Catalase Detection via Membrane-Based Pressure Sensors
Abstract
1. Introduction
2. Results and Discussion
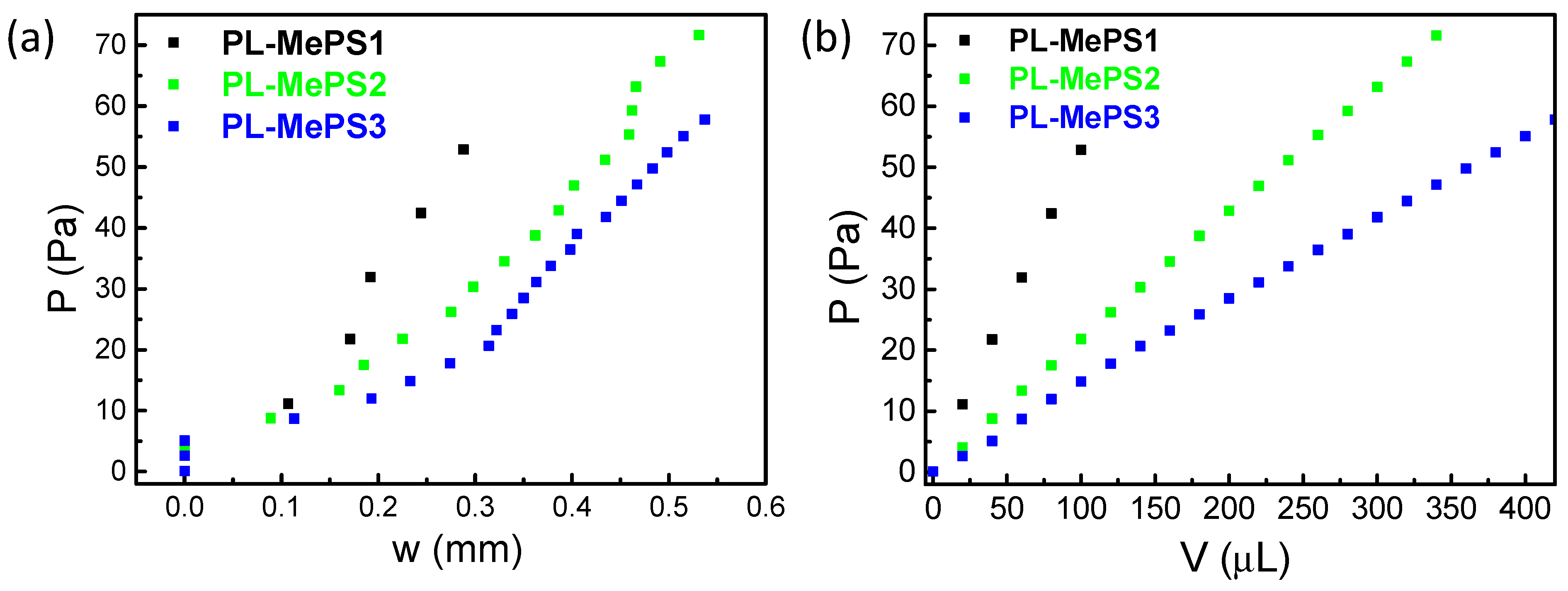
2.1. Bulge Tests Theory
2.2. Plasma-Induced Effects on Membranes
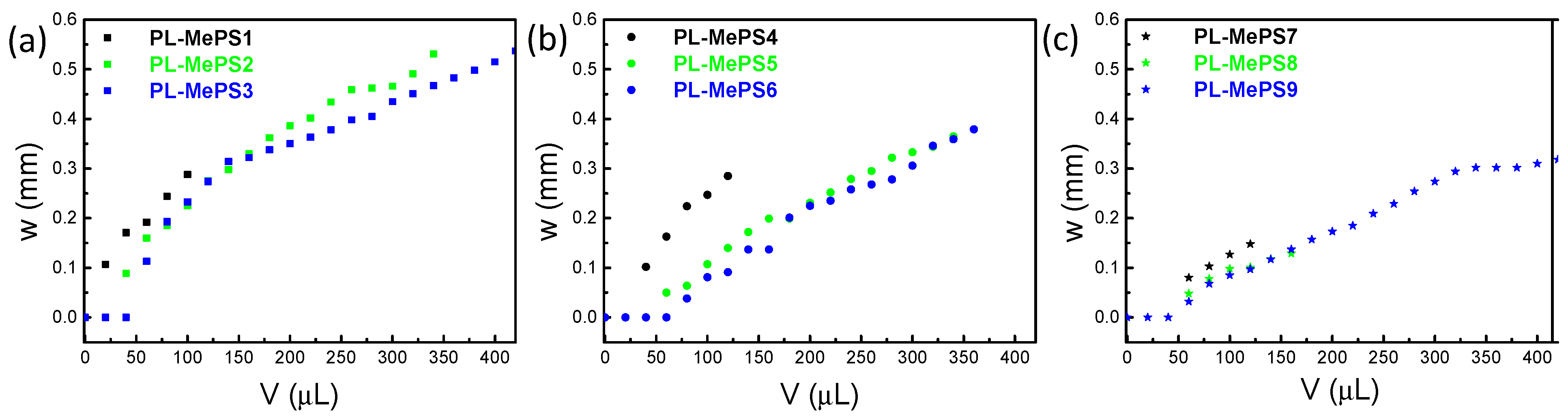
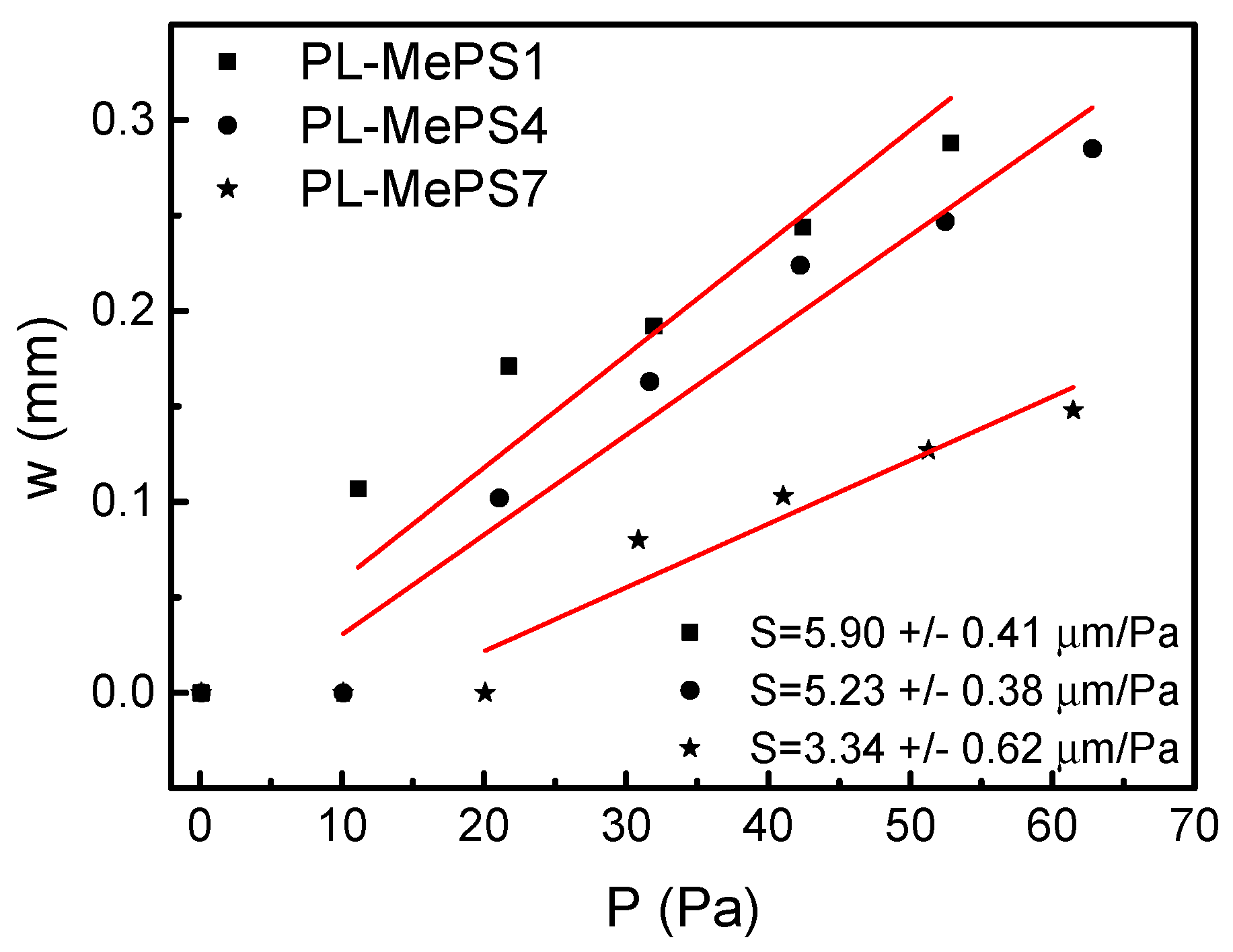
2.3. MePS Calibrations
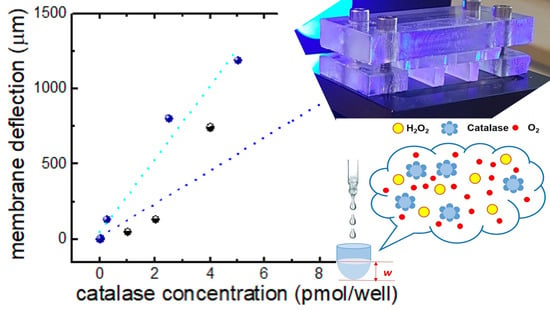
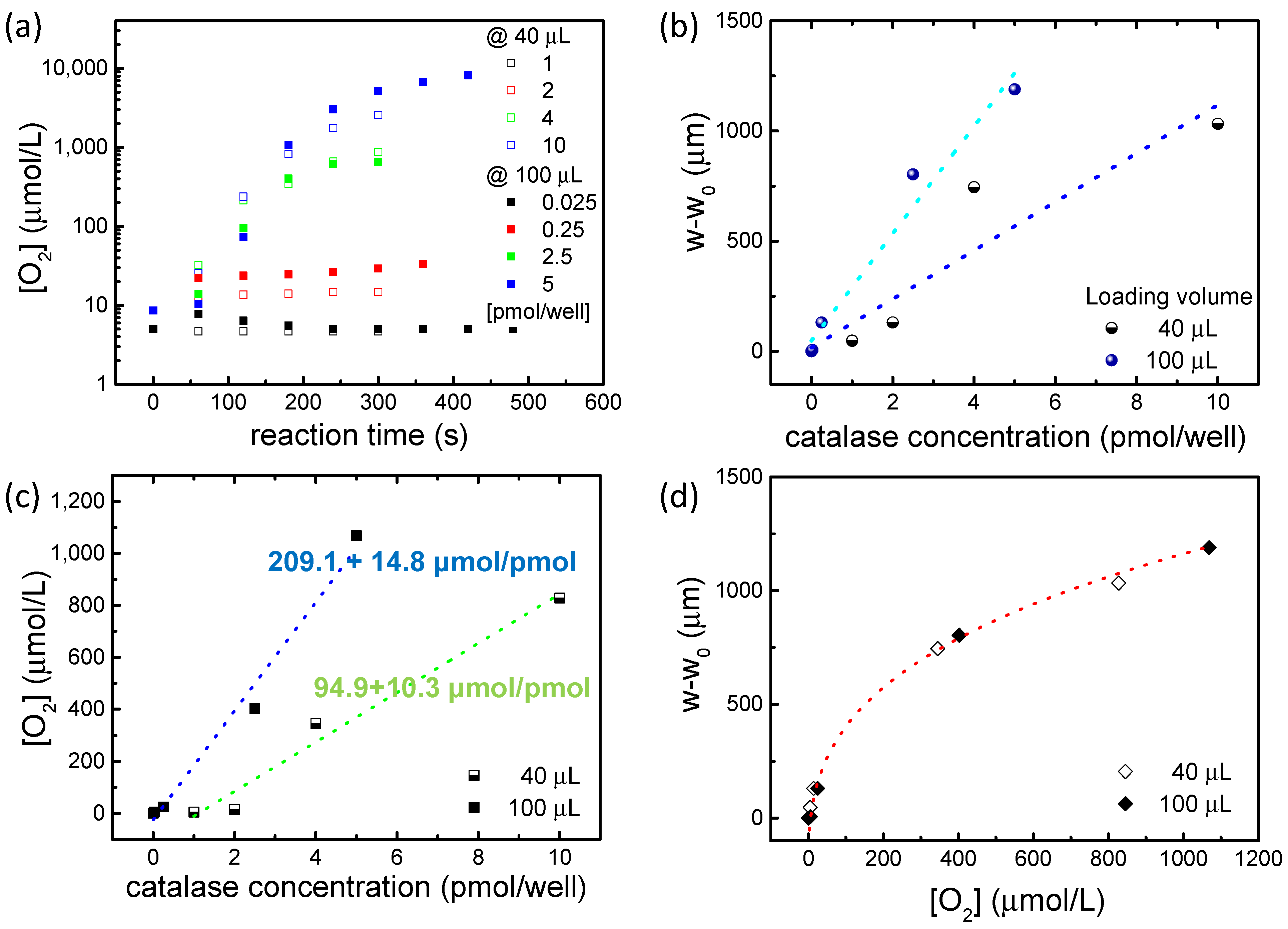
2.4. Catalase Studies
3. Materials and Methods
3.1. Fabrication of MePS Components
3.2. MePS Assembly
3.3. Bulge Test
3.4. Catalase Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, D.; Chesler, N.C.; Beebe, D.J. A Method for Dynamic System Characterization Using Hydraulic Series Resistance. Lab Chip 2006, 6, 639–644. [Google Scholar] [CrossRef]
- Shen, F.; Ai, M.; Li, Z.; Lu, X.; Pang, Y.; Liu, Z. Pressure Measurement Methods in Microchannels: Advances and Applications. Microfluid. Nanofluid. 2021, 25, 39. [Google Scholar] [CrossRef]
- Mao, Y.; Ji, B.; Chen, G.; Hao, C.; Zhou, B.; Tian, Y. Robust and Wearable Pressure Sensor Assembled from AgNW-Coated PDMS Micropillar Sheets with High Sensitivity and Wide Detection Range. ACS Appl. Nano Mater. 2019, 2, 3196–3205. [Google Scholar] [CrossRef]
- Lee, K.; Lee, S.S.; Lee, J.A.; Lee, K.-C.; Ji, S. Carbon Nanotube Film Piezoresistors Embedded in Polymer Membranes. Appl. Phys. Lett. 2010, 96, 013511. [Google Scholar] [CrossRef]
- Jian, M.; Xia, K.; Wang, Q.; Yin, Z.; Wang, H.; Wang, C.; Xie, H.; Zhang, M.; Zhang, Y. Flexible and Highly Sensitive Pressure Sensors Based on Bionic Hierarchical Structures. Adv. Funct. Mater. 2017, 27, 1606066. [Google Scholar] [CrossRef]
- Huang, J.; Li, D.; Zhao, M.; Ke, H.; Mensah, A.; Lv, P.; Tian, X.; Wei, Q. Flexible Electrically Conductive Biomass-Based Aerogels for Piezoresistive Pressure/Strain Sensors. Chem. Eng. J. 2019, 373, 1357–1366. [Google Scholar] [CrossRef]
- Escudero, P.; Yeste, J.; Pascual-Izarra, C.; Villa, R.; Alvarez, M. Color Tunable Pressure Sensors Based on Polymer Nanostructured Membranes for Optofluidic Applications. Sci. Rep. 2019, 9, 3259. [Google Scholar] [CrossRef]
- Choi, J.H.; Shim, T.S. Real-Time Pressure Monitoring System for Microfluidic Devices Using Deformable Colloidal Crystal Membrane. Lab Chip 2019, 19, 3954–3961. [Google Scholar] [CrossRef]
- Chaudhury, A.R.; Pantazis, A.K.; Chronis, N. An Image Contrast-Based Pressure Sensor. Sens. Actuators A Phys. 2016, 245, 63–67. [Google Scholar] [CrossRef]
- Wang, X.; Yang, B.; Liu, J.; Zhu, Y.; Yang, C.; He, Q. A Flexible Triboelectric-Piezoelectric Hybrid Nanogenerator Based on P(VDF-TrFE) Nanofibers and PDMS/MWCNT for Wearable Devices. Sci. Rep. 2016, 6, 36409. [Google Scholar] [CrossRef]
- Zizzari, A.; Bianco, M.; Miglietta, R.; Del Mercato, L.L.; Carraro, M.; Sorarù, A.; Bonchio, M.; Gigli, G.; Rinaldi, R.; Viola, I.; et al. Catalytic Oxygen Production Mediated by Smart Capsules to Modulate Elastic Turbulence under a Laminar Flow Regime. Lab Chip 2014, 14, 4391–4397. [Google Scholar] [CrossRef] [PubMed]
- Ariati, R.; Sales, F.; Souza, A.; Lima, R.A.; Ribeiro, J. Polydimethylsiloxane Composites Characterization and Its Applications: A Review. Polymers 2021, 13, 4258. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhang, Y.S.; Santiago, G.T.-D.; Alvarez, M.M.; Ribas, J.; Jonas, S.J.; Weiss, P.S.; Andrews, A.M.; Aizenberg, J.; Khademhosseini, A. Interplay between Materials and Microfluidics. Nat. Rev. Mater. 2017, 2, 17016. [Google Scholar] [CrossRef]
- Niu, X.; Peng, S.; Liu, L.; Wen, W.; Sheng, P. Characterizing and Patterning of PDMS-Based Conducting Composites. Adv. Mater. 2007, 19, 2682–2686. [Google Scholar] [CrossRef]
- Sollier, E.; Murray, C.; Maoddi, P.; Di Carlo, D. Rapid Prototyping Polymers for Microfluidic Devices and High Pressure Injections. Lab Chip 2011, 11, 3752–3765. [Google Scholar] [CrossRef] [PubMed]
- Bianco, M.; Zizzari, A.; Priore, P.; Moroni, L.; Metrangolo, P.; Frigione, M.; Rella, R.; Gaballo, A.; Arima, V. Lab-on-a-Brane for Spheroid Formation. Biofabrication 2019, 11, 021002. [Google Scholar] [CrossRef] [PubMed]
- Karrock, T.; Gerken, M. Pressure Sensor Based on Flexible Photonic Crystal Membrane. Biomed. Opt. Express 2015, 6, 4901–4911. [Google Scholar] [CrossRef] [PubMed]
- Zizzari, A.; Bianco, M.; Del Mercato, L.L.; Sorarù, A.; Carraro, M.; Pellegrino, P.; Perrone, E.; Monteduro, A.G.; Bonchio, M.; Rinaldi, R.; et al. Highly Sensitive Membrane-Based Pressure Sensors (MePS) for Real-Time Monitoring of Catalytic Reactions. Anal. Chem. 2018, 90, 7659–7665. [Google Scholar] [CrossRef] [PubMed]
- Zizzari, A.; Bianco, M.; del Mercato, L.L.; Carraro, M.; Bonchio, M.; Frigione, M.; Montagna, F.; Gigli, G.; Viola, I.; Arima, V. Self-Powered Catalytic Microfluidic Platforms for Fluid Delivery. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 257–262. [Google Scholar] [CrossRef]
- Zizzari, A.; Cesaria, M.; Bianco, M.; del Mercato, L.L.; Carraro, M.; Bonchio, M.; Rella, R.; Arima, V. Mixing Enhancement Induced by Viscoelastic Micromotors in Microfluidic Platforms. Chem. Eng. J. 2020, 391, 123572. [Google Scholar] [CrossRef]
- Tao, Z.; Raffel, R.A.; Souid, A.-K.; Goodisman, J. Kinetic Studies on Enzyme-Catalyzed Reactions: Oxidation of Glucose, Decomposition of Hydrogen Peroxide and Their Combination. Biophys. J. 2009, 96, 2977–2988. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Guan, Z.; Jia, S.; Lei, Z.; Lin, S.; Zhang, H.; Ma, Y.; Tian, Z.-Q.; Yang, C.J. Au@pt Nanoparticle Encapsulated Target-Responsive Hydrogel with Volumetric Bar-Chart Chip Readout for Quantitative Point-of-Care Testing. Angew. Chem. Int. Ed. 2014, 53, 12503–12507. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Tice, J.D.; Ismagilov, R.F. A Microfluidic System for Controlling Reaction Networks in Time. Angew. Chem. Int. Ed. 2003, 42, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.F.; Jia, S.; Ahmed, M.G.; Li, X.; Lin, L.; Chen, X.; Zhu, Z.; Yang, C. Visual Quantitative Detection of Circulating Tumor Cells with Single-Cell Sensitivity Using a Portable Microfluidic Device. Small 2019, 15, 1804890. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, Y.; Qi, W.; Li, Y.; Xuan, J.; Wang, P.; Qin, L. Integrative Volumetric Bar-Chart Chip for Rapid and Quantitative Point-of-Care Detection of Myocardial Infarction Biomarkers. Lab Chip 2016, 16, 2955–2962. [Google Scholar] [CrossRef]
- Ma, Y.; Mao, Y.; Huang, D.; He, Z.; Yan, J.; Tian, T.; Shi, Y.; Song, Y.; Li, X.; Zhu, Z.; et al. Portable Visual Quantitative Detection of Aflatoxin B 1 Using a Target-Responsive Hydrogel and a Distance-Readout Microfluidic Chip. Lab Chip 2016, 16, 3097–3104. [Google Scholar] [CrossRef]
- Liu, R.; Huang, Y.; Ma, Y.; Jia, S.; Gao, M.; Li, J.; Zhang, H.; Xu, D.; Wu, M.; Chen, Y.; et al. Design and Synthesis of Target-Responsive Aptamer-Cross-Linked Hydrogel for Visual Quantitative Detection of Ochratoxin A. ACS Appl. Mater. Interfaces 2015, 7, 6982–6990. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, Y.; Chen, Y.; Wu, X.; Fang, L.; Zhu, Z.; Yang, C.J. Target-Responsive DNAzyme Cross-Linked Hydrogel for Visual Quantitative Detection of Lead. Anal. Chem. 2014, 86, 11434–11439. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, G.; Qi, W.; Li, Y.; Song, Y. A Versatile Quantitation Platform Based on Platinum Nanoparticles Incorporated Volumetric Bar-Chart Chip for Highly Sensitive Assays. Biosens. Bioelectron. 2016, 85, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xia, X.; Wu, X.; Wang, P.; Qin, L. Integration of Platinum Nanoparticles with a Volumetric Bar-Chart Chip for Biomarker Assays. Angew. Chem. Int. Ed. 2014, 53, 12451–12455. [Google Scholar] [CrossRef]
- Galasso, M.; Gambino, S.; Romanelli, M.G.; Donadelli, M.; Scupoli, M.T. Browsing the Oldest Antioxidant Enzyme: Catalase and Its Multiple Regulation in Cancer. Free Radic. Biol. Med. 2021, 172, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Heit, C.; Marshall, S.; Singh, S.; Yu, X.; Charkoftaki, G.; Zhao, H.; Orlicky, D.J.; Fritz, K.S.; Thompson, D.C.; Vasiliou, V. Catalase Deletion Promotes Prediabetic Phenotype in Mice. Free Radic. Biol. Med. 2017, 103, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, C.; Zamocky, M.; Sandoval, J.M.; Verrax, J.; Calderon, P.B. Regulation of Catalase Expression in Healthy and Cancerous Cells. Free Radic. Biol. Med. 2015, 87, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Peña-Oyarzun, D.; Bravo-Sagua, R.; Diaz-Vega, A.; Aleman, L.; Chiong, M.; Garcia, L.; Bambs, C.; Troncoso, R.; Cifuentes, M.; Morselli, E.; et al. Autophagy and Oxidative Stress in Non-Communicable Diseases: A Matter of the Inflammatory State? Free Radic. Biol. Med. 2018, 124, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Yan, L.-J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- And Age-Associated Degenerative Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Wu, H.; Niu, G.; Ren, W.; Jiang, L.; Zhao, J.; Quan, Y.; Ren, M.X.; Yu, W.; Zhang, Y.; Cao, X.; et al. Highly sensitive label-free detection of analytes at different scales using uniform graphene-nanopyramids hybrid SERS system. Sens. Actuators B Chem. 2022, 354, 131205. [Google Scholar] [CrossRef]
- de Abreu Franchini, R.; Costa Matos, M.; Matos, R.C. Amperometric Determination of Catalase in Brazilian Commercial Honeys. Anal. Lett. 2011, 44, 232–240. [Google Scholar] [CrossRef]
- Leng, Y.; Cheng, J.; Liu, C.; Wang, D.; Lu, Z.; Ma, C.; Zhang, M.; Dong, Y.; Xing, X.; Yao, L.; et al. A rapid reduction of Au (I→0) strategy for the colorimetric detection and discrimination of proteins. Mikrochim. Acta 2021, 188, 249. [Google Scholar] [CrossRef]
- Rim, J.; Jang, C.H. Detection of catalase activity with aldehyde-doped liquid crystals confined in microcapillaries. Anal. Biochem. 2018, 560, 19–23. [Google Scholar] [CrossRef]
- Lu, S.; Guo, Y.; Qi, L.; Hu, Q.; Yu, L. Highly Sensitive and Label-Free Detection of Catalase by a H2O2-Responsive Liquid Crystal Sensing Platform. Sens. Actuators B Chem. 2021, 344, 130279. [Google Scholar] [CrossRef]
- Béfahy, S.; Lipnik, P.; Pardoen, T.; Nascimento, C.; Patris, B.; Bertrand, P.; Yunus, S. Thickness and Elastic Modulus of Plasma Treated PDMS Silica-like Surface Layer. Langmuir 2010, 26, 3372–3375. [Google Scholar] [CrossRef] [PubMed]
- Bowden, N.; Huck, W.T.S.; Paul, K.E.; Whitesides, G.M. The Controlled Formation of Ordered, Sinusoidal Structures by Plasma Oxidation of an Elastomeric Polymer. Appl. Phys. Lett. 1999, 75, 2557–2559. [Google Scholar] [CrossRef]
- Arima, V.; Bianco, M.; Zacheo, A.; Zizzari, A.; Perrone, E.; Marra, L.; Rinaldi, R. Fluoropolymers Coatings on Polydimethylsiloxane for Retarding Swelling in Toluene. Thin Solid Films 2012, 520, 2293–2300. [Google Scholar] [CrossRef]
- Thangawng, A.L.; Ruoff, R.S.; Swartz, M.A.; Glucksberg, M.R. An Ultra-Thin PDMS Membrane as a Bio/Micro-Nano Interface: Fabrication and Characterization. Biomed. Microdevices 2007, 9, 587–595. [Google Scholar] [CrossRef]
- Tan, J.L.; Tien, J.; Pirone, D.M.; Gray, D.S.; Bhadriraju, K.; Chen, C.S. Cells Lying on a Bed of Microneedles: An Approach to Isolate Mechanical Force. Proc. Natl. Acad. Sci. USA 2003, 100, 1484–1489. [Google Scholar] [CrossRef] [PubMed]
- Brown, X.Q.; Ookawa, K.; Wong, J.Y. Evaluation of Polydimethylsiloxane Scaffolds with Physiologically-Relevant Elastic Moduli: Interplay of Substrate Mechanics and Surface Chemistry Effects on Vascular Smooth Muscle Cell Response. Biomaterials 2005, 26, 3123–3129. [Google Scholar] [CrossRef]
- Armani, D.; Liu, C.; Aluru, N. Re-Configurable Fluid Circuits by PDMS Elastomer Micromachining. In Proceedings of the IEEE Micro Electro Mechanical Systems (MEMS), Orlando, FL, USA, 21 January 1999; pp. 222–227. [Google Scholar]
- Gray, D.S.; Tien, J.; Chen, C.S. Repositioning of Cells by Mechanotaxis on Surfaces with Micropatterned Young’s Modulus. J. Biomed. Mater. Res. A 2003, 66, 605–614. [Google Scholar] [CrossRef]
- Loock, H.-P.; Wentzell, P.D. Detection Limits of Chemical Sensors: Applications and Misapplications. Sens. Actuators B Chem. 2012, 173, 157–163. [Google Scholar] [CrossRef]
- Jiang, C.; Markutsya, S.; Pikus, Y.; Tsukruk, V.V. Freely Suspended Nanocomposite Membranes as Highly Sensitive Sensors. Nat. Mater. 2004, 3, 721–728. [Google Scholar] [CrossRef]
- Chueh, B.-H.; Huh, D.; Kyrtsos, C.R.; Houssin, T.; Futai, N.; Takayama, S. Leakage-Free Bonding of Porous Membranes into Layered Microfluidic Array Systems. Anal. Chem. 2007, 79, 3504–3508. [Google Scholar] [CrossRef]
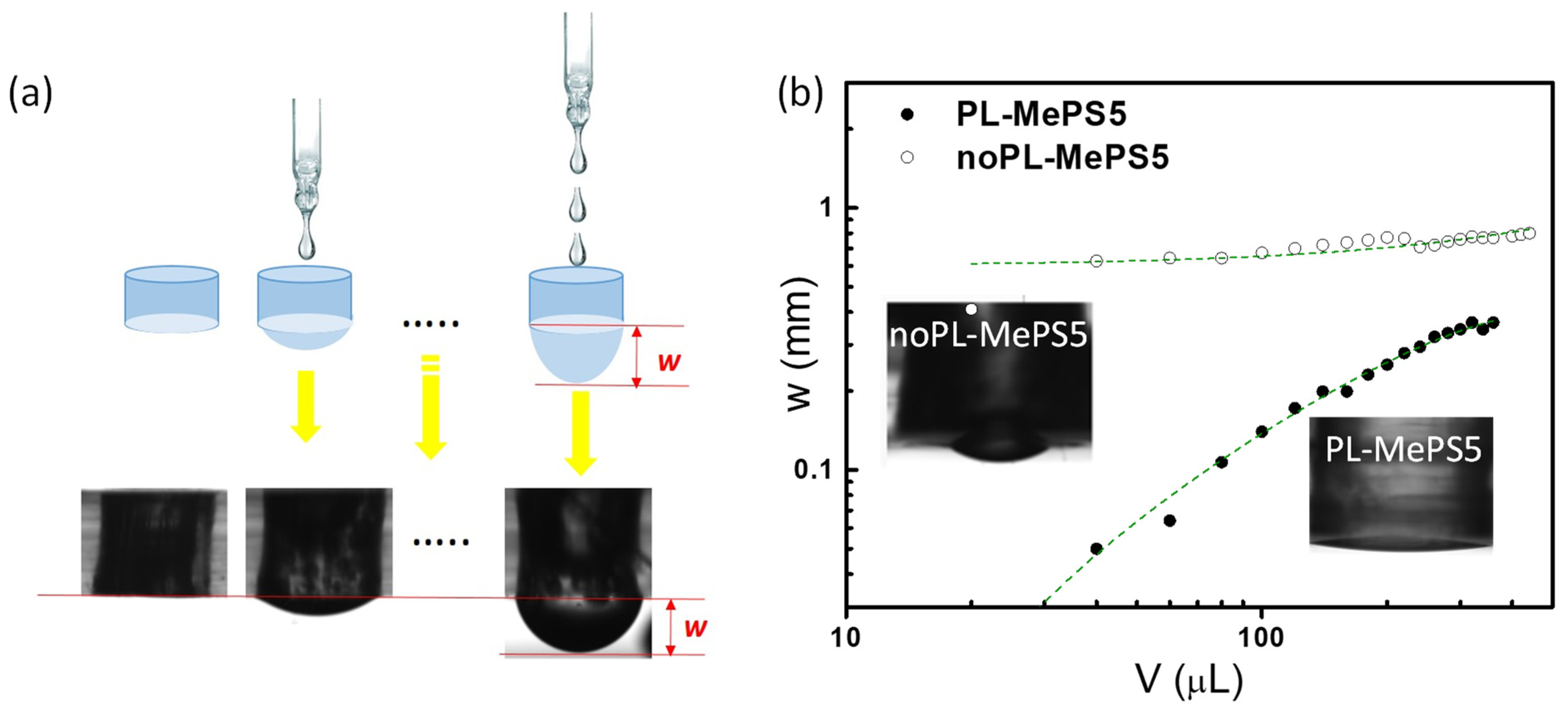

| MePS Chips | 2r (mm) | d (µm) | Vin (µL) |
|---|---|---|---|
| PL-MePS1 | 5 | 2 | 120 |
| noPL-MePS1 | 5 | 2 | 120 |
| PL-MePS2 | 8 | 2 | 340 |
| PL-MePS3 | 10 | 2 | 500 |
| noPL-MePS3 | 10 | 2 | 500 |
| PL-MePS4 | 5 | 10 | 120 |
| PL-MePS5 | 8 | 10 | 340 |
| noPL-MePS5 | 8 | 10 | 340 |
| PL-MePS6 | 10 | 10 | 500 |
| PL-MePS7 | 5 | 50 | 120 |
| PL-MePS8 | 8 | 50 | 340 |
| PL-MePS9 | 10 | 50 | 500 |
| d (µm) | 2r = 5 mm | 2r = 8 mm | 2r = 10 mm |
|---|---|---|---|
| 2 | PL-MePS1 | PL-MePS2 | PL-MePS3 |
| E = 1.75 ± 0.90 Mpa σ0 = 0.094 ± 0.003 MPa Σ = 0.525 ± 0.003 Pa/µL | E = 3.28 ± 0.46 MPa σ0 = 0.220 ± 0.055 MPa Σ = 0.2090 ± 0.0008 Pa/µL | E = 5.32 ± 0.92 MPa σ0 = 0.310 ± 0.094 MPa Σ = 0.1370 ± 0.0008 Pa/µL | |
| 10 | PL-MePS4 | PL-MePS5 | PL-MePS6 |
| E = 1.51 ± 0.98 Mpa σ0 = 0.004 ± 0.002 MPa S = 0.525 ± 0.002 Pa/µL | E = 1.73 ± 0.67 Mpa σ0 = 0.051 ± 0.007 MPa S = 0.2070 ± 0.0003 Pa/µL | E = 2.11 ± 0.74 Mpa σ0 = 0.068 ± 0.008 MPa S = 0.1360 ± 0.0004 Pa/µL | |
| 50 | PL-MePS7 | PL-MePS8 | PL-MePS9 |
| E = 1.23 ± 0.78 Mpa σ0 = 0.003 ± 0.001 MPa S = 0.513 ± 0.002 Pa/µL | E = 1.43 ± 0.81 MPa σ0 = 0.074 ± 0.003 MPa S = 0.2060 ± 0.0008 Pa/µL | E = 1.59 ± 0.51 MPa σ0 = 0.011 ± 0.002 MPa S = 0.1340 ± 0.0002 Pa/µL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianco, M.; Zizzari, A.; Perrone, E.; Mangiullo, D.; Mazzeo, M.; Viola, I.; Arima, V. Catalase Detection via Membrane-Based Pressure Sensors. Molecules 2024, 29, 1506. https://doi.org/10.3390/molecules29071506
Bianco M, Zizzari A, Perrone E, Mangiullo D, Mazzeo M, Viola I, Arima V. Catalase Detection via Membrane-Based Pressure Sensors. Molecules. 2024; 29(7):1506. https://doi.org/10.3390/molecules29071506
Chicago/Turabian StyleBianco, Monica, Alessandra Zizzari, Elisabetta Perrone, Diego Mangiullo, Marco Mazzeo, Ilenia Viola, and Valentina Arima. 2024. "Catalase Detection via Membrane-Based Pressure Sensors" Molecules 29, no. 7: 1506. https://doi.org/10.3390/molecules29071506
APA StyleBianco, M., Zizzari, A., Perrone, E., Mangiullo, D., Mazzeo, M., Viola, I., & Arima, V. (2024). Catalase Detection via Membrane-Based Pressure Sensors. Molecules, 29(7), 1506. https://doi.org/10.3390/molecules29071506