Antimicrobial Activity of Individual Volatile Compounds from Various Essential Oils
Abstract
1. Introduction
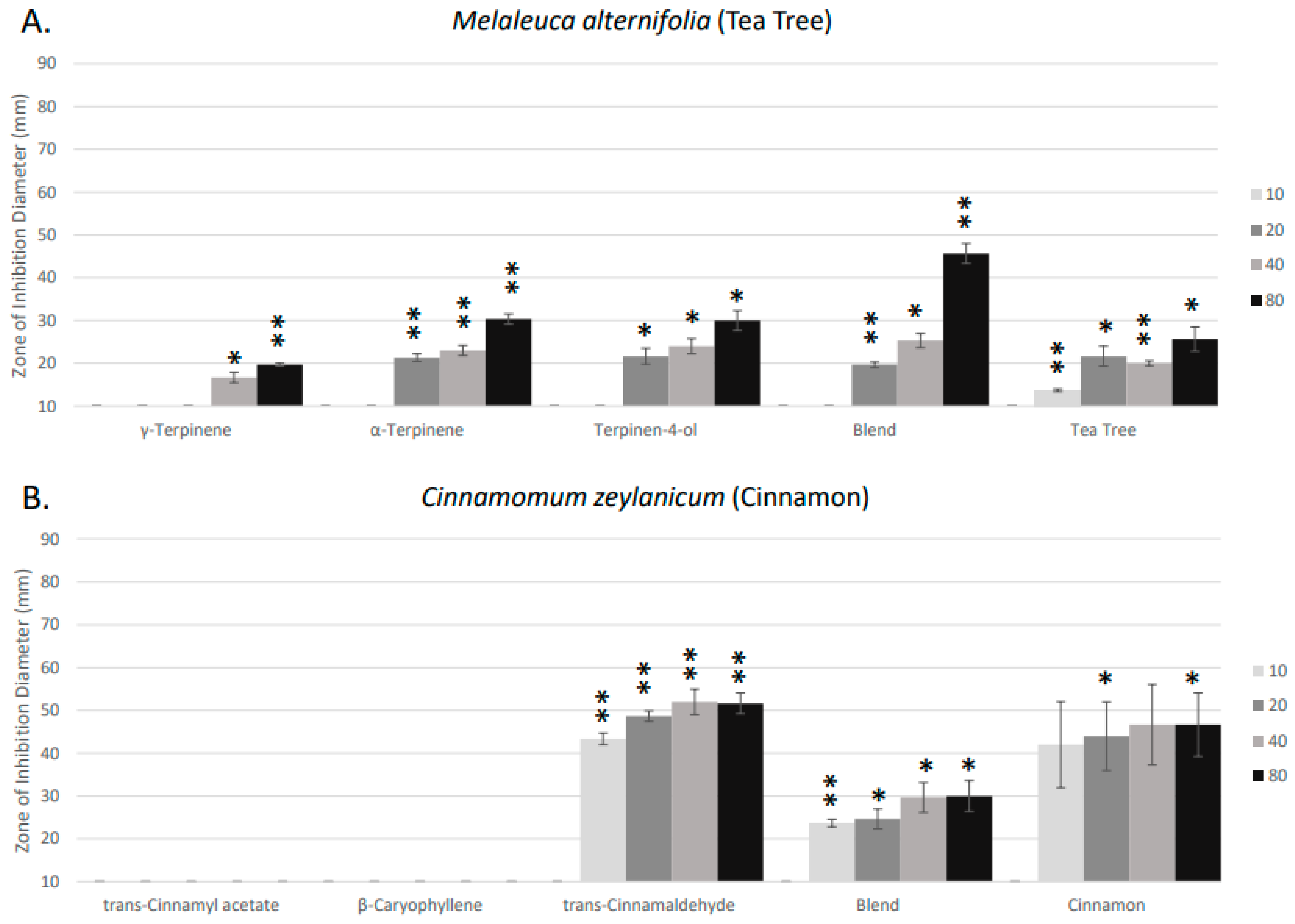
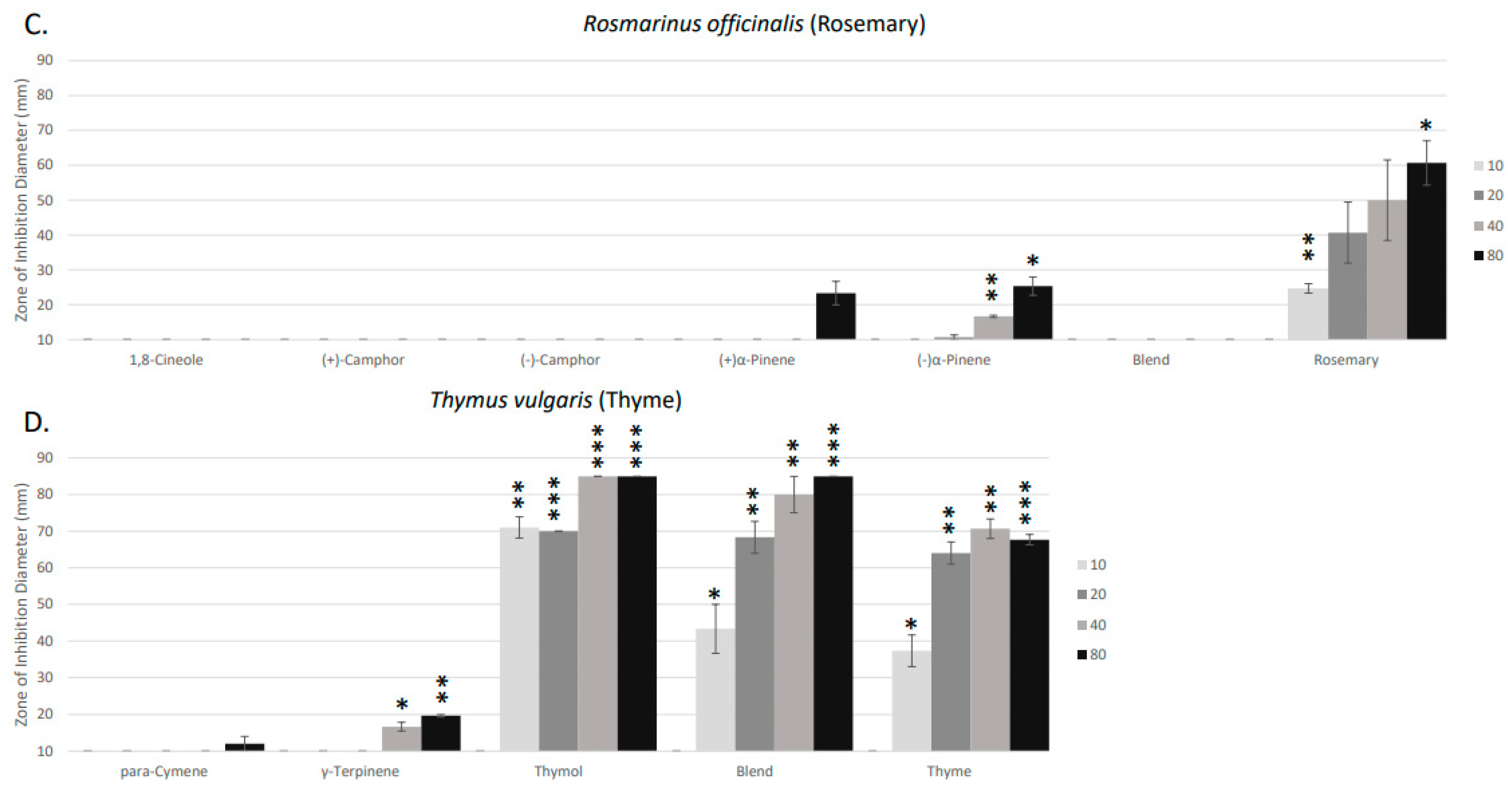
2. Results
3. Materials and Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol. 2009. Available online: https://asm.org/getattachment/2594ce26-bd44-47f6-8287-0657aa9185ad/Kirby-Bauer-Disk-DiffusionSusceptibility-Test-Protocol-pdf.pdf (accessed on 20 January 2024).
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Hajar, R. The Air of History (Part II) Medicine in the Middle Ages. Heart Views Off. J. Gulf Heart Assoc. 2012, 13, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.D.; Fetherston, J.D. Yersinia pestis--etiologic agent of plague. Clin. Microbiol. Rev. 1997, 10, 35–66. [Google Scholar] [CrossRef]
- Nautiyal, C.S.; Chauhan, P.S.; Nene, Y.L. Medicinal smoke reduces airborne bacteria. J. Ethnopharmacol. 2007, 114, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Rossiter, S.E.; Fletcher, M.H.; Wuest, W.M. Natural Products as Platforms to Overcome Antibiotic Resistance. Chem. Rev. 2017, 117, 12415–12474. [Google Scholar] [CrossRef] [PubMed]
- CDC. National Infection & Death Estimates for AR. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/drugresistance/national-estimates.html (accessed on 6 July 2023).
- Kohlert, C.; van Rensen, I.; März, R.; Schindler, G.; Graefe, E.U.; Veit, M. Bioavailability and pharmacokinetics of natural volatile terpenes in animals and humans. Planta Medica 2000, 66, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Horváth, G.; Ács, K. Essential oils in the treatment of respiratory tract diseases highlighting their role in bacterial infections and their anti-inflammatory action: A review. Flavour Fragr. J. 2015, 30, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.; Takizawa, T.; Yamaguchi, H. Antibacterial activity of essential oils and their major constituents against respiratory tract pathogens by gaseous contact. J. Antimicrob. Chemother. 2001, 47, 565–573. [Google Scholar] [CrossRef]
- Maruzzella, J.C.; Sicurella, N.A. Antibacterial Activity of Essential Oil Vapors. J. Am. Pharm. Assoc. (Sci. Ed.) 1960, 49, 692–694. [Google Scholar] [CrossRef]
- Kienholz, M. On the antibacterial effect of ethereal oils. Arzneim. Forsch. 1959, 9, 519–521. [Google Scholar]
- Abers, M.; Schroeder, S.; Goelz, L.; Sulser, A.; Rose, T.S.; Puchalski, K.; Langland, J. Antimicrobial activity of the volatile substances from essential oils. BMC Complement. Med. Ther. 2021, 21, 124. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid. Based Complement. Altern. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef]
- Moo, C.-L.; Yang, S.-K.; Osman, M.-A.; Yuswan, M.H.; Loh, J.-Y.; Lim, W.-M.; Lim, S.-H.-E.; Lai, K.-S. Antibacterial Activity and Mode of Action of β-caryophyllene on Bacillus cereus. Pol. J. Microbiol. Pol. Tow. Mikrobiol. Pol. Soc. Microbiol. 2020, 69, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Effects of melaleuca alternifolia (tea tree) essential oil and the major monoterpene component terpinen-4-ol on the development of single- and multistep antibiotic resistance and antimicrobial susceptibility. Antimicrob. Agents Chemother. 2012, 56, 909–915. [Google Scholar] [CrossRef]
- Boren, K.; Crown, A.; Carlson, R. Multidrug and Pan-Antibiotic Resistance—The Role of Antimicrobial and Synergistic Essential Oils: A Review. Nat. Prod. Commun. 2020, 15, 1934578X20962595. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control. 2019, 8, 118. [Google Scholar] [CrossRef]
- Rosato, A.; Carocci, A.; Catalano, A.; Clodoveo, M.L.; Franchini, C.; Corbo, F.; Carbonara, G.G.; Carrieri, A.; Fracchiolla, G. Elucidation of the synergistic action of Mentha Piperita essential oil with common antimicrobials. PLoS ONE 2018, 13, e0200902. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [PubMed]
- Mahizan, N.A.; Yang, S.-K.; Moo, C.L.; Song, A.A.-L.; Chong, C.-M.; Chong, C.-W.; Abushelaibi, A.; Lim, S.-H.-E.; Lai, K.-S. Terpene Derivatives as a Potential Agent against Antimicrobial Resistance (AMR) Pathogens. Molecules 2019, 24, 2631. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Van Vuuren, S.; Viljoen, A. Unravelling the complex antimicrobial interactions of essential oils—the case of Thymus vulgaris (thyme). Molecules 2014, 19, 2896–2910. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wu, N.; Fu, Y.-J.; Wang, W.; Luo, M.; Zhao, C.-J.; Zu, Y.-G.; Liu, X.-L. Chemical composition and antimicrobial activity of the essential oil of Rosemary. Environ. Toxicol. Pharmacol. 2011, 32, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Moo, C.-L.; Osman, M.A.; Yang, S.-K.; Yap, W.-S.; Ismail, S.; Lim, S.-H.; Chong, C.-M.; Lai, K.-S. Antimicrobial activity and mode of action of 1,8-cineol against carbapenemase-producing Klebsiella pneumoniae. Sci. Rep. 2021, 11, 20824. [Google Scholar] [CrossRef]
- de Araújo, A.C.J.; Freitas, P.R.; Barbosa, C.R.d.S.; Muniz, D.F.; de Almeida, R.S.; de Menezes, I.R.A.; Ribeiro-Filho, J.; Tintino, S.R.; Coutinho, H.D.M. In Vitro and In Silico Inhibition of Staphylococcus aureus Efflux Pump NorA by α-Pinene and Limonene. Curr. Microbiol. 2021, 78, 3388–3393. [Google Scholar] [CrossRef] [PubMed]
- Azerad, R. 1,8-cineole: Chemical and biological oxidation reactions and products. Chempluschem 2014, 79, 634–655. [Google Scholar] [CrossRef]
- Barton, A.F.M.; Dell, B.; Knight, A.R. Herbicidal activity of cineole derivatives. J. Agric. Food Chem. 2010, 58, 10147–10155. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; del Mar Contreras, M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. Phytother. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.; Meier, A.; Guggenheim, B. The antimicrobial activity of essential oils and essential oil components towards oral bacteria. Oral Microbiol. Immunol. 1994, 9, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2019, 60, 3042–3053. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; Del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Veldhuizen, E.J.A.; Tjeerdsma-van Bokhoven, J.L.M.; Zweijtzer, C.; Burt, S.A.; Haagsman, H.P. Structural Requirements for the Antimicrobial Activity of Carvacrol. J. Agric. Food Chem. 2006, 54, 1874–1879. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, L.; Figueiredo, P.; Souza, H.; Sousa, A.; Andrade-Júnior, F.; Medeiros, D.; Nóbrega, J.; Silva, D.; Martins, E.; Barbosa-Filho, J.; et al. Terpinen-4-ol as an Antibacterial and Antibiofilm Agent against Staphylococcus aureus. Int. J. Mol. Sci. 2020, 21, 4531. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Park, J.C.; Chong, Y. Aromatic hydroxyl group plays a critical role in antibacterial activity of the curcumin analogues. Nat. Prod. Commun. 2012, 7, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Firmino, D.F.; Cavalcante, T.T.A.; Gomes, G.A.; Firmino, N.C.S.; Rosa, L.D.; De Carvalho, M.G.; Catunda, F.E.A., Jr. Antibacterial and antibiofilm activities of cinnamomum sp. essential oil and cinnamaldehyde: Antimicrobial activities. Sci. World J. 2018, 2018, 7405736. [Google Scholar] [CrossRef]
- Pauli, A.; Kubeczka, K.-H. Antimicrobial Properties of Volatile Phenylpropanes. Nat. Prod. Commun. 2010, 5, 1387–1394. [Google Scholar] [CrossRef]
- Nogueira, J.O.e.; Campolina, G.A.; Batista, L.R.; Alves, E.; Caetano, A.R.S.; Brandão, R.M.; Nelson, D.L.; Cardoso, M.D.G. Mechanism of action of various terpenes and phenylpropanoids against Escherichia coli and Staphylococcus aureus. FEMS Microbiol. Lett. 2021, 368, fnab052. [Google Scholar] [CrossRef] [PubMed]
- Abu-Eittah, R.H.; Khedr, M.K.; Goma, M.; Zordok, W. The structure of cinnamic acid and cinnamoyl azides, a unique localized π system: The electronic spectra and DFT-treatment. Int. J. Quantum Chem. 2012, 112, 1256–1272. [Google Scholar] [CrossRef]
- Autelitano, A.; Minassi, A.; Pagani, A.; Taglialatela-Scafati, O.; Appendino, G. The reaction of cinnamaldehyde and cinnam(o)yl derivatives with thiols. Acta Pharm. Sin. B 2017, 7, 523–526. [Google Scholar] [CrossRef]
- Chang, S.T.; Chen, P.F.; Chang, S.C. Antibacterial activity of leaf essential oils and their constituents from Cinnamomum osmophloeum. J. Ethnopharmacol. 2001, 77, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Miladi, H.; Zmantar, T.; Kouidhi, B.; Al Qurashi, Y.M.A.; Bakhrouf, A.; Chaabouni, Y.; Mahdouani, K.; Chaieb, K. Synergistic effect of eugenol, carvacrol, thymol, p-cymene and γ-terpinene on inhibition of drug resistance and biofilm formation of oral bacteria. Microb. Pathog. 2017, 112, 156–163. [Google Scholar] [CrossRef]

| Lot # | Plant Part | 5–10% | 10–20% | 20–30% | >30% | |
|---|---|---|---|---|---|---|
| Wintergreen | 211901A | Methyl Salicylate | ||||
| Tea Tree | 2222711A | Leaf | ND | α-Terpinene | γ-Terpinene | Terpinen-4-ol |
| Cinnamon | 171326A | Bark | β-Caryophyllene | trans-Cinnamyl acetate | ND | trans-Cinnamaldehyde |
| Rosemary | 171319A | Flower/Leaf | ND | α-Pinene Camphor | ND | 1,8-Cineole |
| Thyme | 222369A | Leaf | ND | γ-Terpinene para-Cymene | ND | Thymol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandes, A.; Dunning, M.; Langland, J. Antimicrobial Activity of Individual Volatile Compounds from Various Essential Oils. Molecules 2024, 29, 1811. https://doi.org/10.3390/molecules29081811
Brandes A, Dunning M, Langland J. Antimicrobial Activity of Individual Volatile Compounds from Various Essential Oils. Molecules. 2024; 29(8):1811. https://doi.org/10.3390/molecules29081811
Chicago/Turabian StyleBrandes, Adriana, Mareshah Dunning, and Jeffrey Langland. 2024. "Antimicrobial Activity of Individual Volatile Compounds from Various Essential Oils" Molecules 29, no. 8: 1811. https://doi.org/10.3390/molecules29081811
APA StyleBrandes, A., Dunning, M., & Langland, J. (2024). Antimicrobial Activity of Individual Volatile Compounds from Various Essential Oils. Molecules, 29(8), 1811. https://doi.org/10.3390/molecules29081811






