Aminated Rapeseed Husks (Brassica napus) as an Effective Sorbent for Removing Anionic Dyes from Aqueous Solutions
Abstract
1. Introduction
2. Results and Discussion
2.1. Characteristics of Sorbents Tested (FTIR Analysis and C/N Elemental Analysis)
2.2. The Effect of pH on Dye Sorption Effectiveness
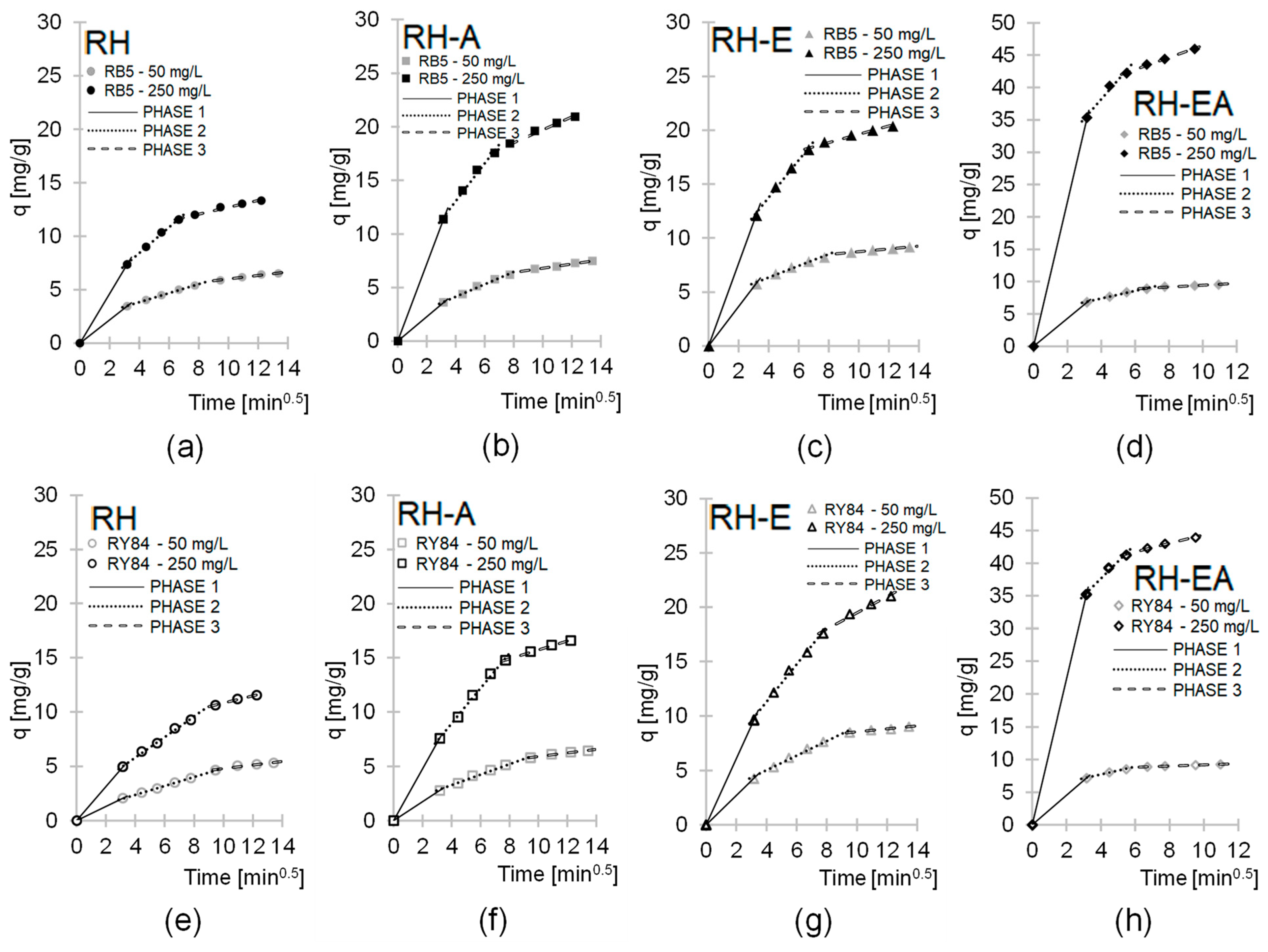
2.3. Dye Sorption Kinetics
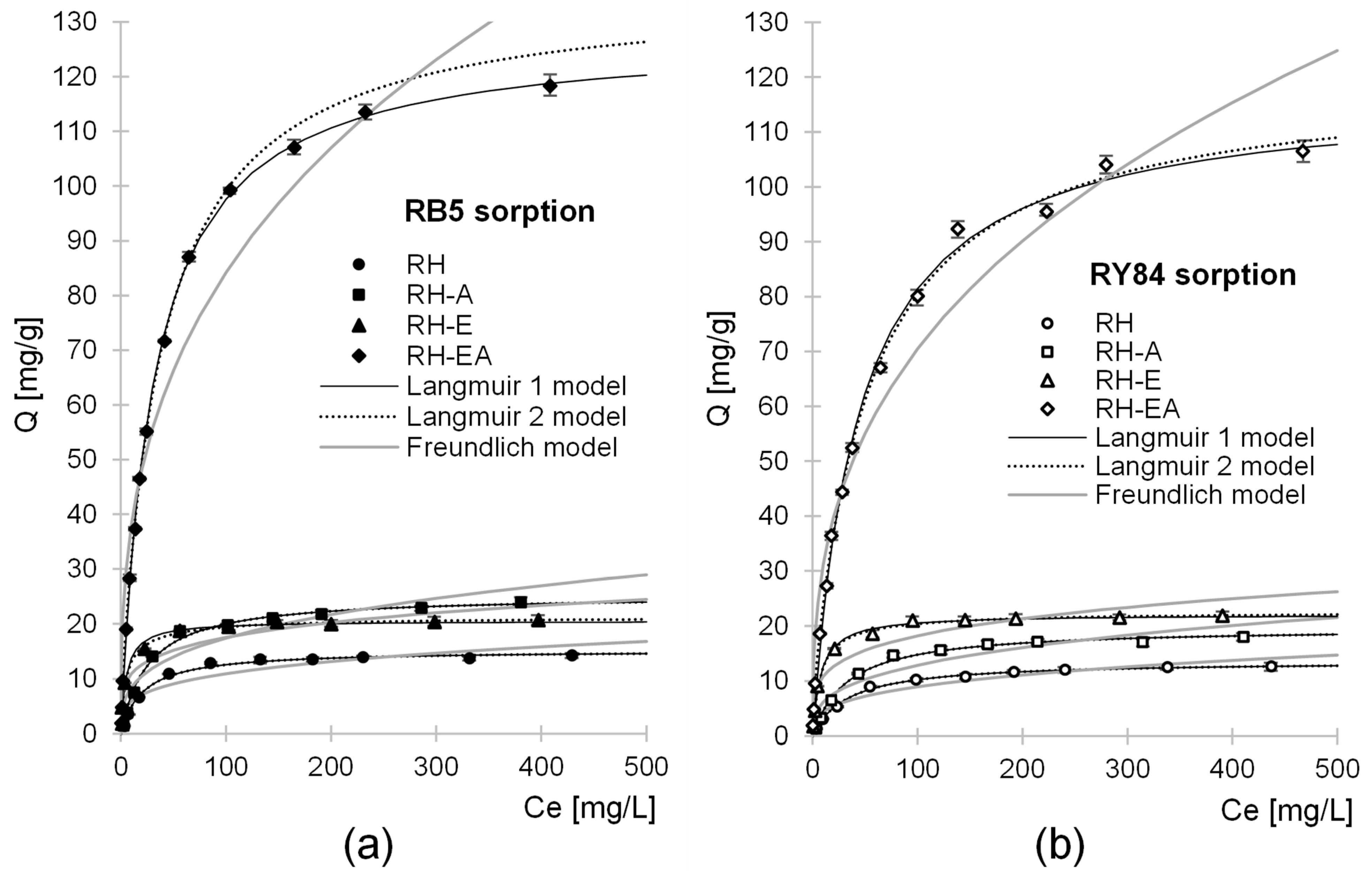
2.4. Maximum Sorption Capacity
3. Materials
3.1. Rapeseed Husks
3.2. Dyes
3.3. Chemical Reagents
3.4. Laboratory Equipment
4. Methods
4.1. Preparation of Rapeseed Husks (RH)
4.2. Preparation of Aminated Rapeseed Husks (RH-A)
4.3. Preparation of Rapeseed Husks Modified with Epichlorohydrin (RH-E)
4.4. Preparation of Aminated Rapeseed Husks Pre-Activated with Epichlorohydrin (RH-EA)
4.5. Analyses of pH Effect on Dye Sorption Effectiveness
4.6. Analyses of Dye Sorption Kinetics
4.7. Analyses of the Maximum Sorption Capacity of the Sorbents Used in the Study
4.8. Comments to Section 4.5, Section 4.6 and Section 4.7
4.9. FTIR Analysis of the Tested Sorbents
4.10. Elemental Analysis of the Tested Sorbents
4.11. Data Analysis
- QS—mass of sorbed dye [mg/g]
- C0—initial concentration of dye [mg/L]
- CS—concentration of dye after sorption [mg/L]
- V—volume of the solution [L]
- m—mass of the sorbent [g].
- q—instantaneous value of the sorbed dye [mg/g]
- qe—the amount of dye sorbed at the equilibrium state [mg/g]
- t—time of sorption [min]
- k1—pseudo-first-order adsorption rate constant [1/min]
- k2—pseudo-second-order adsorption rate constant [g/(mg·min)]
- kid—intramolecular diffusion model adsorption rate constant [mg/(g·min0.5)].
- Q—mass of the sorbed dye [mg/g]
- Qmax–maximum sorption capacity in Langmuir equation [mg/g]
- b1—maximum sorption capacity of sorbent (type I active sites) [mg/g]
- b2—maximum sorption capacity of sorbent (type II active sites) [mg/g]
- KC—constant in Langmuir equation [L/mg]
- K1,K2—constants in Langmuir 2 equation [L/mg]
- K—the equilibrium sorption constant in Freundlich model
- n—Freundlich equilibrium constant
- C—concentration of the dye remaining in the solution [mg/L].
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Batool, F.; Kanwal, S.; Kanwal, H.; Noreen, S.; Hodhod, M.S.; Mustaqeem, M.; Sharif, G.; Naeem, H.K.; Zahid, J.; Gaafar, A.R.Z. Ecofriendly Synthesis of Magnetic Composites Loaded on Rice Husks for Acid Blue 25 Decontamination: Adsorption Kinetics, Thermodynamics, and Isotherms. Molecules 2023, 28, 7124. [Google Scholar] [CrossRef]
- Zhao, J.; Dang, Z.; Muddassir, M.; Raza, S.; Zhong, A.; Wang, X.; Jin, J. A New Cd(II)-Based Coordination Polymer for Efficient Photocatalytic Removal of Organic Dyes. Molecules 2023, 28, 6848. [Google Scholar] [CrossRef]
- Thamer, B.M.; Al-aizari, F.A.; Abdo, H.S. Enhanced Adsorption of Textile Dyes by a Novel Sulfonated Activated Carbon Derived from Pomegranate Peel Waste: Isotherm, Kinetic and Thermodynamic Study. Molecules 2023, 28, 7712. [Google Scholar] [CrossRef]
- Georgianos, P.; Pournara, A.D.; Andreou, E.K.; Armatas, G.S.; Manos, M.J. Composite Materials Based on a Zr4+ MOF and Aluminosilicates for the Simultaneous Removal of Cationic and Anionic Dyes from Aqueous Media. Molecules 2023, 28, 815. [Google Scholar] [CrossRef]
- Paluch, D.; Bazan-Wozniak, A.; Wolski, R.; Nosal-Wiercińska, A.; Pietrzak, R. Removal of Methyl Red from Aqueous Solution Using Biochar Derived from Fennel Seeds. Molecules 2023, 28, 7786. [Google Scholar] [CrossRef]
- Sultana, M.; Rownok, M.H.; Sabrin, M.; Rahaman, M.H.; Alam, S.M.N. A Review on Experimental Chemically Modified Activated Carbon to Enhance Dye and Heavy Metals Adsorption. Clean. Eng. Technol. 2022, 6, 100382. [Google Scholar] [CrossRef]
- Wawrzyniak, A.; Wiśniewska, M.; Nowicki, P. Carbon Adsorbents Obtained from Pistachio Nut Shells Used as Potential Ingredients of Drinking Water Filters. Molecules 2023, 28, 4497. [Google Scholar] [CrossRef] [PubMed]
- Nasruddin, M.N.; Fahmi, M.R.; Abidin, C.Z.A.; Yen, T.S. Regeneration of Spent Activated Carbon from Wastewater Treatment Plant Application. J. Phys. Conf. Ser. 2018, 1116, 032022. [Google Scholar] [CrossRef]
- Rios, R.D.F.; Bueno, P.J.B.; Terra, J.C.S.; Moura, F.C.C. Influence of the Surface Modification of Granular-Activated Carbon Synthesized from Macauba on Heavy Metal Sorption. Environ. Sci. Pollut. Res. Int. 2023, 30, 31881–31894. [Google Scholar] [CrossRef] [PubMed]
- Filipkowska, U.; Jόźwiak, T.; Szymczyk, P.; Kuczajowska-Zadrożna, M. The Use of Active Carbon Immobilised on Chitosan Beads for RB5 and BV10 Dye Removal from Aqueous Solutions. Prog. Chem. Appl. Chitin Deriv. 2017, 22, 14–26. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Filipkowska, U.; Szymczyk, P.; Zyśk, M. Effect of the Form and Deacetylation Degree of Chitosan Sorbents on Sorption Effectiveness of Reactive Black 5 from Aqueous Solutions. Int. J. Biol. Macromol. 2017, 95, 1169–1178. [Google Scholar] [CrossRef]
- da Silva Alves, D.C.; Healy, B.; Pinto, L.A.d.A.; Cadaval, T.R.S.; Breslin, C.B. Recent Developments in Chitosan-Based Adsorbents for the Removal of Pollutants from Aqueous Environments. Molecules 2021, 26, 594. [Google Scholar] [CrossRef]
- Nerdy, N.; Lestari, P.; Simorangkir, D.; Aulianshah, V.; Yusuf, F.; Bakri, T.K. Comparison of chitosan from crab shell waste and shrimp shell waste as natural adsorbent against heavy metals and dyes. Int. J. Appl. Pharm. 2022, 14, 181–185. [Google Scholar] [CrossRef]
- Thambiliyagodage, C.; Jayanetti, M.; Mendis, A.; Ekanayake, G.; Liyanaarachchi, H.; Vigneswaran, S. Recent Advances in Chitosan-Based Applications—A Review. Materials 2023, 16, 2073. [Google Scholar] [CrossRef]
- Shelke, B.N.; Jopale, M.K.; Kategaonkar, A.H. Exploration of Biomass Waste as Low Cost Adsorbents for Removal of Methylene Blue Dye: A Review. J. Indian. Chem. Soc. 2022, 99, 100530. [Google Scholar] [CrossRef]
- Szymczyk, P.; Filipkowska, U.; Jóźwiak, T.; Kuczajowska-Zadrożna, M. Use of Sawdust Immobilised on Chitosan for Disposal of Dyes from Water Solutions. Prog. Chem. Appl. Chitin Deriv. 2017, 22, 207–219. [Google Scholar] [CrossRef]
- Ucun, H. Equilibrium, Thermodynamic and Kinetics of Reactive Black 5 Biosorption on Loquat (Eriobotrya Japonica) Seed. Sci. Res. Essays 2011, 6, 4113–4124. [Google Scholar] [CrossRef][Green Version]
- Chen, J.; An, L.; Heo, J.W.; Bae, J.H.; Jeong, H.; Kim, Y.S. Utilization of Aminated Lignin as an Adsorbent to Remove Cationic and Anionic Dyes from Aqueous Solutions. J. Wood Chem. Technol. 2022, 42, 114–124. [Google Scholar] [CrossRef]
- Józwiak, T.; Filipkowska, U.; Kowalkowska, A.; Struk-Sokoowska, J.; Werbowy, D. The Influence of Amination of Sorbent Based on Buckwheat (Fagopyrum Esculentum) Husks on the Sorption Effectiveness of Reactive Black 5 Dye. J. Environ. Chem. Eng. 2021, 9, 105092. [Google Scholar] [CrossRef]
- Raboanatahiry, N.; Li, H.; Yu, L.; Li, M. Rapeseed (Brassica Napus): Processing, Utilization, and Genetic Improvement. Agronomy 2021, 11, 1776. [Google Scholar] [CrossRef]
- Carré, P.; Quinsac, A.; Citeau, M.; Fine, F. A Re-Examination of the Technical Feasibility and Economic Viability of Rapeseed Dehulling. OCL Oilseeds Fats 2015, 22, D304. [Google Scholar] [CrossRef]
- Boucher, J.; Chabloz, C.; Lex, O.; Marison, I.W. Oleaginous Seeds, Press-Cake and Seed Husks for the Biosorption of Metals. J. Water Supply Res. Technol. AQUA 2008, 57, 489–499. [Google Scholar] [CrossRef]
- Carre, P.; Citeau, M.; Robin, G.; Estorges, M. Hull Content and Chemical Composition of Whole Seeds, Hulls and Germs in Cultivars of Rapeseed (Brassica Napus). OCL 2016, 23, A302. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Vrouwenvelder, J.S.; Saikaly, P.E. Physicochemical Properties of Extracellular Polymeric Substances Produced by Three Bacterial Isolates From Biofouled Reverse Osmosis Membranes. Front. Microbiol. 2021, 12, 668761. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret Ftir Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Bhavsar, P.S.; Dalla Fontana, G.; Zoccola, M. Sustainable Superheated Water Hydrolysis of Black Soldier Fly Exuviae for Chitin Extraction and Use of the Obtained Chitosan in the Textile Field. ACS Omega 2021, 6, 8884–8893. [Google Scholar] [CrossRef]
- Hospodarova, V.; Singovszka, E.; Stevulova, N. Characterization of Cellulosic Fibers by FTIR Spectroscopy for Their Further Implementation to Building Materials. Am. J. Anal. Chem. 2018, 09, 303–310. [Google Scholar] [CrossRef]
- Rana, R.; Langenfeld-Heyser, R.; Finkeldey, R.; Polle, A. FTIR Spectroscopy, Chemical and Histochemical Characterisation of Wood and Lignin of Five Tropical Timber Wood Species of the Family of Dipterocarpaceae. Wood Sci. Technol. 2010, 44, 225–242. [Google Scholar] [CrossRef]
- Stępień, E.; Kamińska, A.; Surman, M.; Karbowska, D.; Wróbel, A.; Przybyło, M. Fourier-Transform InfraRed (FT-IR) Spectroscopy to Show Alterations in Molecular Composition of EV Subpopulations from Melanoma Cell Lines in Different Malignancy. Biochem. Biophys. Rep. 2021, 25, 100888. [Google Scholar] [CrossRef]
- Kukula-Koch, W.; Grzybek, M.; Strachecka, A.; Jaworska, A.; Ludwiczuk, A. ATR-FTIR-Based Fingerprinting of Some Cucurbitaceae Extracts: A Preliminary Study. Acta Soc. Bot. Pol. 2018, 87, 3579. [Google Scholar] [CrossRef]
- Sivakumar, S.; Khatiwada, C.P.; Sivasubramanian, J.; Jini, P.; Prabu, N.; Venkateson, A.; Soundararajan, P. FT-IR Study of Green Tea Leaves and Their Diseases of Arunachal Pradesh, North East, India. Afr. J. Parasitol. Res. 2013, 3, 166–172. [Google Scholar]
- Al-Wahaibi, L.H.; Govindarajan, M.; El-Emam, A.A.; Attia, M.I. Spectroscopic (FT-IR, FT-Raman, UV, 1H and 13C NMR) Insights, Electronic Profiling and DFT Computations on ({(E)-[3-(1H-Imidazol-1-Yl)-1-Phenylpropylidene] Amino}oxy)(4-Nitrophenyl)Methanone, an Imidazole-Bearing Anti-Candida Agent. Open Chem. 2018, 16, 50–63. [Google Scholar] [CrossRef]
- Navarro, R.; Guzmán, J.; Saucedo, I.; Revilla, J.; Guibal, E. Recovery of Metal Ions by Chitosan: Sorption Mechanisms and Influence of Metal Speciation. Macromol. Biosci. 2003, 3, 552–561. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Filipkowska, U.; Brym, S.; Kopeć, L. Use of Aminated Hulls of Sunflower Seeds for the Removal of Anionic Dyes from Aqueous Solutions. Int. J. Environ. Sci. Technol. 2020, 17, 1211–1224. [Google Scholar] [CrossRef]
- Kowalkowska, A.; Jóźwiak, T. Utilization of Pumpkin (Cucurbita pepo) Seed Husks as a Low-Cost Sorbent for Removing Anionic and Cationic Dyes from Aqueous Solutions. Desalination Water Treat. 2019, 171, 397–407. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Filipkowska, U.; Brym, S.; Zyśk, M. The Use of Aminated Cotton Fibers as an Unconventional Sorbent to Remove Anionic Dyes from Aqueous Solutions. Cellulose 2020, 27, 3957–3969. [Google Scholar] [CrossRef]
- Mook, W.T.; Aroua, M.K.; Szlachta, M. Palm Shell-Based Activated Carbon for Removing Reactive Black 5 Dye: Equilibrium and Kinetics Studies. Bioresources 2016, 11, 1432–1447. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Hameed, B.H. Fixed-Bed Adsorption of Reactive Azo Dye onto Granular Activated Carbon Prepared from Waste. J. Hazard. Mater. 2010, 175, 298–303. [Google Scholar] [CrossRef]
- Paczyńska, K.; Jóźwiak, T.; Filipkowska, U. The Effect of Modifying Canadian Goldenrod (Solidago canadensis) Biomass with Ammonia and Epichlorohydrin on the Sorption Efficiency of Anionic Dyes from Water Solutions. Materials 2023, 16, 4586. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Filipkowska, U.; Walczak, P. The Use of Aminated Wheat Straw for Reactive Black 5 Dye Removal from Aqueous Solutions as a Potential Method of Biomass Valorization. Energies 2022, 15, 6257. [Google Scholar] [CrossRef]
- Osma, J.F.; Saravia, V.; Toca-Herrera, J.L.; Couto, S.R. Sunflower Seed Shells: A Novel and Effective Low-Cost Adsorbent for the Removal of the Diazo Dye Reactive Black 5 from Aqueous Solutions. J. Hazard. Mater. 2007, 147, 900–905. [Google Scholar] [CrossRef]
- Güzel, F.; Sayğili, H.; Akkaya Sayğili, G.; Koyuncu, F. New Low-Cost Nanoporous Carbonaceous Adsorbent Developed from Carob (Ceratonia siliqua) Processing Industry Waste for the Adsorption of Anionic Textile Dye: Characterization, Equilibrium and Kinetic Modeling. J. Mol. Liq. 2015, 206, 244–255. [Google Scholar] [CrossRef]
- do Nascimento, B.F.; de Araujo, C.M.B.; do Nascimento, A.C.; da Costa, G.R.B.; Gomes, B.F.M.L.; da Silva, M.P.; da Silva Santos, R.K.; da Motta Sobrinho, M.A. Adsorption of Reactive Black 5 and Basic Blue 12 Using Biochar from Gasification Residues: Batch Tests and Fixed-Bed Breakthrough Predictions for Wastewater Treatment. Bioresour. Technol. Rep. 2021, 15, 100767. [Google Scholar] [CrossRef]
- Ip, A.W.M.; Barford, J.P.; McKay, G. A Comparative Study on the Kinetics and Mechanisms of Removal of Reactive Black 5 by Adsorption onto Activated Carbons and Bone Char. Chem. Eng. J. 2010, 157, 434–442. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Filipkowska, U.; Bakuła, T.; Bralewska-Piotrowicz, B.; Karczmarczyk, K.; Gierszewska, M.; Olewnik-Kruszkowska, E.; Szyryńska, N.; Lewczuk, B. The Use of Chitin from the Molts of Mealworm (Tenebrio Molitor) for the Removal of Anionic and Cationic Dyes from Aqueous Solutions. Materials 2023, 16, 545. [Google Scholar] [CrossRef]
- Nabil, G.M.; El-Mallah, N.M.; Mahmoud, M.E. Enhanced Decolorization of Reactive Black 5 Dye by Active Carbon Sorbent-Immobilized-Cationic Surfactant (AC-CS). J. Ind. Eng. Chem. 2014, 20, 994–1002. [Google Scholar] [CrossRef]
- Eren, Z.; Acar, F.N. Adsorption of Reactive Black 5 from an Aqueous Solution: Equilibrium and Kinetic Studies. Desalination 2006, 194, 1–10. [Google Scholar] [CrossRef]
- Hamzeh, Y.; Ashori, A.; Azadeh, E.; Abdulkhani, A. Removal of Acid Orange 7 and Remazol Black 5 Reactive Dyes from Aqueous Solutions Using a Novel Biosorbent. Mater. Sci. Eng. C 2012, 32, 1394–1400. [Google Scholar] [CrossRef]
- Munagapati, V.S.; Yarramuthi, V.; Kim, Y.; Lee, K.M.; Kim, D.S. Removal of Anionic Dyes (Reactive Black 5 and Congo Red) from Aqueous Solutions Using Banana Peel Powder as an Adsorbent. Ecotoxicol. Environ. Saf. 2018, 148, 601–607. [Google Scholar] [CrossRef]
- Heibati, B.; Rodriguez-Couto, S.; Amrane, A.; Rafatullah, M.; Hawari, A.; Al-Ghouti, M.A. Uptake of Reactive Black 5 by Pumice and Walnut Activated Carbon: Chemistry and Adsorption Mechanisms. J. Ind. Eng. Chem. 2014, 20, 2939–2947. [Google Scholar] [CrossRef]
- Uçar, D.; Armağan, B. The Removal of Reactive Black 5 from Aqueous Solutions by Cotton Seed Shell. Water Environ. Res. 2012, 84, 323–327. [Google Scholar] [CrossRef]
- Felista, M.M.; Wanyonyi, W.C.; Ongera, G. Adsorption of Anionic Dye (Reactive Black 5) Using Macadamia Seed Husks: Kinetics and Equilibrium Studies. Sci. Afr. 2020, 7, e00283. [Google Scholar] [CrossRef]
- Indhu, S.; Muthukumaran, K. Removal and Recovery of Reactive Yellow 84 Dye from Wastewater and Regeneration of Functionalised Borassus Flabellifer Activated Carbon. J. Environ. Chem. Eng. 2018, 6, 3111–3121. [Google Scholar] [CrossRef]
- Şahin, E. Interpretation of Sorption Kinetics for Mixtures of Reactive Dyes on Wool. Turk. J. Chem. 2005, 29, 617–625. [Google Scholar]
- Harikishore Kumar Reddy, D.; Vijayaraghavan, K.; Kim, J.A.; Yun, Y.S. Valorisation of Post-Sorption Materials: Opportunities, Strategies, and Challenges. Adv. Colloid Interface Sci. 2017, 242, 35–58. [Google Scholar] [CrossRef]
- Ronda, A.; Della Zassa, M.; Martín-Lara, M.A.; Calero, M.; Canu, P. Combustion of a Pb(II)-Loaded Olive Tree Pruning Used as Biosorbent. J. Hazard. Mater. 2016, 308, 285–293. [Google Scholar] [CrossRef]
- McKay, G. Use of Adsorbents for the Removal of Pollutants from Wastewaters, 1st ed.; CRC Press: London, UK, 1996; ISBN 0849369207. [Google Scholar]
- Feng, Y.; Dionysiou, D.D.; Wu, Y.; Zhou, H.; Xue, L.; He, S.; Yang, L. Adsorption of Dyestuff from Aqueous Solutions through Oxalic Acid-Modified Swede Rape Straw: Adsorption Process and Disposal Methodology of Depleted Bioadsorbents. Bioresour. Technol. 2013, 138, 191–197. [Google Scholar] [CrossRef]
- Chwastowski, J.; Guzik, M.; Bednarz, S.; Staroń, P. Upcycling Waste Streams from a Biorefinery Process—A Case Study on Cadmium and Lead Biosorption by Two Types of Biopolymer Post-Extraction Biomass. Molecules 2023, 28, 6345. [Google Scholar] [CrossRef]
- Zafar, S.; Khalid, N.; Daud, M.; Mirza, M.L. Kinetic Studies of the Adsorption of Thorium Ions onto Rice Husk from Aqueous Media: Linear and Nonlinear Approach. Nucl. 2015, 52, 14–19. [Google Scholar]
- Wang, J.; Guo, X. Rethinking of the Intraparticle Diffusion Adsorption Kinetics Model: Interpretation, Solving Methods and Applications. Chemosphere 2022, 309, 136732. [Google Scholar] [CrossRef]
- Ghosal, P.S.; Gupta, A.K. Development of a Generalized Adsorption Isotherm Model at Solid-Liquid Interface: A Novel Approach. J. Mol. Liq. 2017, 240, 21–24. [Google Scholar] [CrossRef]
- Wang, J.; Wei, Y.; Ma, Z. Modified Dual-Site Langmuir Adsorption Equilibrium Models from A GCMC Molecular Simulation. Appl. Sci. 2020, 10, 1311. [Google Scholar] [CrossRef]




| Type of Sorbent | Carbon Content [%] | Nitrogen Content [%] | N/C Ratio |
|---|---|---|---|
| RH | 42.51 ± 0.16 | 1.052 ± 0.010 | 0.0247 |
| RH-A | 42.25 ± 0.14 | 1.075 ± 0.008 | 0.0254 |
| RH-E | 43.48 ± 0.20 | 1.043 ± 0.004 | 0.0240 |
| RH-EA | 43.39 ± 0.18 | 1.131 ± 0.003 | 0.0261 |
| Sorbent | Dye | Dye Conc. | Pseudo-First-Order Model | Pseudo-Second-Order Model | Exp. Data | Equil. Time | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| k1 | qe, cal. | R2 | k2 | qe, cal. | R2 | qe, exp. | ||||
| [mg/L] | [1/min] | [mg/g] | - | [g/mg·min] | [mg/g] | - | [mg/g] | [min] | ||
| RH | RB5 | 50 | 0.0495 | 6.22 | 0.9488 | 0.0108 | 6.84 | 0.9881 | 6.53 | 180 |
| 250 | 0.0618 | 13.00 | 0.9783 | 0.0069 | 14.10 | 0.9985 | 13.44 | 150 | ||
| RY84 | 50 | 0.0286 | 5.23 | 0.9687 | 0.0060 | 6.03 | 0.9874 | 5.35 | 180 | |
| 250 | 0.0365 | 11.32 | 0.9709 | 0.0039 | 12.74 | 0.9912 | 11.54 | 150 | ||
| RH-A | RB5 | 50 | 0.0459 | 7.17 | 0.9633 | 0.0084 | 7.93 | 0.9936 | 7.50 | 180 |
| 250 | 0.0600 | 20.26 | 0.9717 | 0.0043 | 22.02 | 0.9975 | 21.05 | 150 | ||
| RY84 | 50 | 0.0371 | 6.25 | 0.9703 | 0.0072 | 7.04 | 0.9933 | 6.46 | 180 | |
| 250 | 0.0448 | 16.29 | 0.9849 | 0.0036 | 18.04 | 0.9956 | 16.63 | 150 | ||
| RH-E | RB5 | 50 | 0.0792 | 8.78 | 0.9622 | 0.0140 | 9.42 | 0.9951 | 9.24 | 180 |
| 250 | 0.0738 | 19.83 | 0.9810 | 0.0058 | 21.27 | 0.9988 | 20.44 | 150 | ||
| RY84 | 50 | 0.0446 | 8.77 | 0.9728 | 0.0067 | 9.71 | 0.9950 | 9.06 | 180 | |
| 250 | 0.0420 | 20.47 | 0.9713 | 0.0026 | 22.77 | 0.9942 | 21.02 | 150 | ||
| RH-EA | RB5 | 50 | 0.1070 | 9.36 | 0.9796 | 0.0207 | 9.86 | 0.9982 | 9.64 | 120 |
| 250 | 0.1370 | 45.30 | 0.9889 | 0.0062 | 47.18 | 0.9997 | 46.35 | 90 | ||
| RY84 | 50 | 0.1402 | 9.10 | 0.9903 | 0.0328 | 9.46 | 0.9998 | 9.30 | 120 | |
| 250 | 0.1537 | 43.39 | 0.9924 | 0.0081 | 44.91 | 0.9998 | 44.28 | 90 | ||
| Sorbent | Dye | Dye Conc. | Phase 1 | Phase 2 | Phase 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| kd1 * | Dur. Time | R2 | kd2 | Dur. Time | R2 | kd3 | Dur. Time | R2 | |||
| [mg/L] | * | [min] | - | * | [min] | - | * | [min] | - | ||
| RH | RB5 | 50 | 1.0907 | 10 | 0.(9) | 0.4250 | 50 | 0.9988 | 0.1648 | 120 | 0.9834 |
| 250 | 2.3211 | 10 | 0.(9) | 1.1989 | 35 | 0.9951 | 0.3184 | 105 | 0.9751 | ||
| RY84 | 50 | 0.6514 | 10 | 0.(9) | 0.4097 | 80 | 0.9997 | 0.1670 | 90 | 0.9279 | |
| 250 | 1.5748 | 10 | 0.(9) | 0.9419 | 50 | 0.9965 | 0.3311 | 90 | 0.9864 | ||
| RH-A | RB5 | 50 | 1.1416 | 10 | 0.(9) | 0.5767 | 50 | 0.9919 | 0.1947 | 120 | 0.9946 |
| 250 | 3.5955 | 10 | 0.(9) | 1.7621 | 35 | 0.9888 | 0.6045 | 105 | 0.9869 | ||
| RY84 | 50 | 0.8791 | 10 | 0.(9) | 0.4828 | 80 | 0.9903 | 0.1624 | 90 | 0.9815 | |
| 250 | 2.3970 | 10 | 0.(9) | 1.6056 | 50 | 0.9951 | 0.4099 | 90 | 0.9940 | ||
| RH-E | RB5 | 50 | 1.8215 | 10 | 0.(9) | 0.5362 | 50 | 0.9836 | 0.1296 | 120 | 0.9907 |
| 250 | 3.8200 | 10 | 0.(9) | 1.7271 | 35 | 0.9912 | 0.3822 | 105 | 0.9771 | ||
| RY84 | 50 | 1.3503 | 10 | 0.(9) | 0.6725 | 80 | 0.9872 | 0.1367 | 90 | 0.9870 | |
| 250 | 3.0453 | 10 | 0.(9) | 1.7225 | 50 | 0.9948 | 0.7566 | 90 | 0.9795 | ||
| RH-EA | RB5 | 50 | 2.1598 | 10 | 0.(9) | 0.5897 | 35 | 0.9892 | 0.1496 | 75 | 0.9364 |
| 250 | 11.189 | 10 | 0.(9) | 3.0029 | 20 | 0.9744 | 0.9229 | 60 | 0.9971 | ||
| RY84 | 50 | 2.2705 | 10 | 0.(9) | 0.5945 | 20 | 0.9915 | 0.0951 | 90 | 0.9993 | |
| 250 | 11.140 | 10 | 0.(9) | 2.6307 | 20 | 0.9858 | 0.6610 | 60 | 0.9887 | ||
| Sorbent | Dye | Langmuir 1 Model | Langmuir 2 Model | Freundlich Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Qmax | Kc | R2 | Qmax | b1 | K1 | b2 | K2 | R2 | k | n | R2 | ||
| [mg/g] | [L/mg] | - | mg/g | mg/g | L/mg | mg/g | L/mg | - | - | - | - | ||
| RH | RB5 | 15.20 | 0.048 | 0.9916 | 15.20 | 7.60 | 0.048 | 7.60 | 0.048 | 0.9916 | 3.19 | 0.267 | 0.8506 |
| RY84 | 13.65 | 0.030 | 0.9974 | 13.65 | 6.77 | 0.030 | 6.88 | 0.030 | 0.9974 | 2.16 | 0.309 | 0.9162 | |
| RH-A | RB5 | 26.26 | 0.038 | 0.9926 | 26.26 | 12.63 | 0.038 | 12.63 | 0.038 | 0.9926 | 4.09 | 0.315 | 0.8918 |
| RY84 | 19.67 | 0.030 | 0.9935 | 19.67 | 9.83 | 0.030 | 9.84 | 0.03 | 0.9935 | 2.94 | 0.320 | 0.8870 | |
| RH-E | RB5 | 20.54 | 0.201 | 0.9891 | 21.24 | 15.70 | 0.303 | 5.54 | 0.032 | 0.9926 | 6.75 | 0.207 | 0.8746 |
| RY84 | 22.08 | 0.134 | 0.9948 | 22.58 | 20.35 | 0.153 | 2.23 | 0.014 | 0.9954 | 6.42 | 0.227 | 0.8712 | |
| RH-EA | RB5 | 127.72 | 0.032 | 0.9991 | 135.83 | 130.30 | 0.026 | 5.53 | 0.955 | 0.9993 | 17.15 | 0.346 | 0.9376 |
| RY84 | 117.23 | 0.023 | 0.9954 | 119.98 | 114.23 | 0.019 | 5.75 | 2.70 | 0.9978 | 13.71 | 0.356 | 0.9554 | |
| DYE | Sorbent | Sorption Capacity [mg/g] | pH of Sorption | Duration of Sorption [min] | Source |
|---|---|---|---|---|---|
| RB5 | Chitosan flakes DD = 90% | 451.5 | 4 | 720 | [11] |
| Activated carbon Filtrasorb 400 (commercial) | 198.0 | 5.2 | 400 | [44] | |
| Aminated rapeseed husks (activated with epichlorohydrin) | 135.8 | 3 | 120 | This work | |
| Chitin flakes from snow crab shells (prod. by BioLog Heppe) | 131.6 | 3 | 360 | [45] | |
| Activated carbon (powder) | 125.8 | 2 | 240 | [10] | |
| Aminated wheat straw (activated with epichlorohydrin) | 91.0 | 3 | 210 | [40] | |
| Aminated buckwheat hulls (activated with epichlorohydrin) | 85.2 | 3 | 300 | [19] | |
| Aminated goldenrod biomass (activated with epichlorohydrin) | 71.3 | 3 | 120 | [39] | |
| Activated carbon modified with SPC | 69.9 | 2 | <60 | [46] | |
| Activated carbon (powdered) | 58.8 | - | - | [47] | |
| Aminated sunflower seed shells (activated with epichlorohydrin) | 51.0 | 3 | 240 | [34] | |
| Activated carbon from bamboo | 39.0 | 2 | 60 | [38] | |
| Activated carbon from Carob tree | 36.9 | 2 | 120 | [42] | |
| Biochar from gasification residues | 35.7 | - | 90 | [43] | |
| Rape stalks (waste) | 32.8 | 2.5 | 30 | [48] | |
| Banana peel (powder) | 26.9 | 3 | 60 | [49] | |
| Activated carbon from palm shell | 25.1 | 2 | 300 | [37] | |
| Wood (walnut) activated carbon | 19.3 | 5 | 400 | [50] | |
| Wheat straw | 15.7 | 7 | 195 | [40] | |
| Rapeseed husks | 15.2 | 3 | 180 | This work | |
| Beech sawdust | 13.9 | 3 | 1440 | [16] | |
| Seed scales of Eriobotrya japonica | 13.8 | 3 | 150 | [17] | |
| Cotton seed husks | 12.9 | 2 | 30 | [51] | |
| Buckwheat hulls | 4.43 | 3 | 300 | [19] | |
| Sunflower seed shells | 2.9 | 3 | 210 | [34] | |
| Cotton fibers | 2.7 | 3 | 240 | [36] | |
| Goldenrod biomass | 2.3 | 3 | 150 | [39] | |
| Macadamia seed husks | 1.2 | 3 | 510 | [52] | |
| Sunflower biomass | 1.1 | 2 | 210 | [41] | |
| Pumpkin seed husks | 1.0 | 3 | 60 | [35] | |
| RY84 | Aminated rapeseed husks (activated with epichlorohydrin) | 114.2 | 3 | 120 | This work |
| Aminated sunflower seed hulls (activated with epichlorohydrin) | 63.3 | 3 | 240 | [34] | |
| Aminated goldenrod biomass (activated with epichlorohydrin) | 59.3 | 3 | 120 | [39] | |
| Aminated cotton fibers (activated with epichlorohydrin) | 43.3 | 2 | 240 | [36] | |
| Activated carbon from the Borassus flabellifer plant | 40.0 | - | - | [53] | |
| Cotton fibers | 15.9 | 2 | 240 | [36] | |
| Rapeseed husks | 13.7 | 3 | 180 | This work | |
| Wool | 11.0 | 7 | 180 | [54] | |
| Sunflower seed husks | 4.2 | 2 | 90 | [34] | |
| Goldenrod biomass | 2.3 | 3 | 180 | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jóźwiak, T.; Filipkowska, U. Aminated Rapeseed Husks (Brassica napus) as an Effective Sorbent for Removing Anionic Dyes from Aqueous Solutions. Molecules 2024, 29, 843. https://doi.org/10.3390/molecules29040843
Jóźwiak T, Filipkowska U. Aminated Rapeseed Husks (Brassica napus) as an Effective Sorbent for Removing Anionic Dyes from Aqueous Solutions. Molecules. 2024; 29(4):843. https://doi.org/10.3390/molecules29040843
Chicago/Turabian StyleJóźwiak, Tomasz, and Urszula Filipkowska. 2024. "Aminated Rapeseed Husks (Brassica napus) as an Effective Sorbent for Removing Anionic Dyes from Aqueous Solutions" Molecules 29, no. 4: 843. https://doi.org/10.3390/molecules29040843
APA StyleJóźwiak, T., & Filipkowska, U. (2024). Aminated Rapeseed Husks (Brassica napus) as an Effective Sorbent for Removing Anionic Dyes from Aqueous Solutions. Molecules, 29(4), 843. https://doi.org/10.3390/molecules29040843







