Impact of Various Essential Oils on the Development of Pathogens of the Fusarium Genus and on Health and Germination Parameters of Winter Wheat and Maize
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soto-Gómez, D.; Pérez-Rodríguez, P. Sustainable agriculture through perennial grains: Wheat, rice, maize, and other species. A review. Agric. Ecosyst. Environ. 2022, 325, 107747. [Google Scholar] [CrossRef]
- Grote, U.; Fasse, A.; Nguyen, T.T.; Erenstein, O. Food Security and the Dynamics of Wheat and Maize Value Chains in Africa and Asia. Front. Sustain. Food Syst. 2021, 4, 617009. [Google Scholar] [CrossRef]
- Sharma, R.K.; Kumar, S.; Vatta, K.; Bheemanahalli, R.; Dhillon, J.; Reddy, K.N. Impact of recent climate change on corn, rice, and wheat in southeastern USA. Sci. Rep. 2022, 12, 16928. [Google Scholar] [CrossRef] [PubMed]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Singh, R.P.; Singh, P.K.; Rutkoski, J.; Hodson, D.P.; He, X.; Jørgensen, L.N.; Hovmøller, M.S.; Huerta-Espino, J.; Jørgenssen, L.N. Disease Impact on Wheat Yield Potential and Prospects of Genetic Control. Annu. Rev. Phytopathol. 2016, 54, 303–322. [Google Scholar] [CrossRef]
- Mueller, D.S.; Wise, K.A.; Sisson, A.J.; Allen, T.W.; Bergstrom, G.C.; Bosley, D.B.; Bradley, C.A.; Broders, K.D.; Byamukama, E.; Chilvers, M.I.; et al. Corn yield loss estimates due to diseases in the United States and Ontario, Canada from 2012 to 2015. Plant Health Prog. 2016, 17, 211–222. [Google Scholar] [CrossRef]
- Valverde-Bogantes, E.; Bianchini, A.; Herr, J.R.; Rose, D.J.; Wegulo, S.N.; Hallen-Adams, H.E. Recent population changes of Fusarium head blight pathogens: Drivers and implications. Can. J. Plant Pathol. 2019, 42, 315–329. [Google Scholar] [CrossRef]
- Karlsson, I.; Persson, P.; Friberg, H. Fusarium Head Blight from a Microbiome Perspective. Front. Microbiol. 2021, 12, 628373. [Google Scholar] [CrossRef]
- Petrucci, A.; Khairullina, A.; Sarrocco, S.; Jensen, D.F.; Jensen, B.; Jørgensen, H.J.L.; Collinge, D.B. Understanding the mechanisms underlying biological control of Fusarium diseases in cereals. Eur. J. Plant Pathol. 2023, 167, 453–476. [Google Scholar] [CrossRef]
- Sawinska, Z.; Małecka, I. Economical aspects of disease control in winter wheat. J. Plant Prot. Res. 2006, 46, 255–260. [Google Scholar]
- Moumni, M.; Brodal, G.; Romanazzi, G. Recent innovative seed treatment methods in the management of seedborne pathogens. Food Secur. 2023, 15, 1365–1382. [Google Scholar] [CrossRef]
- Zargaryan, N.Y.; Kekalo, A.Y.; Nemchenko, V.V. Infection of Grain Crops with Fungi of the Genus Fusarium. In BIO Web of Conferences; EDP Sciences: Les Ulis, France, 2021; Volume 36, p. 04008. [Google Scholar] [CrossRef]
- Kazan, K.; Gardiner, D.M. Fusarium crown rot caused by Fusarium pseudograminearum in cereal crops: Recent progress and future prospects. Mol. Plant Pathol. 2018, 19, 1547–1562. [Google Scholar] [CrossRef]
- Shude, S.P.; Yobo, K.S.; Mbili, N.C. Progress in the management of Fusarium head blight of wheat: An overview. S. Afr. J. Sci. 2020, 116, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kryczyński, S.; Weber, Z. Fitopatologia. T. 2. Choroby Roślin Uprawnych [Diseases of Cultivated Plants]; PWRiL: Poznań, Poland, 2021; ISBN 978-83-09-01077-7. [Google Scholar]
- Morrison, E.; Kosiak, B.; Ritieni, A.; Aastveit, A.H.; Uhlig, S.; Bernhoft, A. Mycotoxin production by Fusarium avenaceum strains isolated from norwegian grain and the cytotoxicity of rice culture extracts to porcine kidney epithelial cells. J. Agric. Food Chem. 2002, 50, 3070–3075. [Google Scholar] [CrossRef] [PubMed]
- Holtz, M.D.; Chang, K.F.; Hwang, S.F.; Gossen, B.D.; Strelkov, S.E. Characterization of Fusarium avenaceum from lupin in central Alberta: Genetic diversity, mating type and aggressiveness. Can. J. Plant Pathol. 2011, 33, 61–76. [Google Scholar] [CrossRef]
- Munkvold, G.P. Fusarium species and their associated mycotoxins. In Mycotoxigenic Fungi: Methods and Protocols, Methods in Molecular Biology; Moretti, A., Sueca, A., Eds.; Springer: New York, NY, USA, 2017; Volume 1542, pp. 51–106. [Google Scholar]
- Schiwek, S.; Alhussein, M.; Rodemann, C.; Budragchaa, T.; Beule, L.; von Tiedemann, A.; Karlovsky, P. Fusarium culmorum Produces NX-2 Toxin Simultaneously with Deoxynivalenol and 3-Acetyl-Deoxynivalenol or Nivalenol. Toxins 2022, 14, 456. [Google Scholar] [CrossRef] [PubMed]
- Castiblanco, V.; Marulanda, J.J.; Würschum, T.; Miedaner, T. Candidate gene based association mapping in Fusarium culmorum for field quantitative pathogenicity and mycotoxin production in wheat. BMC Genet. 2017, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Scherm, B.; Balmas, V.; Spanu, F.; Pani, G.; Delogu, G.; Pasquali, M.; Migheli, Q. Fusarium culmorum: Causal Agent of Foot and Root Rot and Head Blight on Wheat. Mol. Plant Pathol. 2013, 14, 323–341. [Google Scholar] [CrossRef]
- Montibus, M.; Vitrac, X.; Coma, V.; Loron, A.; Pinson-Gadais, L.; Ferrer, N.; Verdal-Bonnin, M.-N.; Gabaston, J.; Waffo-Téguo, P.; Richard-Forget, F.; et al. Screening of Wood/Forest and Vine By-Products as Sources of New Drugs for Sustainable Strategies to Control Fusarium graminearum and the Production of Mycotoxins. Molecules 2021, 26, 405. [Google Scholar] [CrossRef]
- Reed, H.; Mueller, B.; Groves, C.L.; Smith, D.L. Presence and Correlation of Fusarium graminearum and Deoxynivalenol Accumulation in Silage Corn Plant Parts. Plant Dis. 2022, 106, 87–92. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, L.; Yang, X.; Shen, G.; Wang, S.; Teng, H.; Yang, C.; Liu, X.; Wang, X.; Zhao, J.; et al. Fusarium Species Associated with Maize Leaf Blight in Heilongjiang Province, China. J. Fungi 2022, 8, 1170. [Google Scholar] [CrossRef] [PubMed]
- Mohd, M.H.; Nor, N.M.I.M.; Azuddin, N.F.; Zakaria, L. Mycotoxin production by Fusarium proliferatum and Fusarium fujikuroi causing stem rot of Hylocereus polyrhizus in Malaysia. Malays. Appl. Biol. 2023, 52, 13–22. [Google Scholar] [CrossRef]
- Sunani, S.K.; Bashyal, B.M.; Rawat, K.; Manjunatha, C.; Sharma, S.; Prakash, G.; Krishnan, S.G.; Singh, A.K.; Aggarwal, R. Development of PCR and loop mediated isothermal amplification assay for the detection of bakanae pathogen Fusarium fujikuroi. Eur. J. Plant Pathol. 2019, 154, 715–725. [Google Scholar] [CrossRef]
- Niehaus, E.-M.; Münsterkötter, M.; Proctor, R.H.; Brown, D.W.; Sharon, A.; Idan, Y.; Oren-Young, L.; Sieber, C.M.; Novak, O.; Pencik, A.; et al. Comparative “Omics” of the Fusarium Fujikuroi Species Complex Highlights Differences in Genetic Potential and Metabolite Synthesis. Genome Biol. Evol. 2016, 8, 3574–3599. [Google Scholar] [CrossRef] [PubMed]
- Tadasanahaller, P.S.; Bashyal, B.M.; Yadav, J.; Krishnan Subbaiyan, G.; Ellur, R.K.; Aggarwal, R. Identification and Characterization of Fusarium fujikuroi Pathotypes Responsible for an Emerging Bakanae Disease of Rice in India. Plants 2023, 12, 1303. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Fischer, S.; Egan, D.; Doohan, F.M. Biological control of Fusarium seedling blight disease of wheat and barley. Phytopathology 2006, 96, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Inbaia, S.; Farooqi, A.; Ray, R.V. Aggressiveness and mycotoxin profile of Fusarium avenaceum isolates causing Fusarium seedling blight and Fusarium head blight in UK malting barley. Front. Plant Sci. 2023, 14, 1121553. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Bae, J.S.; Bergstrom, G.C.; Jander, G. Fusarium graminearum-induced shoot elongation and root reduction in maize seedlings correlate with later seedling blight severity. Plant Direct. 2018, 2, e00075. [Google Scholar] [CrossRef] [PubMed]
- Tia, V.E.; Gueu, S.; Cissé, M.; Tuo, Y.; Gnago, A.J.; Konan, E. Bio-insecticidal effects of essential oil nano-emulsion of Lippia multiflora Mold. on major cabbage pests. J. Plant Prot. Res. 2021, 61, 103–109. [Google Scholar] [CrossRef]
- Kesraoui, S.; Andrés, M.F.; Berrocal-Lobo, M.; Soudani, S.; Gonzalez-Coloma, A. Direct and Indirect Effects of Essential Oils for Sustainable Crop Protection. Plants 2022, 11, 2144. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; El Gendy, A.E.-N.G.; Assaeed, A.M.; Al-Rowaily, S.L.; Alharthi, A.S.; Mohamed, T.A.; Nassar, M.I.; Dewir, Y.H.; Elshamy, A.I. Phytotoxic Effects of Plant Essential Oils: A Systematic Review and Structure-Activity Relationship Based on Chemometric Analyses. Plants 2021, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, M.; Mehdizadeh, L. Chemistry of essential oils and factors influencing their constituents. In Soft Chemistry and Food Fermentation; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: London, UK, 2017; pp. 379–419. [Google Scholar]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Abu-Darwish, M.S.; Cabral, C.; Ferreira, I.V.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Al-bdour, T.H.; Salgueiro, L. Essential oil of common sage (Salvia officinalis L.) from Jordan: Assessment of safety in mammalian cells and its antifungal and anti-inflammatory potential. Biomed Res. Int. 2013, 2013, 538940. [Google Scholar] [CrossRef] [PubMed]
- Al-Snafi, A.E. Medical importance of Cupressus sempervirens—A review. IOSR J. Pharm. 2016, 6, 66–76. [Google Scholar]
- Allaq, A.A.; Sidik, N.J.; Abdul-Aziz, A.; Ahmed, I.A. Cumin (Cuminum cyminum L.): A review of its ethnopharmacology, phytochemistry. Biomed. Res. Ther. 2020, 7, 9. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil-New Insights into Selected Therapeutic Applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef] [PubMed]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: A review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Gull-e-laala, K.; Irshad, G.; Naz, F.; Hafiz, A.A. Microencapsulation of Eucalyptus globulus essential oil anti-fungal sachet against blue mold on peaches. J. Plant Prot. 2023, 63, 428–439. [Google Scholar] [CrossRef]
- Medina-Romero, Y.M.; Hernandez-Hernandez, A.B.; Rodriguez-Monroy, M.A.; Canales-Martínez, M.M. Essential oils of Bursera morelensis and Lippia graveolens for the development of a new biopesticides in postharvest control. Sci. Rep. 2021, 11, 20135. [Google Scholar] [CrossRef]
- Chang, Y.; Harmon, P.F.; Treadwell, D.D.; Carrillo, D.; Sarkhosh, A.; Brecht, J.K. Biocontrol Potential of Essential Oils in Organic Horticulture Systems: From Farm to Fork. Front. Nutr. 2022, 8, 1275. [Google Scholar] [CrossRef]
- Isman, M.B. Bioinsecticides based on plant essential oils: A short overview. Z Nat. C J. Biosci. 2020, 75, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Franz, C.; Novak, J. Sources of essential oils. In Handbook of Essential Oils: Science, Technology, and Applications; Baser, K.H.C., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 39–82. [Google Scholar]
- Koul, O.; Walia, S.; Dhaliwal, G.S. Essential oils as green pesticides: Potential and constraints. Biopestic. Int. 2008, 4, 63–84. [Google Scholar]
- Czaja, K.; Góralczyk, K.; Struci’nski, P.; Hernik, A.; Korcz, W.; Minorczyk, M.; Łyczewska, M.; Ludwicki, J.K. Biopesticides–towards increased consumer safety in the European Union. Pest Manag. Sci. 2015, 71, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Rosell, G.; Quero, C.; Coll, J.; Guerrero, A. Biorational insecticides in pest management. J. Pestic. Sci. 2008, 33, 103–121. [Google Scholar] [CrossRef]
- Edwards, S.G. Pydiflumetofen Co-Formulated with Prothioconazole: A Novel Fungicide for Fusarium Head Blight and Deoxynivalenol Control. Toxins 2022, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Marrone, P. Pesticidal natural products—Status and future potential. Pest Manag. Sci. 2019, 75, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Pavoni, L.; Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. An Overview of Micro- and Nanoemulsions as Vehicles for Essential Oils: Formulation, Preparation and Stability. Nanomaterials 2020, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Proto, M.R.; Biondi, E.; Baldo, D.; Levoni, M.; Filippini, G.; Modesto, M.; Di Vito, M.; Bugli, F.; Ratti, C.; Minardi, P.; et al. Essential Oils and Hydrolates: Potential Tools for Defense against Bacterial Plant Pathogens. Microorganisms 2022, 10, 702. [Google Scholar] [CrossRef] [PubMed]
- Blažytė-Čereškienė, L.; Apšegaitė, V.; Radžiutė, S.; Mozūraitis, R.; Būda, V.; Pečiulytė, D. Electrophysiological and behavioural responses of Ips typographus (L.) to trans-4-thujanol—A host tree volatile compound. Ann. For. Sci. 2016, 73, 247–256. [Google Scholar] [CrossRef]
- Kačániová, M.; Galovičová, L.; Valková, V.; Ďuranová, H.; Borotová, P.; Štefániková, J.; Vukovic, N.L.; Vukic, M.; Kunová, S.; Felsöciová, S.; et al. Chemical Composition and Biological Activity of Salvia officinalis Essential Oil. Acta Hortic. Regiotect. 2021, 24, 81–88. [Google Scholar] [CrossRef]
- Longaray Delamare, A.P.; Moschen-Pistorello, I.T.; Artico, L.; Atti-Serafini, L.; Echeverrigaray, S. Antibacterial activity of the essential oils of Salvia officinalis L. and Salvia triloba L. cultivated in South Brazil. Food Chem. 2007, 100, 603–608. [Google Scholar] [CrossRef]
- Wanner, J.; Bail, S.; Jirovetz, L.; Buchbauer, G.; Schmidt, E.; Gochev, V.; Girova, T.; Atanasova, T.; Stoyanova, A. Chemical composition and antimicrobial activity of cumin oil (Cuminum cyminum, Apiaceae). Nat. Prod. Commun. 2010, 5, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Rivas da Silva, A.C.; Lopes, P.M.; Barros de Azevedo, M.M.; Costa, D.C.; Alviano, C.S.; Alviano, D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules 2012, 25, 6305–6316. [Google Scholar] [CrossRef] [PubMed]
- Galovičová, L.; Čmiková, N.; Schwarzová, M.; Vukic, M.D.; Vukovic, N.L.; Kowalczewski, P.Ł.; Bakay, L.; Kluz, M.I.; Puchalski, C.; Obradovic, A.D.; et al. Biological Activity of Cupressus sempervirens Essential Oil. Plants 2023, 12, 1097. [Google Scholar] [CrossRef]
- Turkez, H.; Aydin, E. In vitro assessment of cytogenetic and oxidative effects of alpha-pinene. Toxicol. Ind. Health 2016, 32, 168–176. [Google Scholar] [CrossRef]
- Anžlovar, S.; Likar, M.; Koce, J.D. Antifungal potential of thyme essential oil as a preservative for storage of wheat seeds. Acta Bot. Croat. 2017, 76, 64–71. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; Blázquez, M.A. Phytotoxic Effects of Commercial Eucalyptus citriodora, Lavandula angustifolia, and Pinus sylvestris Essential Oils on Weeds, Crops, and Invasive Species. Molecules 2019, 24, 2847. [Google Scholar] [CrossRef]
- Ismail, A.; Hamrouni, L.; Hanana, M.; Jamoussi, B. Review on the phytotoxic effects of essential oils and their individual components: News approach for weed management. Int. J. Appl. Biol. Pharm. Technol. 2013, 4, 96–114. [Google Scholar]
- Caruso, C.; Chilosi, G.; Caporale, C.; Leonardi, L.; Bertini, L.; Magro, P.; Buonocore, V. Induction of pathogenesis-related proteins in germinating wheat seeds infected with Fusarium culmorum. Plant Sci. 1999, 140, 87–97. [Google Scholar] [CrossRef]
- Tufan, F.; Uçarl, C.; Tunalı, B.; Gürel, F. Analysis of early events in barley (Hordeum vulgare L.) roots in response to Fusarium culmorum infection. Eur. J. Plant Pathol. 2017, 148, 343–355. [Google Scholar] [CrossRef]
- Taylor, A.G.; Salanenka, Y.A. Seed treatments: Phytotoxicity amelioration and tracer uptake. Seed Sci. Res. 2012, 22, S86–S90. [Google Scholar] [CrossRef]
- Grzanka, M.; Sobiech, Ł.; Danielewicz, J.; Horoszkiewicz-Janka, J.; Skrzypczak, G.; Sawinska, Z.; Radzikowska, D.; Switek, S. Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops. Open Chem. 2021, 19, 884–893. [Google Scholar] [CrossRef]
- Bota, V.; Sumalan, R.M.; Obistioiu, D.; Negrea, M.; Cocan, I.; Popescu, I.; Alexa, E. Study on the Sustainability Potential of Thyme, Oregano, and Coriander Essential Oils Used as Vapours for Antifungal Protection of Wheat and Wheat Products. Sustainability 2022, 14, 4298. [Google Scholar] [CrossRef]
- Faghih-Imani, M.H.; Taheri, P.; Tarighi, S. Antifungal and virulence-modulating effects of thyme essential oil against Fusarium spp., causing wheat diseases. Appl. Microbiol. Theory Technol. 2020, 1, 1–17. [Google Scholar] [CrossRef]
- Palfi, M.; Konjevoda, P.; Karolina Vrandečić, J.C. Antifungal activity of essential oils on mycelial growth of Fusarium oxysporum and Bortytis cinerea. Emirates J. Food Agric. 2019, 31, 544–554. [Google Scholar] [CrossRef]
- Yilar, M.; Kadioglu, I. Antifungal Activities of some Salvia Species Extracts on Fusarium oxysporum f. sp. radicis-lycopersici (Forl) Mycelium Growth In-Vitro. Egypt. J. Pest Cont. 2016, 26, 115–118. [Google Scholar]
- Kedia, A.; Prakash, B.; Mishra, P.K.; Dwivedy, A.K.; Dubey, N.K. Trachyspermum ammi L. essential oil as plant based preservative in food system. Ind. Crops Prod. 2015, 69, 104–109. [Google Scholar] [CrossRef]
- Isman, M.B.; Miresmailli, S.; Machial, C. Commercial opportunities for pesticides based on plant essential oils in agriculture, industry and consumer products. Phytochem. Rev. 2011, 10, 197–204. [Google Scholar] [CrossRef]
- Grzanka, M.; Sobiech, L.; Stuper-Szablewska, K.; Danielewicz, J.; Skrzypczak, G. Effect of selected essential oils on the efficacy of volunteer oilseed rape control and to phytotoxicity in maize plants. Chil. J. Agric. Res. 2022, 82, 88–96. [Google Scholar] [CrossRef]


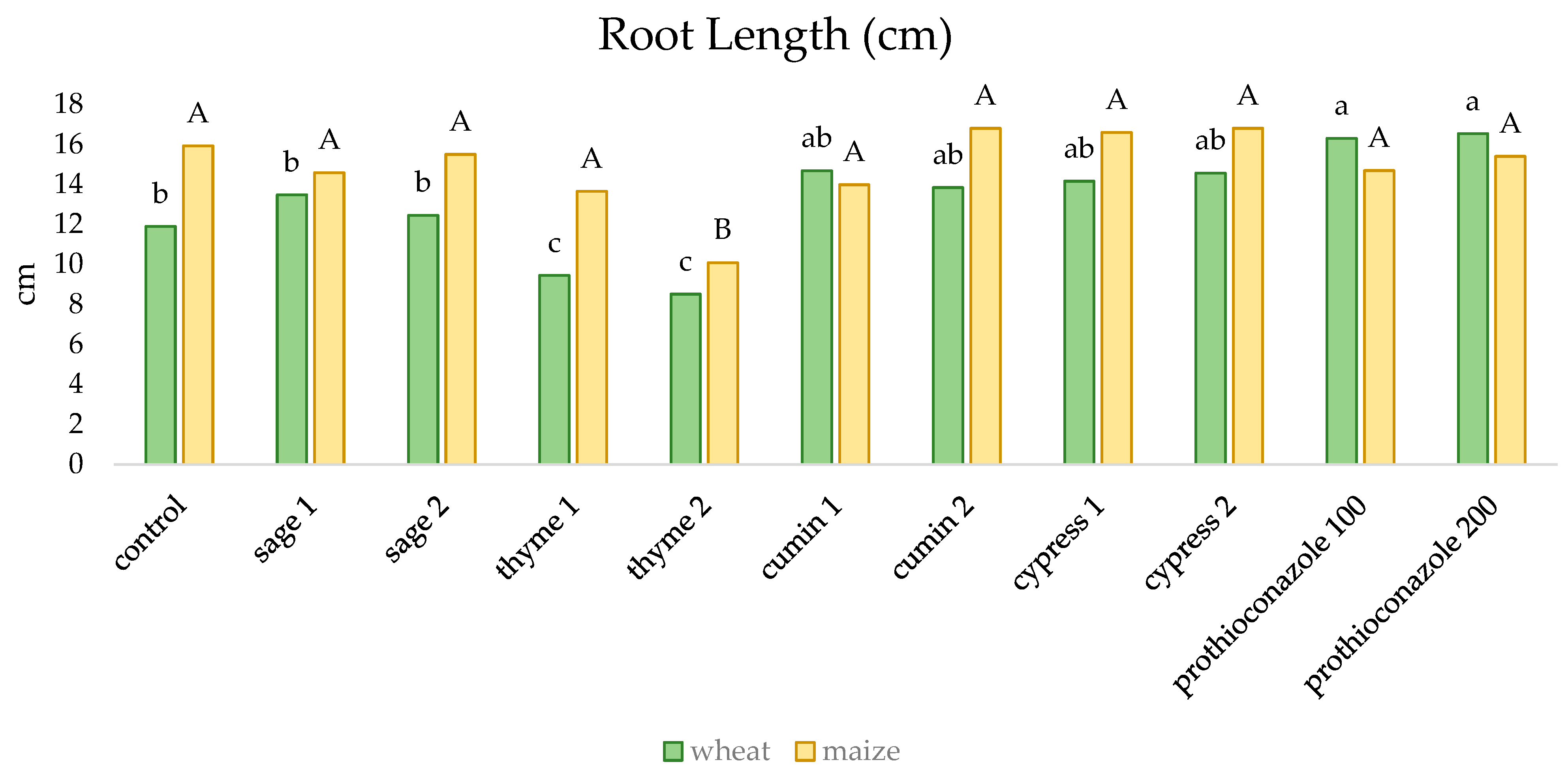
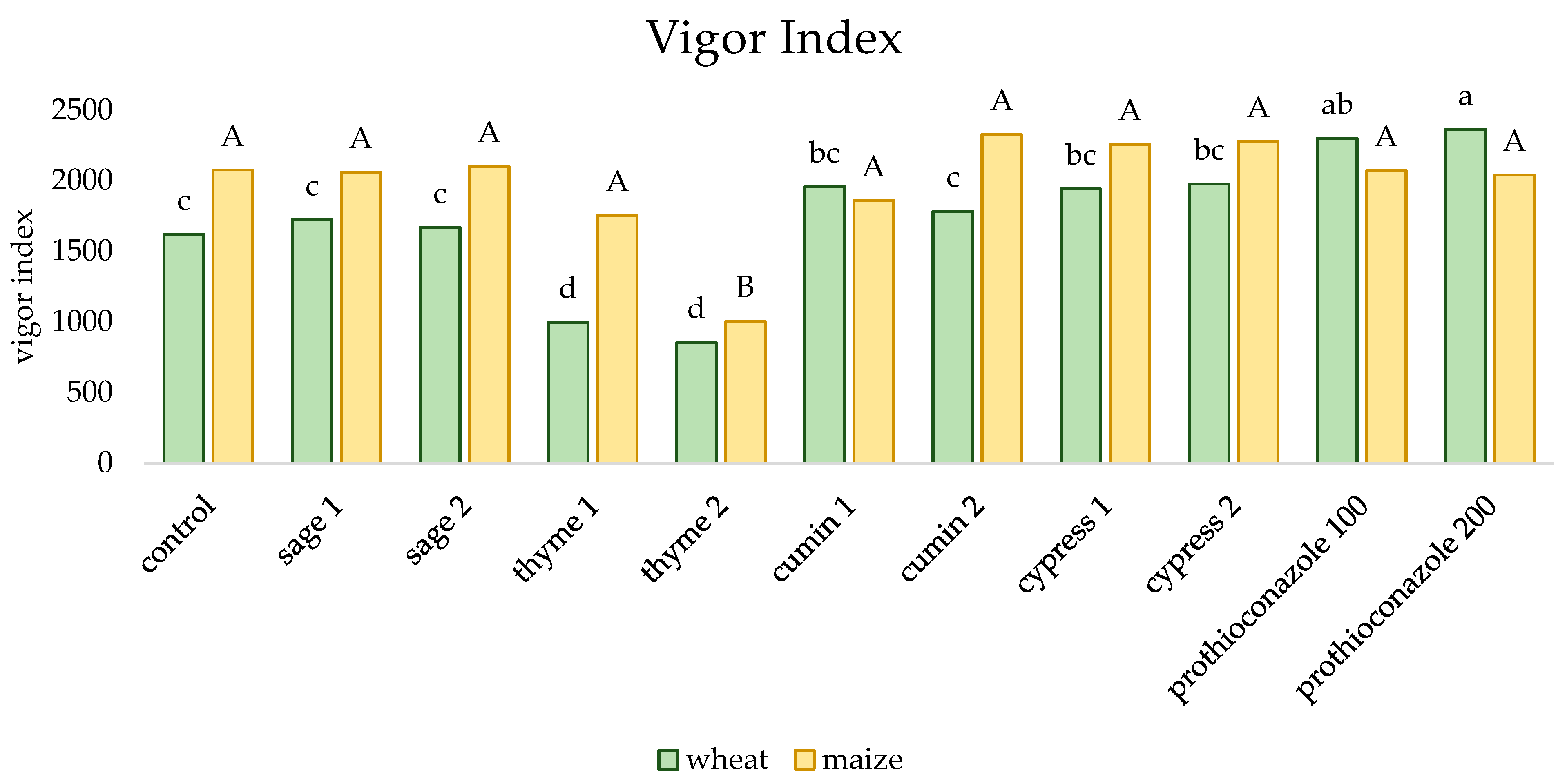
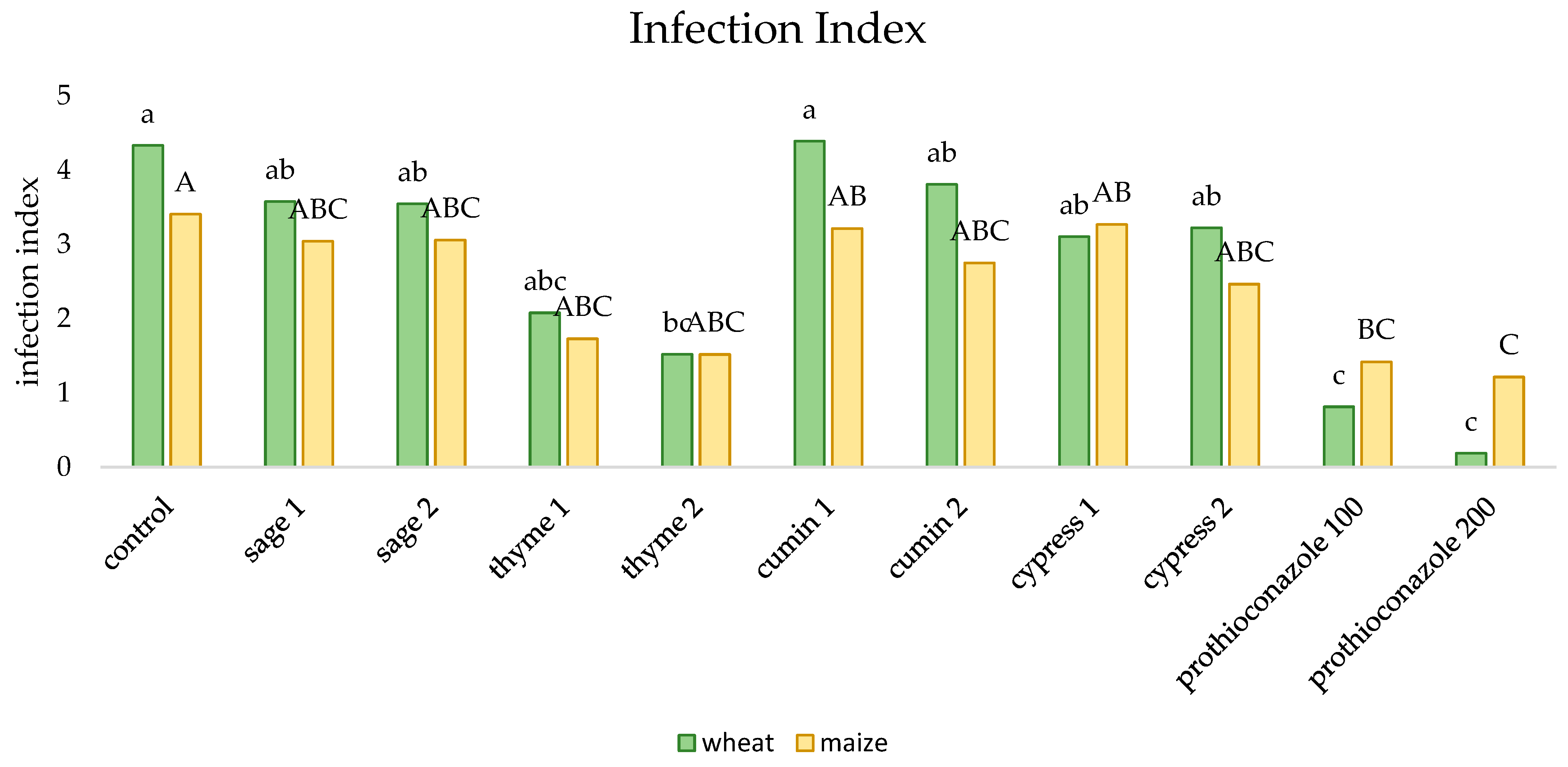
| Sage | Thyme | Cumin | Cypress | ||||
|---|---|---|---|---|---|---|---|
| Chemical Compounds | % | Chemical Compounds | % | Chemical Compounds | % | Chemical Compounds | % |
| α-Thujone | 38.8 | Trans-Thujanol | 36.1 | ß-Pinene | 58.3 | α-Pinene | 44.7 |
| Camphor | 28.1 | α-Thujene | 12.4 | Myrcene | 26.6 | δ-3-Carene | 22.8 |
| α-Pinene | 6.2 | Linalool | 10.3 | Cuminal | 6.8 | Limonene | 6.4 |
| β-Thujone | 5.2 | Terpinen-4-ol | 14.1 | ||||
| Camphene | 3.2 | Cis-Thujanol | 5.9 | ||||
| Treatment | Dose per 200 L of Water | F. culmorum | F. graminearum | F. fujikuori | F. avenaceum | |
|---|---|---|---|---|---|---|
| No. | Name | Surface of the Mycelium (mm) | ||||
| 1 | control | - | 90.0 a | 90.0 a | 90.0 a | 90.0 a |
| 2 | sage | 1% | 10.3 d | 0.0 d | 26.3 d | 10.8 d |
| 3 | sage | 2% | 7.0 de | 0.0 d | 7.8 e | 4.0 fg |
| 4 | thyme | 1% | 0.0 e | 0.0 d | 0.0 f | 0.0 g |
| 5 | thyme | 2% | 0.0 e | 0.0 d | 0.0 f | 0.0 g |
| 6 | cumin | 1% | 1.8 e | 0.0 d | 2.8 f | 0.0 g |
| 7 | cumin | 2% | 4.3 de | 0.0 d | 0.0 f | 0.0 g |
| 8 | cypress | 1% | 69.3 b | 69.8 b | 76.8 b | 81.3 b |
| 9 | cypress | 2% | 40.0 c | 34.3 c | 55.3 c | 51.0 c |
| 10 | prothioconazole | 0.33 l | 7.8 de | 8.3 d | 6.3 e | 8.3 de |
| 11 | prothioconazole | 0.65 l | 0.0 e | 6.0 d | 0.0 f | 6.0 ef |
| HSD (0.05) | 5.64 | 6.90 | 3.09 | 3.37 | ||
| Treatment | Dose per 200 mL of Water or 100 kg of Grain (mL) | Winter Wheat | Maize | |||
|---|---|---|---|---|---|---|
| No. | Name | Germination Energy (%) | Germination Capacity (%) | Germination Energy (%) | Germination Capacity (%) | |
| 1 | control | - | 92.0 a | 96.0 a | 94.0 a | 94.0 a |
| 2 | sage | 1 | 91.0 a | 93.0 a | 95.0 a | 97.0 a |
| 3 | sage | 2 | 96.0 a | 96.0 a | 94.0 a | 96.0 a |
| 4 | thyme | 1 | 67.0 b | 78.0 b | 94.0 a | 94.0 a |
| 5 | thyme | 2 | 47.0 c | 72.0 b | 64.0 b | 68.0 b |
| 6 | cumin | 1 | 96.0 a | 96.0 a | 94.0 a | 94.0 a |
| 7 | cumin | 2 | 94.0 a | 94.0 a | 98.0 a | 98.0 a |
| 8 | cypress | 1 | 95.0 a | 95.0 a | 97.0 a | 97.0 a |
| 9 | cypress | 2 | 94.0 a | 96.0 a | 95.0 a | 97.0 a |
| 10 | prothioconazole | 100 | 98.0 a | 98.0 a | 97.0 a | 98.0 a |
| 11 | prothioconazole | 200 | 99.0 a | 99.0 a | 96.0 a | 96.0 a |
| HSD (0.05) | 8.49 | 8.75 | 8.95 | 10.25 | ||
| SD | 5.88 | 6.06 | 6.20 | 7.10 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danielewicz, J.; Grzanka, M.; Sobiech, Ł.; Jajor, E.; Horoszkiewicz, J.; Korbas, M.; Blecharczyk, A.; Stuper-Szablewska, K.; Matysiak, K. Impact of Various Essential Oils on the Development of Pathogens of the Fusarium Genus and on Health and Germination Parameters of Winter Wheat and Maize. Molecules 2024, 29, 2376. https://doi.org/10.3390/molecules29102376
Danielewicz J, Grzanka M, Sobiech Ł, Jajor E, Horoszkiewicz J, Korbas M, Blecharczyk A, Stuper-Szablewska K, Matysiak K. Impact of Various Essential Oils on the Development of Pathogens of the Fusarium Genus and on Health and Germination Parameters of Winter Wheat and Maize. Molecules. 2024; 29(10):2376. https://doi.org/10.3390/molecules29102376
Chicago/Turabian StyleDanielewicz, Jakub, Monika Grzanka, Łukasz Sobiech, Ewa Jajor, Joanna Horoszkiewicz, Marek Korbas, Andrzej Blecharczyk, Kinga Stuper-Szablewska, and Kinga Matysiak. 2024. "Impact of Various Essential Oils on the Development of Pathogens of the Fusarium Genus and on Health and Germination Parameters of Winter Wheat and Maize" Molecules 29, no. 10: 2376. https://doi.org/10.3390/molecules29102376
APA StyleDanielewicz, J., Grzanka, M., Sobiech, Ł., Jajor, E., Horoszkiewicz, J., Korbas, M., Blecharczyk, A., Stuper-Szablewska, K., & Matysiak, K. (2024). Impact of Various Essential Oils on the Development of Pathogens of the Fusarium Genus and on Health and Germination Parameters of Winter Wheat and Maize. Molecules, 29(10), 2376. https://doi.org/10.3390/molecules29102376







