Abstract
Artemisia japonica Thunb. has been used as a traditional Chinese medicine and a vegetable for thousands of years in China. However, there are few reports on the chemical composition and biological activity of its leaves. Thus, this study aimed to evaluate the chemical composition, antioxidant and anti-inflammatory effects of water extracts of A. japonica leaves and their underlying mechanisms. A total of 48 compounds were identified in the water extract using UPLC-QTOF-MS2 analysis, with phenolic acids, particularly chlorogenic acid compounds, being the predominant components. The ethyl acetate fraction (EAF) contained most of the total phenolic content (385.4217 mg GAE/g) and displayed superior antioxidant capacity with the IC50DPPH•, IC50ABTS•+, and OD0.5reducing power at 10.987 μg/mL, 43.630 μg/mL and 26.883 μg/mL, respectively. Furthermore, EAF demonstrated potent antioxidant and anti-inflammatory effects in LPS-induced RAW264.7 cells by upregulating the Nrf2/HO-1 signal pathway. These findings highlight that A. japonica leaves possess remarkable abilities to mitigate inflammation and oxidative stress, suggesting their potential utilization as medicinal agents and food additives for promoting human health.
1. Introduction
Artemisia L. is a large genus comprising over 500 species, which are mainly found in northern temperate regions, such as North America, Europe, and Asia [1]. The Artemisia species plays an important role in the traditional herbal medicine of various countries and is frequently utilized for the treatment of hepatitis, malaria, cancer, infections, and inflammation diseases [1]. Most Artemisia plants are rich in essential oil and are used as aromatic plants, which have high application value in the cosmetics and pharmaceutical industry [1,2]. Besides the high-value essential oils, Artemisia plants (especially leaves) are also rich in hydrophilic phenolic acids and flavonoids, and the high content of total phenolic compounds in the aqueous extracts of Artemisia plants contributes to their high antioxidant activities [3]. Numerous studies have provided evidence of beneficial health effects of consumption of these phenolic compounds due to their antioxidant, anti-inflammation, and vasodilation activities [4], which makes Artemisia plants potentially functional foods. Indeed, in addition to being widely used as folk herbal medicine in the treatment of various diseases, many Artemisia species are also used as food, spices, condiments, and beverages, on which Trendafilova A. et al. have made a systematic review [5].
Artemisia japonica Thunb. (A. japonica) is a perennial herb of the Artemisia genus, mainly distributed in Asia. According to the Xian Dai Ben Cao Gang Mu, the whole herb of A. japonica can be used as medicine, which has the effects of clearing away heat, relieving exterior, cooling blood, and killing insects, and is mainly used for treating cold, fever, strain, cough, hot flashes, heatstroke, malaria, hypertension, aphthous, scabies, and eczema in China [6]. The chemical composition of essential oil from the leaves of A. japonica has been extensively studied [7,8]. A series of terpenoids, phenolic acids, and flavonoids were also identified by column chromatography, but most of which were hydrophobic components and lacked pharmacological evaluation [9,10]. On the contrary, several pharmacological studies of A. japonica showed that its aqueous extract had great anti-inflammatory, anti-oxidation, and hemostatic activities [11,12]. Genotoxicity and maximum tolerance tests in mice also showed that the water extract of A. japonica had low toxicity and high safety [11,12]. Moreover, the purified polysaccharide from A. japonica could significantly alleviate collagen-induced arthritis in mice [13]. These results, at least in part, reveal the effectiveness of A. japonica as a traditional medicine and the safety of A. japonica as an edible and food additive in China, Korea, and Japan [5,14]. However, to the best of our knowledge, no systematic study has been reported on the chemical profile of the water extracts of A. japonica. Therefore, additional research is needed to uncover the bioactive components in the water extracts of A. japonica.
In the present study, A. japonica leaves were extracted with water, and the extract was successively partitioned with ethyl acetate and n-butanol. The content of total phenolics and their antioxidant, anti-inflammatory, and potential mechanisms were analyzed, and the chemical compositions of water extract were investigated by the UPLC-QTOF-MS2 technique. We believe that the results from this study will provide sufficient evidence for the further study and utilization of A. japonica.
2. Results and Discussion
2.1. Chemical Composition
The chemical profiles of A. japonica were characterized by UPLC-QTOF-MS2 analysis. A total of 48 compounds were detected in the water extract (Table 1, Figure 1). Four compounds were identified by comparing the retention time, high-resolution molecular ions ([M-H]−), and major MS2 fragment ions of the compounds with those of the standards (Table 1). For example, compound 3 had the same retention time, molecular ion ([M-H]−) at m/z 353, and major fragment at m/z 191 ([quinic acid-H]− ion) and 179 ([caffeic acid-H]− ion) with the authenticated standard chlorogenic acid, indicating that compound 3 was chlorogenic acid. Similarly, compounds 29, 31, and 36 were positively identified as isochlorogenic acid B, isochlorogenic acid A, and isochlorogenic acid C (Table 1).

Table 1.
Chemical components in the water extract of A. japonica.
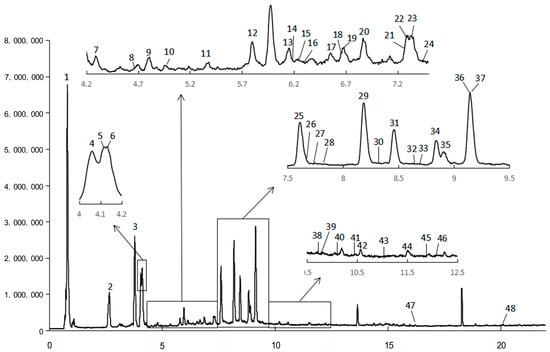
Figure 1.
MS chromatography of water extract (WE) of the leaves of A. japonica recorded by UPLC-QTOF-MS2.
The rest of the 44 compounds were tentatively identified according to the retention time, experimental and theoretical molecular ions ([M-H]−), major MS2 fragment ions, and the MS data in the literature, especially those reported on Artemisia species [15,16,17,18,19,20]. For instance, compounds 2, 3, 4, and 9 displayed a [M-H]− ion at m/z 353, and its MS2 spectrum exhibited a base peak at m/z 191, 191, 179, and 191, respectively. According to the reports, quinic acid was substituted at the 1-OH or 5-OH position and produced a base peak at m/z 191 [20]. As compound 3 has been authenticated by the standard as 5-O-caffeoylquinic acid, therefore, compounds 2 or 9 are likely to be 1-O-caffeoylquinic acid. Research indicated that 5-O-caffeoylquinic acid has greater hydrophobicity than 1-O-caffeoylquinic acid, which ensures that the two isomers can be differentiated on reversed-phase packing [21]; therefore, compound 2 was tentatively characterized as 1-O-caffeoylquinic acid. In addition, Zhang Peijie et al. showed that the order of peaks of 3-OH, 4-OH, and 5-OH substitutes of quinic acid on the reversed-phase column was 5-OH, 4-OH, and 3-OH substitutes; meanwhile, the MS2 spectrum of 3-O-caffeoylquinic acid only gave a [quinic acid–H]− ion at m/z 191 [18]. Therefore, compounds 4 and 9 were tentatively characterized as 4-O-caffeoylquinic acid and 3-O-caffeoylquinic acid (Table 1). Another example is that compounds 8, 13, and 14 all give a [M-H]− ion at m/z 367, with typical MS2 fragments at m/z 193 ([ferulic acid-H]−), indicating that the three compounds were feruloylquinic acid isomers. Similar to caffeic acid modification, the elution order of 3-OH, 4-OH, and 5-OH modification of quinic acid by ferulic acid on the reversed-phase column was 5-OH, 4-OH, and 3-OH substitutes [18,20]. Therefore, compounds 8, 13, and 14 were tentatively characterized as 5-O-feruloylquinic acid, 4-O-feruloylquinic acid, and 3-O-feruloylquinic acid. Moreover, compounds 28, 29, 31, 36, and 42 exhibited a [M-H]− ion at m/z 515 and the major fragments at 353 (loss of caffeoyl), 191 ([M-H]− of quinic acid), and 179 ([M-H]− of caffeic acid), indicating that they were dicaffeoylquinic acid isomers. Compounds 29, 31, and 36 were further identified with the authentic standards as 3,4-O-dicaffeoylquinic acid, 3,5-O-dicaffeoylquinic acid, and 4,5-O-dicaffeoylquinic acid, respectively. The elution order on the reversed-phase column of these three compounds was in accordance with the order reported by Clifford et al. [21]. Additionally, compounds 40, 41, and 43 manifested [M-H]− ion at m/z 529 and displayed characteristic fragments either at m/z 367 ([feruloylquinic acid-H]−), 335 ([caffeoylquinic acid-H2O-H]−), 179 ([caffeic acid-H]−), or 173 ([quinic acid-H2O-H]−), indicating that they were caffeoylferuloylquinic acid isomers. Consequently, a total of 48 compounds, including 21 phenolic acids, 18 flavonoids, 3 terpinoids, 2 phenolics, 2 phenylpropanoids, and 2 organic acids, were tentatively identified from the water extract of A. japonica. Due to the lack of research on the water-soluble components of A. japonica, only three of the above-identified compounds (19, 33, and 48) have been reported in A. japonica. However, except for the compound vitexnegheteroin M (39), all other compounds have been reported in other Artemisia plants [15,16,17,18,19,20].
2.2. Fraction Characterization
The MS chromatography results depicted in Figure 1 and Table 1 indicate that A. japonica is rich in phenolic acids and flavonoids, especially chlorogenic acids, which is confirmed by comparing with the standards of chlorogenic acids using the HPLC method (Figure 2). In order to better reveal the anti-inflammatory and antioxidant effects of these components on A. japonica, sequential partitioning of the water extract of A. japonica leaves was performed using ethyl acetate and n-butanol. As shown in Table 2, most of the components in WE were distributed in water fraction (WF), followed by the n-butanol fraction (BF). The fractions were further characterized by HPLC, and the content of chlorogenic acids was determined. As illustrated in Figure 2 and Table 2, isochlorogenic acids in WE subfractions were predominantly distributed in EAF, while CA was concentrated within BF. The different distribution of these components may contribute to varying pharmacological activities exhibited by different fractions.
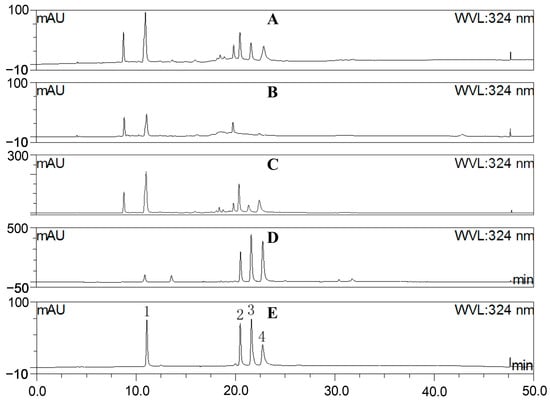
Figure 2.
HPLC characterization of (A) the water extract of A. japonica (WE); (B) the water fraction of WE (WE-WF); (C) the n-butanol fraction of WE (WE-BF); (D) the ethyl acetate fraction of WE (WE-EAF); and (E) standards of chlorogenic acids (1. chlorogenic acid; 2. isochlorogenic acid B; 3. isochlorogenic acid A; and 4. isochlorogenic acid C).

Table 2.
Extraction yield and chlorogenic acids content in different fractions.
2.3. Total Phenolics
The total phenolic content (TPC) in crude extracts and its fractions is presented in Figure 3. The TPC in WE reached 149.442 mg GAE/g, while the TPC for different fractions followed the following order: EAF > BF > WE > WF. Notably, the EAF exhibited the highest TPC value of 385.4217 mg GAE/g. These findings highlight A. japonica leaves as a promising source of polyphenols, with ethyl acetate serving as an effective solvent for their enrichment.
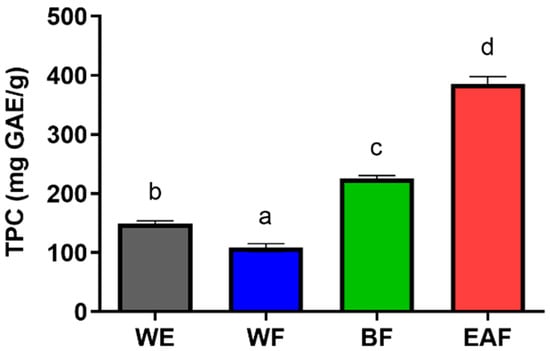
Figure 3.
Quantification of total phenolic content in extracts obtained from leaves of A. japonica. WE: the water extract of A. japonica; WF: the water fraction of WE; BF: the n-butanol fraction of WE; EAF: the ethyl acetate fraction of WE. Columns marked with different letters are significantly different from each other (p < 0.05).
2.4. Comparison of Antioxidant Activity
Radical scavenging ability assays (DPPH• and ABTS+•) and reducing power assays were used to evaluate the antioxidant activities of the crude extract and fractions of A. japonica. For better evaluating the antioxidant activity of the crude extract and fractions of A. japonica, Vitamin C (Vc), Trolox (Tr), and Butylated hydroxyanisole (BHA), which are commonly utilized as positive controls for assessing the antioxidant activity of drugs, were all employed as positive controls. The results are presented in Figure 4. It was observed that the scavenging activity of free radicals and reducing the power of the three positive controls showed significant activity, and the overall activity trend followed the following descending order: BHA > Vc > Tr, among which the reducing power of Tr was significantly different from the other two (p < 0.05).
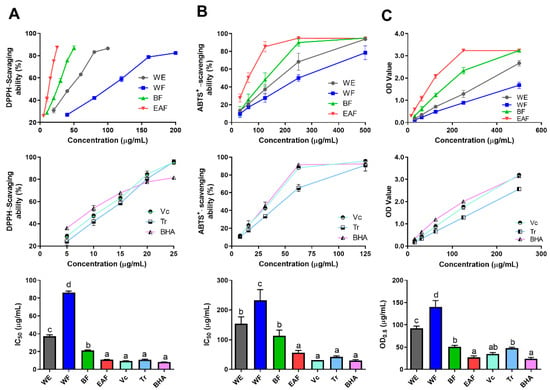
Figure 4.
Free radical scavenging ability of the crude extract and fractions of A. japonica by the (A) DPPH•, (B) ABTS+•, and (C) Reducing power assay. WE: the water extract of A. japonica; WF: the water fraction of WE; BF: the n-butanol fraction of WE; EAF: the ethyl acetate fraction of WE; Vc: vitamin C; Tr: trolox; BHA: butylated hydroxyanisole. The data are presented as the mean ± SD; n = 3. Columns marked with different letters are significantly different from each other (p < 0.05).
As depicted in Figure 4, all fractions showed potential antioxidant activity in a dose-dependent manner, and the antioxidant activity showed a downward trend of EAF > BF > WE > WF in general. In particular, EAF exhibited the strongest free radical scavenging ability and reducing power activity, which were equivalent to those of positive control (Vc and BHA), and with the IC50 DPPH•, IC50 ABTS•+, and OD0.5 reducing power were at 10.987 μg/mL, 43.630 μg/mL, and 26.883 μg/mL, respectively, while WF gave the lowest antioxidant activity. The results above imply that hot water extraction is a potential method to obtain antioxidant components of A. japonica, and the active ingredients are more easily enriched in ethyl acetate and n-butanol fractions.
2.5. Anti-Inflammatory Effects on Lipopolysaccharide (LPS)-Induced RAW264.7 Cells
LPS stimulation can induce RAW264.7 cells to express and release a variety of proinflammatory cytokines [22]. The levels of interleukin 6 (IL-6) and tumor necrosis factor-ɑ (TNF-ɑ) in the supernatant of RAW264.7 cells were determined after LPS exposure. As shown in Figure 5A,B, the basal levels of IL-6 and TNF-α in RAW264.7 cells were 18.987 pg/mL and 573.315 pg/mL, respectively. Stimulation with 100 ng/mL of LPS for 24 h led to a 1025.7-fold increase in IL-6 and a 51.3-fold increase in TNF-α. Pretreatment with coelonin at 5 μg/mL, previously reported as an effective anti-inflammatory compound [23], dramatically downregulated the expressions of IL-6 and TNF-α. Except for WF, pretreatment with WE and other fractions of A. japonica resulted in a concentration-dependent reduction in IL-6 and TNF-ɑ levels. EAF exhibited significant anti-inflammatory activity, leading to an 82.045% decrease in IL-6 and a 67.38% decrease in TNF-ɑ when treated with 60 μg/mL EAF.
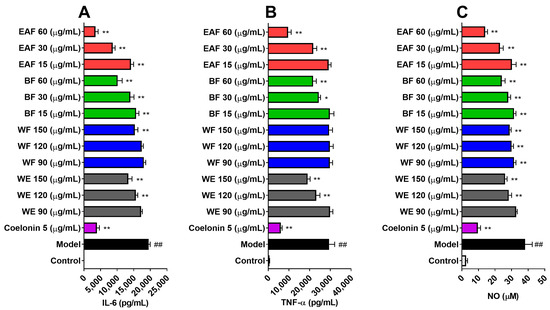
Figure 5.
The inhibitory effect of A. japonica extracts on LPS-induced inflammatory cytokines and nitric oxide (NO) in RAW264.7 cells. The levels of (A) IL-6, (B) TNF-α, and (C) NO in the supernatant of RAW264.7 cells treated with 100 ng/mL LPS for 24 h were measured after pretreatment with various concentrations of crude extract and fractions of A. japonica. The data are presented as the mean ± SD; n = 3. ##, p < 0.01 compare with control; *, p < 0.05, **, p < 0.01, compare with Model.
2.6. Effects on NO and Reactive Oxygen Species (ROS) Production on LPS-Induced RAW264.7 Cells
NO is a multi-effector molecule, which is closely related to inflammation and oxidative stress [24]. LPS can induce over NO and superoxide anion (O2−) production on RAW264.7 cells through upregulation of inducible nitric oxide synthase (iNOS) and NADPH oxidase, respectively, and these two can form peroxynitrite (ONOO−), which mediates the cytotoxic effect of NO [25]. These excessive ROS will damage DNA, lipids, protein, and mitochondria, leading to cell damage and even death. Therefore, inhibition of these ROS can protect cells from oxidative stress. Consistent with previous reports [25], stimulation with 100 ng/mL LPS significantly augmented the levels of NO in the supernatant (from 2.574 μM to 38.130 μM, as shown in Figure 5C), and increased ROS levels within the cells (Figure 6). However, pretreatment with different concentrations of WE and fractions derived from A. japonica effectively attenuated NO and ROS levels in a dose-dependent manner. Notably, the NO inhibition and ROS-scavenging active ingredients were mainly enriched in the n-butanol and ethyl acetate fractions. Particularly, the ROS scavenging efficacy of EAF was further evaluated via flow cytometry (Figure 6B), which reconfirmed the remarkable ability of EAF to scavenge ROS.
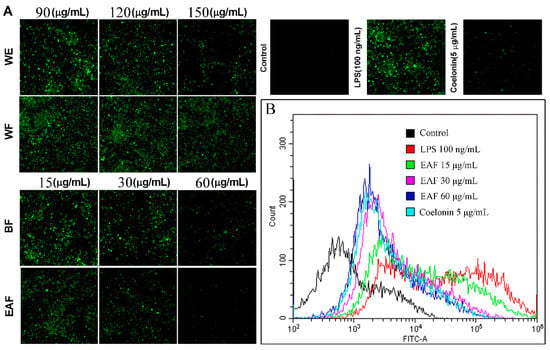
Figure 6.
The extracts of A. japonica exhibited a dose-dependent inhibition on the levels of ROS in RAW264.7 cells induced by LPS. RAW264.7 cells were pretreated with or without different concentrations of crude extract and fractions of A. japonica for 1 h, and following stimulated with 100 ng/mL of LPS for 24 h. After being stained with 10 μM DCFH-DA for 30 min, cells were (A) photographed with ImageXpress Micro XLS system and (B) determined by flow cytometry.
2.7. The Impact of EAF on the Expression of Genes Associated with Inflammation and Oxidative Stress
The binding of LPS to the Toll-like receptor 4 (TLR4) receptor on macrophage surfaces can activate the NF-κB pathway, leading to upregulation of gene expressions such as IL-6, TNF-ɑ, and iNOS, thereby inducing an inflammatory reaction in the body [26]. Simultaneously, LPS can also induce ROS outbreak and oxidative stress in cells by activating the NOX2/ROS pathway [27], interfering with mitochondria [28], and downregulating antioxidant enzymes [29]. An appropriate inflammatory reaction and oxidative stress are beneficial for pathogen elimination, while excessive or chronic inflammation and oxidative stress may contribute to diseases like fibrosis [30], diabetes [31], cancer [32], and Alzheimer’s disease [33]. Those compounds possess antioxidant and anti-inflammatory activities and hold potential value in drug development, as well as functional food additives that promote human health. Evidently, EAF exhibits significant inhibitory activity against LPS-induced secretion of inflammatory factors and the production of free radicals in RAW264.7 cells. This highlights its key role as an anti-inflammatory and antioxidant agent derived from A. japonica, with promising prospects for further development and application. Therefore, exploring its molecular mechanism is highly significant.
As shown in Figure 7A,B, the mRNA levels of IL-6 and TNF-ɑ were significantly upregulated by LPS stimulation. However, this upregulation was dose-dependently inhibited by EAF pretreatment, which is consistent with the observed changes in protein levels (Figure 5). These findings provide further evidence for the anti-inflammatory activity of EAF. Moreover, exposure to LPS dramatically increased iNOS mRNA expression (Figure 7C), which is known to be a major source of nitrogen free radicals. Notably, EAF exhibited a strong inhibitory effect on iNOS expression, explaining the corresponding decrease in NO level in the supernatant following EFA treatment (Figure 5). Macrophage oxidative stress is not only caused by excessive production of free radicals but is also associated with an imbalance in the antioxidant system [34]. In line with this concept, superoxide dismutase 1 (SOD1) and catalase (CAT) mRNA levels were significantly decreased upon LPS exposure (Figure 7D,E). Additionally, glutathione peroxidase 4 (Gpx4) mRNA level was also reduced (Figure 7F), all of which could be reversed by 60 μg/mL of EAF pretreatment (Figure 7D,E).
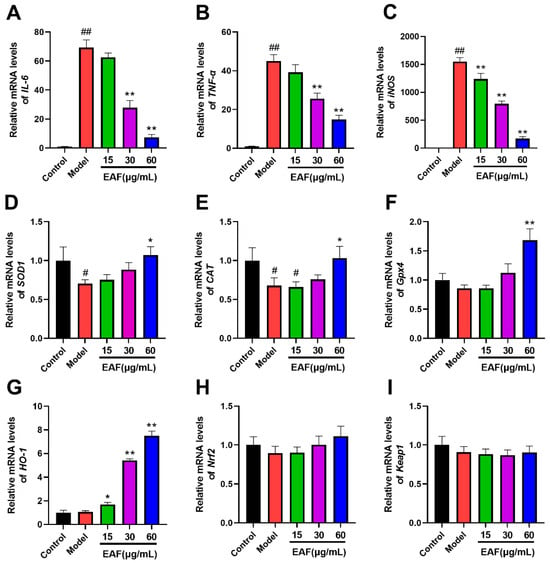
Figure 7.
The impact of EAF on the expression of genes related to inflammation and antioxidant activity was assessed using qPCR. Following a 1 h treatment with either vehicle or varying concentrations of EAF, the cells were exposed to LPS at a concentration of 100 ng/mL for 6 h. Subsequently, total RNA was extracted and subjected to qPCR analysis, with expression data normalized to the reference gene β-actin. (A–I) represents the relative expression levels of IL-6, TNF-ɑ, iNOS, SOD1, CAT, Gpx4, HO-1, Nrf2 and Keap1 genes, respectively. Data represent the mean ± SD; n = 3. #, p < 0.05, ##, p < 0.01 compare with control; *, p < 0.05, **, p < 0.01, compare with Model.
Interestingly, EAF treatment significantly promoted the mRNA level of heme oxygenase-1 (HO-1), with a statistically significant difference observed at 15 μg/mL (Figure 7G). Moreover, the high-dose treatment group at 60 μg/mL exhibited a remarkable increase in HO-1 mRNA level, which was approximately seven-fold higher than that of the LPS model group (Figure 7G). HO-1 is a well-known phase II antioxidant enzyme that can convert hemoglobin into carbon monoxide (CO), ferrous ions (Fe2+), and biliverdin, thereby exerting potent antioxidant, anti-inflammatory, and anti-apoptotic effects [35]. Consequently, we speculated that EAF may exhibit its antioxidative activity by upregulating the expression of HO-1.
2.8. EAF Exerts Antioxidant and Anti-Inflammatory Effects through the Regulation of the Nrf2/HO-1 Pathway
HO-1 functions as an inducible antioxidant enzyme, which is usually regulated by the nuclear transcription factor Nrf2. Nrf2, a member of the Cap’n’collar (CNC)-BZIP transcription factor family, plays a crucial role in regulating cellular responses to oxidative stress. In normal physiological conditions, Nrf2 is bound to Kelch-like epichlorohydrin-related proteins (Keap1) and remains in a state of low activity. However, under conditions of oxidative stress or other pathological stimuli, modifications occur on the cysteine residues of Keap1, leading to phosphorylation of Nrf2. Consequently, Nrf2 dissociates from the complex and translocates into the nucleus, where it interacts with accessory proteins and recognizes an antioxidant response element (ARE). This interaction activates downstream antioxidant-related genes, including HO-1, SOD, glutamate-cysteine ligase (GCL), and NAD(P)H-quinone oxidoreductase 1 (NQO1). The activation facilitates the removal of reactive oxygen species (ROS) while promoting balance within the body’s antioxidant system [35]. Therefore, we quantified the mRNA levels of Nrf2 and Keap1. However, the results indicated that there were no significant changes in the expression levels of both Nrf2 and Keap1 mRNA following exposure to LPS or EAF treatment (Figure 7H,I).
In order to validate the regulatory effect of EAF on the Nrf2/HO-1 pathway, a Western blot experiment was conducted. The results confirmed that treatment with EAF did not effectively enhance the protein level of Nrf2. However, it dose-dependently promoted the expression of HO-1, particularly in the 60 μg/mL treatment group, which exhibited a 3.454-fold increase compared to the LPS treatment group (Figure 8A). Since Nrf2 is acknowledged as a pivotal regulatory transcription factor for HO-1, immunofluorescence analysis was performed to examine the nuclear translocation of Nrf2. As depicted in Figure 8B, Nrf2 predominantly localized in the cytoplasm in both control and LPS-treated groups; however, after treatment with 60 μg/mL EAF, there was a significant elevation observed in nuclear levels of Nrf2. This suggests that EAF contains components that facilitate the activation of Nrf2.
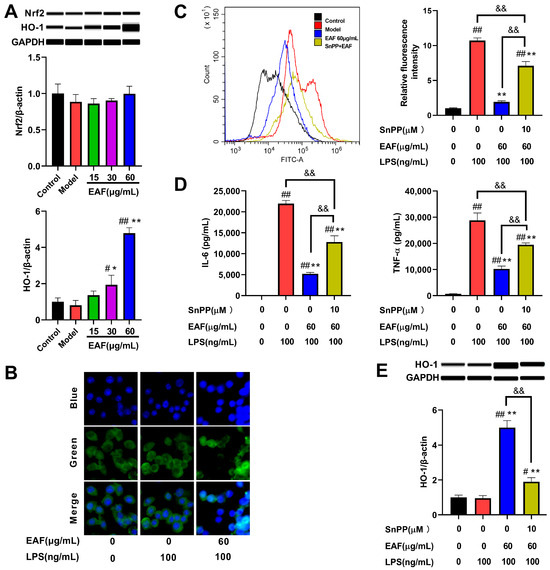
Figure 8.
EAF exerts antioxidant and anti-inflammatory effects through the regulation of the Nrf2/HO-1 pathway. The RAW264.7 cells were pretreated with the vehicle, different concentrations of EAF, or the HO-1 inhibitor Snpp (10 μM) combined with EAF (60 μg/mL) for 1 h. Subsequently, they were exposed to 100 ng/mL LPS for either 2 h (to detect Nrf2 nuclear translocation) or 24 h (to assess protein expression, cytokine levels, and ROS levels). (A) Protein levels of Nrf2 and HO-1 detected by Simple Western. (B) Immunofluorescence analysis of Nrf2 nuclear translocation. (C) Flow cytometry analysis of the effect of HO-1 inhibitor intervention on the ROS scavenging activity of EAF. (D) Effects of HO-1 inhibitor intervention on the anti-inflammatory activity of EAF. (E) The impact of an HO-1 inhibitor on the expression of HO-1 protein induced by EAF. Data represent the mean ± SD, n = 3. #, p < 0.05, ##, p < 0.01 compare with control; *, p < 0.05, **, p < 0.01, compare with Model; &&, p < 0.01 compare between two groups.
Recent studies have demonstrated that ROS could activate NF-κB, thereby promoting inflammatory responses and exacerbating tissue damage [36]. Consequently, antioxidant medications can ameliorate excessive inflammatory responses while concurrently reducing oxidative stress within the body, thus facilitating tissue repair [37]. Similarly, the Nrf2/HO-1 pathway not only eliminates ROS but also effectively regulates inflammatory responses [35]. Those compounds that promote HO-1 expression, such as notopterol, nepetoidin B, and ramelteon, can inhibit inflammation, but their effects can be weakened by HO-1 inhibitors [38,39,40]. Therefore, we tested whether EAF exerts antioxidant effects and alleviates inflammation by upregulating the Nrf2/HO-1 pathway. As demonstrated in Figure 8C, exposure to LPS significantly increased intracellular ROS levels. However, pretreatment with 60 μg/mL EAF effectively inhibited this increase in ROS levels, while the scavenging activity of EAF against ROS was found to be attenuated by intervention with an HO-1 inhibitor SnPP. Furthermore, the inhibitory effect of EAF on the secretion of IL-6 and NF-ɑ inflammatory factors induced by LPS was also significantly reduced when co-treated with an HO-1 inhibitor (Figure 8D). These changes in the efficacy of EAF were consistent with alterations observed in the protein level of HO-1 after using SnPP (Figure 8E). Collectively, these findings confirm that EAF alleviates oxidative stress and inflammatory response induced by LPS at least partially through modulation of the Nrf2/HO-1 signaling pathway.
The above results showed that the antioxidant and anti-inflammatory components of A. japonica were mainly distributed in the EAF fraction, which is rich in chlorogenic acids, and the total contents of chlorogenic acid (CA), isochlorogenic acid A (IAA), isochlorogenic acid B (IAB), and isochlorogenic acid C (IAC) reached 519.667 mg/g. Numerous publications have shown that AC [41] and IAA [42] have strong antioxidant and cell protective effects. Chlorogenic acid, as the most abundant and effective dietary phenolic compound, not only exhibits remarkable antioxidant activity but also possesses anti-inflammatory properties [41]. According to the literature, the anti-inflammatory effect of chlorogenic acid in various cell inflammation models ranges from 1.77 μg/mL to 708.62 μg/mL [43,44,45]. Therefore, chlorogenic acids are one of the active components of EAF. The current study indicated that besides direct scavenging free radicals, EAF also showed remarkable antioxidant and anti-inflammatory activities in LPS-induced RAW264.7 cells by partially upregulating the Nrf2/HO-1 signal pathway (Figure 9). However, few publications have reported on the ability of these chlorogenic acids to effectively activate Nrf2 nuclear translocation and promote HO-1 expression for alleviating oxidative stress and inflammation in LPS-induced macrophage cells. Therefore, further investigation is warranted to elucidate the constituents contributing to the antioxidant and anti-inflammatory effects of EAF.
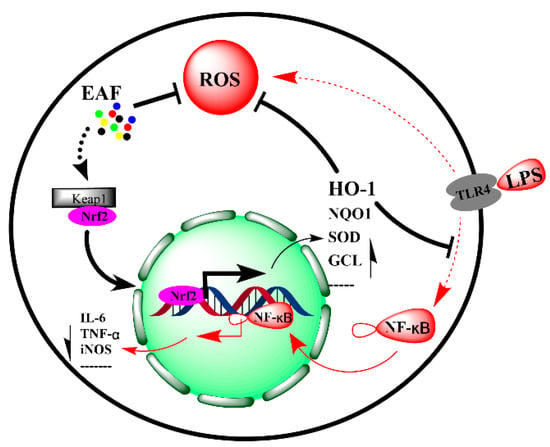
Figure 9.
Molecular mechanism of EAF alleviating LPS-induced oxidative stress and inflammatory response in RAW264.7 cells by upregulating Nrf2/HO-1 pathway.
3. Materials and Methods
3.1. Chemicals and Reagents
Chlorogenic acid (CA), butylated hydroxyanisole (BHA), and 2,2-azinobis(3-ethylbenzoth-iazoline-6-sulfonic acid ammonium salt) (ABTS) were purchased from Bide pharmatech Ltd. (Shanghai, China), isochlorogenic acid A (IAA), isochlorogenic acid B (IAB), and isochlorogenic acid C (IAC) were purchased from Chengdu Push Biotechnology Co., Ltd. (Chengdu, China), Folin–Ciocalteu’s phenol reagent was purchased from Solarbio Science & Technology Co., Ltd. (Beijin, China), gallic acid (GAE), 2,2-diphenyl-1-picrylhydrazyl radical (DPPH), trolox (Tr), vitamin C (Vc), 2′,7′-dichlorofluorescin diacetate (DCFH-DA), and thirty percent hydrogen peroxide (30%-H2O2) were obtained from Meryer Chemical Technology Co., Ltd. (Shanghai, China). The HPLC-grade acetonitrile and formic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ultrapure water was prepared using a Milli-Q purification system (Millipore Laboratory, Bedford, MA, USA). Other chemicals or solvents were of analytical grade and used without further purification.
3.2. Plant Material
The leaves of A. japonica were collected from Shouning County, Ningde City (Fujian Province, China) in May 2019 and identified by Professor Zhishan Ding of Zhejiang Chinese Medical University. The fresh leaves were cleaned and dried in a constant-temperature oven at 60 °C, then powdered and sieved through an 80 mesh screen. Finally, the powder was sealed and stored in a drier at −40 °C.
3.3. Sample Preparation
Leaves powder (10.0 g) was reflux extracted with 100 mL water for 60 min, filtered, and the residue was re-extracted following the same conditions. The supernatants were combined and concentrated to 10 mL with a vacuum rotary evaporator at 45 °C; then, 15 mL ethanol was added. The mixture was placed at 4 °C overnight, then centrifuged at 12,000 rpm for 10 min, and the supernatant was vacuum-dried at 40 °C to obtain the water extract of A. japonica (WE). A total of 2.0 g WE was suspended in 40 mL distilled water and partitioned sequentially with ethyl acetate (3 × 40 mL) and n-butanol (3 × 40 mL), respectively. The three fractions were then evaporated to dry under reduced pressure to give ethyl acetate fraction (EAF), n-butanol fraction (BF), and water fraction (WF). These fractions were stored at −40 °C.
3.4. HPLC Characterization
All extracts were prepared with 50% acetonitrile at a concentration of 1 mg/mL and analyzed by HPLC using the UltiMate 3000 high-performance liquid chromatography system (Dionex Corp., Sunnyvale, CA, USA). Method S1 (Supplementary Materials) contains specific chromatographic conditions.
3.5. UPLC-QTOF-MS2 Conditions
The samples were subjected to the UPLC-QTOF-MS2 system, which consisted of an ACQUITY ultraperformance liquid chromatography instrument (Waters Corporation, Milford, MA, USA) and a SYNAPT G2-Si Q-TOF Mass Spectrometer (Waters Corporation, Manchester, UK), to reveal the chemical composition of A. japonica leaves. The chromatographic separation conditions and mass spectrometry analysis methods are detailed in Method S2 (Supplementary Materials).
3.6. Determination of Total Phenolic Content (TPC)
The TPC in fractions was determined by the Folin–Ciocalteu’s method as reported by [15] with gallic acid as standard (Y = 9.3253X + 0.0052, R2 = 0.9998). The TPC was expressed as a milligram of gallic acid equivalent per gram of fraction (mg GAE/g). The whole sample was tested in triplicate.
3.7. Radical Scanvenging Ability Assays
3.7.1. DPPH• Scanvenging Ability
The DPPH• scavenging activity was determined following the method of [15]. Briefly, 100 μL of the sample was mixed with an equal volume of freshly prepared DPPH• solution (0.1 mM). After incubating in the dark at room temperature for 30 min, the absorbance was recorded at 510 nm. Vc, Te, and BHA were used as positive controls. IC50 value was calculated, and the whole sample was tested in triplicate.
3.7.2. Reducing Power
The reducing power activity was determined according to the method reported by [46]. Absorbance was measured at 700 nm, and the OD0.5 value (the concentration of sample absorbance of 0.500) was calculated. Vc, Te, and BHA were used as positive controls, and all the tests were made in triplicate.
3.7.3. ABTS+• Scavenging Activity
The ABTS+• scavenging activity was conducted following the reference [15] and with modification. Briefly, 20 μL of the sample was mixed with 180 μL of freshly prepared ABTS+• solution, and the absorbance was measured at 734 nm after 6 min. Using Vc, Te, and BHA as positive controls, the IC50 value was calculated.
3.8. Anti-Inflammatory and Reactive Oxygen Species (ROS) Scavenging Activities on LPS-Induced RAW264.7 Cells
3.8.1. Nitric Oxide (NO) and Cytokines Level in Supernatants
RAW264.7 cells (American Type Culture Collection) were cultured in DMEM media supplemented with 10% FBS and 1% penicillin-streptomycin. RAW264.7 cells (5 × 105 cells/mL) were seeded in 96-well plates and incubated overnight. The drugs and extracts of A. japonica were prepared as high-concentration stock solutions using DMSO, followed by dilution into a series of treatment concentrations with serum-containing DMEM medium to achieve a final DMSO content of 0.1%. The medium containing 0.1% DMSO was utilized as the reagent control, while the coelonin treatment group at a concentration of 5 μg/mL was used as the positive control. Subsequently, the medium in the 96-well plate was discarded and replaced with the aforementioned diluted drugs. After incubation for 1 h, LPS at a final concentration of 100 ng/mL was added to stimulate the cells for 24 h. Then, the culture supernatants were collected for detection of the NO level using the Griess reagent method [47], and the interleukin-6 (IL-6) and tumor necrosis factor-ɑ (TNF-ɑ) levels in the supernatants were analyzed by cytometric beads array (CBA) method [23].
3.8.2. Determination of Intracellular ROS
RAW264.7 cells were seeded in 96-well and 6-well plates and treated as NO assay. After 24 h treatment, cells were stained with 2′,7′-dichlorofluorescin diacetate (DCFH-DA, 10 μM) for 30 min at 37 °C. The medium was then removed and washed with serum-free DMEM three times. Cells in 96-well plates were photographed by ImageXpress Micro XLS system (Molecular Devices LLC, Sunnyvale, CA, USA). Cells in 6-well plates were collected and resuspended in PBS and immediately analyzed on a BD Accuri™ C6 flow cytometer (BD, Ann Arbor, MI, USA).
3.9. Real-Time Fluorescence Quantitative PCR (qPCR) Analysis
RAW264.7 cells (5 × 105 cells/mL) were seeded in 6-well plates and pretreated with either vehicle (containing 0.1% DMSO in the medium) or various concentrations of extracts, with or without the HO-1 inhibitor (SnPP) for 1 h. Subsequently, the cells were exposed to 100 ng/mL LPS for 6 h. Following our previously published protocol [23], the cells were collected, and total RNA was extracted with Trizol reagent (BS259A; Biosharp, Hefei, China). The RNA concentration was measured using a trace nucleic acid detector (Thermo Fisher Scientific, Waltham, MA, USA), and cDNA was obtained using a reverse transcription kit (RiboBio, Guangzhou, China). The mRNA expression levels of IL-6, TNF-ɑ, inducible nitric oxide synthase (iNOS), Kelch-1ike ECH-associated protein l (Keap1), nuclear factor erythroid 2-related factor 2 (Nrf2), catalase (CAT), superoxide dismutase 1 (SOD1), glutathione peroxidase 4 (Gpx4), and heme oxygenase (HO-1) were detected through qPCR using specific primers listed in Table S1. The expression levels of each gene were calculated relative to β-actin according to the 2−ΔΔCT method and normalized relative to the control group.
3.10. Western Blot Analysis
Treated cells were harvested and lysed with RIPA buffer (78503; Thermo Fisher Scientific) supplemented with a phosphatase inhibitor (41659200; Roche) and a protease inhibitor (18065900; Roche, Mannheim, Germany). Nuclear and cytoplasmic proteins were separated using a nucleoprotein and plasma protein extraction kit (P0027; Beyotime, Beijing, China). The protein levels were subsequently determined using a Simple Western System (ProteinSimple, San Jose, CA, USA). Briefly, the protein concentrations were adjusted with buffer solution, mixed with the loading buffer, denatured, and subjected to capillary electrophoresis. After the blocking step was performed, followed by incubation with primary antibodies, such as anti-GAPDH (A19056; ABclonal, Wuhan, China), anti-Nrf2 (A0674; Abclonal, Wuhan, China), or anti-HO-1 (A1346; ABclonal, Wuhan, China), and then incubated with secondary antibody. Finally, the samples were imaged and quantified using Simple Western System software 6.3.0 (ProteinSimple, San Jose, CA, USA) [23].
3.11. Immunofluorescence Analysis
RAW264.7 cells were preincubated with a vehicle solution (containing 0.1% DMSO in the medium) or with EAF (60 μg/mL) for 1 h, followed by stimulation with LPS (100 ng/mL) for 2 h. The cells were fixed using 4% paraformaldehyde and permeabilized with Triton-100 (0.1%). After overnight incubation with the primary antibody against Nrf2, the cells were washed and further incubated with goat anti-rabbit IgG H&L (Alexa Fluor® 488) (ab150073; Abcam, Cambridge, MA, USA) for an additional hour. After DAPI (C1002; Beyotime, Shanghai, China) staining, the cells were imaged with an ImageXpress Micro Confocal High-Content Cell Imaging System (Molecular Devices).
3.12. Statistical Analysis
Data were presented as the mean ± standard deviation (SD) in triplicate. Statistical significance was considered at p < 0.05, followed by a one-way analysis of variance (ANOVA) with Tukey’s post hoc test. IC50 values, statistical analyses, and figures were prepared using GraphPad Prism software (Version 6.0, Graphpad Software Inc., San Diego, CA, USA).
4. Conclusions
In summary, the water extract of A. japonica is abundant in phenolic compounds that are primarily enriched in the ethyl acetate fraction, which exhibits the most potent free radical scavenging activity in vitro and also possesses significant antioxidant and anti-inflammatory properties in LPS-induced RAW264.7 cells. Notably, the ethyl acetate fraction prominently activates the Nrf2/HO-1 pathway and partially relies on this pathway to alleviate oxidative stress and inflammatory responses induced by LPS. However, the specific active compound responsible for these effects remains unknown. Apparently, these findings provide substantial support for the potential application of A. japonica as a medicinal and health food additive to enhance human well-being by mitigating oxidative stress and inflammatory risks.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules29061375/s1, Method S1. HPLC characterization. Method S2. UPLC-QTOF-MS2 conditions. Table S1: Primer list for qPCR.
Author Contributions
Y.Y., conceptualization, methodology, data curation, writing—original draft; X.L., methodology, writing—original draft; M.C., methodology, writing—original draft; X.W., methodology, writing—original draft; M.L., data curation, formal analysis, validation; F.J., investigation, software, funding acquisition; X.Z., conceptualization, investigation, software; C.Z., conceptualization, supervision, data curation, project administration; S.L., conceptualization, data curation, formal analysis, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The present research was carried out with the financial support of the Natural Science Foundation of Zhejiang Province [grant number: LY23H280004].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
We thank the public platform of the Medical Research Center, Academy of Chinese Medical Sciences, Zhejiang Chinese Medical University for its instrumentation and equipment.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. Genus: A review of bioactive essential oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef] [PubMed]
- Guetat, A.; Al-Ghamdi, F.A.; Osman, A.K. The genus Artemisia L. In the northern region of Saudi Arabia: Essential oil variability and antibacterial activities. Nat. Prod. Res. 2017, 31, 598–603. [Google Scholar] [CrossRef]
- Koyuncu, I. Evaluation of anticancer, antioxidant activity and phenolic compounds of Artemisia absinthium L. Extract. Cell Mol. Biol. 2018, 64, 25–34. [Google Scholar] [CrossRef]
- Landete, J.M. Updated knowledge about polyphenols: Functions, bioavailability, metabolism, and health. Crit. Rev. Food Sci. Nutr. 2012, 52, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Trendafilova, A.; Moujir, L.M.; Sousa, P.; Seca, A. Research advances on health effects of edible Artemisia species and some sesquiterpene lactones constituents. Foods 2020, 10, 65. [Google Scholar] [CrossRef]
- Huang, T.K.; Ding, Z.D.; Zhao, S.X. Xian Dai Ben Cao Gang Mu: Volume I; Beijing China Medical Science Press: Beijing, China, 2001; p. 1325. ISBN 7-5067-1821-9. [Google Scholar]
- Rashmi, T.R.; Francis, M.S.; Murali, S. Essential oil composition of Artemisia japonica Thunb. from kerala. J. Pharmacogn. Phytochem. 2014, 3, 160–163. [Google Scholar]
- Joshi, R.K. Volatile oil composition of Artemisia japonica Thunb. from western himalaya of uttarakhand. J. Pharmacogn. Phytochem. 2015, 3, 96–97. [Google Scholar]
- Kwon, H.C.; Lee, K.R. Phytochemical constituents of Artemisia japonica ssp. Littoricola. Arch. Pharm. Res. 2001, 24, 194–197. [Google Scholar] [CrossRef]
- Giang, P.M.; Binh, N.T.; Matsunami, K.; Son, P.T. Three new eudesmanes from Artemisia japonica. Nat. Prod. Res. 2014, 28, 631–635. [Google Scholar] [CrossRef]
- Zhang, D.H.; Cheng, P.F.; Ling, L. Antioxidation and genetic toxicity of Artemisia japonica extract. Nat. Prod. RD 2011, 23, 39–42. [Google Scholar]
- Huang, T.H.; Lu, X.M.; Chen, S.L. Pharmacodynamics research and safety evaluation of the folk herb—Artemisia japonica Thumb. J. Chengdu Univ. Tradit. Chin. Med. 2010, 33, 77–79. [Google Scholar]
- Li, N.; Shi, C.; Shi, S.; Wang, H.; Yan, J.; Wang, S. An inulin-type fructan isolated from Artemisia japonica and its anti-arthritic effects. J. Funct. Food 2017, 29, 29–36. [Google Scholar] [CrossRef]
- Zhang, D.H. Study on processing technology for Artemisia japonica tea. Food Ferment. Ind. 2008, 32, 168–171. [Google Scholar]
- Zhang, L.; Tu, Z.C.; Wang, H.; Fu, Z.F.; Wen, Q.H.; Fan, D. Metabolic profiling of antioxidants constituents in Artemisia selengensis leaves. Food Chem. 2015, 186, 123–132. [Google Scholar] [CrossRef]
- Zhang, L.B.; Duan, J.A.; Lv, J.L. Phytochemistry and bioactivities of sesquiterpenoids from the Artemisia species. J. Chin. Pharm. Sci. 2017, 26, 317–334. [Google Scholar] [CrossRef]
- Yu, H.H.; Gao, X.Y. Identification of chemical components in capillary wormwood herb by UPLC-Q-TOF/MS. Cent South Pharm. 2019, 17, 656–661. [Google Scholar]
- Zhang, P.J.; Cao, Y.; Zhang, K.; Song, Y.L.; Li, J.; Tang, L. Chemical profiling of Artemisia rupestris using HPLC-IT-TOF-MS. Chin. Pharm. J. 2020, 45, 4658–4666. [Google Scholar]
- Zhang, L.; Tu, Z.C.; Yuan, T.; Wang, H.; Fu, Z.F.; Wen, Q.H.; Wang, X.Q. Solvent optimization, antioxidant activity, and chemical characterization of extracts from Artemisia selengnesis turcz. Ind. Crop. Prod. 2014, 56, 223–230. [Google Scholar] [CrossRef]
- Gu, D.; Yang, Y.; Abdulla, R.; Aisa, H.A. Characterization and identification of chemical compositions in the extract of Artemisia rupestris L. by liquid chromatography coupled to quadrupole time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, M.; Muroi, M.; Tanamoto, K.; Suzuki, T.; Azuma, H.; Ikeda, H. Molecular mechanisms of macrophage activation and deactivation by lipopolysaccharide: Roles of the receptor complex. Pharmacol. Ther. 2003, 100, 171–194. [Google Scholar] [CrossRef]
- Jiang, F.S.; Li, M.Y.; Wang, H.Y.; Ding, B.; Zhang, C.C.; Ding, Z.S.; Yu, X.B.; Lv, G.Y. Coelonin, an anti-inflammation active component of Bletilla striata and its potential mechanism. Int. J. Mol. Sci. 2019, 20, 4422. [Google Scholar] [CrossRef]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar]
- Zielonka, J.; Sikora, A.; Joseph, J.; Kalyanaraman, B. Peroxynitrite is the major species formed from different flux ratios of co-generated nitric oxide and superoxide: Direct reaction with boronate-based fluorescent probe. J. Biol. Chem. 2010, 285, 14210–14216. [Google Scholar] [CrossRef]
- Beutler, B.; Du, X.; Poltorak, A. Identification of toll-like receptor 4 (TLR4) as the sole conduit for LPS signal transduction: Genetic and evolutionary studies. J. Endotoxin Res. 2001, 7, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Banskota, S.; Wang, H.; Kwon, Y.H.; Gautam, J.; Haq, S.; Grondin, J.; Steinberg, G.R.; Khan, W.I. Inhibition of nadph oxidase (NOX) 2 mitigates colitis in mice with impaired macrophage AMPK function. Biomedicines 2023, 11, 1443. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Jiang, Y.; Wang, Y.; Huo, R.; Ma, N.; Shen, X.; Chang, G. Beta-carotene targets IP3R/GRP75/VDAC1-MCU axis to renovate LPS-induced mitochondrial oxidative damage by regulating STIM1. Free Radic. Biol. Med. 2023, 205, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, Z.; Wang, X.; Duan, F.; Xiong, L.; Li, J.; Tian, J.; Jia, L.; Gao, H. Premna microphylla Turcz leaf pectin exhibited antioxidant and anti-inflammatory activities in LPS-stimulated RAW264.7 macrophages. Food Chem. 2021, 349, 129164. [Google Scholar] [CrossRef] [PubMed]
- Antar, S.A.; Ashour, N.A.; Marawan, M.E.; Al-Karmalawy, A.A. Fibrosis: Types, effects, markers, mechanisms for disease progression, and its relation with oxidative stress, immunity, and inflammation. Int. J. Mol. Sci. 2023, 24, 4004. [Google Scholar] [CrossRef] [PubMed]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019, 70, 809–824. [Google Scholar]
- Neganova, M.; Liu, J.; Aleksandrova, Y.; Klochkov, S.; Fan, R. Therapeutic influence on important targets associated with chronic inflammation and oxidative stress in cancer treatment. Cancers 2021, 13, 6062. [Google Scholar] [CrossRef] [PubMed]
- Andronie-Cioara, F.L.; Ardelean, A.I.; Nistor-Cseppento, C.D.; Jurcau, A.; Jurcau, M.C.; Pascalau, N.; Marcu, F. Molecular mechanisms of neuroinflammation in aging and alzheimer’s disease progression. Int. J. Mol. Sci. 2023, 24, 1869. [Google Scholar] [CrossRef] [PubMed]
- Crimi, E.; Sica, V.; Slutsky, A.S.; Zhang, H.; Williams-Ignarro, S.; Ignarro, L.J.; Napoli, C. Role of oxidative stress in experimental sepsis and multisystem organ dysfunction. Free Radic. Res. 2006, 40, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Y.; Cai, Y.; Li, D.; He, J.; Feng, Z.; Xu, Q. NAT10 regulates the LPS-induced inflammatory response via the NOX2-ROS-NF-kappaB pathway in macrophages. Biochim. Biophys. Acta-Mol. Cell Res. 2023, 1870, 119521. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, S.; Li, X.; Lin, T.; Qin, T. Astringin protects LPS-induced toxicity by suppressing oxidative stress and inflammation via suppression of PI3K/AKT/NF-kappaB pathway for pediatric acute lung injury. Naunyn-Schmiedebergs Arch. Pharmacol. 2023, 396, 2369–2377. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; He, D.; Hu, J.; Yang, S.; Gao, X.; Cui, M.; Li, Z.; Wang, H.; Huang, B.; Fu, S.; et al. Notopterol inhibits LPS-induced inflammation in BV-2 cells via AKT/Nrf2/HO-1 signaling axis. Int. Immunopharmacol. 2023, 120, 110334. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, J.Y.; Yang, H.S.; Choe, J.S.; Hwang, I.G. Nepetoidin b from salvia plebeia r. Br. Inhibits inflammation by modulating the NF-kappaB and Nrf2/HO-1 signaling pathways in macrophage cells. Antioxidants 2021, 10, 1208. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, Y.; Lu, D.; Huang, T.; Yan, K.; Wang, W.; Gao, J. Ramelteon protects against human pulmonary microvascular endothelial cell injury induced by lipopolysaccharide (LPS) via activating nuclear factor erythroid 2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) pathway. Bioengineered 2022, 13, 1518–1529. [Google Scholar] [CrossRef]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food. Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef]
- Wang, H.N.; Shen, Z.; Liu, Q.; Hou, X.Y.; Cao, Y.; Liu, D.H.; Jiang, H.; Du, H.Z. Isochlorogenic acid (ICGA): Natural medicine with potentials in pharmaceutical developments. Chin. J. Nat. Med. 2020, 18, 860–871. [Google Scholar] [PubMed]
- Krakauer, T. The polyphenol chlorogenic acid inhibits Staphylococcal exotoxin-induced inflammatory cytokines and chemokines. Immunopharmacol. Immunotoxicol. 2002, 24, 113–119. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, Y.W.; Park, Y.; Lee, H.J.; Kim, K.W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW264.7 cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Park, S.Y.; Park, Y.L.; Myung, D.S.; Rew, J.S.; Joo, Y.E. Chlorogenic acid suppresses lipopolysaccharide induced nitric oxide and interleukin1β expression by inhibiting JAK2/STAT3 activation in RAW264.7 cells. Mol. Med. Rep. 2017, 16, 9224–9232. [Google Scholar] [CrossRef]
- Liao, W.C.; Lai, Y.C.; Yuan, M.C.; Hsu, Y.L.; Chan, C.F. Antioxidative activity of water extract of sweet potato leaves in Taiwan. Food Chem. 2011, 127, 1224–1228. [Google Scholar] [CrossRef]
- Brindisi, M.; Bouzidi, C.; Frattaruolo, L.; Loizzo, M.R.; Tundis, R.; Dugay, A.; Deguin, B.; Cappello, A.R.; Cappello, M.S. Chemical profile, antioxidant, anti-inflammatory, and anti-cancer effects of Italian Salvia rosmarinus Spenn. methanol leaves extracts. Antioxidants 2020, 9, 826. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).