO3:5Nb2O5 glass, monitored at the 574 nm wavelength, are shown at Figure 4a. The sharp excitation peaks seen at 324 nm, 337 nm, 349 nm, 363 nm, 385 nm, 424 nm, 452 nm and 471 nm can be attributed to the transitions of 6H15/2 → 6P3/2, 6H15/2 → 4I9/2, 6H15/2 → 4P7/2, 6H15/2 → 6P5/2, 6H15/2 → 4I13/2, 6H15/2 → 4G11/2, 6H15/2 → 4I15/2, 6H15/2 → 4F9/2, respectively [39]. From the observed spectra, the intense band at 385 nm (6H15/2 → 4I13/2) was selected for photoluminescence measurements, as it shows the highest intensity.
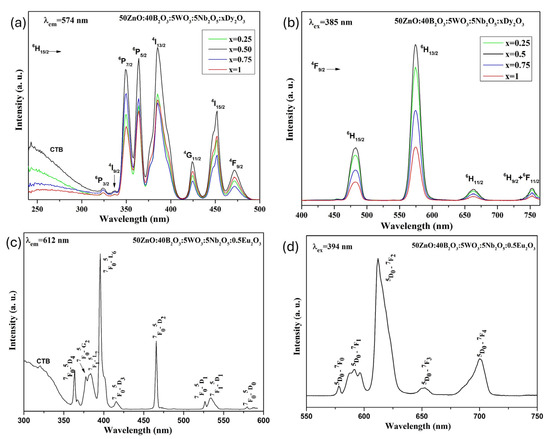
Figure 4.
(a) Excitation spectra of Dy3+-doped 50ZnO:40B2O3:5WO3:5Nb2O5 glass; (b) emission spectra of Dy3+-doped 50ZnO:40B2O3:5WO3:5Nb2O5 glass; (c) excitation spectrum of Eu3+-doped 50ZnO:40B2O3:5WO3:5Nb2O5 glass; (d) emission spectrum of Eu3+-doped 50ZnO:40B2O3:5WO3:5Nb2O5 glass.
These wavelengths are appropriate for excitation using blue LED chips (430–470 nm) and commercial near-ultraviolet light-emitting diodes (LEDs) (250–400 nm). In addition, it can be seen that the excitation spectra show a broad band below 320 nm, which corresponds to the ligand-to-metal charge transfer states (LMCT) of O2− → Dy3+ from the oxygen 2p excited state to the Dy3+ 4f state [40], and O2− → W6+ in WO4 groups [41] and O2− → Nb5+ in NbOn groups (NbOn = NbO6, NbO4) [42]. The overlapping of bands in this spectral region makes it impossible to distinguish the individual contribution of these transitions. The existence of the host lattice absorption in the excitation spectra indicates the presence of energy transfer from the WO4 and NbOn structural polyhedra to the active Dy3+ ions. This mechanism is known as host-sensitized luminescence [41,43]. Figure 4b shows the visible emission spectra of the Dy3+-doped 50ZnO:40B2O3:5WO3:5Nb2O5 glass under excitation at a wavelength of 385 nm. The obtained glasses are characterized by strong luminescence at 482 and 574 nm corresponding to the 4F9/2 → 6H15/2 (blue) and 4F9/2 → 6H13/2 (yellow) transitions, respectively, and weaker luminescence at 663 nm and 753 nm due to the 4F9/2 → 6H11/2 (red) and 4F9/2 → 6H9/2 + 6F11/2 (red) transitions. From Figure 4b it is clear that the emission intensity strongly depends on the Dy3+ concentration and increases with increasing Dy2O3 content, reaching a maximum at 0.5 mol%, after which it gradually decreases. This fact may be attributed to the concentration quenching effect [44]. As the Dy3+ concentration rises, the distance between Dy3+ reduces, enabling non-radiative energy transfer between neighboring Dy3+ ions. The occurrence of crossing-relaxation phenomena between these ions reduces the jump from the 4F9/2 energy level to 6HJ levels (J = 15/2, 13/2, 11/2 and 9/2) and 6F11/2, leading to fluorescence quenching and weakening of the emission intensity.
To study the local symmetry around rare earth ions, dysprosium can be used as a spectroscopic probe. Among all the observed emission transitions, the blue emission transition (4F9/2 → 6H15/2) at 482 nm is an allowed magnetic dipole (MD) in nature and is independent of a local crystal field environment, while the yellow emission transition at 574 nm (4F9/2 → 6H13/2) is identified as an electric dipole (ED) and is forced by the crystal field environment in the vicinity of the Dy3+ ions. When the yellow emission is dominant in the spectrum, the Dy3+ ions are located at low-symmetry sites. Conversely, when the blue emission is stronger in the PL spectrum, the Dy3+ ions are located at high-symmetry sites (with inversion symmetry center) [45,46]. In our case, Dy3+ ions are located in the more asymmetrical environment, as evidenced by the greater intensity of the 4F9/2 → 6H13/2 transition compared to the 4F9/2 → 6H15/2 emission transition.
In our previous article, the same glass composition (50ZnO:40B2O3:5WO3:5Nb2O5) with different Eu3+ doping concentrations (0–10 mol%) was obtained [28]. As an example, in Figure 4c,d can be seen the excitation and emission spectra of 0.5 Eu3+-doped glass. We established an increase in europium emission intensity due to the appearance of strong host lattice absorption, resulting in non-radiative energy transfer from the WO4 and NbOn structural polyhedra to the active Eu3+ ion. This fact, along with the present results concerning Dy3+-doped glass (Figure 4a,b), shows that 50ZnO:40B2O3:5WO3:5Nb2O5 glass matrix is suitable for hosting both active ions and can serve as a host sensitizer. Therefore, inspired by the above-mentioned observations, in the present study, we are synthesizing Dy3+/Eu3+ co-doped glass with different active ion concentrations, which will be excited simultaneously to verify the resulting color of emission.
Furthermore, Figure 5 shows the overlap between the excitation spectra of Eu3+-doped glass (black line), monitored at 612 nm wavelength and emission spectra of Dy3+-doped glass (red line) at 385 nm excitation. Within the wavelength range of 450–500 nm, a spectral overlap is observed, which indicates that Eu3+ can be sensitized by Dy3+ in the obtained Dy3+/Eu3+ co-doped 50ZnO:40B2O3:5WO3:5Nb2O5 luminescent glass [47].
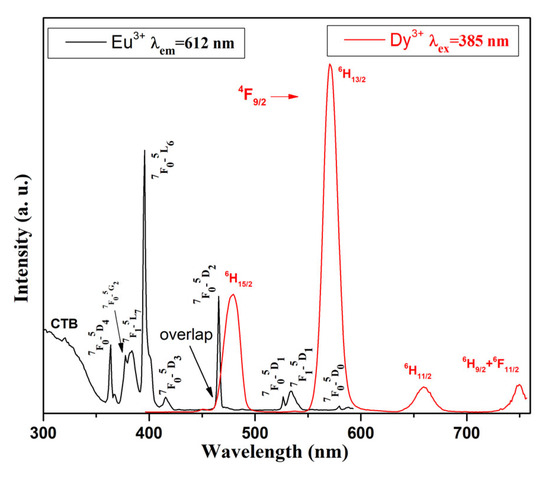
Figure 5.
Overlap between excitation spectra of Eu3+-doped 50ZnO:40B2O3:5WO3:5Nb2O5 glass (black line) and emission spectra of Dy3+-doped 50ZnO:40B2O3:5WO3:5Nb2O5 glass (red line).
Figure 6 shows the excitation spectra of the 0.5Dy3+/0.5Eu3+ co-doped 50ZnO:40B2O3:5WO3:5Nb2O5 glass at 612 nm (a) and at 574 nm (b) excitation wavelengths. When monitoring the most intensive transition for Dy3+ (4F9/2 → 6H13/2) at 574 nm, the excitation spectra of 0.5Dy3+/0.5Eu3+ co-doped glass consists of only Dy3+ bands (Figure 6b). In contrast, when monitoring the most prominent emission of Eu3+ (5D0 → 7F2) at 612 nm, the excitation spectrum consists of all Eu3+ transitions along with several low-intensity Dy3+ bands: 6H15/2 → 6P7/2 (349 nm), 6H15/2 → 4G11/2 (424 nm), and 6H15/2 → 4I15/2 (452 nm). The appearance of excitation wavelengths of Dy3+, when monitoring the Eu3+ emission at 612 nm, provides evidence that energy transfer from Dy3+ to Eu3+ occurs. As can be seen from Figure 6, for both Dy3+ and Eu3+, excitation peaks are located at around 363 nm.
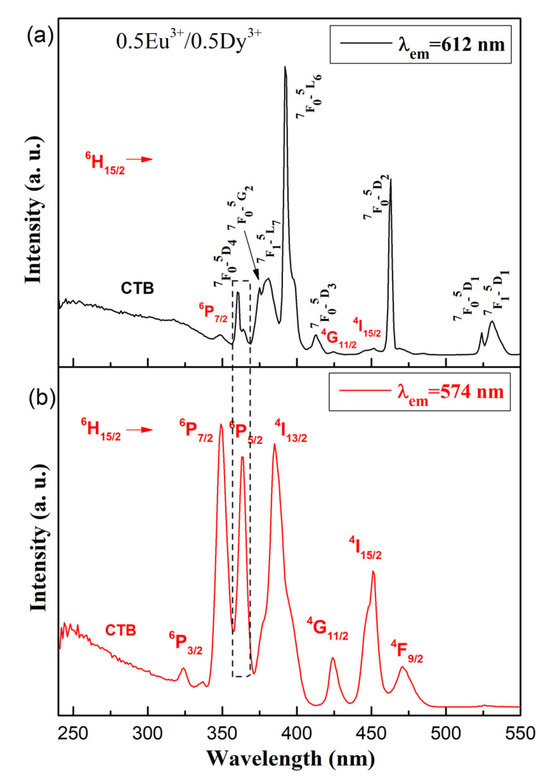
Figure 6.
Excitation spectra of the 0.5Dy3+/0.5Eu3+ co-doped 50ZnO:40B2O3:5WO3:5Nb2O5 glass under (a) 612 nm (Eu3+) and (b) 575 (Dy3+) excitations.
Hence, in order to achieve better co-emission from Dy3+ and Eu3+ in the co-doped glass, the 363 nm wavelength was chosen as the excitation source.
As can be seen from Figure 4a, the maximum emission intensity is observed at a Dy3+ concentration of 0.5 mol%. Based on this observation, we synthesized co-doped glass with 0.5 mol% Dy3+ and varying Eu3+ concentrations ranging from 0.25 to 1.0 mol%. The emission spectra of 0.5Dy3+/xEu3+ (x = 0, 0.25, 0.5, 0.75, and 1.0 mol%) co-doped glass and single Eu3+-doped glass under 363 nm excitation wavelength are shown in Figure 7. The characteristic peaks located at 482, 574 and 663 nm belong to the Dy3+ transitions 4F9/2 → 6H15/2, 4F9/2 → 6H13/2 and 4F9/2 → 6H11/2, respectively. In addition to the dysprosium, the 5D0 → 7F1, 5D0 → 7F2 and 5D0 → 7F4 emission peaks of europium are also present at 597 nm, 612 nm and 700 nm.
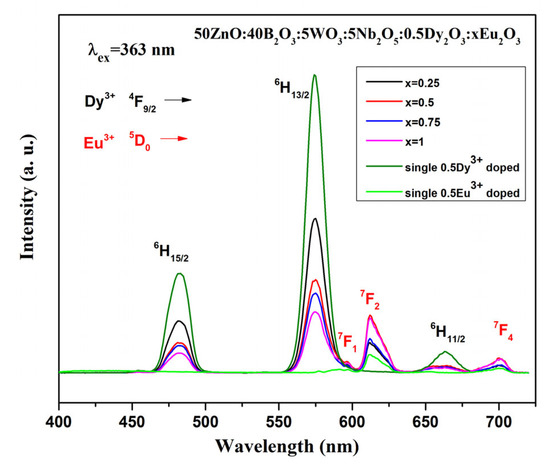
Figure 7.
Emission spectra of 50ZnO:40B2O3:5WO3:5Nb2O5:0.5Dy2O3:xEu2O3 (x = 0, 0.25, 0.5, 0.75 and 1 mol%) and single Eu3+-doped glasses.
The intensity of europium emission peaks in the co-doped samples is most prominent at a concentration of 0.5 mol% Eu3+ ion (Figure 7). No further increase in intensity is observed at 1.0 mol%. The intensity of the Dy3+ transitions decreases progressively with increasing Eu3+ concentration, while the intensity of Eu3+ emission bands increases. This observation can be attributed to the fact that Dy3+ sensitizes Eu3+ and thus enhances the emission intensity of Eu3+. Additional evidence for the occurring energy transfer is that the single Eu3+-doped glass is characterized by lower emission intensity.
Figure 8 shows the energy level diagram of Dy3+ and Eu3+ ions within the obtained luminescent glass. Under 363 wavelength of excitation, the ground state of Dy3+ ions was excited to the 6P5/2 excitation state and subsequently decays non-radiatively to the 4F9/2 state. From there, they emit radiatively to the 6HJ levels (J = 15/2, 13/2, 11/2 and 9/2) and 6F11/2. A portion of the excitation energy of the 4F9/2 state is absorbed by Eu3+ ions and is promoted to the 5D2 level, and after that, via non-radiative transitions, they populate the lower 5D1,0 levels and further decay radiatively to the 7FJ (J = 0, 1, 2, 3) ground states.
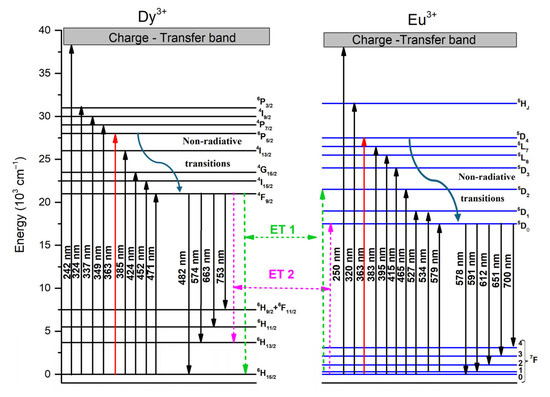
Figure 8.
Energy transfer diagram of Dy3+ and Eu3+ co-doped glass.
The energy transfer mechanisms that can occur are illustrated in Figure 8 and are as follows [48]:
ET1: 4F9/2 [Dy3+] + 7F1 [Eu3+] → 6H15/2 [Dy3+] + 5D2 [Eu3+]
ET2: 4F9/2 [Dy3+]+7F0 [Eu3+] → 6H13/2 [Dy3+] + 5D0 [Eu3+]
To estimate the actual emission colors, the Commission International de l’Eclairage (CIE) 1931 chromaticity diagram was applied (Figure 9) [49]. The color calculator program SpectraChroma (Version 1.0.1) was employed to determine the chromaticity coordinates from the emission spectra (CIE coordinate calculator) [50]. Table 5 displays the calculated values. The emission color of the single Dy3+ (0.25–1 mol%)-doped glass is yellow-orange. Their values are almost identical and appear indistinguishable in the figure. When the resulting 0.5 mol% Dy3+-doped glass is co-doped with Eu3+ at a concentration of 0.25–1 mol%, the emission color becomes darker orange, and for a single Eu3+-doped glass, the color is pure red. The obtained results show that the investigated glass has the potential to be used as orange-emitting materials, with tunable color output, depending on the rare earth doping proportion.
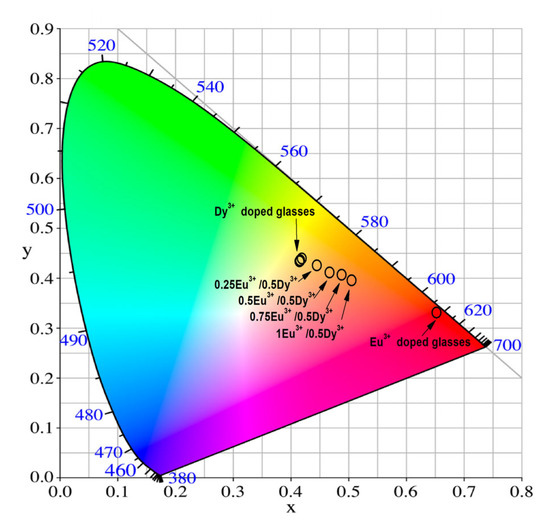
Figure 9.
CIE chromaticity coordinates of Dy3+-doped glass, Eu3+-doped glass and Dy3+/Eu3+ co-doped 50ZnO:40B2O3:5WO3:5Nb2O5 glass.

Table 5.
CIE chromaticity coordinates of Dy3+ and Eu3+ single-doped and Dy3+/Eu3+ co-doped 50ZnO:40B2O3:5WO3:5Nb2O5 glass.
3. Materials and Methods
Glasses with nominal composition 50ZnO:40B2O3:5WO3:5Nb2O5:xDy2O3 (x = 0.25, 0.5, 0.75, 1 mol%) and 50ZnO:40B2O3:5WO3:5Nb2O5:0.5Dy2O3:xEu2O3 (x = 0.25, 0.5, 0.75, 1 mol%) were obtained by the conventional melt-quenching method, using commercial powders of reagent grade WO3, ZnO, H3BO3, Nb2O5, Dy2O3 and Eu2O3 as starting materials. The homogenized batches were melted at 1250 °C for 30 min in a platinum crucible in air. The melts were cast into a graphite mold to produce bulk glass samples. Then, the glasses were transferred to a laboratory electric furnace, annealed at 490 °C (a temperature 10 °C below the glass transition temperature), and cooled to room temperature at a very slow cooling rate of about 0.5 °C/min. The phase of the samples was established by X-ray phase analysis with a Bruker D8 Advance diffractometer (Karlsruhe, Germany) using Cu Kα radiation in the 10° < 2θ < 80° range. The differential scanning calorimetry (DSC) was performed using NETZSCH Jupiter STA 449 F5 apparatus (Selb, Germany) with a Pt/Pt-Rh thermocouple in a platinum crucible with a cover. The sample was heated from room temperature up to 800 °C at a heating rate of 10 K/min under an atmosphere of argon. The density of the obtained glasses at room temperature was measured by Archimedes’ principle using toluene (ρ = 0·867 g/cm3) as an immersion liquid on a Mettler Toledo electronic balance (Parramatta, NSW, USA) of sensitivity 10−4 g. The Raman spectra were recorded on a Via Qontor Raman Confocal Microscope (Renishaw plc, Wotton-under-Edge, UK) with a laser wavelength of 532 nm (Nd:YAG-Laser, London, UK). Photoluminescence (PL) excitation and emission spectra at room temperature were measured for all glasses with a Spectrofluorometer FluoroLog3-22, Horiba, Jobin Yvon (Longjumeau, France).
4. Conclusions
In summary, 50ZnO:40B2O3:5WO3:5Nb2O5 luminescent glasses containing Dy3+ and Dy3+/Eu3+ were prepared by the standard melt-quenching method. The densities are in the range of 3.887–3.991 g/cm3. The glass transition temperature for all glasses is over 500 °C, while the crystallization temperature, Tc, varies between 695 and 730°C. All glasses are characterized by a high thermal stability, i.e., ΔT = Tc − Tg between 178 and 210 °C. Raman analysis revealed that the glass network consists of [WO4]2− and [NbO4]3− tetrahedral units, NbO6 octahedra, and metaborate units, [BØ2O]−. The existence of Dy3+ → Eu3+ energy transfer was confirmed by the fact that in the co-doped glasses, intensity of the Dy3+ transitions decreases progressively with increasing Eu3+ concentration, while the intensity of Eu3+ emission bands increases. Depending on the rare earth doping content, the researched glasses may be used as orange light-emitting materials. The CIE coordinates may be modified by altering the dopant ratio and the obtained color shifted from yellow-orange to the dark orange light region.
Author Contributions
Conceptualization, A.Y., R.I.; methodology, M.M., A.Y.; software, A.Y., M.M., L.A.; validation, R.I.; formal analysis, M.M., A.Y.; investigation, M.M., A.Y., L.A., P.P.; resources, L.A.; data curation, R.I.; writing—original draft preparation, M.M., A.Y.; writing—review and editing, R.I.; visualization, M.M., A.Y.; supervision, R.I.; project administration, L.A.; funding acquisition, L.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
Research equipment of distributed research infrastructure INFRAMAT (part of Bulgarian National roadmap for research infrastructures) supported by the Bulgarian Ministry of Education and Science.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, S.; Qin, S.; Xiao, Y.; Liu, Z.; Hu, X.; Xiao, Z.; Huang, D.; Han, L.; Ye, X. Near-infrared luminescent materials: A review of their practical applications and prospective advancements. Dalton Trans. 2025, 54, 6717–6740. [Google Scholar] [CrossRef] [PubMed]
- Schröder, F.; Reetz, S.; Jüstel, T. On the Energy Dependence of the PL of RE Ions in LuBO3:RE (RE = Ce, Eu, Gd, or Tb). Crystals 2024, 14, 341. [Google Scholar] [CrossRef]
- Dahiya, J.; Hooda, A.; Agarwal, A.; Khasa, S. Tuneable colour flexibility in Dy3+&Eu3+co-doped lithium fluoride bismuth borate glass system for solid state lighting applications. J. Non-Cryst. Solids 2022, 576, 121237. [Google Scholar]
- Krishna Reddy, D.V.; Sambasiva Rao, T.; Taherunnisa, S.; Suchocki, A.; Zhydachevskyy, Y.; Piaseckic, M.; Rami Reddy, M. Tunable white light by varying excitations in yttrium alumino bismuth borosilicate glasses co-doped with Dy3+-Eu3+ for cool WLED applications. J. Non-Cryst. Solids 2019, 513, 167–182. [Google Scholar] [CrossRef]
- An, J.; Zhang, S.; Liu, R.; Hu, G.; Zhang, Z.; Qiu, Y.; Zhou, Y.F.; Su, Z.Z. Luminescent properties of Dy3+/Eu3+ doped fluorescent glass for white LED based on oxyfluoride matrix. J. Rare Earths 2021, 39, 26–32. [Google Scholar] [CrossRef]
- Yanga, J.; Sohn, I. Compositional dependence of thermophysical properties in binary alkaline earth borate melts: Insights from structure in short-range and intermediate-range order. J. Mater. Sci. Technol. 2022, 131, 195–203. [Google Scholar] [CrossRef]
- Boussetta, A.; Al-Syadi, A.M.; Damak, K.; Ersundu, A.E.; Ersundu, M.C.; Ramadan, E.; Alshehri, A.M.; Hussein, K.I.; Maalej, R.; Yousef, E.S. Investigation of Thermal and Spectroscopic Properties of Tellurite-Based Glasses Doped with Rare-Earth Oxides for Infrared Solid-State Lasers. Materials 2024, 17, 3717. [Google Scholar] [CrossRef]
- Milewska, K.; Maciejewski, M.; Žitňan, M.; Velázquez, J.J.; Galusek, D.; Sadowski, W.; Kościelska, B. Tunable emission and energy transfer of B2O3–Bi2O3–AlF3 glass system doped with Eu3+/Dy3+. J. Lumin. 2024, 269, 120440. [Google Scholar] [CrossRef]
- Hussain, S.K.; Yu, J.S. Bluish-green emission of novel BaAl2Ge2O8:Eu2+ phosphors under near-ultraviolet excitation. J. Rare Earths 2025, 43, 30–38. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Chen, J.; Wang, X.; Gong, S.; Zhang, X.; Huisheng, L.; Chi, Y.; Sun, X.; Mahadevan, C.K. Towards white light emission in Dy3+/Sm3+ co-doped niobio silicate glasses via adjusting doping concentration and excitation wavelength for w-LED applications. Ceram. Int. 2024, 50, 25548–25557. [Google Scholar] [CrossRef]
- Zissis, G. Energy consumption and environmental and economic impact of lighting: The current situation. In Handbook of Advanced Lighting Technology; Karlicek, R., Sun, C.C., Zissis, G., Ma, R., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Erol, E.; Vahedigharehchopogh, N.; Kıbrıslı, O.; Ersundu, M.C.; Ersundu, A.E. Recent progress in lanthanide-doped luminescent glasses for solid-state lighting applications—A review. J. Phys. Condens. Matter. 2021, 33, 483001. [Google Scholar] [CrossRef] [PubMed]
- Lewandowskia, T.; Seweryński, C.; Walasa, M.; Łapiński, M.; Synak, A.; Sadowski, W.; Kościelska, B. Structural and luminescent study of TeO2-BaO-Bi2O3-Ag glass systemdoped with Eu3+ and Dy3+ for possible color-tunable phosphor. Opt. Mater. 2018, 79, 390–396. [Google Scholar] [CrossRef]
- Zhang, F.; Cao, Q.; Lu, J.; Tao, H.; Yang, S.; Yao, S.; Fu, Q.; Ma, Z.; Dai, W.; Zhao, H. Adjusting photoluminescence of high thermostability Dy3+/Eu3+ co-doped borosilicate glass for white LED device. Ceram. Int. 2024, 50, 35557–35567. [Google Scholar] [CrossRef]
- Diaz-Torres, L.A.; De la Rosa, E.; Salas, P.; Romero, V.H.; Angeles-Chavez, C. Efficient photoluminescence of Dy3+ at low concentrations in nanocrystalline ZrO2. J. Solid State Chem. 2008, 181, 75–80. [Google Scholar] [CrossRef]
- Blasse, G.B.; Grabmaier, C. Luminescent Materials; Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar]
- Fhoula, M.; Koubaa, T.; Dammak, M. White photoluminescence and energy transfer properties of Dysprosium and Europium singly and codoped Na2ZnP2O7 phosphors. Optics Laser Technol. 2020, 130, 106352. [Google Scholar] [CrossRef]
- Griebenow, K.; Phuong-Truong, M.; Munoz, F.; Klement, R.; Galusek, D. Tuning the fluorecence of Dy3+ via the structure of borophosphate glasses. Sci. Rep. 2023, 13, 1919. [Google Scholar] [CrossRef]
- Griebenow, K.; Munoz, F.; Tagiara, N.S.; Klement, R.; Prnova, A.; Wolfrum, B.; Kamitsos, E.I.; Duran, A.; Galusek, D. Structure and fluorescence properties of Dy doped alkaline earth borophosphate glasses. Int. J. Appl. Glass Sci. 2021, 12, 472–484. [Google Scholar] [CrossRef]
- Zhu, Z.; Tao, C.; Wang, Z.; Yang, Z.; Li, P. Luminescence and energy transfer of warm white-emitting phosphor Mg2Y2Al2Si2O12:Dy3+,Eu3+ for white LEDs. RSC Adv. 2021, 11, 32707–32716. [Google Scholar] [CrossRef]
- Teixeira, M.M.; Pinatti, I.M.; Laranjeira, J.A.S.; Fabris, G.S.L.; Teodoro, M.D.; Rosa, I.L.V.; Simoes, A.Z.; Andrés, J.; Sambrano, J.R.; Longo, E. Photoluminescence of Ca10V6O25:Eu3+: A theoretical and experimental approach. J. Alloys Compd. 2024, 980, 173525. [Google Scholar] [CrossRef]
- Parauha, Y.R.; Halwar, D.K.; Dhoble, S.J. Photoluminescence properties of Eu3+-doped Na2CaSiO4 phosphor prepared by wet-chemical synthesis route. Displays 2022, 75, 102304. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, D.A.; Meza-Rocha, A.N.; Caldino, U.; Lozada-Morales, R.; Alvarez, E.; Zayas, M.E. Reddish-orange, neutral and warm white emissions in Eu3+, Dy3+ and Dy3+/Eu3+ doped CdO-GeO2-TeO2 glasses. Solid State Sci. 2016, 61, 70–76. [Google Scholar] [CrossRef]
- Yu, P.; Guo, W.; Zhang, R.; Su, L.; Xu, J. White and tunable light emission in Eu3+, Dy3+ codoped phosphate glass. Opt. Mater. 2021, 114, 110939. [Google Scholar] [CrossRef]
- Mukamil, S.; Sarumaha, C.; Wabaidur, S.M.; Islam, M.A.; Khattak, S.A.; Kothan, S.; Shoaib, M.; Khan, I.; Ullah, I.; Kaewkhao, J.; et al. Investigation of color tenability of Dy3+ & Eu3+ Co-doped bismuth borate glasses for lighting applications. Mater. Chem. Phys. 2022, 288, 126422. [Google Scholar]
- Meza-Rocha, A.N.; Speghini, A.; Bettinelli, M.; Caldiñ, U. White light generation through Zn(PO3)2 glass activated with Eu3+ and Dy3+. J. Lumin. 2016, 176, 235–239. [Google Scholar] [CrossRef]
- Li, W.; Chen, Z.; Zhang, B.; Zhao, P.; Fan, Y. Luminescence properties and thermal stability of Dy3+/Eu3+ co-doped single-component BaZn2(PO4)2 glass-ceramic phosphors. J. Lumin. 2022, 246, 118824. [Google Scholar] [CrossRef]
- Aleksandrov, L.; Milanova, M.; Yordanova, A.; Iordanova, R.; Nedyalkov, N.; Petrova, P.; Tagiara, N.S.; Palles, D.; Kamitsos, E.I. Synthesis, structure and luminescence properties of Eu3+-doped 50ZnO.40B2O3.5WO3.5Nb2O5 glass. Phys. Chem. Glasses Eur. J. Glass Sci. Technol. B 2023, 64, 101–109. [Google Scholar]
- Aryal, P.; Kesavulu, C.R.; Kim, H.J.; Lee, S.W.; Kang, S.J.; Kaewkhao, J.; Chanthima, N.; Damdee, B. Optical and luminescence characteristics of Eu3 +- doped B2O3:SiO2:Y2O3:CaO glasses for visible red laser and scintillation material applications. J. Rare Earth 2018, 36, 482–491. [Google Scholar] [CrossRef]
- Villegas, M.A.; Fernández Navarro, J.M. Physical and structural properties of glasses in the TeO2–TiO2–Nb2O5 system. J. Eur. Ceram. Soc. 2007, 27, 2715–2723. [Google Scholar] [CrossRef]
- El Jouad, M.; Touhtouh, S.; Addou, M.; Ollier, N.; Sahraoui, B. Red luminescence and ultraviolet light generation of europium doped zincoxide thin films for optoelectronic applications. EPJ Appl. Phys. 2020, 91, 10501. [Google Scholar] [CrossRef]
- Iordanova, R.; Milanova, M.; Aleksandrov, L.; Shinozaki, K. Structural study of WO3-La2O3-B2O3-Nb2O5 glasses. J. Non-Cryst. Solids 2020, 543, 120132. [Google Scholar] [CrossRef]
- Yao, Z.; Möncke, D.; Kamitsos, E.I.; Houizot, P.; Célarié, F.; Rouxel, T.; Wondraczek, L. Structure and mechanical properties of cooper-lead and copper-zinc borate glasses. J. Non-Cryst. Solids 2016, 435, 55–68. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, M.; Ma, Q.; Wang, Q. The structure, spectra and properties of Dy2O3 modified diamagentic lead bismuth-germanium glasses. J. Non-Cryst. Solids 2019, 507, 46–55. [Google Scholar] [CrossRef]
- Milanova, M.; Aleksandrov, L.; Yordanova, A.; Iordanova, R.; Tagiara, N.S.; Herrmann, A.; Gao, G.; Wondraczek, L.; Kamitsos, E.I. Structural and luminescence behavior of Eu3+ ions in ZnO-B2O3-WO3 glasses. J. Non-Cryst. Solids 2023, 600, 122006. [Google Scholar] [CrossRef]
- Daturi, M.; Busca, G.; Borel, M.M.; Leclaire, A.; Piaggio, P. Vibrational and XRD Study of the System CdWO4-CdMoO4. J. Phys. Chem. B 1997, 101, 4358–4369. [Google Scholar] [CrossRef]
- Winterstein-Beckmann, A.; Möncke, D.; Palles, D.; Kamitsos, E.I.; Wondraczek, L. Structure and Properties of Orthoborate Glasses in the Eu2O3–(Sr,Eu)O–B2O3 Quaternary. J. Phys. Chem. B 2015, 119, 3259–3272. [Google Scholar] [CrossRef]
- He, F.; He, Z.; Xie, J.; Li, Y. IR and Raman Spectra Properties of Bi2O3-ZnO-B2O3-BaO Quaternary Glass System. Am. J. Anal. Chem. 2014, 5, 1142–1150. [Google Scholar] [CrossRef]
- Carnall, W.T.; Fields, P.R.; Rajnak, K. Electronic energy levels in the trivalent lanthanide aquo ions. I. Pr3+, Nd3+, Pm3+, Sm3+, Dy3+, Ho3+, Er3+, and Tm3+. J. Chem. Phys. 1968, 49, 4424–4442. [Google Scholar] [CrossRef]
- Chemingui, S.; Ferhi, M.; Horchani-Naifer, K.; Férid, M. Synthesis and luminescence characteristics of Dy3+ doped KLa(PO3)4. J. Lumin. 2015, 166, 82–87. [Google Scholar] [CrossRef]
- Dutta, P.S.; Khanna, A. Eu3+ activated molybdate and tungstate based red phosphors with charge transfer band in blue region. ECS J. Solid State Sci. Technol. 2013, 2, R3153–R3167. [Google Scholar] [CrossRef]
- Zeng, H.; Song, J.; Chen, D.; Yuan, S.; Jiang, X.; Cheng, Y.; Chen, G. Three-photon-excited upconversion luminescence of niobium ions doped silicate glass by a femtosecond laser irradiation. Opt. Express 2008, 16, 6502–6506. [Google Scholar] [CrossRef]
- Thieme, C.; Herrmann, A.; Kracker, M.; Patzig, C.; Höche, T.; Rüssel, C. Microstructure investigation and fluorescence properties of europium-doped scheelite crystals in glass-ceramics made under different synthesis conditions. J. Lumin. 2021, 238, 118244. [Google Scholar] [CrossRef]
- Ju, G.F.; Hu, Y.H.; Chen, L.; Wang, X.J.; Mu, Z.F. Concentration quenching of persistent luminescence. Physica B 2013, 415, 1–4. [Google Scholar] [CrossRef]
- Su, Q.; Pei, Z.; Chi, L.; Zhang, H.; Zhang, Z.; Zou, F. The yellow-to-blue intensity ratio (Y/B) of Dy3+ emission. J. Alloys Compd. 1993, 192, 25–27. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Xu, S.; Cheng, L.; Sun, J.; Zhang, J.; Zhang, X.; Chen, B. Luminescence properties of color-tunable YNbO4: Dy3+, Tm3+ phosphors. J. Asian Ceram. Soc. 2020, 8, 1066–1075. [Google Scholar] [CrossRef]
- Vijayalakshmi, L.; Kumar, K.N.; Baek, J.D. Multicolor and warm white luminescence from Dy3+/Eu3+ co-activated glasses for indoor and solid-state lighting applications. Ceram. Int. 2023, 49, 5013–5021. [Google Scholar] [CrossRef]
- Liu, R.; Wang, D.; Chen, M.; Liu, L.; Zhou, Y.; Zeng, F.; Su, Z. Luminescence, energy transfer properties of Dy3+/Eu3+ coactivated neutral and warm white emissions GSBG glasses. J. Lumin. 2021, 237, 118180. [Google Scholar] [CrossRef]
- Smith, T.; Guild, J. The CIE colorimetric standards and their use. Trans. Opt. Soc. 1931, 33, 73. [Google Scholar] [CrossRef]
- Paolini, T.B. SpectraChroma (Version 1.0.1) [Computer Software]. 2021. Available online: https://zenodo.org/records/4906590 (accessed on 7 June 2021).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).