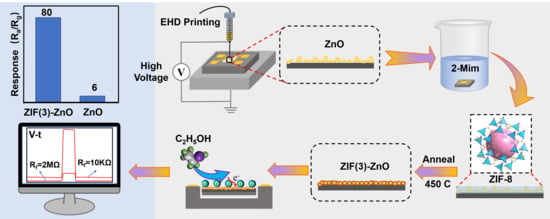
In Situ-Derived N-Doped ZnO from ZIF-8 for Enhanced Ethanol Sensing in ZnO/MEMS Devices
Abstract
1. Introduction
2. Results and Discussion
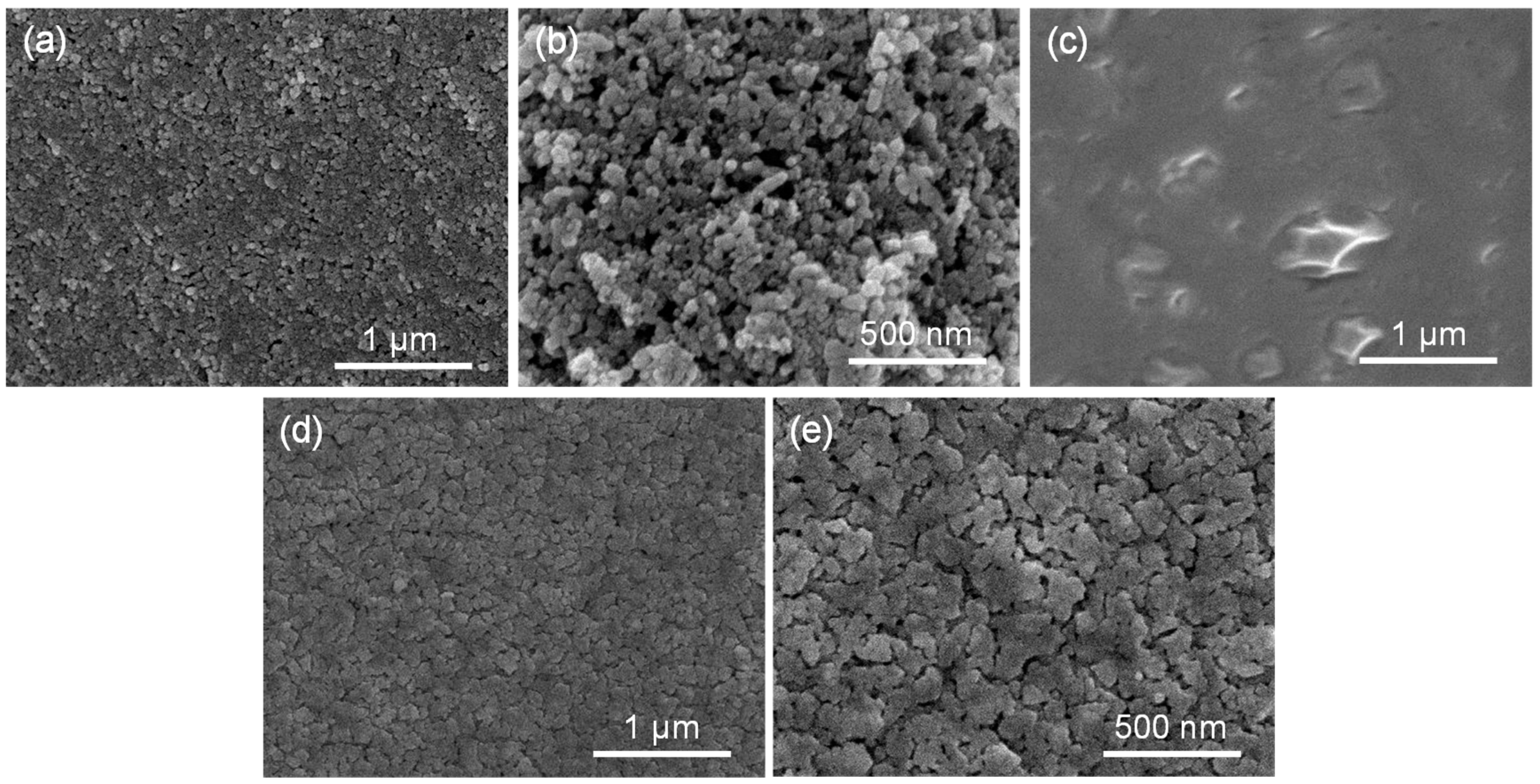
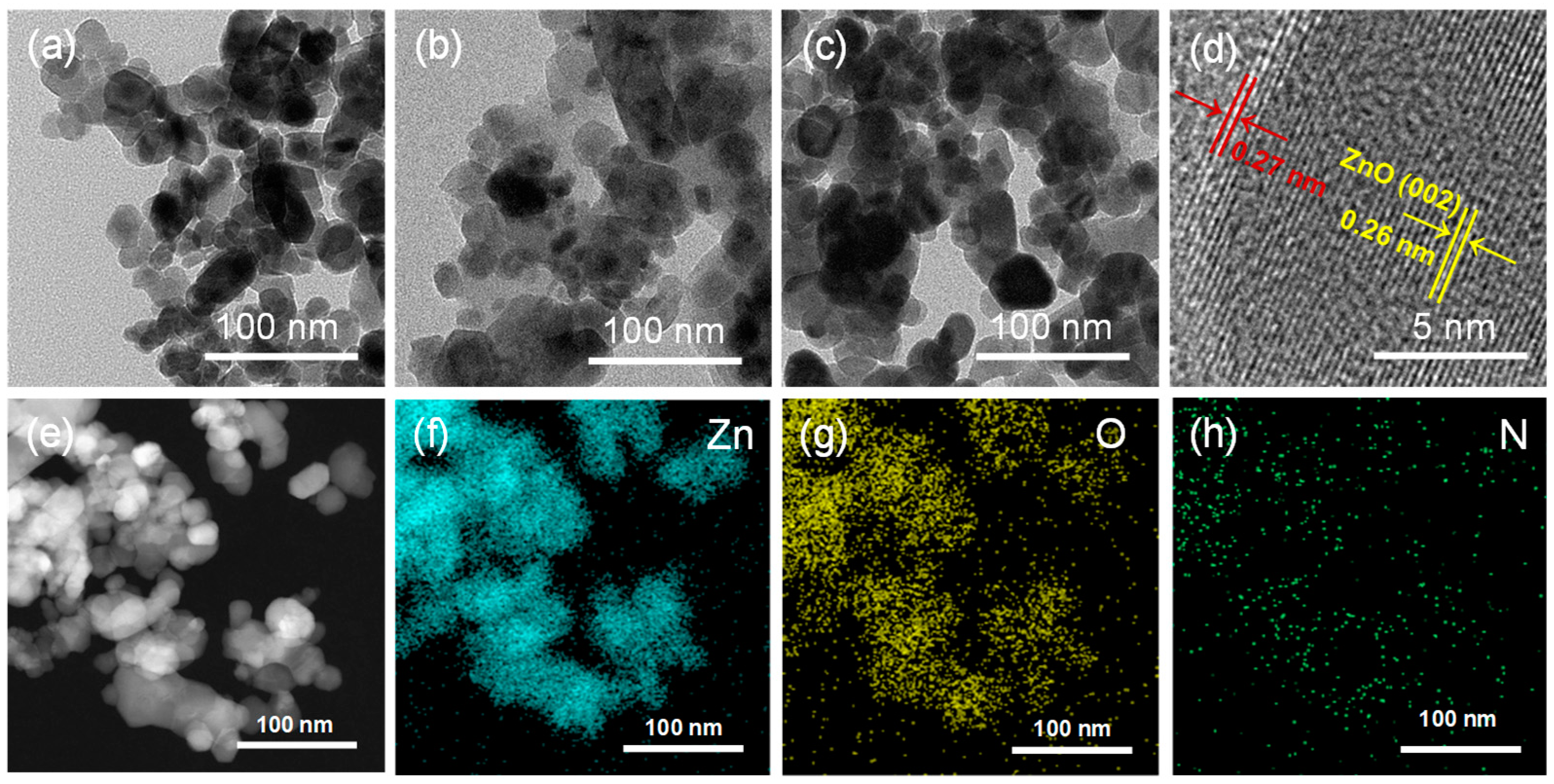
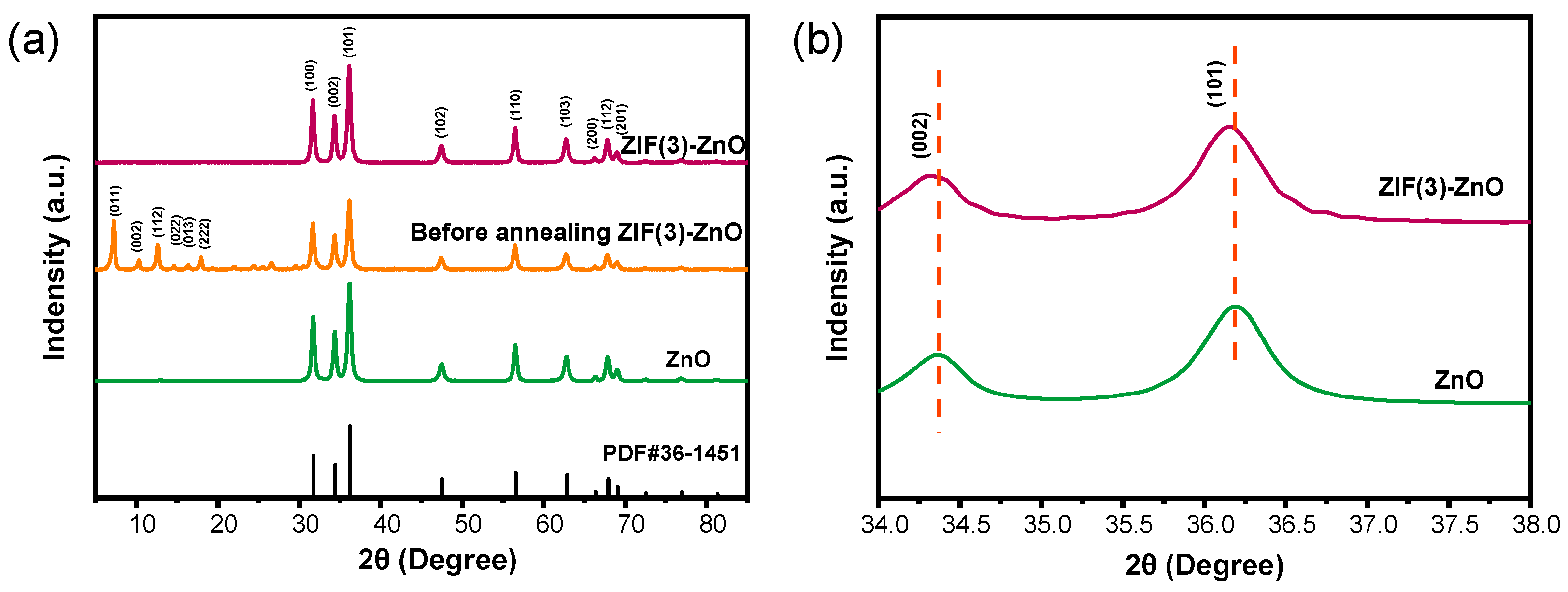
2.1. The Microstructure and Surface Properties of Materials
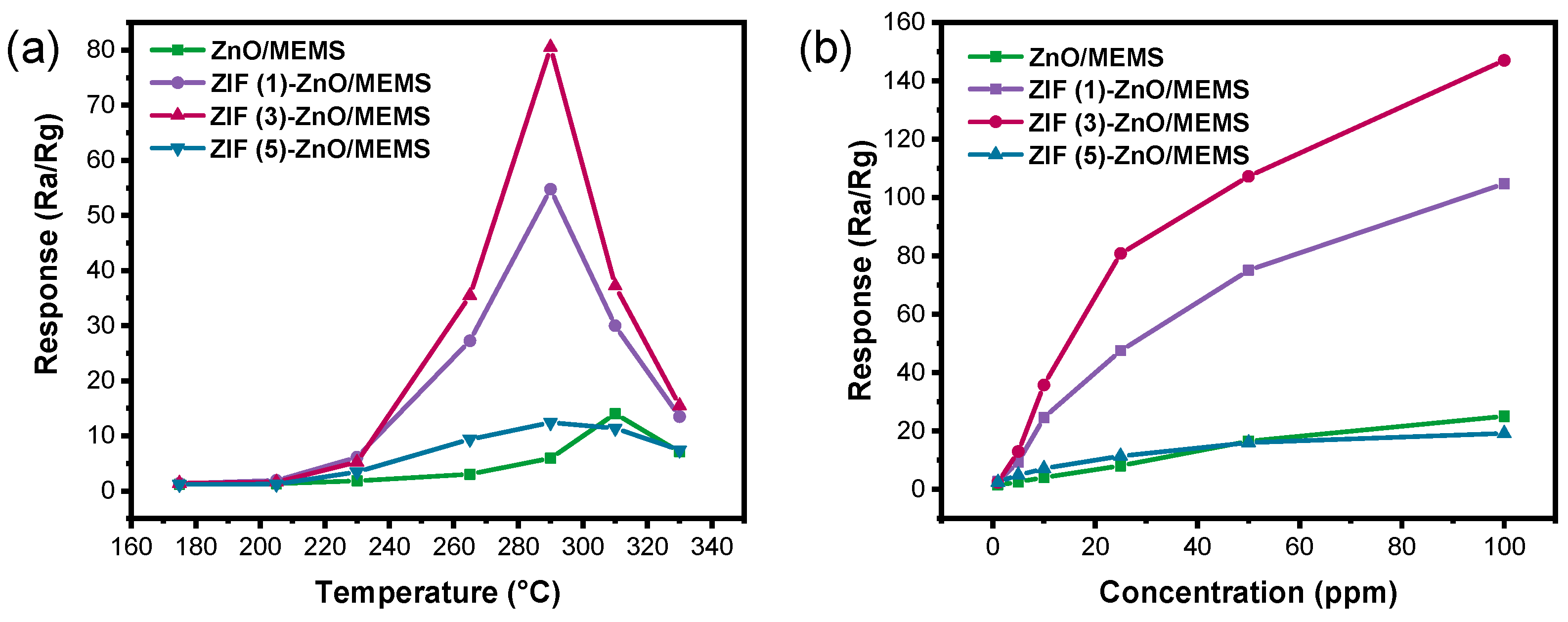
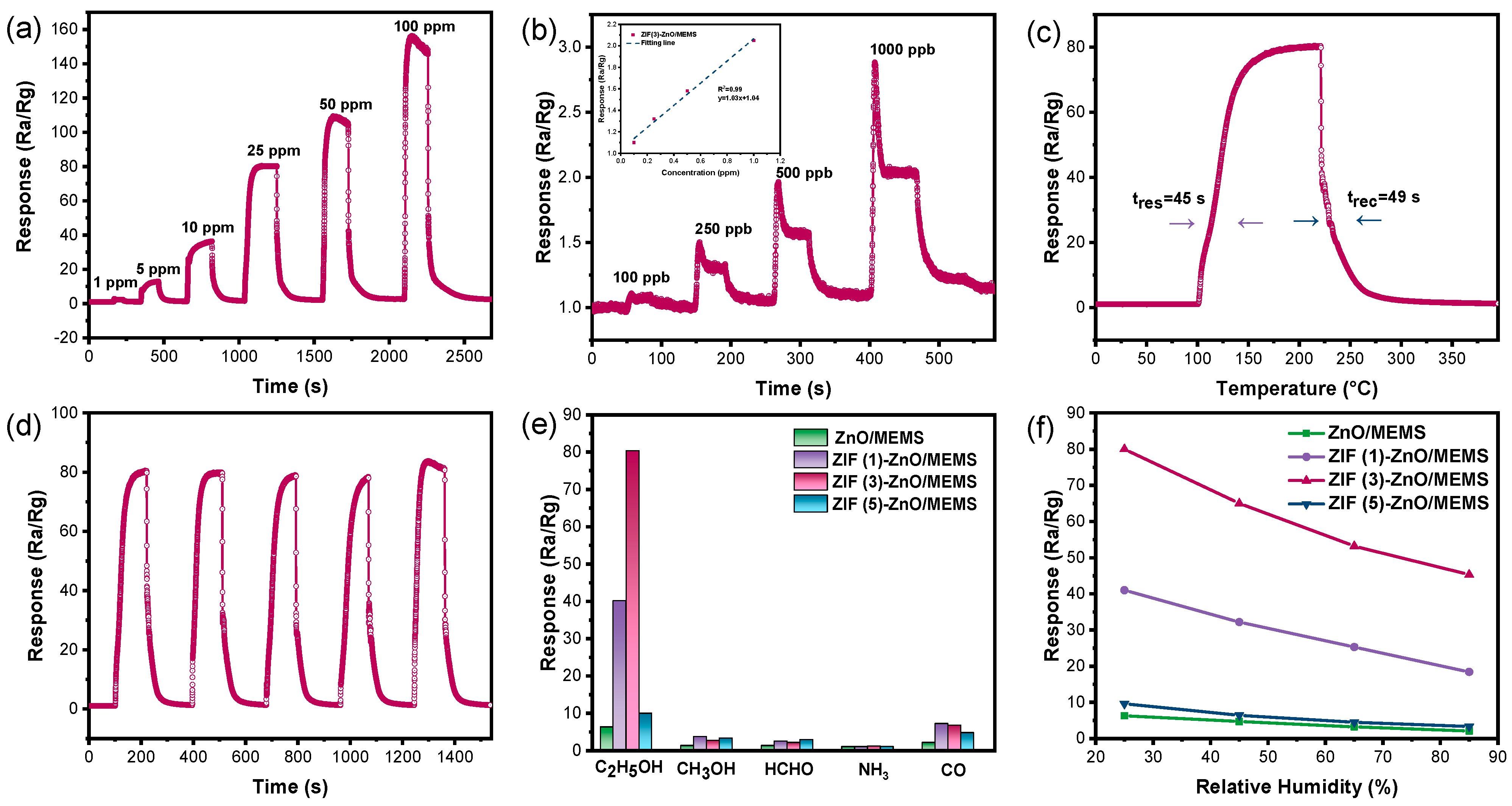
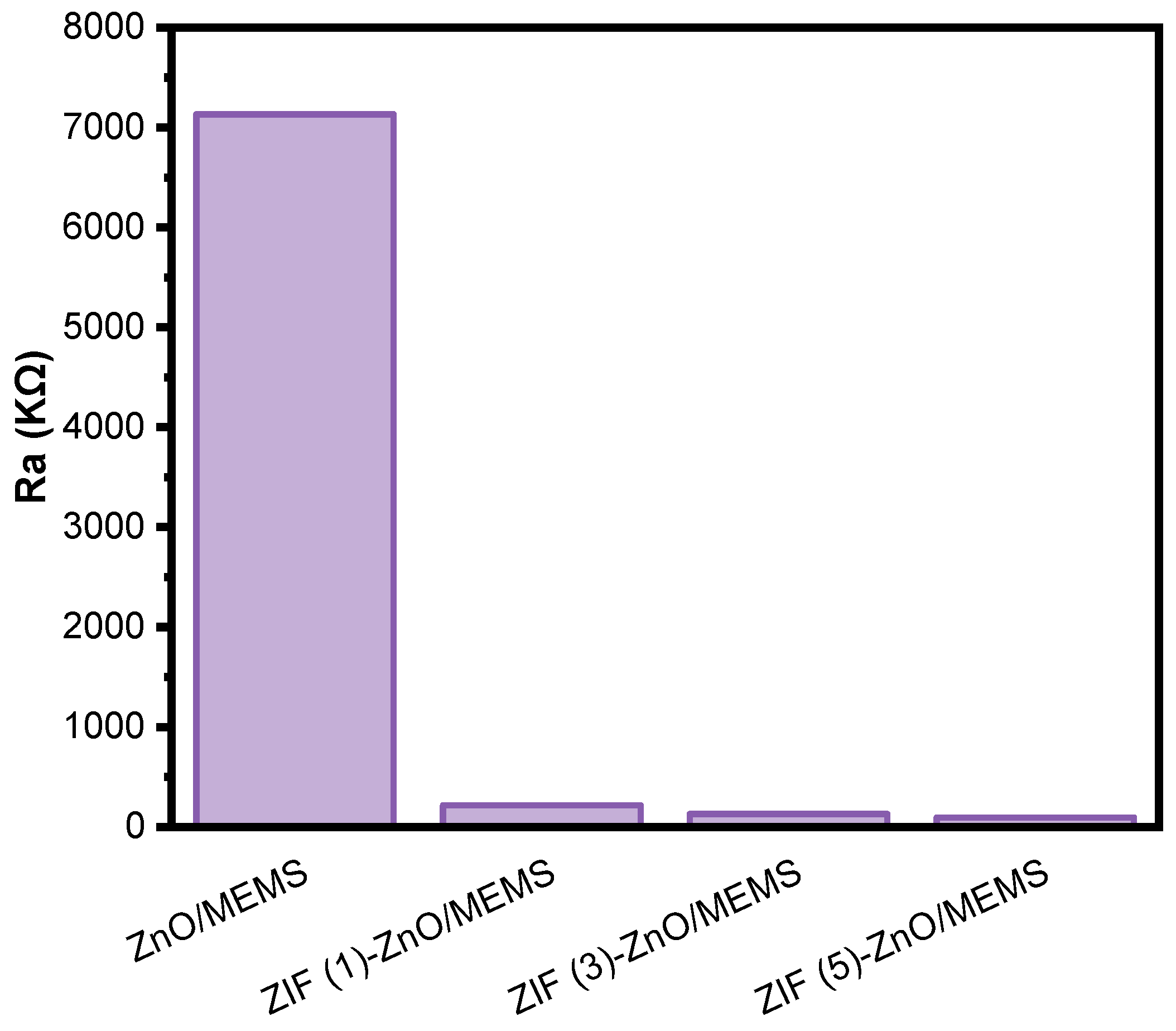
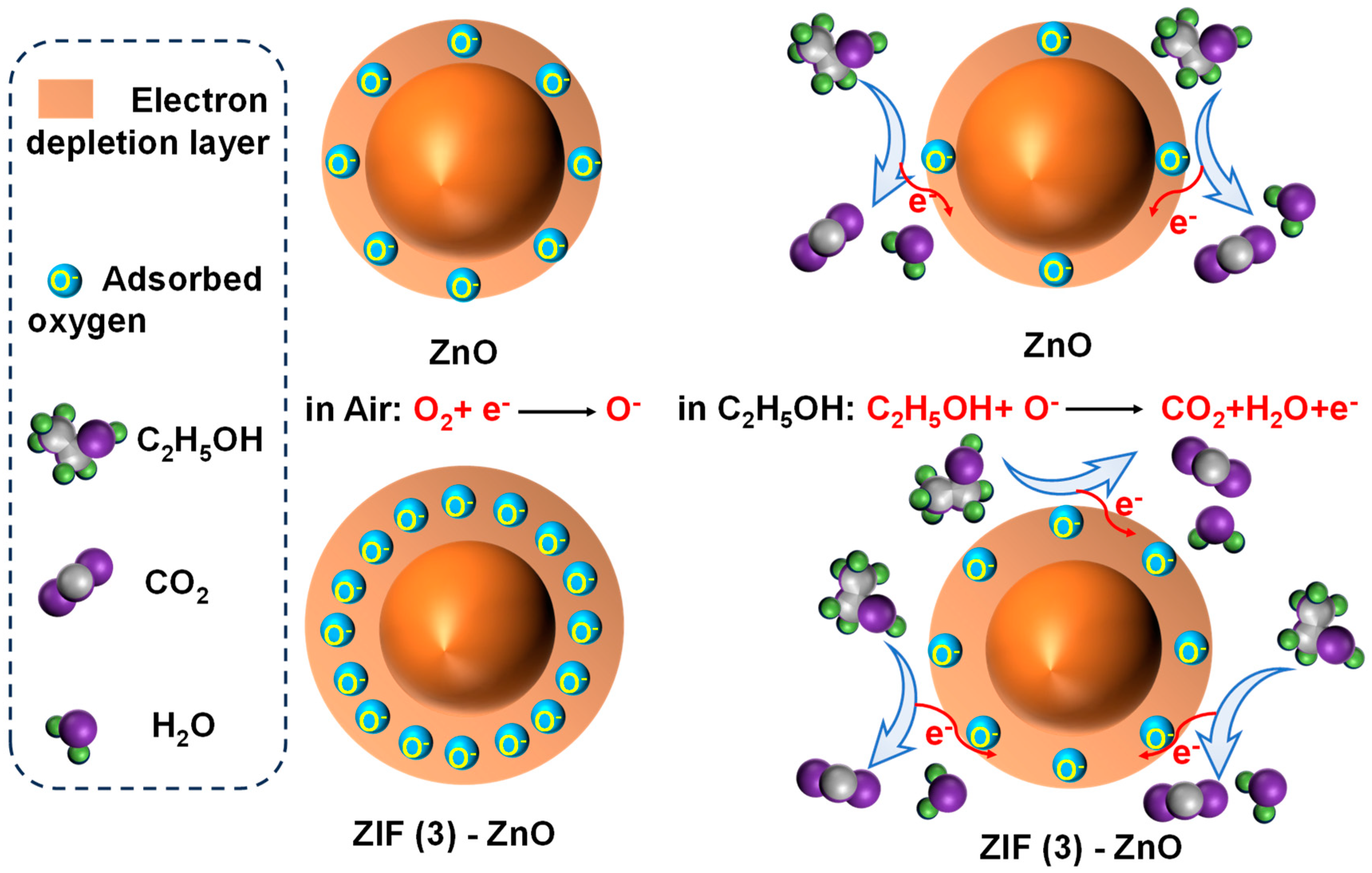
2.2. The Performances of Ethanol
3. Experimental Methods
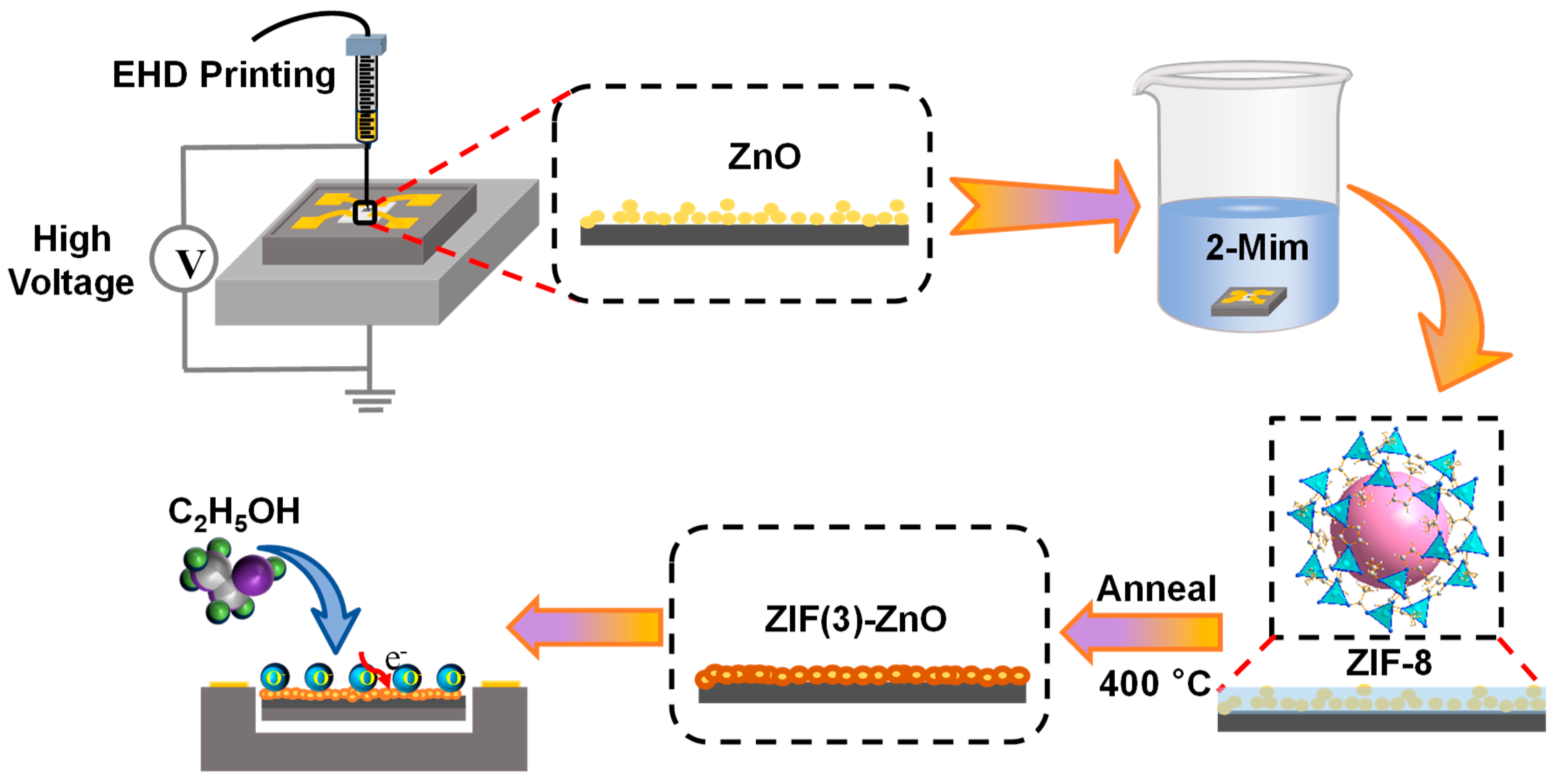
3.1. Preparation of ZnO Slurry and EHD Printing
3.2. In Situ Growth of Nitrogen-Doped ZnO Derived from ZIF-8 on ZnO/MEMS Chips
3.3. Characterization
3.4. Gas Sensitivity Performance Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.X.; Zhang, Y.N.; Liu, S.T.; Shi, B.F.; Wang, L.F.; Zhao, Y. Fiber optic room temperature ethanol sensor based on ZnSnO3/TiO2 with UV radiation sensitization. Sens. Actuators B 2024, 399, 134814. [Google Scholar] [CrossRef]
- Ubando, A.T.; Africa, A.D.M.; Maniquiz-Redillas, M.C.; Culaba, A.B.; Chen, W.H. Reduction of particulate matter and volatile organic compounds in biorefineries: A state-of-the-art review. J. Hazard. Mater. 2021, 403, 123955. [Google Scholar] [CrossRef]
- Li, Q.C.; Chen, D.; Miao, J.M.; Lin, S.J.; Yu, Z.X.; Cui, D.X.; Yang, Z.; Chen, X.P. Highly sensitive sensor based on ordered porous ZnO nanosheets for ethanol detecting application. Sens. Actuators B 2021, 326, 128952. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, K.C.; Liu, K.W.; Xu, J.Y.; Zheng, Z.C. Metal oxide resistive sensors for carbon dioxide detection. Coord. Chem. Rev. 2022, 472, 214758. [Google Scholar] [CrossRef]
- Zhao, S.K.; Shen, Y.B.; Yan, X.X.; Zhou, P.F.; Yin, Y.Y.; Lu, R.; Han, C.; Cui, B.Y.; Wei, D.Z. Complex-surfactant-assisted hydrothermal synthesis of one-dimensional ZnO nanorods for high-performance ethanol gas sensor. Sens. Actuators B 2019, 286, 501–511. [Google Scholar] [CrossRef]
- Shaik, R.; Kampara, R.K.; Kumar, A.; Sharma, C.S.; Kumar, M. Metal oxide nanofibers based chemiresistive H2S gas sensors. Coord. Chem. Rev. 2022, 471, 214752. [Google Scholar] [CrossRef]
- Majhi, S.M.; Mirzaei, A.; Kim, H.W.; Kim, S.S.; Kim, T.W. Recent advances in energy-saving chemiresistive gas sensors: A review. Nano Energy 2021, 79, 105369. [Google Scholar] [CrossRef]
- Zhu, J.X.; Liu, X.M.; Shi, Q.F.; He, T.Y.Y.; Sun, Z.D.; Guo, X.G.; Liu, W.X.; Bin Sulaiman, O.; Dong, B.W.; Lee, C. Development Trends and Perspectives of Future Sensors and MEMS/NEMS. Micromachines 2020, 11, 7. [Google Scholar] [CrossRef]
- Puigcorbé, J.; Vilà, A.; Cerdà, J.; Cirera, A.; Gràcia, I.; Cané, C.; Morante, J.R. Thermo-mechanical analysis of micro-drop coated gas sensors. Sens. Actuators A 2002, 97–98, 379–385. [Google Scholar] [CrossRef]
- Cavicchi, R.E.; Walton, R.M.; Aquino-Class, M.; Allen, J.D.; Panchapakesan, B.J.S.; Chemical, A.B. Spin-on nanoparticle tin oxide for microhotplate gas sensors. Sens. Actuators B Chem. 2001, 77, 145–154. [Google Scholar] [CrossRef]
- Xie, C.S.; Xiao, L.Q.; Hu, M.L.; Bai, Z.K.; Xia, X.P.; Zeng, D.W. Fabrication and formaldehyde gas-sensing property of ZnO-MnO2 coplanar gas sensor arrays. Sens. Actuators B 2010, 145, 457–463. [Google Scholar] [CrossRef]
- Kang, K.; Yang, D.; Park, J.; Kim, S.; Cho, I.; Yang, H.H.; Cho, M.; Mousavi, S.; Choi, K.H.; Park, I. Micropatterning of metal oxide nanofibers by electrohydrodynamic (EHD) printing towards highly integrated and multiplexed gas sensor applications. Sens. Actuators B 2017, 250, 574–583. [Google Scholar] [CrossRef]
- Mkhize, N.; Murugappan, K.; Castell, M.R.; Bhaskaran, H. Electrohydrodynamic jet printed conducting polymer for enhanced chemiresistive gas sensors†. J. Mater. Chem. C 2021, 9, 4591–4596. [Google Scholar] [CrossRef]
- Wu, H.; Yu, J.; Cao, R.; Yang, Y.H.; Tang, Z.N. Electrohydrodynamic inkjet printing of Pd loaded SnO2 nanofibers on a CMOS micro hotplate for low power H2 detection. AIP Adv. 2018, 8, 055307. [Google Scholar] [CrossRef]
- Almaev, A.V.; Kopyev, V.V.; Novikov, V.A.; Chikiryaka, A.V.; Yakovlev, N.N.; Usseinov, A.B.; Karipbayev, Z.T.; Akilbekov, A.T.; Koishybayeva, Z.K.; Popov, A.I. ITO Thin Films for Low-Resistance Gas Sensors. Materials 2023, 16, 342. [Google Scholar] [CrossRef]
- Kambara, H.; Tanaka, H.; Oishi, S.; Tenya, K.; Tsujii, H. Room-temperature reduction at SrRuO3-metal interface in hydrogenous atmosphere detected by interface-sensitive resistance measurement. J. Appl. Phys. 2020, 128, 175306. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Xing, C.Y.; Zhang, X.R.; Liang, Z.L.; Wang, X.K.; Zhang, K.; Wang, Y.T.; Liu, D.; Fan, W.D.; et al. Two alkynyl functionalized Co(II)-MOFs as fluorescent sensors exhibiting selectivity and sensitivity for Fe3+ and nitroaromatic compounds. Chin. Chem. Lett. 2019, 30, 1440–1444. [Google Scholar] [CrossRef]
- Mo, Z.L.; Tai, D.Z.; Zhang, H.; Shahab, A. A comprehensive review on the adsorption of heavy metals by zeolite imidazole framework (ZIF-8) based nanocomposite in water. Chem. Eng. J. 2022, 443, 136320. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, Q.M.; Yang, P.; Huang, Y.; Liu, Y.N.; Yang, H.H.; Yan, J.H. ZIF-8 derived ZnO/Zn6Al2O9/Al2O3 nanocomposite with excellent photocatalytic performance under simulated sunlight irradiation. New J. Chem. 2019, 43, 2990–2999. [Google Scholar] [CrossRef]
- Jo, Y.M.; Jo, Y.K.; Lee, J.H.; Jang, H.W.; Hwang, I.S.; Yoo, D. MOF-Based Chemiresistive Gas Sensors: Toward New Functionalities. Adv. Mater. 2023, 35, 2206842. [Google Scholar] [CrossRef]
- Zhang, N.; Yan, L.M.; Lu, Y.; Fan, Y.Z.; Guo, S.J.; Adimi, S.; Liu, D.L.; Ruan, S.P. Metal-organic frameworks-derived hierarchical ZnO structures as efficient sensing materials for formaldehyde detection. Chin. Chem. Lett. 2020, 31, 2071–2076. [Google Scholar] [CrossRef]
- Qi, T.J.; Yang, X.; Sun, J. Neck-connected ZnO films derived from core-shell zeolitic imidazolate framework-8 (ZIF-8)@ZnO for highly sensitive ethanol gas sensors. Sens. Actuators B 2019, 283, 93–98. [Google Scholar] [CrossRef]
- Wu, C.H.; Xie, D.G.; Mei, Y.J.; Xiu, Z.F.; Poduska, K.M.; Li, D.C.; Xu, B.; Sun, D.F. Unveiling the thermolysis natures of ZIF-8 and ZIF-67 by employing in-situ structural characterization studies. Phys. Chem. Chem. Phys. 2019, 21, 17571–17577. [Google Scholar] [CrossRef]
- Xia, Y.; Pan, A.F.; Gardner, D.W.; Zhao, S.K.; Davey, A.K.; Li, Z.; Zhao, L.B.; Carraro, C.; Maboudian, R. Well-connected ZnO nanoparticle network fabricated by in-situ annealing of ZIF-8 for enhanced sensitivity in gas sensing application. Sens. Actuators B 2021, 344, 130180. [Google Scholar] [CrossRef]
- Zhu, P.F.; Yin, X.H.; Gao, X.H.; Dong, G.H.; Xu, J.K.; Wang, C.Y. Enhanced photocatalytic NO removal and toxic NO2 production inhibition over ZIF-8-derived ZnO nanoparticles with controllable amount of oxygen vacancies. Chin. J. Catal. 2021, 42, 175–183. [Google Scholar] [CrossRef]
- Zhang, X.J.; Chen, Y.; Zhang, S.; Qiu, C.Y. High photocatalytic performance of high concentration Al-doped ZnO nanoparticles. Sep. Purif. Technol. 2017, 172, 236–241. [Google Scholar] [CrossRef]
- Sun, L.; Shao, Q.; Zhang, Y.; Jiang, H.Y.; Ge, S.S.; Lou, S.Q.; Lin, J.; Zhang, J.X.; Wu, S.D.; Dong, M.Y.; et al. N self-doped ZnO derived from microwave hydrothermal synthesized zeolitic imidazolate framework-8 toward enhanced photocatalytic degradation of methylene blue. J. Colloid Interface Sci. 2020, 565, 142–155. [Google Scholar] [CrossRef]
- Zou, R.J.; He, G.J.; Xu, K.B.; Liu, Q.; Zhang, Z.Y.; Hu, J.Q. ZnO nanorods on reduced graphene sheets with excellent field emission, gas sensor and photocatalytic properties. J. Mater. Chem. A 2013, 1, 8445–8452. [Google Scholar] [CrossRef]
- Huang, X.C.; Lin, Y.Y.; Zhang, J.P.; Chen, X.M. Ligand-directed strategy for zeolite-type metal-organic frameworks: Zinc(II) imidazolates with unusual zeolitic topologies. Angew. Chem. Int. Ed. 2006, 45, 1557–1559. [Google Scholar] [CrossRef]
- Liang, P.; Zhang, C.; Sun, H.Q.; Liu, S.M.; Tadé, M.; Wang, S.B. Photocatalysis of C, N-doped ZnO derived from ZIF-8 for dye degradation and water oxidation. RSC Adv. 2016, 6, 95903–95909. [Google Scholar] [CrossRef]
- Sun, S.B.; Chang, X.T.; Li, X.J.; Li, Z.J. Synthesis of N-doped ZnO nanoparticles with improved photocatalytical activity. Ceram. Int. 2013, 39, 5197–5203. [Google Scholar] [CrossRef]
- Wu, C.L. Facile one-step synthesis of N-doped ZnO micropolyhedrons for efficient photocatalytic degradation of formaldehyde under visible-light irradiation. Appl. Surf. Sci. 2014, 319, 237–243. [Google Scholar] [CrossRef]
- Liu, J.J.; Zhang, L.Y.; Fan, J.J.; Zhu, B.C.; Yu, J.G. Triethylamine gas sensor based on Pt-functionalized hierarchical ZnO microspheres. Sens. Actuators B 2021, 331, 129425. [Google Scholar] [CrossRef]
- Yuan, X.J.; Duan, S.L.; Wu, G.Y.; Sun, L.; Cao, G.; Li, D.Y.; Xu, H.M.; Li, Q.; Xia, D.S. Enhanced catalytic ozonation performance of highly stabilized mesoporous ZnO doped g-C3N4 composite for efficient water decontamination. Appl. Catal. A 2018, 551, 129–138. [Google Scholar] [CrossRef]
- Yuan, H.Y.; Aljneibi, S.; Yuan, J.R.; Wang, Y.X.; Liu, H.; Fang, J.; Tang, C.H.; Yan, X.H.; Cai, H.; Gu, Y.D.; et al. ZnO Nanosheets Abundant in Oxygen Vacancies Derived from Metal-Organic Frameworks for ppb-Level Gas Sensing. Adv. Mater. 2019, 31, 1807161. [Google Scholar] [CrossRef]
- Wang, Q.; Hong, J.S.; Zhang, Z.L.; Li, J.D.; Cao, X.D.; Tang, J.H.; Geng, Y.F.; Wang, J.Q.; Li, X.J.; Pei, K.; et al. Visible light-activated ethanol sensor based on flower-like N3-loaded ZnO composites. Sens. Actuators B 2022, 370, 132399. [Google Scholar] [CrossRef]
- Ayeb, K.; Moussa, N.; Nsib, M.F.; Leonardi, S.G.; Neri, G. NO2 sensing properties of N-, F- and NF co-doped ZnO nanoparticles. Mater. Sci. Eng. B 2021, 263, 114870. [Google Scholar] [CrossRef]
- Balasubramani, V.; Chandraleka, S.; Rao, T.S.; Sasikumar, R.; Kuppusamy, M.R.; Sridhar, T.M. Review-Recent Advances in Electrochemical Impedance Spectroscopy Based Toxic Gas Sensors Using Semiconducting Metal Oxides. J. Electrochem. Soc. 2020, 167, 037572. [Google Scholar] [CrossRef]
- Van Zalk, M.; Brinkman, A.; Aarts, J.; Hilgenkamp, H. Interface resistance of YBa2Cu3O7−δ/La0.67Sr0.33MnO3 ramp-type contacts. Phys. Rev. B 2010, 82, 134513. [Google Scholar] [CrossRef]
- Luo, N.; Wang, C.; Zhang, D.; Guo, M.M.; Wang, X.H.; Cheng, Z.X.; Xu, J.Q. Ultralow detection limit MEMS hydrogen sensor based on SnO2 with oxygen vacancies. Sens. Actuators B 2022, 354, 130982. [Google Scholar] [CrossRef]
- Li, Z.J.; Li, H.; Wu, Z.L.; Wang, M.K.; Luo, J.T.; Torun, H.D.; Hu, P.A.; Yang, C.; Grundmann, M.; Liu, X.T.; et al. Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature. Mater. Horiz. 2019, 6, 470–506. [Google Scholar] [CrossRef]
- Ji, H.C.; Zeng, W.; Li, Y.Q. Gas sensing mechanisms of metal oxide semiconductors: A focus review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef]
- Bhatia, S.; Verma, N.; Bedi, R.K. Ethanol gas sensor based upon ZnO nanoparticles prepared by different techniques. Results Phys. 2017, 7, 801–806. [Google Scholar] [CrossRef]
- Shihabudeen, P.K.; Notash, M.Y.; Sardroodi, J.J.; Chaudhuri, A.R. Nitrogen incorporated zinc oxide thin film for efficient ethanol detection. Sens. Actuators B 2022, 358, 131544. [Google Scholar] [CrossRef]
- Jiang, B.; Zhou, T.T.; Zhang, L.; Han, W.J.; Yang, J.Q.; Wang, C.; Sun, Y.F.; Liu, F.M.; Sun, P.; Lu, G.Y. Construction of mesoporous In2O3-ZnO hierarchical structure gas sensor for ethanol detection. Sens. Actuators B 2023, 393, 134203. [Google Scholar] [CrossRef]
- Fu, Y.; Li, C.Z.; Chen, X.; Liu, Y.D.; Wu, H.C.; Jia, R.R.; Shi, L.Y.; Yu, D.Q.; Huang, L. Photochemical fabrication of defect-abundant Pd/SnO2 with promoted performance for dioctyl phthalate gas sensing. New J. Chem. 2023, 47, 10727–10734. [Google Scholar] [CrossRef]
- Cao, F.F.; Li, C.P.; Li, M.J.; Li, H.J.; Huang, X.; Yang, B.H. Direct growth of Al-doped ZnO ultrathin nanosheets on electrode for ethanol gas sensor application. Appl. Surf. Sci. 2018, 447, 173–181. [Google Scholar] [CrossRef]
- Jiang, B.; Tao, W.; Zhao, L.P.; Wang, T.S.; Liu, X.M.; Liu, F.M.; Yan, X.; Sun, Y.F.; Lu, G.Y.; Sun, P. Double-shell ZnO hollow microspheres prepared by template-free method for ethanol detection. Sens. Actuators B Chem. 2023, 385, 133626. [Google Scholar] [CrossRef]
- Zhang, X.; Lan, W.Y.; Xu, J.L.; Luo, Y.T.; Pan, J.; Liao, C.Y.; Yang, L.Y.; Tan, W.H.; Huang, X.T. ZIF-8 derived hierarchical hollow ZnO nanocages with quantum dots for sensitive ethanol gas detection. Sens. Actuators B Chem. 2019, 289, 144–152. [Google Scholar] [CrossRef]
- Li, W.H.; Wu, X.F.; Liu, H.D.; Chen, J.Y.; Tang, W.X.; Chen, Y.F. Hierarchical hollow ZnO cubes constructed using self-sacrificial ZIF-8 frameworks and their enhanced benzene gas-sensing properties. New J. Chem. 2015, 39, 7060–7065. [Google Scholar] [CrossRef]
- Liu, T.T.; Jia, X.H.; Zhang, J.T.; Yang, J.; Wang, S.Z.; Li, Y.; Shao, D.; Feng, L.; Song, H.J. Selective detection of ethanol at low concentration by ZnO@ZIF-8 porous nanosheets. Sens. Actuators B Chem. 2022, 372, 132661. [Google Scholar] [CrossRef]
- Ren, G.J.; Li, Z.M.; Yang, W.T.; Faheem, M.; Xing, J.B.; Zou, X.Q.; Pan, Q.H.; Zhu, G.S.; Du, Y. ZnO@ZIF-8 core-shell microspheres for improved ethanol gas sensing. Sens. Actuators B Chem. 2019, 284, 421–427. [Google Scholar] [CrossRef]
- Fu, H.; Feng, Z.; Liu, S.; Wang, P.; Zhao, C.; Wang, C. Enhanced ethanol sensing performance of N-doped ZnO derived from ZIF-8. Chin. Chem. Lett. 2023, 34, 107425. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, M.; Yan, Y.; Yang, J.; Liu, X.; Jia, R.; Ge, Y.; Li, Z.; Huang, L. In Situ-Derived N-Doped ZnO from ZIF-8 for Enhanced Ethanol Sensing in ZnO/MEMS Devices. Molecules 2024, 29, 1703. https://doi.org/10.3390/molecules29081703
Liang M, Yan Y, Yang J, Liu X, Jia R, Ge Y, Li Z, Huang L. In Situ-Derived N-Doped ZnO from ZIF-8 for Enhanced Ethanol Sensing in ZnO/MEMS Devices. Molecules. 2024; 29(8):1703. https://doi.org/10.3390/molecules29081703
Chicago/Turabian StyleLiang, Meihua, Yong Yan, Jiaxuan Yang, Xiaodong Liu, Rongrong Jia, Yuanyuan Ge, Zhili Li, and Lei Huang. 2024. "In Situ-Derived N-Doped ZnO from ZIF-8 for Enhanced Ethanol Sensing in ZnO/MEMS Devices" Molecules 29, no. 8: 1703. https://doi.org/10.3390/molecules29081703
APA StyleLiang, M., Yan, Y., Yang, J., Liu, X., Jia, R., Ge, Y., Li, Z., & Huang, L. (2024). In Situ-Derived N-Doped ZnO from ZIF-8 for Enhanced Ethanol Sensing in ZnO/MEMS Devices. Molecules, 29(8), 1703. https://doi.org/10.3390/molecules29081703