Silver Nanoparticle-Embedded Hydrogels for Electrochemical Sensing of Sulfamethoxazole Residues in Meat
Abstract
1. Introduction
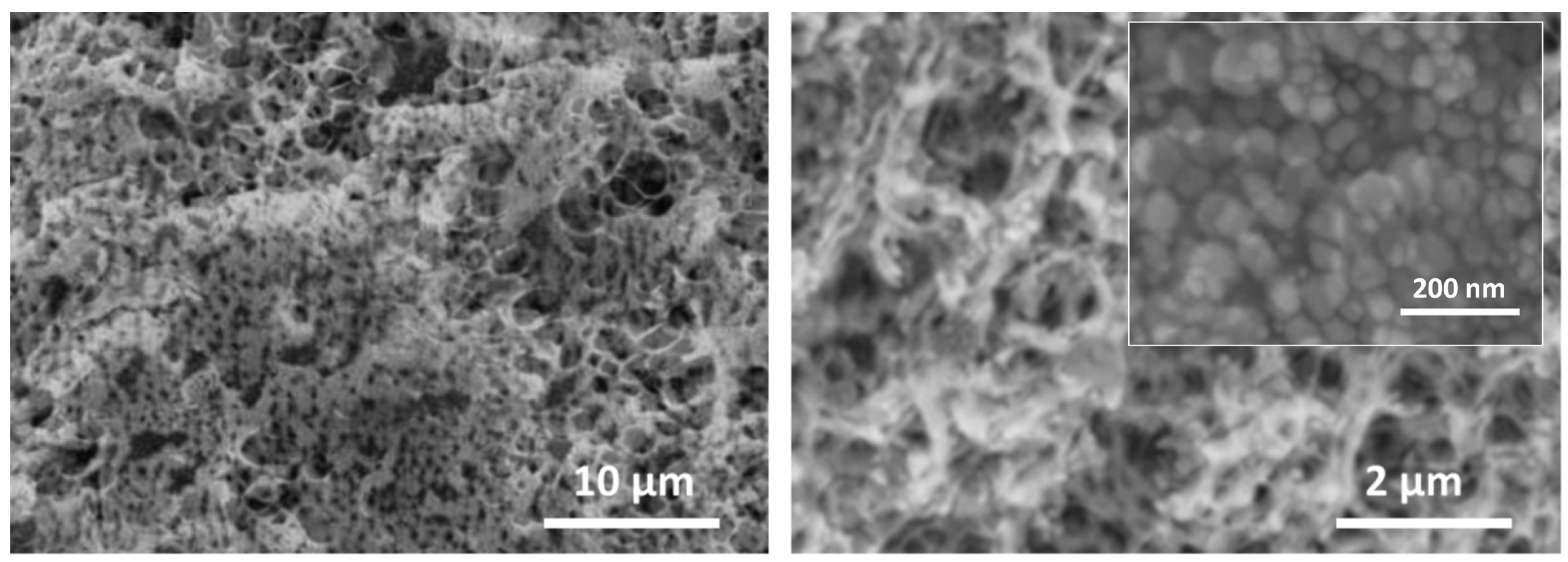
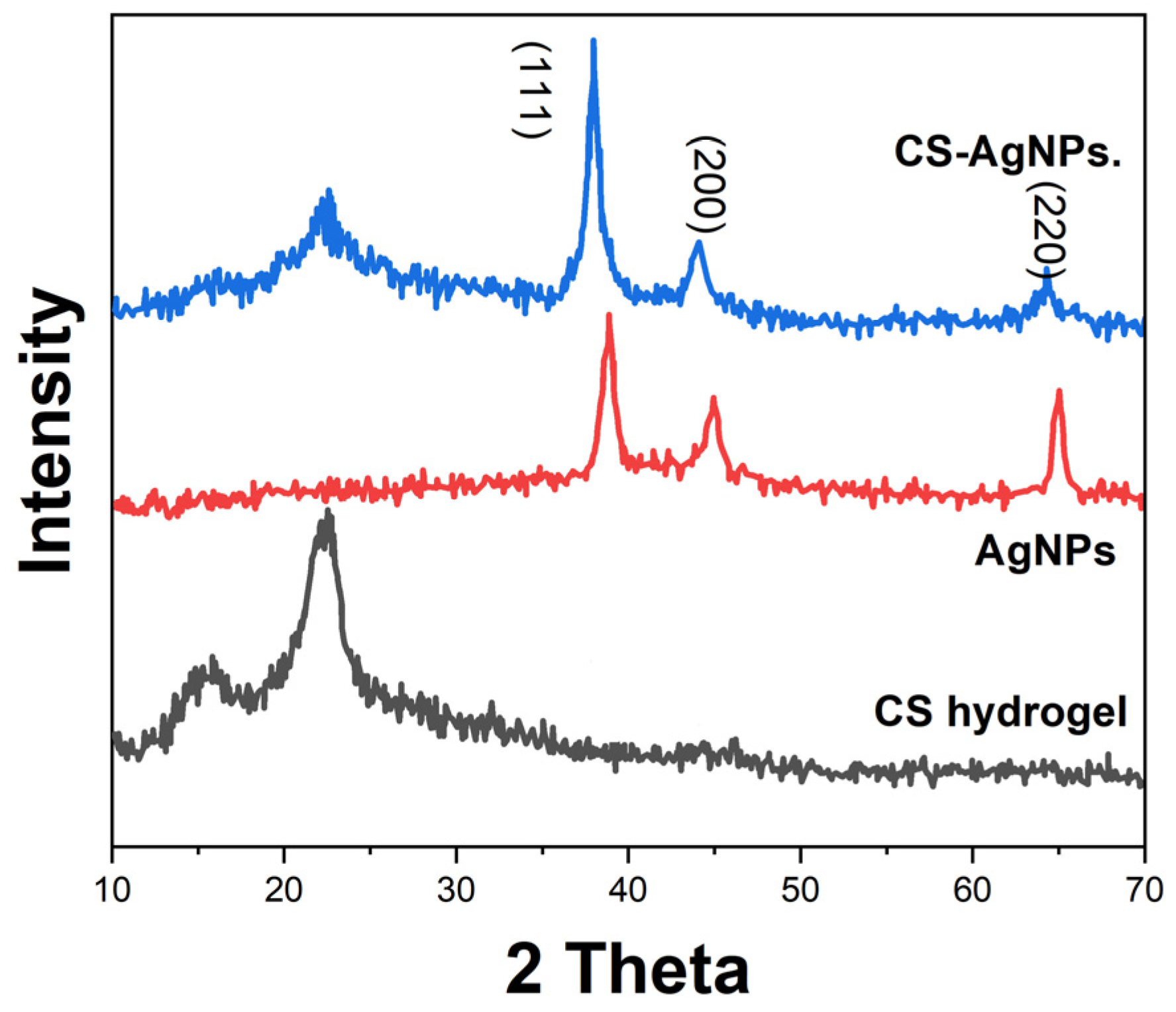
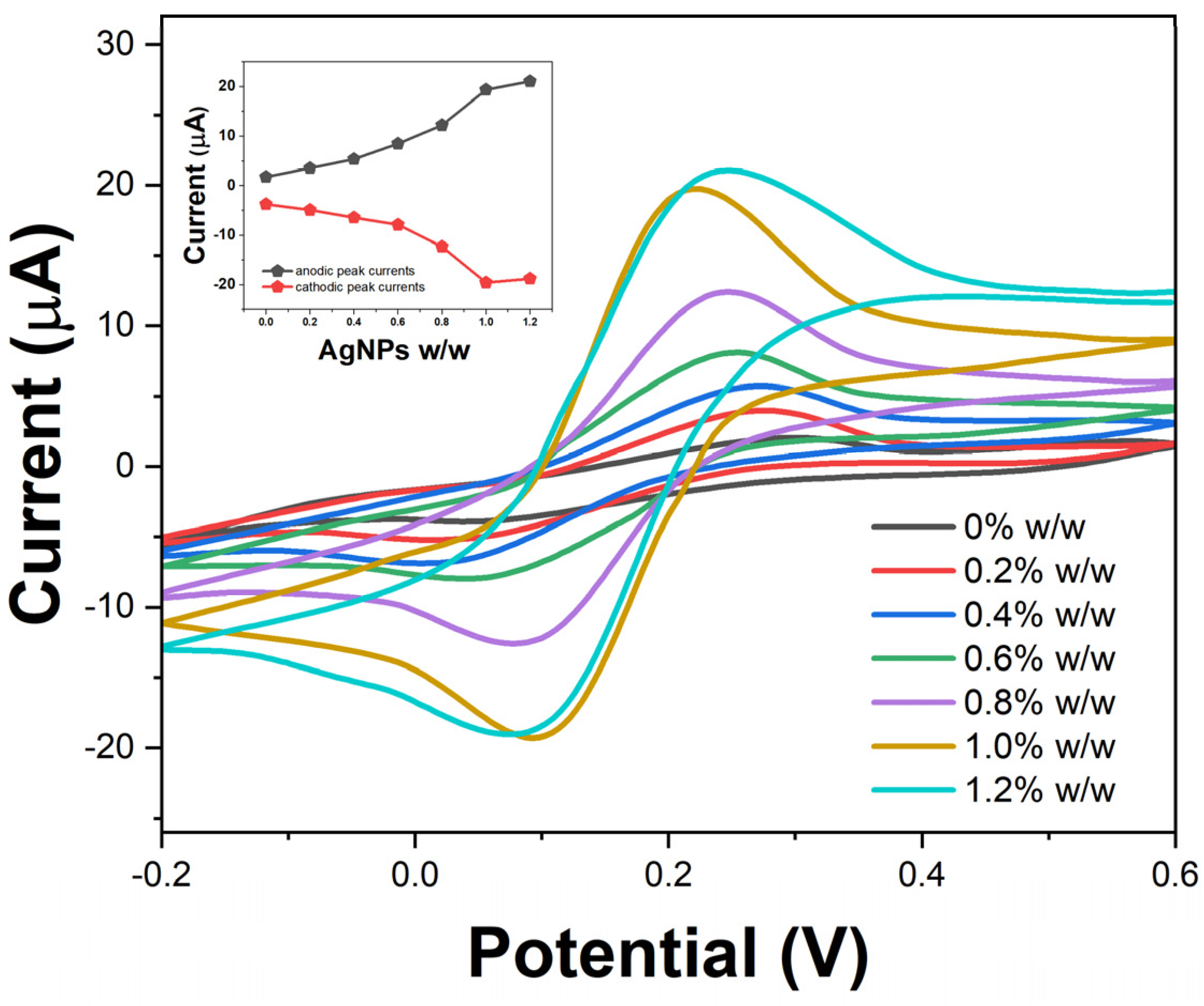
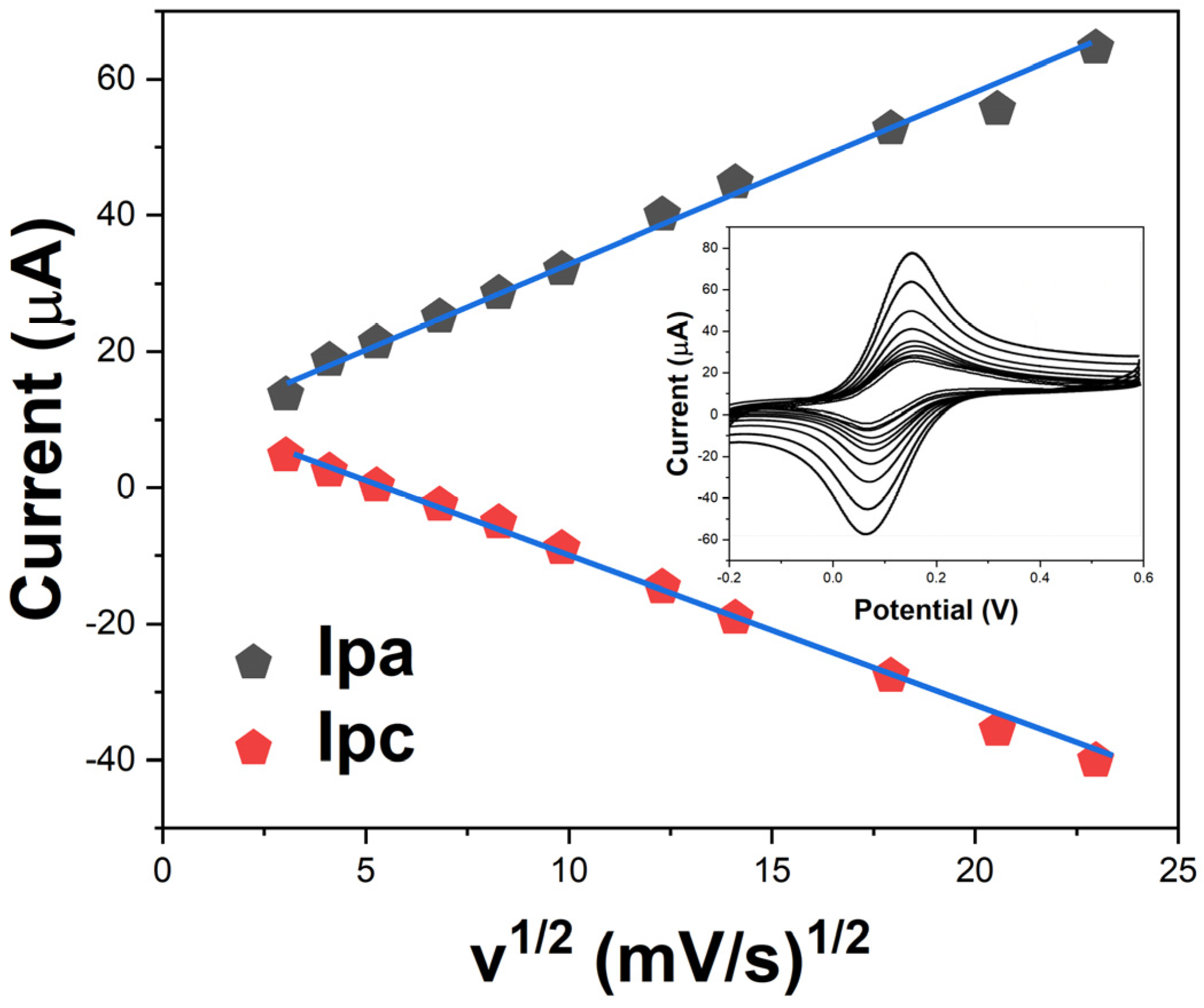
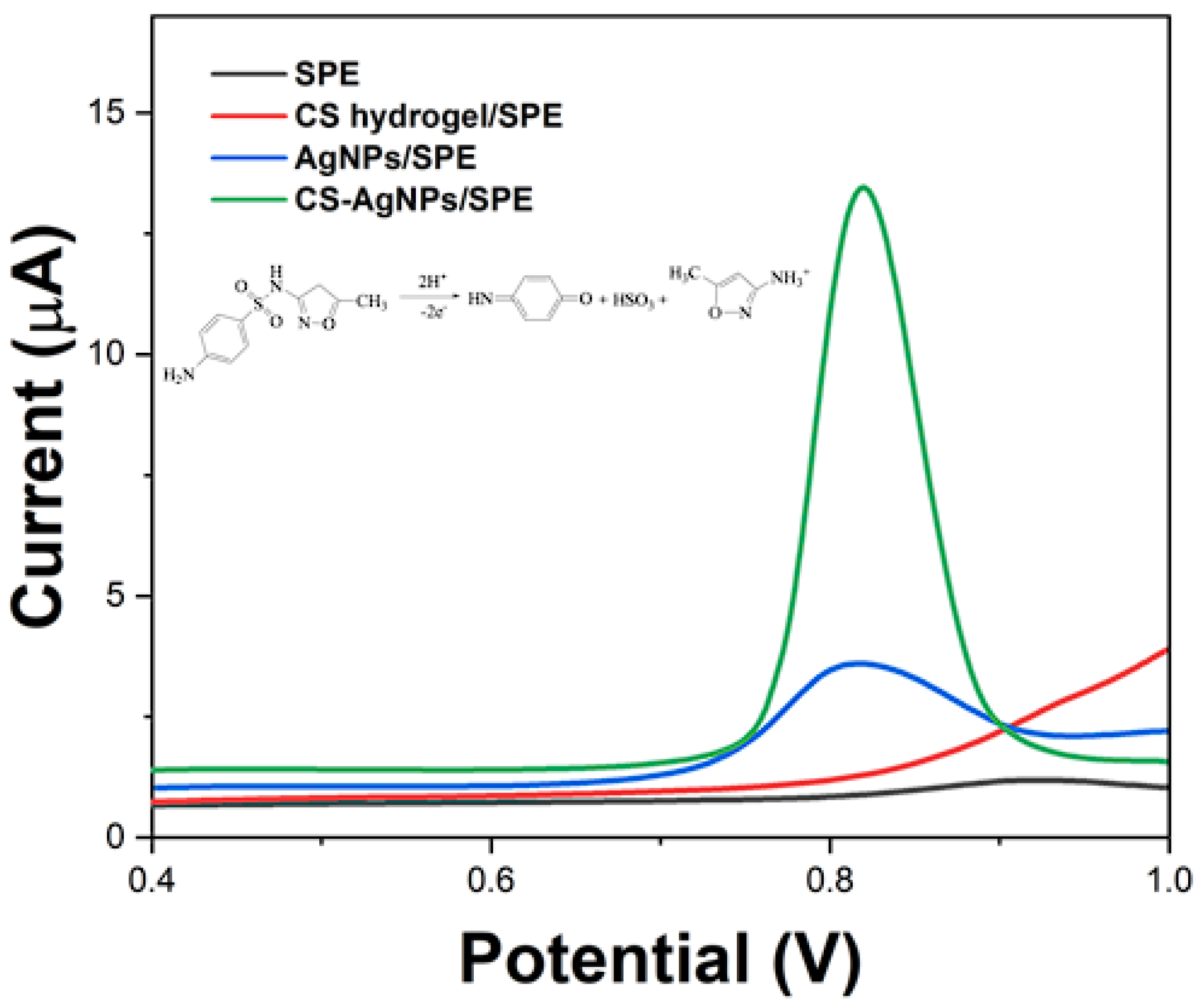
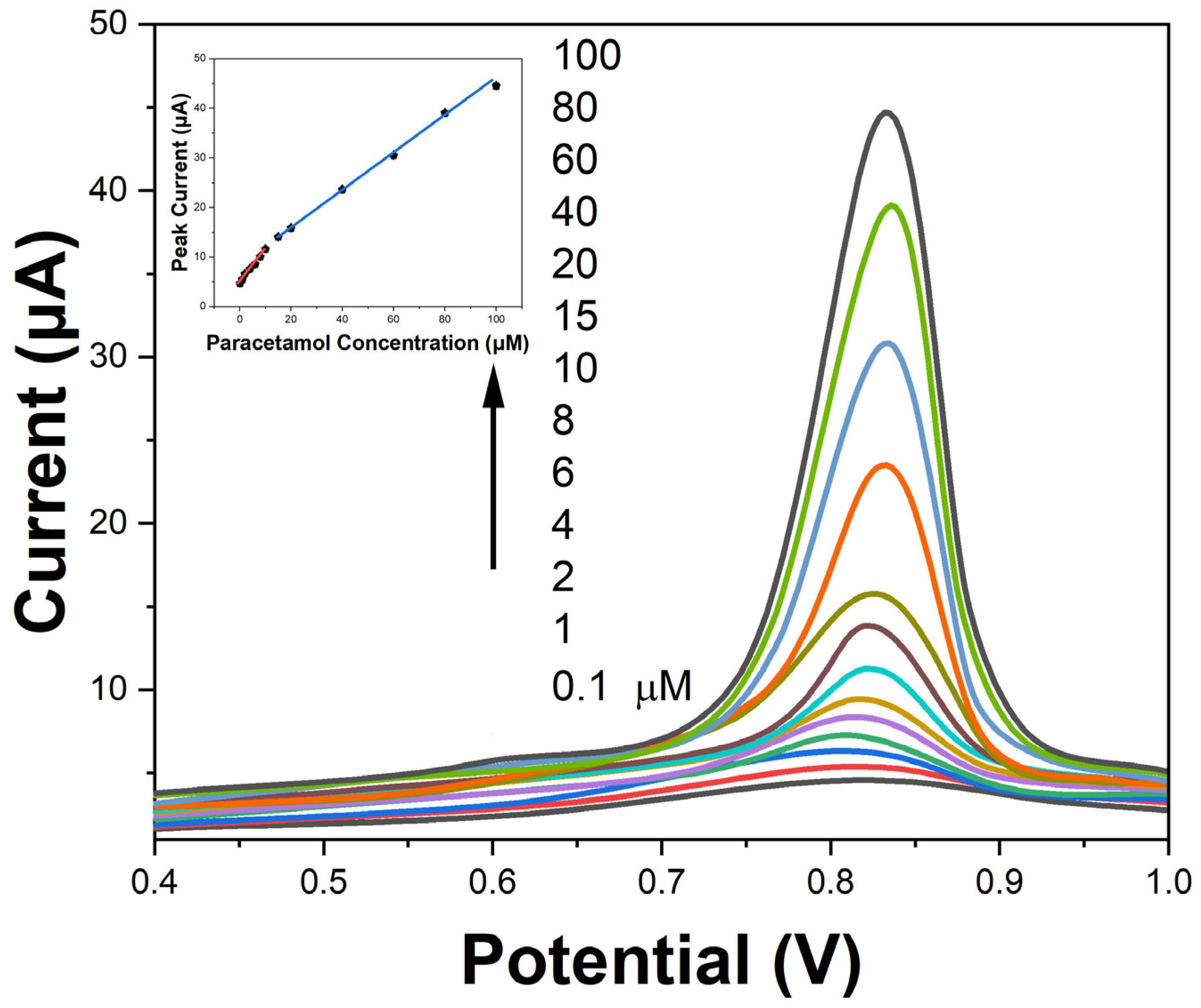
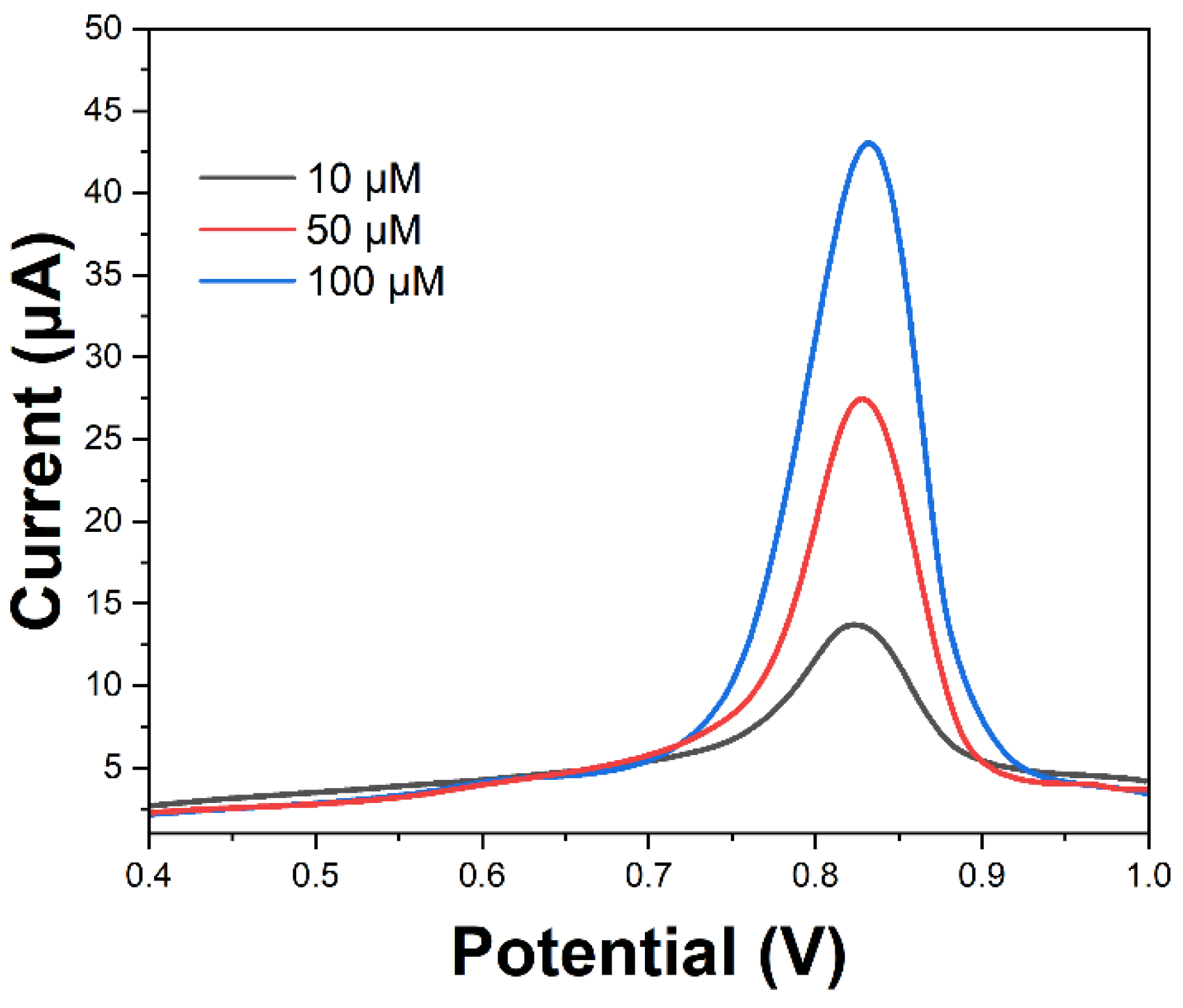
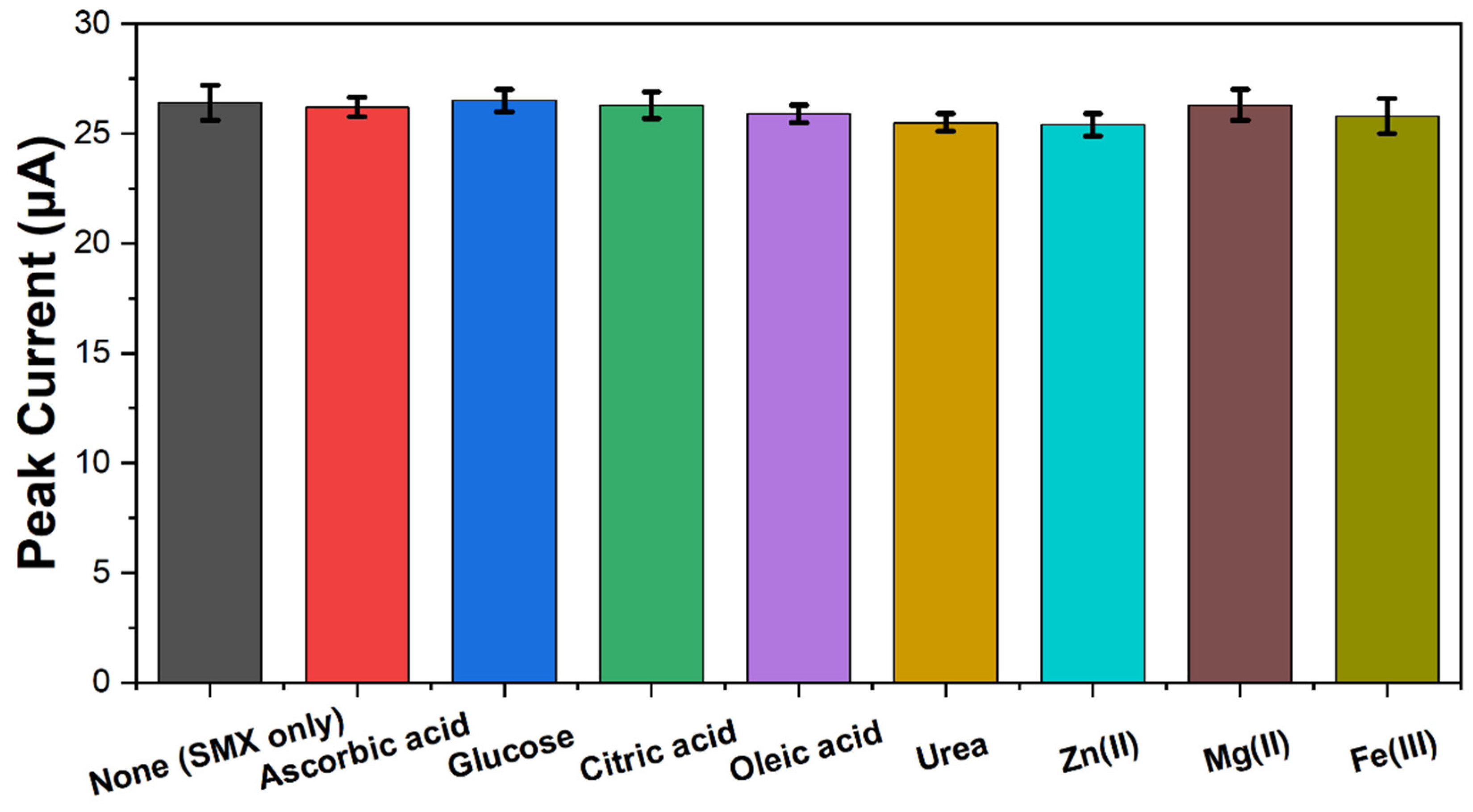
2. Results and Discussion
3. Experimental
3.1. Chemicals and Reagents
3.2. Synthesis of Silver Nanoparticles
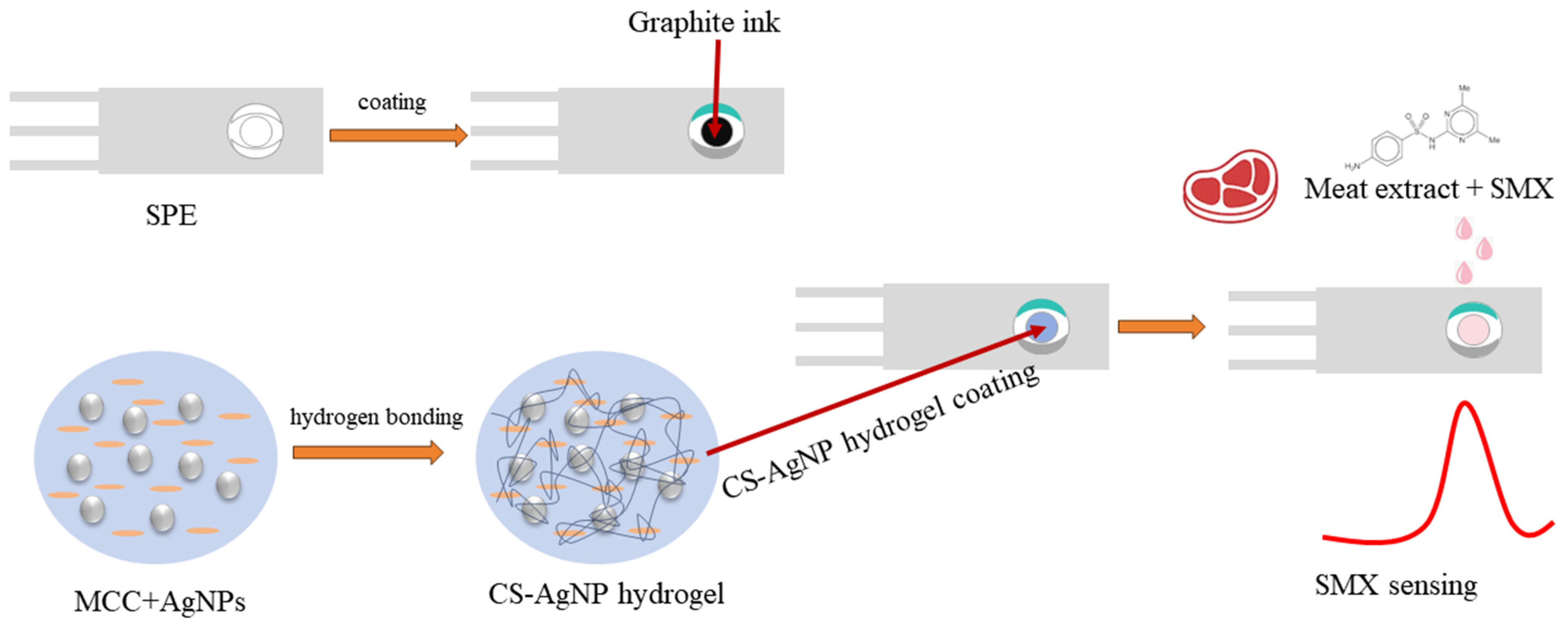
3.3. Preparation of Cellulose–Silver Nanoparticle (CS-AgNPs) Composite Hydrogel
3.4. Development of Disposable Electrochemical Sensors
3.5. Electrochemical Measurements
3.6. Preparation of Spiked Meat Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, B.; Xie, K.; Lee, K. Veterinary Drug Residues in Animal-Derived Foods: Sample Preparation and Analytical Methods. Foods 2021, 10, 555. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Q.; Chen, M.; Zhang, X.; Liu, P. Determination of Veterinary Drug Residues in Food of Animal Origin: Sample Preparation Methods and Analytical Techniques. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 701–724. [Google Scholar] [CrossRef]
- Mingle, C.L.; Darko, G.; Borquaye, L.S.; Asare-Donkor, N.K.; Woode, E.; Koranteng, F. Veterinary Drug Residues in Beef, Chicken, and Egg from Ghana. Chem. Afr. 2021, 4, 339–348. [Google Scholar] [CrossRef]
- Atta, A.H.; Atta, S.A.; Nasr, S.M.; Mouneir, S.M. Current Perspective on Veterinary Drug and Chemical Residues in Food of Animal Origin. Environ. Sci. Pollut. Res. 2022, 29, 15282–15302. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, C.; Xu, Z.; Chakraborty, A. A Coupled Method of On-Line Solid Phase Extraction with the UHPLC–MS/MS for Detection of Sulfonamides Antibiotics Residues in Aquaculture. Chemosphere 2020, 254, 126765. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-P.; Hou, P.-H.; Yang, W.-C.; Wu, C.-F.; Chang, C.-C.; Tsai, M.-Y.; Tsai, H.-P.; Lin, C.-T.; Xue, Y.-J.; Wang, J.-H.; et al. Analytical Detection of Sulfonamides and Organophosphorus Insecticide Residues in Fish in Taiwan. Molecules 2020, 25, 1501. [Google Scholar] [CrossRef]
- Fan, Y.; Huang, L.; Wu, L.; Zhang, C.; Zhu, S.; Xiao, X.; Li, M.; Zou, X. Adsorption of Sulfonamides on Biochars Derived from Waste Residues and Its Mechanism. J. Hazard. Mater. 2021, 406, 124291. [Google Scholar] [CrossRef]
- Chen, A.; Guo, H.; Luan, J.; Li, Y.; He, X.; Chen, L.; Zhang, Y. The Electrospun Polyacrylonitrile/Covalent Organic Framework Nanofibers for Efficient Enrichment of Trace Sulfonamides Residues in Food Samples. J. Chromatogr. A 2022, 1668, 462917. [Google Scholar] [CrossRef]
- Frey, L.; Tanunchai, B.; Glaser, B. Antibiotics Residues in Pig Slurry and Manure and Its Environmental Contamination Potential. A Meta-Analysis. Agron. Sustain. Dev. 2022, 42, 31. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, X.; Ding, S.; Chen, F.; Lv, Y.; Zhang, H.; Zhao, S. Recent Developments in the Electrochemical Determination of Sulfonamides. Curr. Pharm. Anal. 2022, 18, 4–13. [Google Scholar] [CrossRef]
- Chen, Y.; Xia, S.; Han, X.; Fu, Z. Simultaneous Determination of Malachite Green, Chloramphenicols, Sulfonamides, and Fluoroquinolones Residues in Fish by Liquid Chromatography-Mass Spectrometry. J. Anal. Methods Chem. 2020, 2020, e3725618. [Google Scholar] [CrossRef]
- Ning, Y.; Ye, Y.; Liao, W.; Xu, Y.; Wang, W.; Wang, A. Triazine-Based Porous Organic Polymer as Pipette Tip Solid-Phase Extraction Adsorbent Coupled with HPLC for the Determination of Sulfonamide Residues in Food Samples. Food Chem. 2022, 397, 133831. [Google Scholar] [CrossRef] [PubMed]
- Stavroulaki, A.; Tzatzarakis, M.N.; Karzi, V.; Katsikantami, I.; Renieri, E.; Vakonaki, E.; Avgenaki, M.; Alegakis, A.; Stan, M.; Kavvalakis, M.; et al. Antibiotics in Raw Meat Samples: Estimation of Dietary Exposure and Risk Assessment. Toxics 2022, 10, 456. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Kang, H.-S. Multi-Residue Determination of Sulfonamides, Dapsone, Ormethoprim, and Trimethoprim in Fish and Shrimp Using Dispersive Solid Phase Extraction with LC–MS/MS. Food Anal. Methods 2021, 14, 1256–1268. [Google Scholar] [CrossRef]
- Li, Z.B.; Cui, P.L.; Liu, J.; Liu, J.X.; Wang, J.P. Production of Generic Monoclonal Antibody and Development of Chemiluminescence Immunoassay for Determination of 32 Sulfonamides in Chicken Muscle. Food Chem. 2020, 311, 125966. [Google Scholar] [CrossRef]
- Ahmed, S.; Ning, J.; Peng, D.; Chen, T.; Ahmad, I.; Ali, A.; Lei, Z.; Abu bakr Shabbir, M.; Cheng, G.; Yuan, Z. Current Advances in Immunoassays for the Detection of Antibiotics Residues: A Review. Food Agric. Immunol. 2020, 31, 268–290. [Google Scholar] [CrossRef]
- Zou, Y.; Gu, H.; Yang, J.; Zeng, T.; Yang, J.; Zhang, Y. A High Sensitivity Strategy of Nitrite Detection Based on CoFe@NC Nanocubes Modified Glassy Carbon Electrode. Carbon Lett. 2023, 33, 2075–2086. [Google Scholar] [CrossRef]
- Yi, K.; Xu, S.; Cheng, H.; Chen, S.; Jiang, S.; Tu, J. A Label-Free Sensor Based on a Carbon Nanotube-Graphene Platform for the Detection of Non-Hodgkin Lymphoma Genes. Alex. Eng. J. 2023, 84, 93–99. [Google Scholar] [CrossRef]
- Wang, P.; Chen, S.; Guan, Y.; Li, Y.; Jiamali, A. An Electrochemical Sensing Platform Based on Gold Nanostars for the Detection of Alzheimer’s Disease Marker Aβ Oligomers (Aβo). Alex. Eng. J. 2023, 81, 1–6. [Google Scholar] [CrossRef]
- Wang, X.; Shi, S.; Zhang, F.; Li, S.; Tan, J.; Su, B.; Cheng, Q.; Gou, Y.; Zhang, Y. Application of a Nanotip Array-Based Electrochemical Sensing Platform for Detection of Indole Derivatives as Key Indicators of Gut Microbiota Health. Alex. Eng. J. 2023, 85, 294–299. [Google Scholar] [CrossRef]
- Su, J.; Su, X. Determination of Tartrazine in Sports Drinks by a Disposable Electrochemical Sensor Modified with Co2O3. Food Meas. 2023, 17, 5856–5863. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Feng, K.; Wei, C. Fabrication of Graphene-Assisted Voltammetry Platform for the Detection of Nitrate Ions in PM2.5. Carbon Lett. 2023, 33, 2143–2152. [Google Scholar] [CrossRef]
- Tang, M.; Guo, J.; Shen, Z. Rapid Detection of Carbendazim Residue in Tea by Machine Learning Assisted Electrochemical Sensor. Food Meas. 2023, 17, 6363–6369. [Google Scholar] [CrossRef]
- Jiang, B.; Wu, M.; Wu, S.; Ji, M. Study on Anticorrosion of Q235 Building Steel Using Iranian Rock-Graphene Nanocomposite Coating. Alex. Eng. J. 2023, 71, 73–77. [Google Scholar] [CrossRef]
- Gomes, N.O.; Carrilho, E.; Machado, S.A.S.; Sgobbi, L.F. Bacterial Cellulose-Based Electrochemical Sensing Platform: A Smart Material for Miniaturized Biosensors. Electrochim. Acta 2020, 349, 136341. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Georgouvelas, D.; Edlund, U.; Mathew, A.P. CelloZIFPaper: Cellulose-ZIF Hybrid Paper for Heavy Metal Removal and Electrochemical Sensing. Chem. Eng. J. 2022, 446, 136614. [Google Scholar] [CrossRef]
- Acharya, S.; Liyanage, S.; Parajuli, P.; Rumi, S.S.; Shamshina, J.L.; Abidi, N. Utilization of Cellulose to Its Full Potential: A Review on Cellulose Dissolution, Regeneration, and Applications. Polymers 2021, 13, 4344. [Google Scholar] [CrossRef]
- Teow, Y.H.; Kam, L.M.; Mohammad, A.W. Synthesis of Cellulose Hydrogel for Copper (II) Ions Adsorption. J. Environ. Chem. Eng. 2018, 6, 4588–4597. [Google Scholar] [CrossRef]
- Alshehri, S.M.; Aldalbahi, A.; Al-Hajji, A.B.; Chaudhary, A.A.; in het Panhuis, M.; Alhokbany, N.; Ahamad, T. Development of Carboxymethyl Cellulose-Based Hydrogel and Nanosilver Composite as Antimicrobial Agents for UTI Pathogens. Carbohydr. Polym. 2016, 138, 229–236. [Google Scholar] [CrossRef]
- Sethi, S.; Kaith, B.S.; Saruchi; Kumar, V. Fabrication and Characterization of Microwave Assisted Carboxymethyl Cellulose-Gelatin Silver Nanoparticles Imbibed Hydrogel: Its Evaluation as Dye Degradation. React. Funct. Polym. 2019, 142, 134–146. [Google Scholar] [CrossRef]
- Jatoi, A.W.; Kim, I.S.; Ni, Q.Q. A Comparative Study on Synthesis of AgNPs on Cellulose Nanofibers by Thermal Treatment and DMF for Antibacterial Activities. Mater. Sci. Eng. C 2019, 98, 1179–1195. [Google Scholar] [CrossRef] [PubMed]
- Yadollahi, M.; Namazi, H.; Aghazadeh, M. Antibacterial Carboxymethyl Cellulose/Ag Nanocomposite Hydrogels Cross-Linked with Layered Double Hydroxides. Int. J. Biol. Macromol. 2015, 79, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Kwon, G.-J.; Han, S.-Y.; Park, C.-W.; Park, J.-S.; Lee, E.-A.; Kim, N.-H.; Alle, M.; Bandi, R.; Lee, S.-H. Adsorption Characteristics of Ag Nanoparticles on Cellulose Nanofibrils with Different Chemical Compositions. Polymers 2020, 12, 164. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Akter, N.; Rahman, M.M.; Shi, C.; Islam, M.T.; Zeng, H.; Azam, M.S. Mussel-Inspired Immobilization of Silver Nanoparticles toward Antimicrobial Cellulose Paper. ACS Sustain. Chem. Eng. 2018, 6, 9178–9188. [Google Scholar] [CrossRef]
- Wang, S.; Xiang, J.; Sun, Y.; Wang, H.; Du, X.; Cheng, X.; Du, Z.; Wang, H. Skin-Inspired Nanofibrillated Cellulose-Reinforced Hydrogels with High Mechanical Strength, Long-Term Antibacterial, and Self-recovery Ability for Wearable Strain/Pressure Sensors. Carbohydr. Polym. 2021, 261, 117894. [Google Scholar] [CrossRef]
- Kumar, A.S.; Mageswari, G.V.; Nisha, S.; Nellepalli, P.; Vijayakrishna, K. Molecular Orientation and Dynamics of Ferricyanide Ion-Bearing Copoly(Ionic Liquid) Modified Glassy Carbon Electrode towards Selective Mediated Oxidation Reaction of Cysteine versus Ascorbic Acid: A Biomimicking Enzyme Functionality. Electrochim. Acta 2021, 395, 139215. [Google Scholar] [CrossRef]
- Chen, B.; Tao, Q.; Qiao, F.; Fei, Y.; Liu, Y.; Xiong, X.; Liu, S. Temporal Sensing Platform Based on Anodic Dissolution of Ag and Cathodic Biocatalysis of Oxygen Reduction for Staphylococcus Aureus Detection. Food Chem. 2022, 383, 132404. [Google Scholar] [CrossRef]
- Zaidi, S.A.; Shin, J.H. Recent Developments in Nanostructure Based Electrochemical Glucose Sensors. Talanta 2016, 149, 30–42. [Google Scholar] [CrossRef]
- Dirany, A.; Sirés, I.; Oturan, N.; Oturan, M.A. Electrochemical Abatement of the Antibiotic Sulfamethoxazole from Water. Chemosphere 2010, 81, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Misal, S.N.; Lin, M.-H.; Mehraeen, S.; Chaplin, B.P. Modeling Electrochemical Oxidation and Reduction of Sulfamethoxazole Using Electrocatalytic Reactive Electrochemical Membranes. J. Hazard. Mater. 2020, 384, 121420. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, P.; Settu, R.; Chen, S.-M.; Chen, T.-W. Voltammetric Sensing of Sulfamethoxazole Using a Glassy Carbon Electrode Modified with a Graphitic Carbon Nitride and Zinc Oxide Nanocomposite. Microchim. Acta 2018, 185, 396. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Y.-C.; Hong, Y.-T.; Lee, T.-W.; Huang, J.-F. Facile Fabrication of Ascorbic Acid Reduced Graphene Oxide-Modified Electrodes toward Electroanalytical Determination of Sulfamethoxazole in Aqueous Environments. Chem. Eng. J. 2018, 352, 188–197. [Google Scholar] [CrossRef]
- Meshki, M.; Behpour, M.; Masoum, S. Application of Fe Doped ZnO Nanorods-Based Modified Sensor for Determination of Sulfamethoxazole and Sulfamethizole Using Chemometric Methods in Voltammetric Studies. J. Electroanal. Chem. 2015, 740, 1–7. [Google Scholar] [CrossRef]
- Cesarino, I.; Cesarino, V.; Lanza, M.R.V. Carbon Nanotubes Modified with Antimony Nanoparticles in a Paraffin Composite Electrode: Simultaneous Determination of Sulfamethoxazole and Trimethoprim. Sens. Actuators B Chem. 2013, 188, 1293–1299. [Google Scholar] [CrossRef]
- Souza, C.D.; Braga, O.C.; Vieira, I.C.; Spinelli, A. Electroanalytical Determination of Sulfadiazine and Sulfamethoxazole in Pharmaceuticals Using a Boron-Doped Diamond Electrode. Sens. Actuators B Chem. 2008, 135, 66–73. [Google Scholar] [CrossRef]
- Yue, X.; Li, Z.; Zhao, S. A New Electrochemical Sensor for Simultaneous Detection of Sulfamethoxazole and Trimethoprim Antibiotics Based on Graphene and ZnO Nanorods Modified Glassy Carbon Electrode. Microchem. J. 2020, 159, 105440. [Google Scholar] [CrossRef]
- Yari, A.; Shams, A. Silver-Filled MWCNT Nanocomposite as a Sensing Element for Voltammetric Determination of Sulfamethoxazole. Anal. Chim. Acta 2018, 1039, 51–58. [Google Scholar] [CrossRef]
- Pryshchepa, O.; Pomastowski, P.; Buszewski, B. Silver Nanoparticles: Synthesis, Investigation Techniques, and Properties. Adv. Colloid Interface Sci. 2020, 284, 102246. [Google Scholar] [CrossRef]
- Rohde, A.; Hammerl, J.A.; Appel, B.; Dieckmann, R.; Al Dahouk, S. Sampling and Homogenization Strategies Significantly Influence the Detection of Foodborne Pathogens in Meat. BioMed Res. Int. 2015, 2015, e145437. [Google Scholar] [CrossRef]


| Spiked SMX (μg/kg) | Detected (μg/kg) | Recovery (%) | RSD (n = 3) |
|---|---|---|---|
| 10 | 8.6 | 86 | 5.2 |
| 50 | 46 | 92 | 3.8 |
| 100 | 92 | 92 | 1.9 |
| Sensor Interface | Linear Range | Detection Limit (μM) | Reference |
|---|---|---|---|
| Graphitic carbon nitride and zinc oxide nanocomposite | 20 nM–1.1 mM | 6.6 nM | [41] |
| Ascorbic acid-reduced graphene oxide | 0.5–50 μM | 0.04 μM | [42] |
| Fe-doped ZnO nanorods | 2.0–160.0 μM | 30 nM | [43] |
| Carbon nanotube-modified antimony nanoparticles | 0.1–0.7 μM | 24 nM | [44] |
| Boron-doped diamond electrode | 8.01–119 μM | 1.15 μM | [45] |
| Graphene and ZnO nanorods | 1–180 μM | 0.4 μM | [46] |
| Ag/MWCNT | 0.05–70 μM | 0.01 μM | [47] |
| CS-AgNPs | 0.1–100 μM | 0.04 μM | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Yang, N. Silver Nanoparticle-Embedded Hydrogels for Electrochemical Sensing of Sulfamethoxazole Residues in Meat. Molecules 2024, 29, 1256. https://doi.org/10.3390/molecules29061256
Deng Y, Yang N. Silver Nanoparticle-Embedded Hydrogels for Electrochemical Sensing of Sulfamethoxazole Residues in Meat. Molecules. 2024; 29(6):1256. https://doi.org/10.3390/molecules29061256
Chicago/Turabian StyleDeng, Yuanxi, and Ningning Yang. 2024. "Silver Nanoparticle-Embedded Hydrogels for Electrochemical Sensing of Sulfamethoxazole Residues in Meat" Molecules 29, no. 6: 1256. https://doi.org/10.3390/molecules29061256
APA StyleDeng, Y., & Yang, N. (2024). Silver Nanoparticle-Embedded Hydrogels for Electrochemical Sensing of Sulfamethoxazole Residues in Meat. Molecules, 29(6), 1256. https://doi.org/10.3390/molecules29061256






