Abstract
The homogeneous non-catalytic hydrogenation of several types of iso- and syndiotactic cis-1,4 poly(1,3-diene)s with diimide, formed by thermal decomposition of p-toluene-sulfonyl-hydrazide, was examined. Perfectly alternating ethylene/1-alkene copolymers having different tacticity (i.e., isotactic and syndiotactic), which in some cases are difficult to synthesize by simple stereospecific co-polymerization of the corresponding monomers, were obtained. All the copolymers synthesized were fully characterized from a structural, morphological, and rheological point of view through different analytical techniques (FT-IR, NMR, GPC, DSC, RX).
1. Introduction
The stereospecific polymerization of 1,3-dienes with transition metal and lanthanide catalysts can give polymers with different structures (cis-1,4; trans-1,4; 1,2; 3,4; iso-and syndiotactic) from different monomers [1,2,3]. The polymer microstructure mainly depends on the catalyst and monomer structures and particularly on the combination of these two factors [4,5,6]. Thus for instance, the classical ternary system AlEt2Cl/Nd(OCOC7H15)3/Al(iBu)3 [7,8] gives isotactic cis-1,4 polymers from various substituted butadienes such as (E)-1,3-pentadiene [9], (E)-3-methyl-1,3-pentadiene [10,11], 1,3-hexadiene [12], 1,3-heptadiene [13] and 1,3-octadiene [13], while some nickel and cobalt catalysts (e.g., Ni(acac)2-MAO; Co(acac)3-MAO; CoCl2(PtBu2Me)2-MAO) give highly syndiotactic cis-1,4 polymers from the same monomers [13,14,15]. The great availability of all these highly stereoregular polydienes may represent an outstanding source of highly stereoregular olefin copolymers, hardly obtainable through the simple and common stereospecific polymerization of the corresponding monomers. In fact, the hydrogenation of isotactic and syndiotactic cis-1,4 polydienes can give perfectly alternating isotactic and syndiotactic ethylene/α-olefin copolymers, as shown in the following Scheme 1.
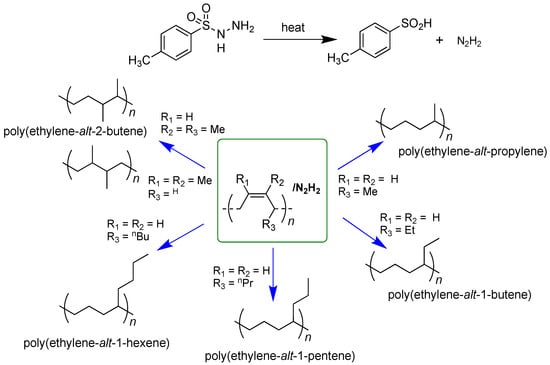
Scheme 1.
Perfectly alternating isotactic and syndiotactic ethylene/a-olefin copolymers by hydrogenation of highly stereoregular iso- and syndiotactic cis-1,4 polydienes.
We have already reported on the synthesis and characterization of isotactic and syndiotactic perfectly alternating ethylene/2-butene [16] and ethylene/propylene [17] copolymers.
In the present paper we are now describing the synthesis and characterization of (i) perfectly alternating isotactic ethylene/1-butene copolymers (E/1-B), obtained by hydrogenation of isotactic cis-1,4 poly(1,3-hexadiene) [12]; (ii) perfectly alternating ethylene/1-pentene copolymers (E/1-P), iso- and syndiotactic, obtained by hydrogenation of cis-1,4 poly(1,3-heptadiene)s, iso- and syndiotactic [13]; (iii) perfectly alternating ethylene/1-hexene copolymers (E/1-H), iso- and syndiotactic, obtained by hydrogenation of cis-1,4 poly(1,3-octadiene)s, iso- and syndiotactic [13].
A wide variety of catalysts and comonomers has been investigated for the ethylene/α-olefin copolymerization [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. Alternating or pseudo-alternating ethylene/α-olefin copolymers were obtained up to now only with metallocene-based catalysts, which, with respect to the classical heterogeneous ZN (Ziegler-Natta) catalysts, exhibit much smaller re values and larger rα-olefin values, allowing indeed for a more uniform comonomer distribution. The hydrogenation of highly stereoregular polydienes may represent a valid alternative to the above processes.
2. Results
As mentioned above, the highly stereoregular cis-1,4, iso- and syndiotactic, polydienes on which this study focused were the following: isotactic cis-1,4 poly(1,3-hexadiene) [hereafter named (cis1,4isoPHX)], syndiotactic cis-1,4 poly(1,3-heptadiene) (cis1,4syPHP), isotactic cis-1,4 poly(1,3-heptadiene) (cis1,4isoPHP), syndiotactic cis-1,4 poly(1,3-octadiene) (cis1,4syPO), and isotactic cis-1,4 poly(1,3-octadiene) (cis1,4isoPO). The syndiotactic cis-1,4 polymers were synthesized with the catalyst system CoCl2(PtBuMe2)2/MAO (MAO = methylalumoxane) [13]; the isotactic cis-1,4 polymers were obtained with the ternary catalyst system AlEt2Cl/Nd(OCOC7H15)3/AliBu3 [10,12,13]. The polymerization data concerning the preparation of all the diene-polymers are summarized in Table 1.

Table 1.
Polymerization of 1,3-dienes to cis-1,4 polymers with neodymium- and cobalt-based catalysts.
The hydrogenation of the above polymers was then examined. The hydrogenation is a type of chemical modification of polymers, allowing to reduce the amount of unsaturation, and leading to polymers with modified and improved properties and to the production of new materials [28]. Nevertheless, the hydrogenation may also be seen as a possible route to provide polymers which can be hardly prepared by a simple conventional polymerization of the corresponding monomers.
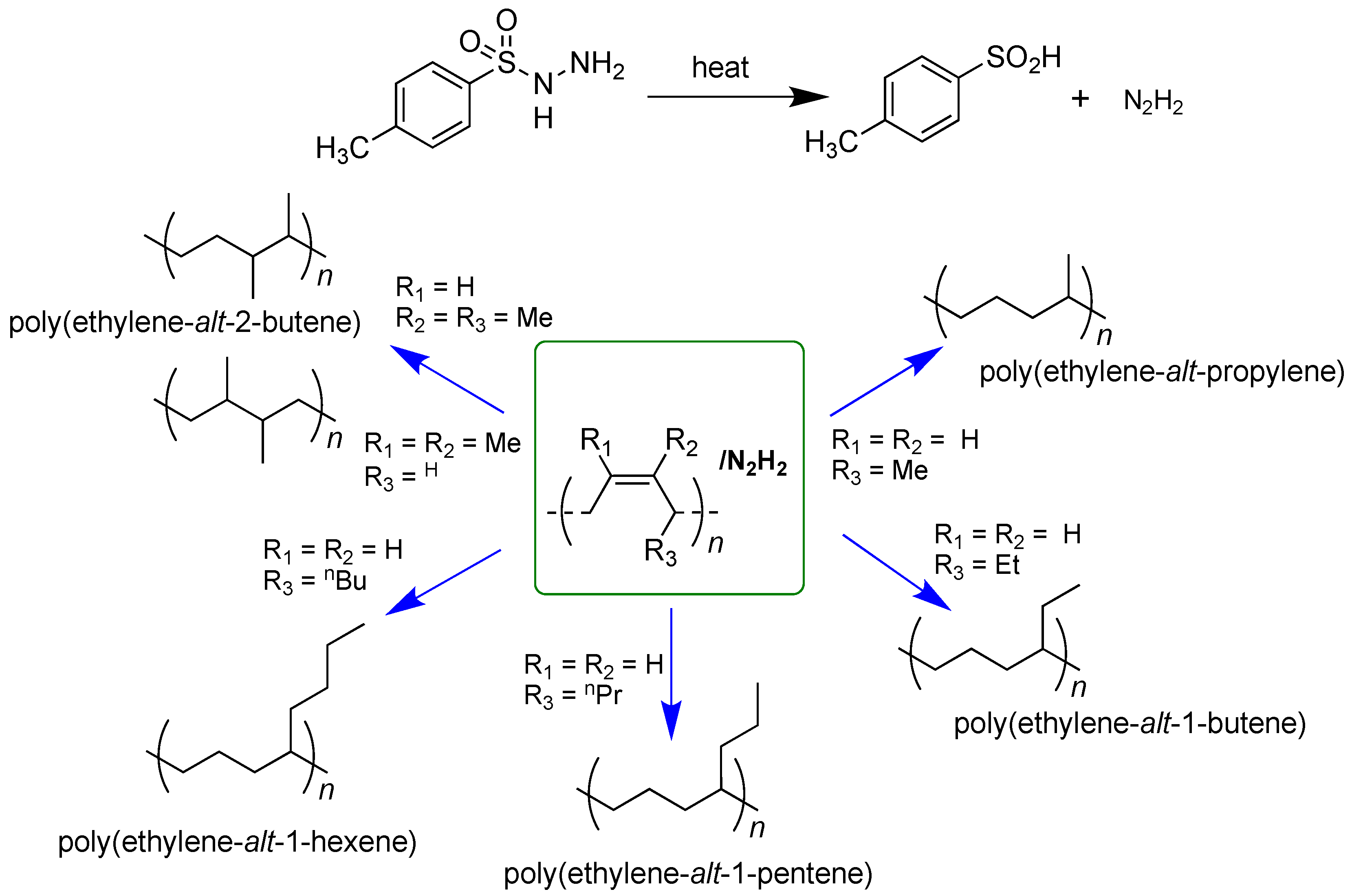
There are different methods to hydrogenate poly(1,3-diene)s, which involve catalytic and non-catalytic processes. The hydrogenation of unsaturated polymers in a non-catalytic way, in which the reaction is promoted by diimide (diazene, NH=NH), turned out to be an attractive process and extremely efficient in the case of 1,3-diene polymers [28,29,35,36,37].
Diimide hydrogenation is a non-catalytic process in which the hydrogenated polymers are formed through the reduction reaction between the diimide and the olefinic group (-C=C-). The diimide molecule (N2H2), as the hydrogenation agent, is generated in situ from the thermal decomposition of p-toluenesulfonyl hydrazide (TSH). The diimide molecule (N2H2) can then release a hydrogen molecule directly to the carbon-carbon double bonds, thus allowing the hydrogenation process.
In this work, we exploited the non-catalytic hydrogenation with diimide in homogeneous conditions at 120 °C, with o-xylene as solvent, of the following highly stereoregular polydienes: isotactic cis-1,4 poly(1,3-hexadiene), isotactic cis-1,4 poly(1,3-heptadiene), isotactic cis-1,4 poly(1,3-octadiene), syndiotactic cis-1,4 poly(1,3-heptadiene), syndiotactic cis-1,4 poly(1,3-octadiene). The hydrogenation process converted the cis-1,4 poly(1,3-hexadiene), poly(1,3-heptadiene)s, and poly(1,3-octadiene)s into perfectly alternating ethylene/1-butene, ethylene/1-pentene and ethylene/1-hexene copolymers (Scheme 1), having iso- or syndiotactic structure as a function of the starting diene polymer, respectively.
Hereafter we will call H(cis1,4isoPHX) the alternating E/1-B copolymers arising from the hydrogenation of isotactic cis-1,4 poly(1,3-hexadiene), H(cis1,4isoPHP) and H(cis1,4syPHP) the alternating E/1-P copolymers arising from the hydrogenation of isotactic and syndiotactic cis-1,4 poly(1,3-heptadiene), H(cis1,4isoPO) and H(cis1,4syPO) the alternating E/1-H copolymers arising from the hydrogenation of isotactic and syndiotactic cis-1,4 poly(1,3-octadiene).
Data concerning tacticity, molecular weight and molecular weight distribution, and glass transition temperature for all the hydrogenated polymers, are summarized in Table 2.

Table 2.
Ethylene-alt-α-olefin copolymers feature.
2.1. Isotactic Alternating Ethylene/1-Butene Copolymer (H(cis1,4isoPHX))
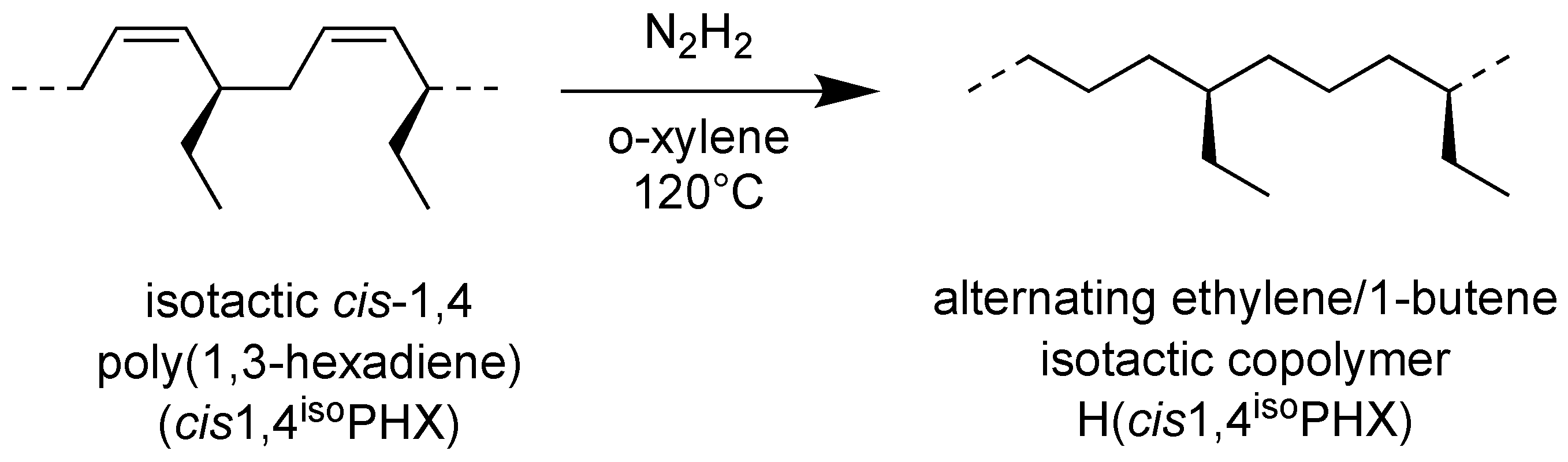
The hydrogenation process of the isotactic cis-1,4 poly(1,3-hexadiene) was illustrated in Scheme 2.
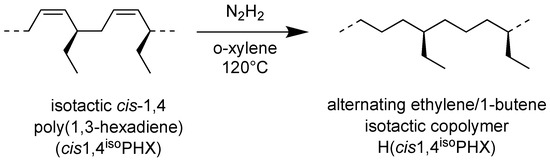
Scheme 2.
Scheme of hydrogenation of isotactic cis-1,4 poly(1,3-hexadiene) (cis1,4isoPHX) to isotactic ethylene-alt-1-butene copolymer H(cis1,4isoPHX).
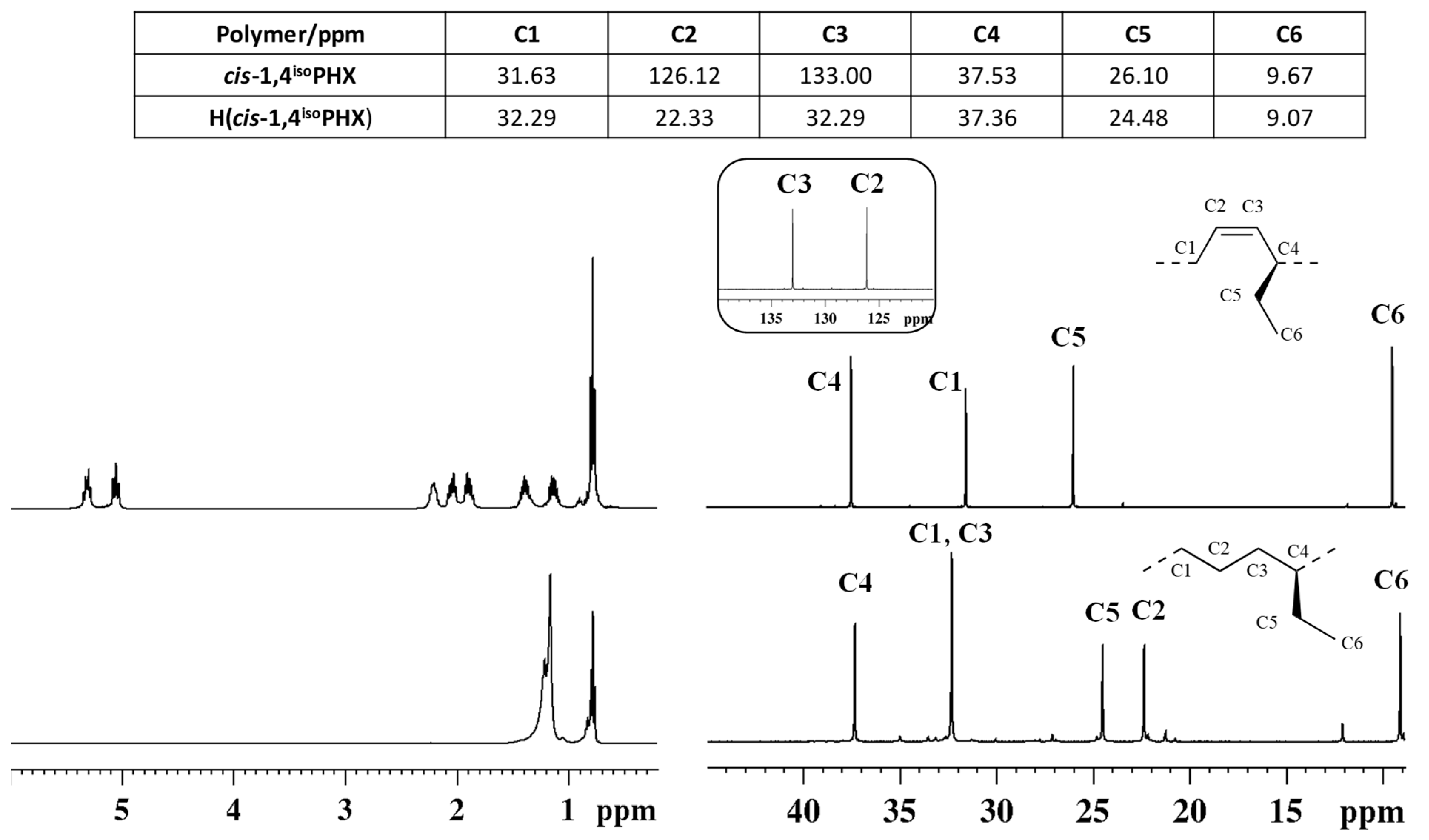
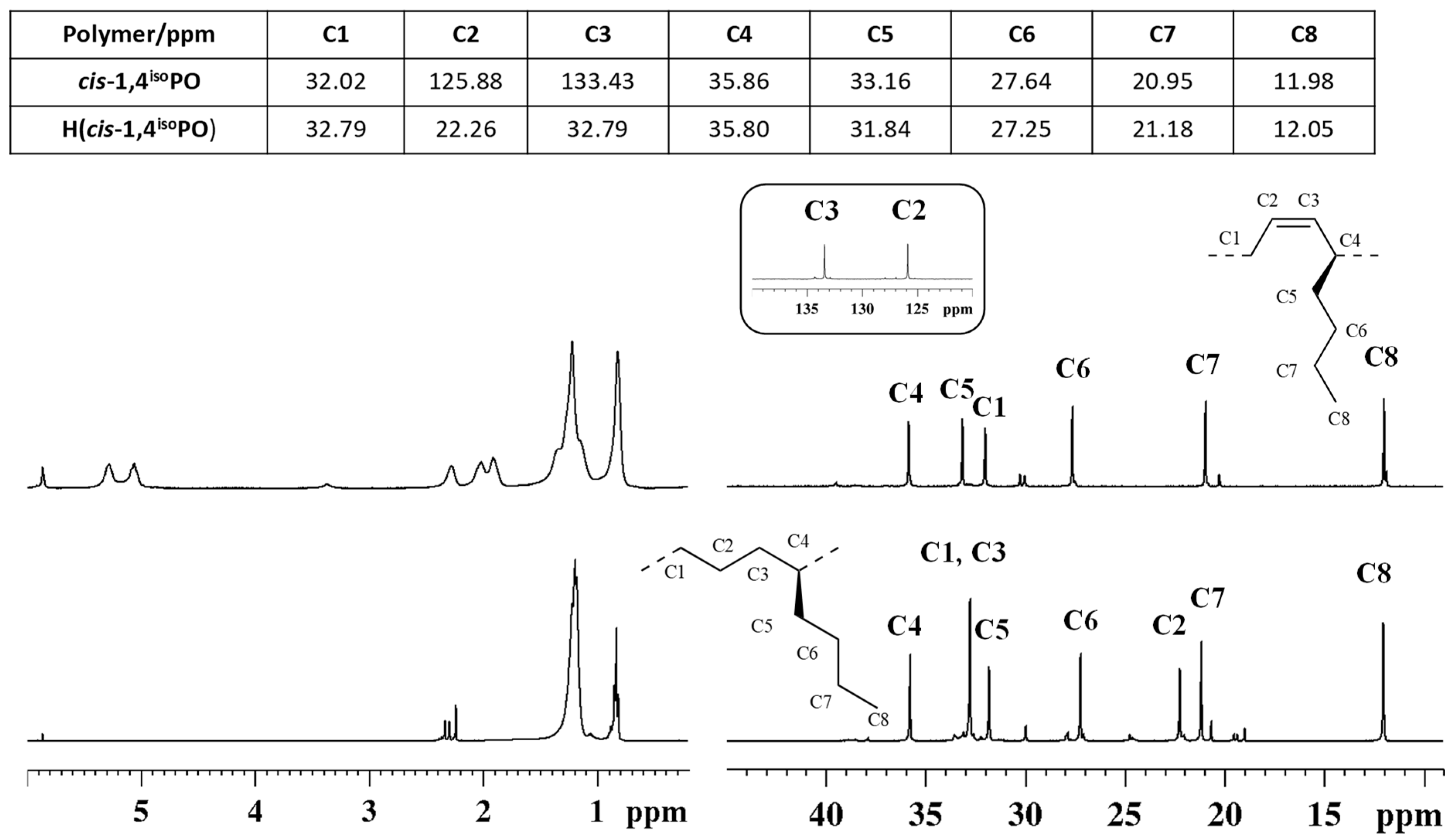
The successful complete hydrogenation of the diene polymer was confirmed by comparison of the Fourier transform infrared (FT-IR) (Figure S1) and 1H and 13C NMR spectra (Figure 1) of the starting cis-1,4 poly(1,3-diene) and of the corresponding hydrogenated product.
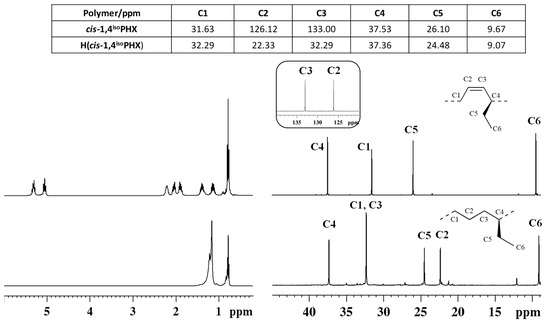
Figure 1.
1H (left) and 13C NMR (right) spectra of isotactic cis-1,4 poly(1,3-hexadiene) ((top), (cis1,4isoPHX)) and its saturated E/1-B copolymer ((bottom), H(cis1,4isoPHX)).
The typical band observed at 751 cm−1 in the FTIR spectrum of cis-1,4 poly(1,3-hexadiene), ascribed to the out-of-plane vibration of the hydrogen atoms adjacent to the double bond in a cis-1,4 unit, is completely absent in the FT-IR spectrum of the corresponding hydrogenated polymer; besides, a new band at 735 cm−1 is observed in the FT-IR spectrum of the hydrogenated polymer, ascribed to the vibration of a –CH2– unit, typical of saturated polyolefins (Figure S1)
The 1H and 13C NMR spectra of the isotactic cis-1,4 poly(1,3-hexadiene) and of the corresponding hydrogenated product are shown in Figure 1. As it is clearly evident, peaks in the olefinic region observed in the 1H NMR (from 5.2 to 5.4 ppm) and 13C NMR (from 120 to 140 ppm) spectra of the diene polymers, and due to the olefinic hydrogen and carbon atoms, are not observed in both the NMR spectra of the hydrogenated polymer, confirming indeed the complete hydrogenation of the diene polymer.
The structure of the ethylene /1-butene copolymer in Figure 1 was determined by means of 1H, 13C, and two-dimensional NMR experiments, such as 1H-13C heteronuclear experiments (HSQC and HMBC in Figure S3) and two-dimensional 1H-1H homonuclear experiments (COSY and TOCSY Figure S4).
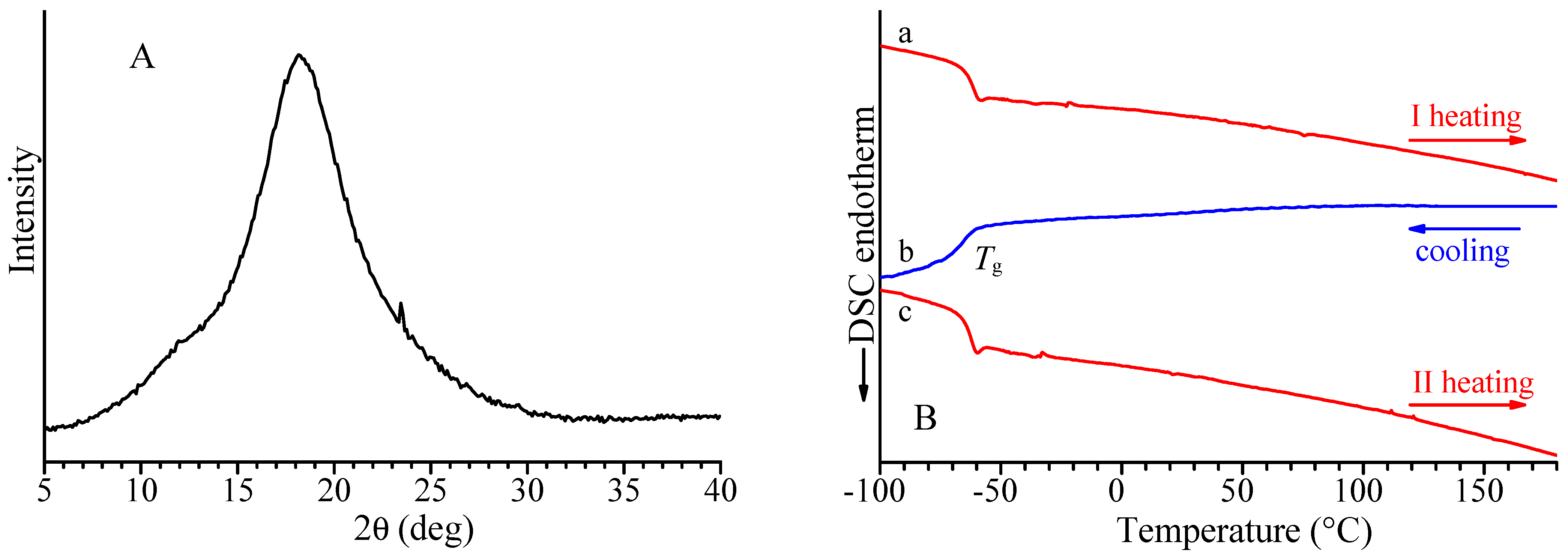
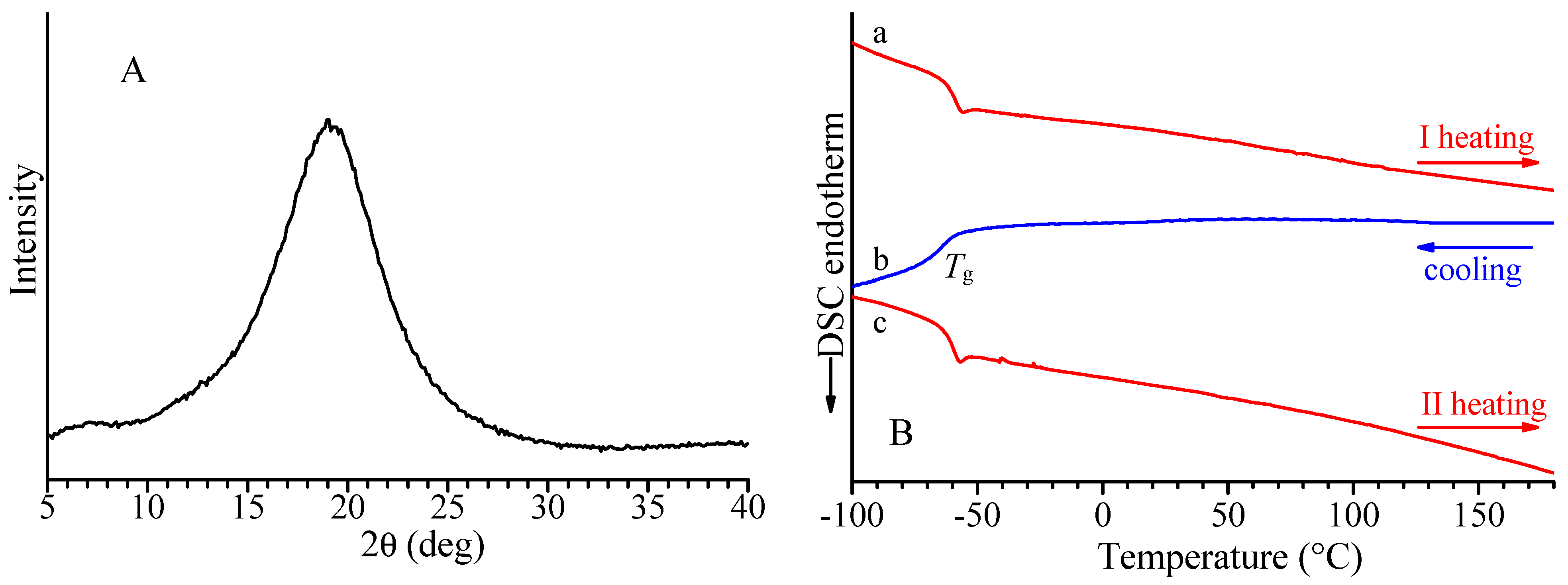
The X-ray diffraction profile of the alternating isotactic ethylene/1-butene copolymer is shown in Figure 2A, and the DSC curves recorded during the first heating, successive cooling from the melt, and second heating of the melt crystallized samples are reported in Figure 2B. Both X-ray diffraction and DSC data indicate the absence of crystallinity in this sample. The alternating isotactic ethylene/1-butene copolymer does not crystallize after annealing or cooling from high temperatures, as confirmed by the absence of exothermic peaks or endothermic peaks in the DSC cooling and heating curves. The DSC shows only a glass transition temperature of −66 °C (Figure 2B and Table 2). As in the case of alternating stereoregular ethylene/propene copolymers prepared from hydrogenation of isotactic and syndiotactic cis-1,4 poly(1,3-pentadiene) [17], the alternating isotactic ethylene/1-butene copolymer is not able to crystallize notwithstanding the regular stereochemical structure.
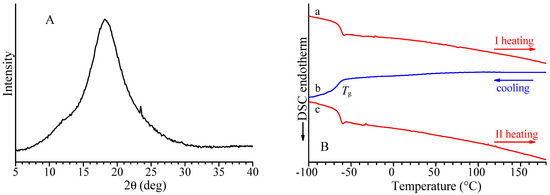
Figure 2.
X-ray powder diffraction profile (A) and DSC curves (B) recorded at 10 °C/min during first heating (a), successive cooling (b), and second heating (c) of alternating E/1-B copolymer (sample H(cis1,4isoPHX)).
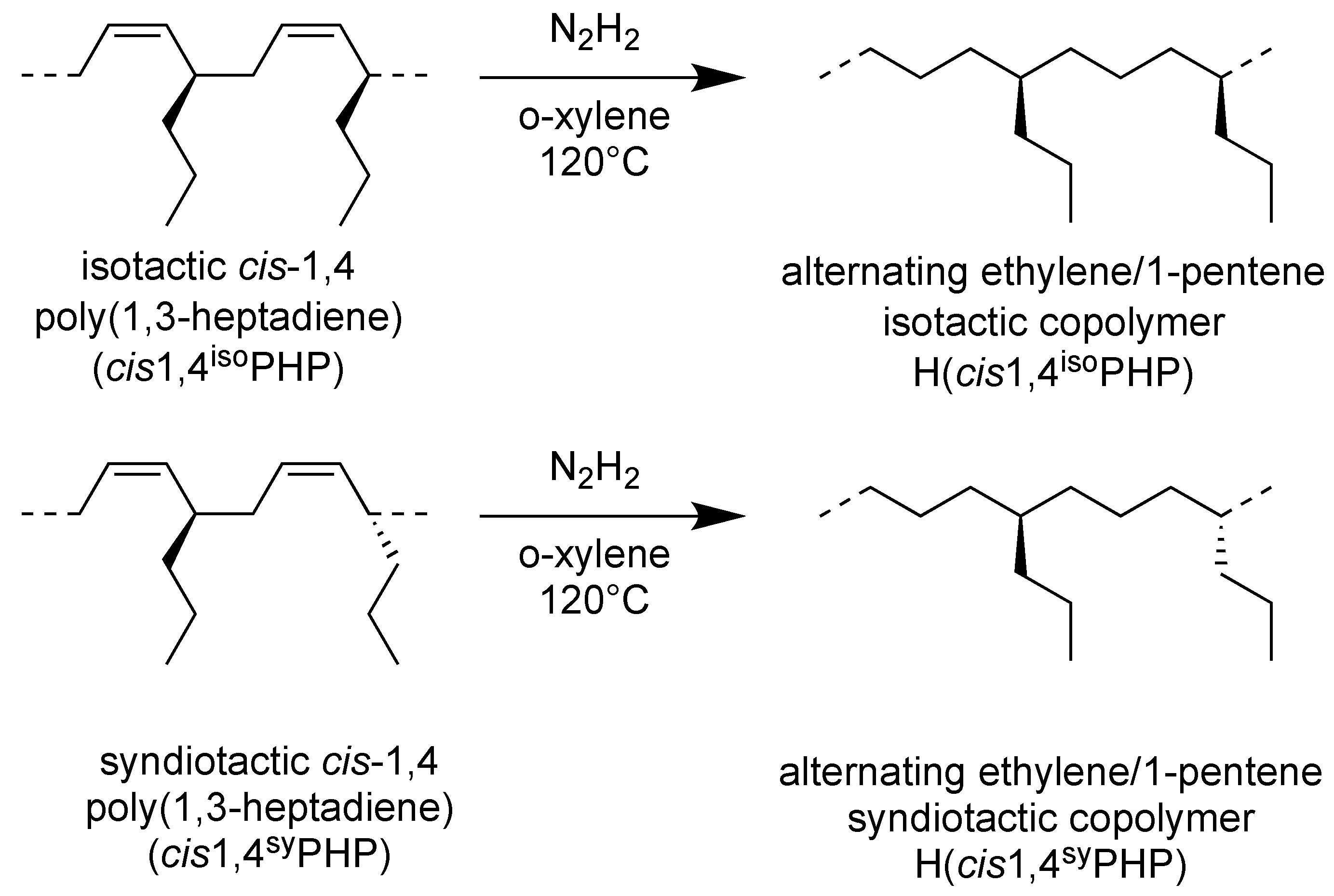
2.2. Iso- and Syndiotactic Alternating Ethylene/1-Pentene Copolymers (H(cis1,4isoPHP) and H(cis1,4syPHP))
The hydrogenation of the isotactic and syndiotactic cis-1,4 poly(1,3-heptadiene)s was shown in Scheme 3.
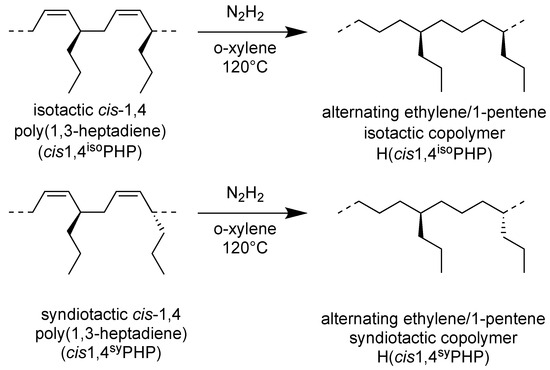
Scheme 3.
Scheme of hydrogenation of (cis1,4isoPHP) and (cis1,4syPHP) to perfectly alternating isotactic and syndiotactic polypentenes (H(cis1,4isoPHP) and H(cis1,4syPHP)).
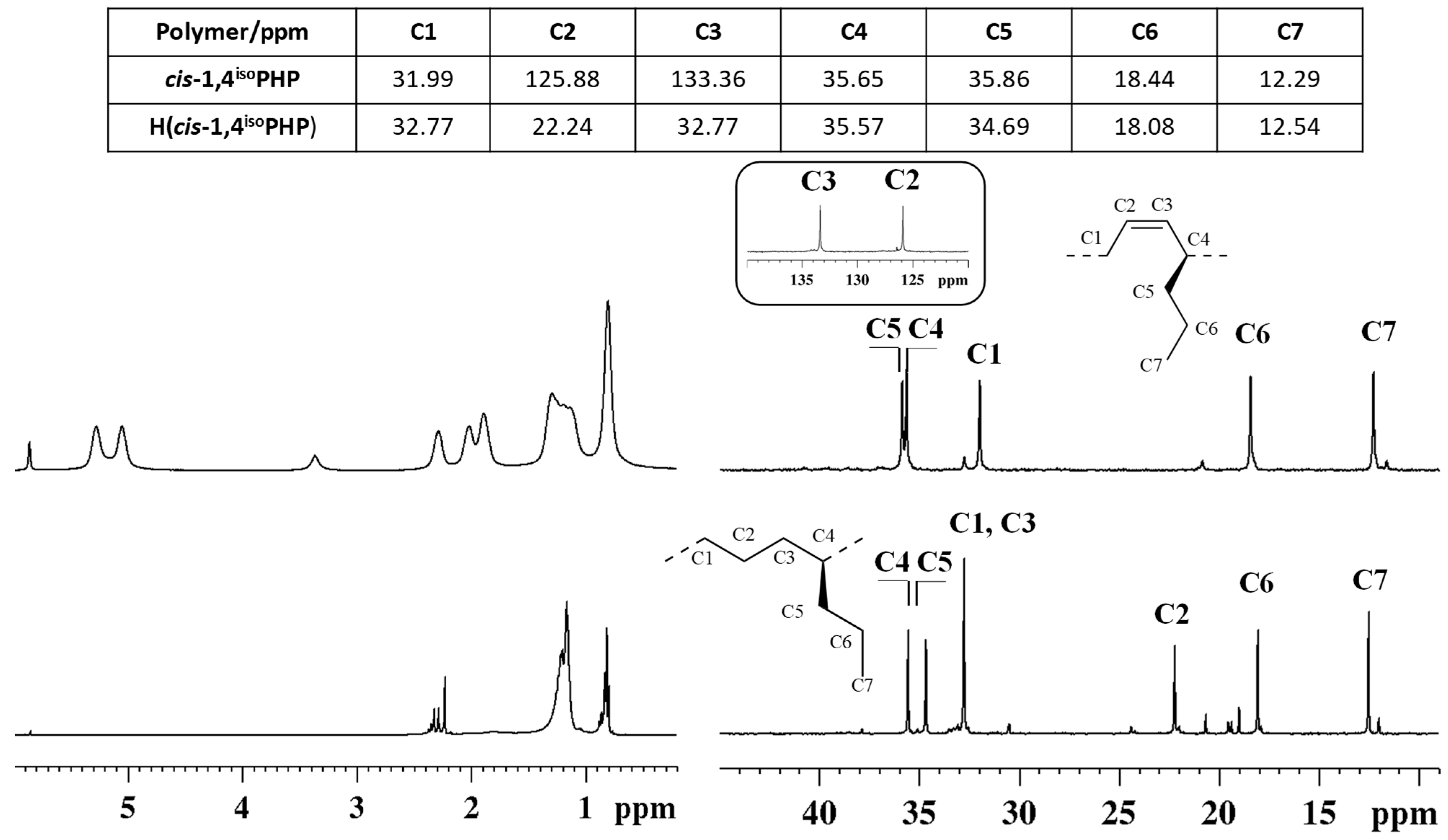
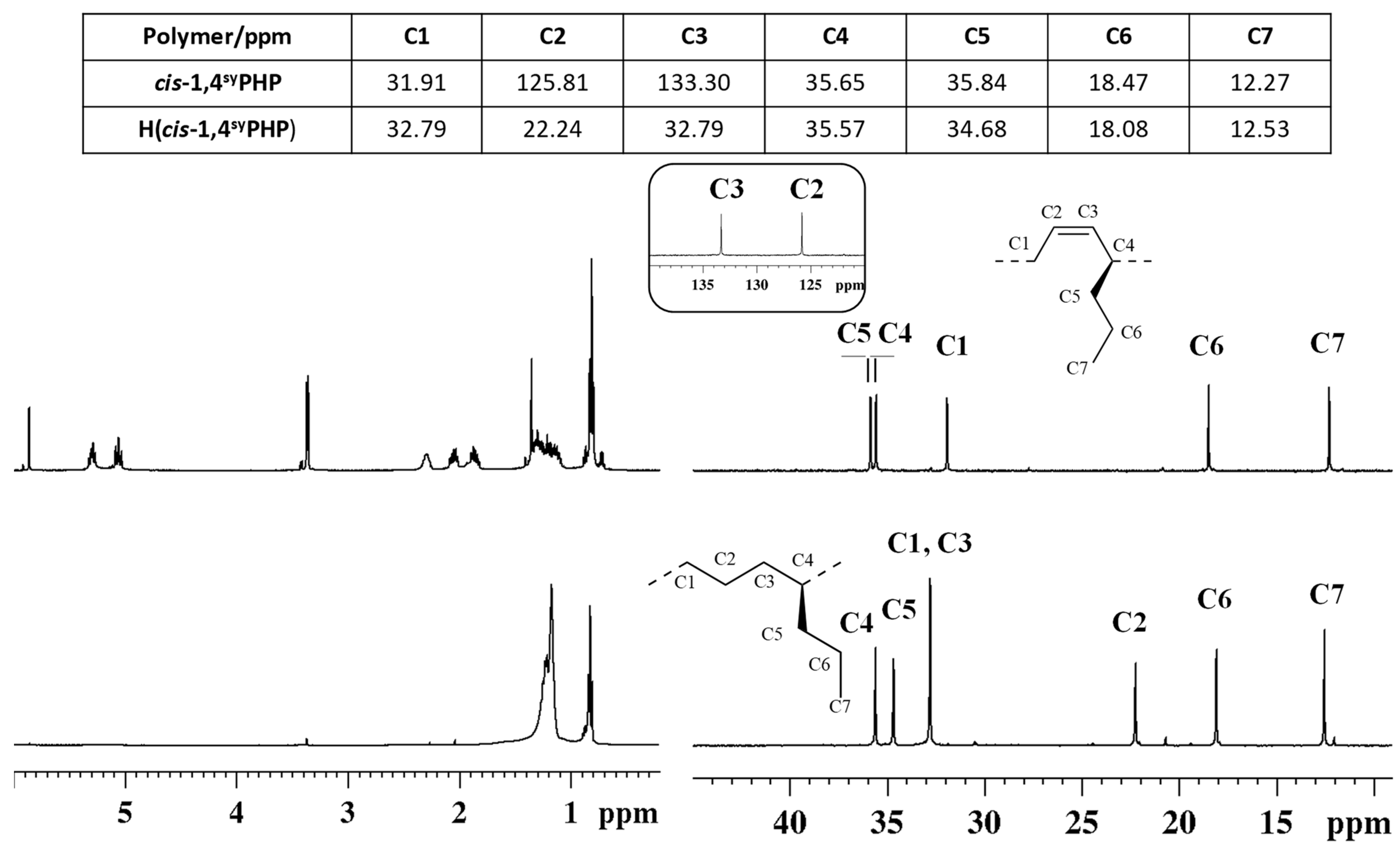
To verify the successful hydrogenation of the diene polymers, FT-IR and 1H and 13C NMR spectra (Figure 3 and Figure 4) of the starting cis-1,4 poly(1,3-diene)s were acquired and compared to the corresponding hydrogenated products.
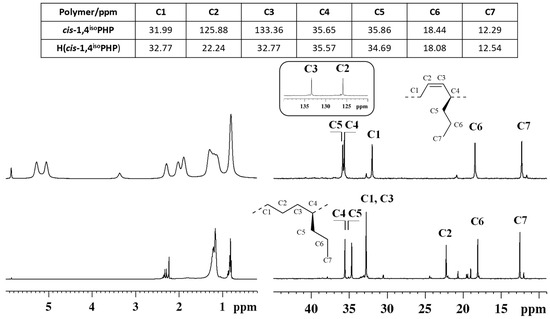
Figure 3.
1H (left) and 13C NMR spectra (right) of isotactic cis-1,4 poly(1,3-heptadiene) (cis-1,4isoPHP) (top) and its saturated E/1-P copolymer (H(cis1,4isoPHP)) (bottom).
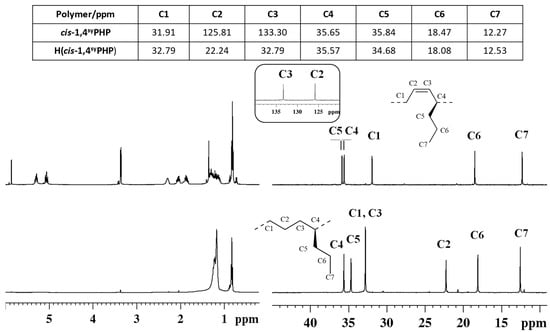
Figure 4.
1H (left) and 13C NMR (right) spectra of syndiotactic cis-1,4 poly(1,3-heptadiene) (cis1,4syPHP) (top) and its saturated E/1-P copolymer (H(cis1,4syPHP)) (bottom).
The peaks attribution of the isotactic and syndiotactic cis-1,4 poly(1,3-heptadiene)s and their saturated E/1-P isotactic and syndiotactic copolymers, are shown in Figure 3 and Figure 4, respectively.
NMR data confirmed the complete hydrogenation reaction for both isotactic and syndiotactic cis-1,4 poly(1,3-heptadiene)s. Furthermore, the structure and tacticity of the resulting ethylene 1-pentene (E/1P) copolymers were analyzed and assigned through two-dimensional NMR experiments reported in Figures S5–S8.
As for the stereoregular E/1-B copolymer, the DSC thermograms and the X-ray diffraction of E/1-P copolymers indicate the both iso- and syndiotactc E/1-P copolymers are amorphous with a Tg of −65 and −63 °C, respectively (Table 2).
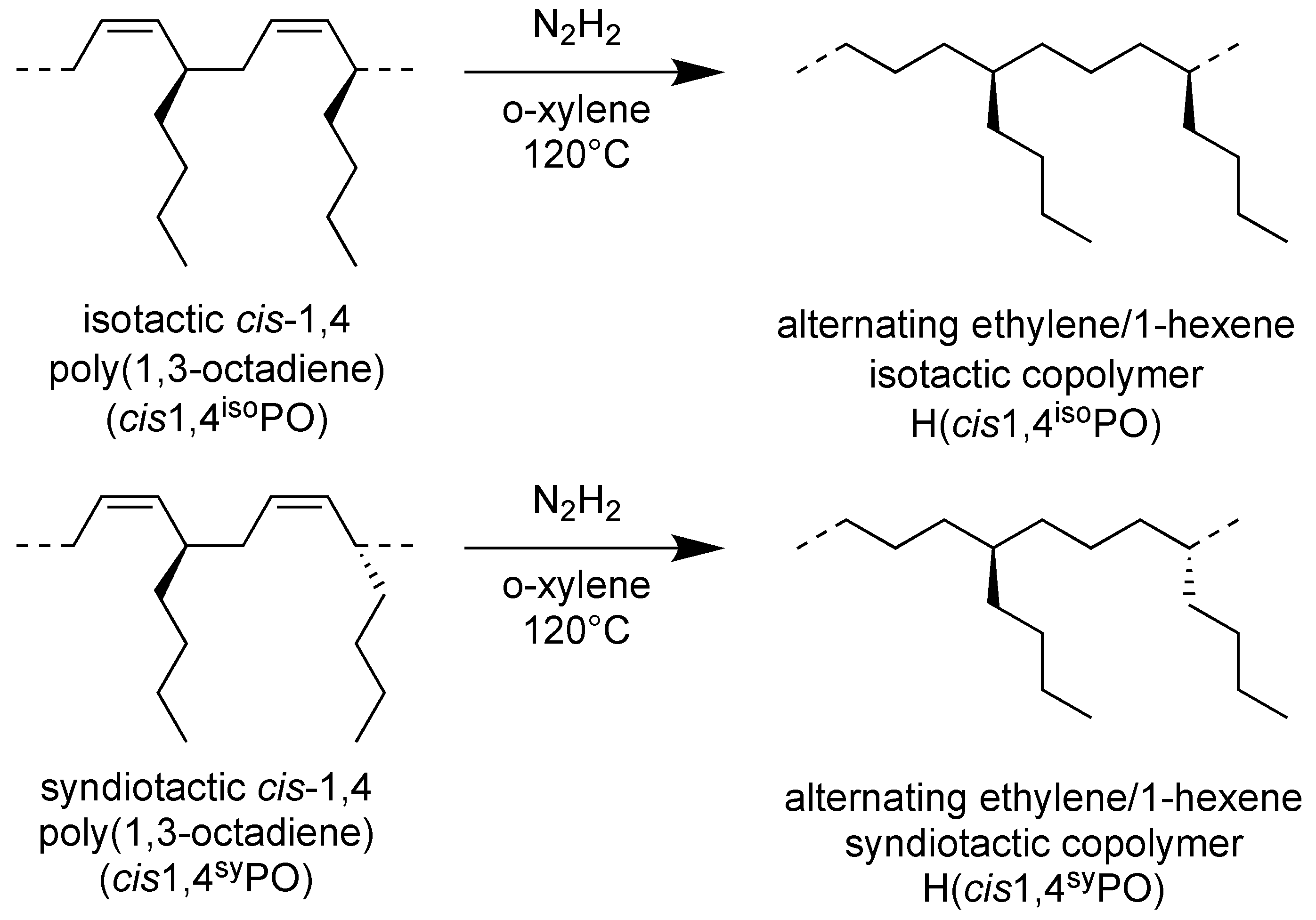
2.3. Iso- and Syndiotactic Alternating Ethylene/1-Hexene Copolymers (H(cis1,4isoPO) and H(cis1,4syndioPO))
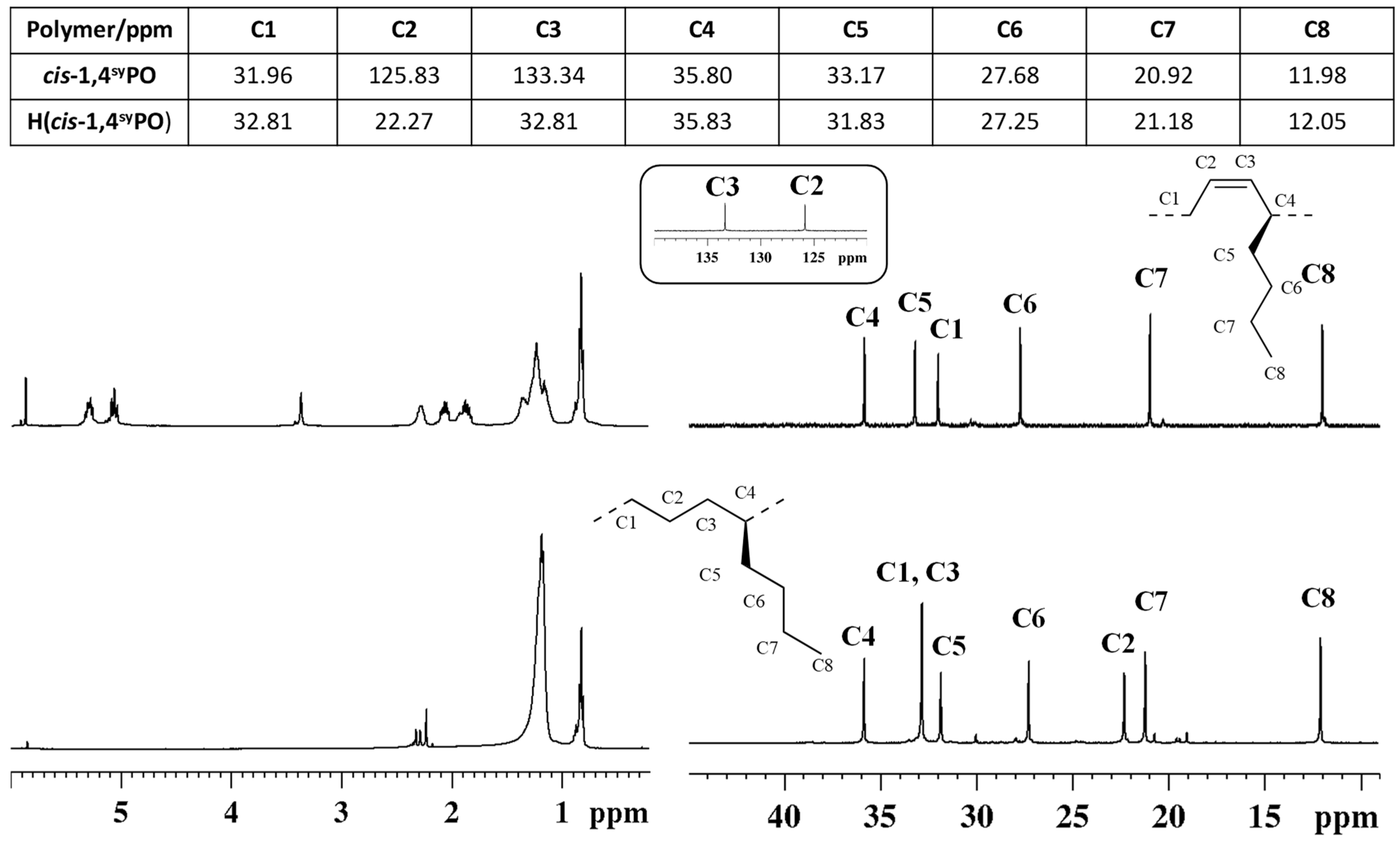
The successful complete hydrogenation of the isotactic and syndiotactic cis-1,4 poly(1,3-octadiene)s to alternating E/1-H copolymers, as reported in Scheme 4, was confirmed by FT-IR (Figure S2) and by 1H and 13C NMR spectra of the starting cis-1,4 poly(1,3-diene)s and of the corresponding hydrogenated products (Figure 5 and Figure 6).
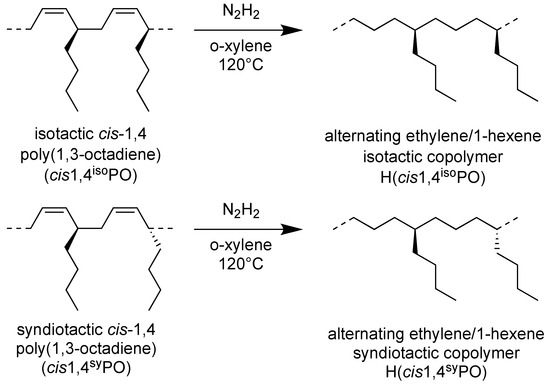
Scheme 4.
Scheme of hydrogenation of isotactic cis-1,4 poly(1,3-octadiene) (cis1,4isoPO) and syndiotactic cis-1,4 poly(1,3-octadiene) (cis1,4syPO) to perfectly alternating ethylene-alt-1-hexene copolymers, isotactic and syndiotactic (H(cis1,4isoPO) and H(cis1,4syPO)).
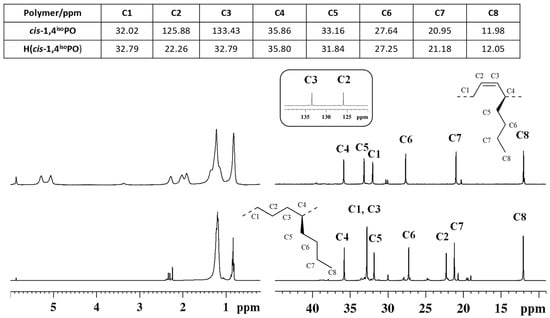
Figure 5.
1H (left) and 13C NMR (right) spectra of isotactic cis-1,4 poly(1,3-octadiene) (cis1,4isoPO) (top) and its saturated E/1-H isotactic copolymer (H(cis1,4isoPO)) (bottom).
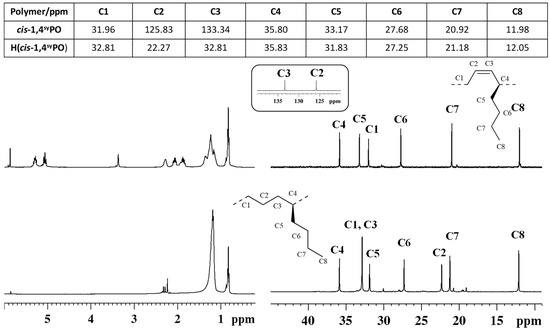
Figure 6.
1H (left) and 13C NMR (right) spectra of syndiotactic cis-1,4 poly(1,3-octadiene) (cis1,4syPO) (top) and its saturated E/1-H syndiotactic copolymer (H(cis1,4syPO)) (bottom).
The peaks in the olefinic regions due to the olefinic hydrogen atoms (from 5.0 to 5.6 ppm) observed in the 1H NMR spectra (Figure 5 and Figure 6) and of olefinic carbon atoms observed in the carbon spectra (at ≈126 and 133 ppm) of the diene polymers are not observed in the spectra of the hydrogenated polymers (Figure 5 and Figure 6), thus confirming indeed the complete hydrogenation reaction.
Also in that case, the polymers microstructure was determined by two dimensional NMR experiments, reported in Figures S9 and S10.
The X-ray diffraction profile of the alternating isotactic ethylene/1-hexene copolymer is shown in Figure 7A, and the DSC curves recorded during the first heating, successive cooling from the melt, and second heating of the melt crystallized samples are reported in Figure 7B. Both X-ray diffraction and DSC data indicate the E/1-H isotactic copolymers are amorphous and are not able to crystallize upon slow cooling from high temperatures or annealing. Similar behavior has been observed for the syndiotactic E/1-H copolymer. The DSC curves show that for both isotactic and syndiotactic E/1-H copolymers, only glass transition temperatures are nearly −60 °C (Figure 7B and Table 2).
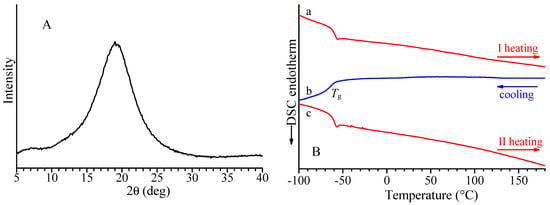
Figure 7.
X-ray powder diffraction profile (A) and DSC curves (B) recorded at 10 °C/min during first heating (a), successive cooling (b), and second heating (c) of alternating E/1-H isotactic copolymer (sample H(cis1,4isoPO)).
3. Materials and Methods
3.1. General Procedures and Materials
Manipulations of air- and/or moisture-sensitive materials were carried out under an inert atmosphere using a dual vacuum/nitrogen line and standard Schlenk-line techniques. Toluene (Sigma-Aldrich, Milano, MI, Italy, ≥99.7% pure) and heptane (Sigma-Aldrich, ≥99% pure) were refluxed over Na for about 8 h and then distilled and stored over molecular sieves under nitrogen. o-Xylene (Sigma-Aldrich, anhydrous grade), p-toluenesulfonylhydrazide (TSH, Sigma-Aldrich), and deuterated solvent for NMR measurements (C2D2Cl4) (Cambridge Isotope Laboratories, Inc., Tewksbury, MA, USA) were used as purchased. 1,3-Hexadiene (Aldrich, 99% pure, mixture of (E) and (Z) isomers), 1,3-heptadiene (Chemsampco, Dallas, TX, USA, 99% pure; predominantly E isomer), and 1,3-octadiene (Chemsampco, 99% pure; predominantly E isomer), were refluxed over calcium hydride for about 4 h, then distilled trap-to-trap, and stored under dry nitrogen. Methylaluminoxane (MAO) (10 wt. % solution in toluene, Sigma-Aldrich) was used as received. Diethylaluminum chloride (AlEt2Cl) (Aldrich, 97% pure), triisobutylaluminum (Al(iBu)3) (Aldrich, 98% pure), and Nd(OCOC7H15)3 (Strem Chemicals, Newburyport, MA, USA) were used as received. CoCl2(PtBu2Me)2 was prepared as described in ref. [38]. Isotactic cis-1,4-poly(E-1,3-hexadiene) (cis1,4isoPHX) [12], isotactic cis-1,4-poly(E-1,3-heptadiene) (cis1,4isoPHP) [13], isotactic cis-1,4-poly(E-1,3-octadiene) (cis1,4isoPO) [13], syndiotactic cis-1,4-poly(E-1,3-heptadiene) (cis1,4syPHP) [13], and syndiotactic cis-1,4-poly(E-1,3-octadiene) (cis1,4syPO) [13], were synthesized as reported in the literature.
3.2. Hydrogenation Procedure
The hydrogenation was carried out in a round-bottom flask equipped with a magnetic stirring, reflux condenser, nitrogen inlet port, and temperature controller. Typically, the specified amount of the diene polymer was dissolved in o-xylene. The reaction mixture was continuously stirred at room temperature until the polymer was completely dissolved. TSH was then added, and the mixture was refluxed by slowly heating to 120 °C. After 3 days, the mixture was allowed to cool spontaneously to room temperature, and TSH was added. This operation is repeated once again. Upon completion of the reaction, the hydrogenated sample was hot-filtered, the volume of the filtered solution was reduced under vacuum, and the dissolved polymer precipitated with methanol and collected by filtration. The polymer was dried under vacuum at room temperature, and then it was extracted with acetone through a Soxhlet method for 10 h in order to remove any excess TSH and byproducts originating from TSH decomposition. The residual polymer was finally dried under a vacuum, dissolved in toluene, precipitated into methanol, and dried again under a vacuum at room temperature to constant weight.
Specifically, for each polymer sample, the hydrogenation conditions were the following:
Cis1,4isoPHX isotactic cis-1,4 poly(1,3-hexadiene): polymer 0.54 g; xylene, 80 mL; first addition of TSH, 4.2 g (2.25 × 10−2 mol); second and third addition of TSH, 7 g (3.74 × 10−2 mol); Yield of hydrogenated polymer H(cis1,4isoPHX): 0.49 g
Cis1,4isoPHP isotactic cis-1,4 poly(1,3-heptadiene): polymer 0.73 g; xylene, 80 mL; first addition of TSH, 5.3 g (2.84 × 10−2 mol); second and third addition of TSH, 9.0 g (4.83 × 10−2 mol); Yield of hydrogenated polymer H(cis1,4isoPHP): 0.70 g
Cis1,4syPHP syndiotactic cis-1,4 poly(1,3-heptadiene): polymer 0.46 g; xylene, 80 mL; first addition of TSH, 3.5 g (1.88 × 10−2 mol); second and third addition of TSH, 7.0 g (3.76 × 10−2 mol); Yield of hydrogenated polymer H(cis1,4syPHP): 0.30 g
Cis1,4isoPO isotactic cis-1,4 poly(1,3-octadiene): polymer 1.08 g; xilene, 125 mL; first addition of THS, 7.7 g (4.76 × 10−2 mol); second and third addition of TSH, 7.7 g (4.76 × 10−2 mol); Yield of hydrogenated polymer H(cis1,4isoPO): 0.96 g
Cis1,4syPO syndiotactic cis-1,4 poly(1,3-octadiene): polymer 0.61 g; xylene, 80 mL; first addition of TSH, 4.3 g (2.3 × 10−2 mol); second and third addition of TSH, 9.0 g (4.83 × 10−2 mol); Yield of hydrogenated polymer H(cis1,4syPO): 0.41 g.
3.3. Polymer Characterization
1H and 13C NMR measurements were carried out on a Bruker (Billerica, MA, USA) Avance 400 spectrometer. The spectra were obtained in C2D2Cl4 at 103 °C (hexamethyldisiloxane, HMDS, as internal standard). The concentration of polymer solutions was about 10 wt %. 13C parameters were: spectral width 17 kHz; 90° pulse 11.0 μs PL1 −5.0 dB, with a delay of 16 s.
The molecular weight averages (Mw) and the molecular weight distribution (Mw/Mn) were obtained by a high-temperature Waters (Milford, MA, USA) GPCV2000 size exclusion chromatography (SEC) system using two online detectors: a differential viscometer and a refractometer. The experimental conditions consisted of three PL Gel Olexis columns, o-DCB as the mobile phase, 0.8 mL min−1 flow rate, and 145 °C temperature. Universal calibration of the SEC system was performed using eighteen narrow Mw/Mn polystyrene standards with molar weights ranging from 162 to 5.6 × 106 g mol−1. For the analysis, about 12 mg of the polymer was dissolved in 5 mL of o-DCB with 0.05% of BHT as the antioxidant.
X-ray powder diffraction profiles were obtained with Ni-filtered Cu Kα radiation by using an Empyrean diffractometer by Malvern Panalytical (Malvern, UK) operating in the reflection geometry with continuous scans of the 2θ angle and scanning rate of 0.02 degree/s
Thermal analysis was performed with a differential scanning calorimeter Mettler-DSC30/2285 (Zurich, Switzerland), equipped with a liquid nitrogen cooling system for measurements at low temperature. The scans were recorded in flowing nitrogen atmosphere at heating or cooling rates of 10 °C/min.
4. Conclusions
Several perfectly alternating ethylene/α-olefin copolymers were prepared by hydrogenation of highly stereoregular cis-1,4 poly(1,3-diene)s. Most of these polymers were completely new, being hardly obtainable by simple stereospecific polymerization of the corresponding α-olefins. All the polymers were fully characterized to determine their (micro)structural and thermal properties.
Some of the polymers obtained exhibited interesting properties for possible application as flexible thermoplastic and elastomeric materials thanks to the very low glass transition temperatures; however, most of the monomers used for the preparation of the stereoregular poly(1,3-diene)s are quite expensive, they will hardly be able to find an industrial application, or, in any case, their possible use should be carefully assessed. However, their use as model polymers, useful for evaluating similar polymers, remains extremely significant.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29061376/s1. FT-IR spectra of the diene polymers and ethylene-alt-α-olefin copolymers; two dimensional NMR spectra of the ethylene-alt-α-olefin copolymers. Figure S1. FTIR spectra of isotactic cis-1,4 poly(1,3-hexadiene) (top, (cis1,4isoPHX)) and its saturated E/1-B copolymer polymer (bottom, H(cis1,4isoPHX); Figure S2. FTIR spectra of (A) isotactic cis-1,4 poly(1,3-octadiene) (cis1,4isoPO) (top) and its saturated E/1-H isotactic copolymer (H(cis1,4isoPO)) (bottom), (B) syndiotactic cis-1,4 poly(1,3-octadiene) (cis1,4syPO) (top) and its saturated E/1-H syndiotactic copolymer (H(cis1,4syPO)) (bottom); Figure S3. Two dimensional 1H-13C HSQC and 1H-13C HMBC spectra of ethylene-alt-1-butene copolymer, H(cis1,4isoPHX); Figure S4. Two dimensional 1H-1H COSY and 1H-1H TOCSY spectra of ethylene-alt-1-butene copolymer, H(cis1,4isoPHX); Figure S5. Two dimensional 1H-13C HSQC and 1H-13C HMBC spectra of ethylene-alt-1-pentene copolymer, H(cis1,4isoPHP); Figure S6. Two dimensional 1H-1H COSY and 1H-1H TOCSY spectra of ethylene-alt-1-pentene copolymer, H(cis1,4isoPHP); Figure S7. Two dimensional 1H-13C HSQC and 1H-13C HMBC spectra of ethylene-alt-1-pentene copolymer, H(cis1,4syPHP); Figure S8. Two dimensional 1H-1H COSY and 1H-1H TOCSY spectra of ethylene-alt-1-pentene copolymer, H(cis1,4syPHX); Figure S9. Two dimensional 1H-13C HSQC and 1H-13C HMBC spectra of ethylene-alt-1-hexene copolymer, H(cis1,4isoPO); Figure S10. Two dimensional 1H-1H COSY and 1H-1H TOCSY spectra of ethylene-alt-1-hexene copolymer, H(cis1,4isoPHO).
Author Contributions
Conceptualization, G.R.; investigation G.R., A.C.B., I.P., C.D.R. and M.S.; writing—original draft preparation, G.R., A.C.B., C.D.R. and M.S.; writing—review and editing, A.C.B. and G.R.; supervision, G.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors are grateful to Daniele Piovani for SEC measurements.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Porri, L.; Giarrusso, A. Conjugated Diene Polymerization. In Comprehensive Polymer Science; Eastmond, G., Edwith, A., Russo, S., Sigwalt, P., Eds.; Pergamon Press Ltd.: Oxford, UK, 1989; Volume 4, Part II; pp. 53–108. [Google Scholar]
- Thiele, S.K.H.; Wilson, D.R. Alternate Transition Metal Complex Based Diene Polymerization. J. Macromol. Sci. Part C Polym. Rev. 2003, 43, 581–628. [Google Scholar] [CrossRef]
- Friebe, L.; Nuyken, O.; Obrecht, W. Neodymium-Based Ziegler/Natta Catalysts and their Application in Diene Polymerization. Adv. Polym. Sci. 2006, 204, 1–154. [Google Scholar]
- Porri, L.; Giarrusso, A.; Ricci, G. Recent views on the mechanism of diolefin polymerization with transition metal initiator systems. Prog. Polym. Sci. 1991, 16, 405–441. [Google Scholar] [CrossRef]
- Ricci, G.; Sommazzi, A.; Masi, F.; Ricci, M.; Boglia, A.; Leone, G. Well Defined Transition Metal Complexes with Phosphorus and Nitrogen Ligands for 1,3-Dienes Polymerization. Coord. Chem. Rev. 2010, 254, 661–676. [Google Scholar] [CrossRef]
- Ricci, G.; Pampaloni, G.; Sommazzi, A.; Masi, F. Dienes polymerization: Where we are and what lies ahead. Macromolecules 2021, 54, 5879–5914. [Google Scholar] [CrossRef]
- Ricci, G.; Boffa, G.; Porri, L. Polymerization of 1,3-dialkenes with neodymium catalysts. Some remarks on the influence of the solvent. Makromol. Chem. Rapid Commun. 1986, 7, 355–359. [Google Scholar] [CrossRef]
- Ricci, G.; Italia, S.; Cabassi, F.; Porri, L. Neodymium catalysts for 1,3-diene polymerization: Influence of the preparation conditions on activity. Polym. Commun. 1987, 28, 223–226. [Google Scholar]
- Purevsuren, B.; Allegra, G.; Meille, S.V.; Farina, A.; Porri, L.; Ricci, G. cis-Isotactic 1,4-polypentadiene. NMR solution characterization and crystal structure of polymers prepared with neodymium-catalytic systems. Polym. J. 1998, 30, 431–434. [Google Scholar] [CrossRef]
- Meille, S.V.; Capelli, S.; Allegra, G.; Ricci, G. Isotactic cis-1,4-poly(3-methyl-1,3-pentadiene): A new conformation for isotactic cis-1,4-polydienes. Macromol. Rapid Commun. 1995, 16, 329–335. [Google Scholar] [CrossRef]
- Ricci, G.; Zetta, L.; Porri, L.; Meille, S.V. Synthesis and characterization of isotactic cis-1,4 Poly(3-methyl-1,3-pentadiene). Macromol. Chem. Phys. 1995, 196, 2785–2793. [Google Scholar] [CrossRef]
- Ricci, G.; Zetta, L.; Meille, S.V. Synthesis and characterization of cis-1,4 iso- and syndiotactic poly(1,3-hexadiene). Gazz. Chim. Ital. 1996, 126, 401–404. [Google Scholar]
- Boccia, A.C.; Leone, G.; Boglia, A.; Ricci, G. Novel Stereoregular cis-1,4 and trans-1,2 Poly(diene)s: Synthesis, Characterization, and Mechanistic Considerations. Polymer 2013, 54, 3492–3503. [Google Scholar] [CrossRef]
- Oliva, L.; Longo, P.; Grassi, A.; Ammendola, P.; Pellecchia, C. Polymerization of 1,3-alkadienes in the presence of Ni- and Ti-based catalytic systems containing methylalumoxane. Makromol. Chem. Rapid Commun. 1990, 11, 519–524. [Google Scholar] [CrossRef]
- Venditto, V.; De Rosa, C.; Guerra, G.; Napolitano, R. X-ray diffraction, conformational analysis and stereoregularity of a crystalline poly(3-methyl-1, 3-pentadiene). Polymer 1992, 33, 3547–3551. [Google Scholar] [CrossRef]
- Ricci, G.; Leone, G.; Boccia, A.C.; Pierro, I.; Zanchin, G.; Mauri, M.; Scoti, M.; Malafronte, A.; Auriemma, F.; De Rosa, C. Perfectly alternating ethylene/2-butene copolymers by hydrogenation of highly stereoregular 1,4 poly(1,3-diene)s: Synthesis and characterization. Macromolecules 2017, 50, 754–761. [Google Scholar] [CrossRef]
- Ricci, G.; Boccia, A.C.; Leone, G.; Pierro, I.; Zanchin, G.; Scoti, M.; Auriemma, F.; De Rosa, C. Isotactic and Syndiotactic Alternating Ethylene/Propylene Copolymers Obtained Through Non-Catalytic Hydrogenation of Highly Stereoregular cis-1,4 Poly(1,3-diene)s. Molecules 2017, 22, 755. [Google Scholar] [CrossRef]
- Simpson, D.M.; Vaughan, G.A. Encyclopedia of Polymer Science and Technology; Mark, H.F., Ed.; Wiley: New York, NY, USA, 2003; Volume 2, pp. 441–482. [Google Scholar]
- Czaia, K.; Bialek, M. Microstructure of ethylene-1-hexene and ethylene-1-octene copolymers obtained over Ziegler–Natta catalysts supported on MgCl2(THF)2. Polymer 2001, 42, 2289–2297. [Google Scholar]
- Kim, I.; Kim, J.H.; Choi, H.K.; Chung, M.C.; Woo, S.I. Comonomer enhancement effect of 1-hexene in ethylene copolymerization catalyzed over MgCl2/THF/TiCl4 catalysts. J. Appl. Polym. Sci. 1993, 48, 721–730. [Google Scholar] [CrossRef]
- Uozumi, T.; Soga, K. Copolymerization of olefins with Kaminsky-Sinn-type catalysts. Makromol. Chem. 1992, 193, 823–831. [Google Scholar] [CrossRef]
- Kokko, E.; Lehmus, P.; Malmberg, A. Organometallic Catalyst and Olefin Polymerization, Catalysts for a New Millenium; Blom, R., Follestad, A., Rytter, E., Tilset, M., Ystenes, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; p. 345. [Google Scholar]
- Galland, G.B.; Quijada, P.; Mauler, R.S.; Menezes, S.C. Determination of reactivity ratios for ethylene/α-olefin copolymerization catalysed by the C2H4[Ind]2ZrCl2/methylaluminoxane system. Macromol. Rapid Commun. 1996, 17, 607–613. [Google Scholar] [CrossRef]
- Dankova, M.; Waymouth, R.M. High Comonomer Selectivity in Ethylene/Hexene Copolymerization by Unbridged Indenyl Metallocenes. Macromolecules 2003, 36, 3815–3820. [Google Scholar] [CrossRef]
- Schneider, M.J.; Suhm, J.; Mulhaupt, R.; Prosenc, M.H.; Brintzinger, H.-H. Influence of Indenyl Ligand Substitution Pattern on Metallocene-Catalyzed Ethene Copolymerization with 1-Octene. Macromolecules 1997, 30, 3164–3168. [Google Scholar] [CrossRef]
- Uozumi, T.; Ahn, C.-H.; Tomisaka, M.; Jin, J.; Tian, G.; Sano, T.; Soga, K. Synthesis of ethylene-α-olefin alternating copolymers with Et(1-Ind)(9-Flu)ZrCl2-MAO catalyst system. Macromol. Chem. Phys. 2000, 201, 1748–1752. [Google Scholar] [CrossRef]
- Kakinuki, K.; Fujiki, M.; Nomura, K. Copolymerization of Ethylene with α-Olefins Containing Various Substituents Catalyzed by Half-Titanocenes: Factors Affecting the Monomer Reactivities. Macromolecules 2009, 42, 4585–4595. [Google Scholar] [CrossRef]
- Singha, N.K.; Bhattacharjee, S.; Sivaram, S. Hydrogenation of Diene Elastomers, Their Properties and Applications: A Critical Review. Rubber Chem. Technol. 1997, 70, 309–367. [Google Scholar] [CrossRef]
- Samran, J.; Phinyocheep, P.; Daniel, P.; Kittipoom, S. Hydrogenation of unsaturated rubbers using diimide as a reducing agent. J. App. Pol. Sc. 2005, 95, 16–27. [Google Scholar] [CrossRef]
- Bravaya, N.M.; Faingol’d, E.E.; Badamshina, E.R.; Sanginov, E.A. Advances in Synthesis of Ethylene–Propylene–Diene Elastomers by Ion-Coordination Polymerization on Single-Site Catalytic Systems of New Generation. Polym. Sci. Ser. C 2020, 62, 1–16. [Google Scholar] [CrossRef]
- Zanchin, G.; Leone, G. Polyolefin thermoplastic elastomers from polymerization catalysis: Advantages, pitfalls and future challenges. Prog. Polym. Sci. 2021, 113, 101342. [Google Scholar] [CrossRef]
- Wang, W.; Qu, S.; Li, X.; Chen, J.; Guo, Z.; Sun, W.-H. Transition metal complex catalysts promoting copolymers of cycloolefin with propylene/higher olefins. Coord. Chem. Rev. 2023, 494, 215351. [Google Scholar] [CrossRef]
- Sun, M.; Xiao, Y.; Liu, K.; Yang, X.; Liu, P.; Jie, S.; Hu, J.; Shi, S.; Wang, Q.; Lim, K.H.; et al. Synthesis and characterization of polyolefin thermoplastic elastomers: A review. Can. J. Chem. Eng. 2023, 101, 4886–4906. [Google Scholar] [CrossRef]
- Mundil, R.; Bravo, C.; Merle, N.; Zinck, P. Coordinative Chain Transfer and Chain Shuttling Polymerization. Chem. Rev. 2024, 124, 210–244. [Google Scholar] [CrossRef] [PubMed]
- Mango, L.A.; Lenz, R.W. Hydrogenation of unsaturated polymers with diimide. Makromol. Chem. 1973, 163, 13. [Google Scholar] [CrossRef]
- Phinyocheep, P.; Pasiri, S.; Tavachai, O. Diimide hydrogenation of isoprene–styrene diblock copolymers. J. Appl. Polym. Sci. 2003, 87, 76. [Google Scholar] [CrossRef]
- Harwood, H.J.; Russel, D.B.; Verthe, J.J.A.; Zymonas, J. Diimide as a reagent for the hydrogenation of unsaturated polymers. Die Makromol. Chem. 1973, 163, 1–12. [Google Scholar] [CrossRef]
- Ricci, G.; Forni, A.; Boglia, A.; Motta, T. Synthesis, structure, and butadiene polymerization behavior of alkylphosphine cobalt(II) complexes. J. Mol. Catal. A Chem. 2005, 226, 235–241. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).