Optimization of Pt(II) and Pt(IV) Adsorption from a Water Solution on Biochar Originating from Honeycomb Biomass
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Characteristics
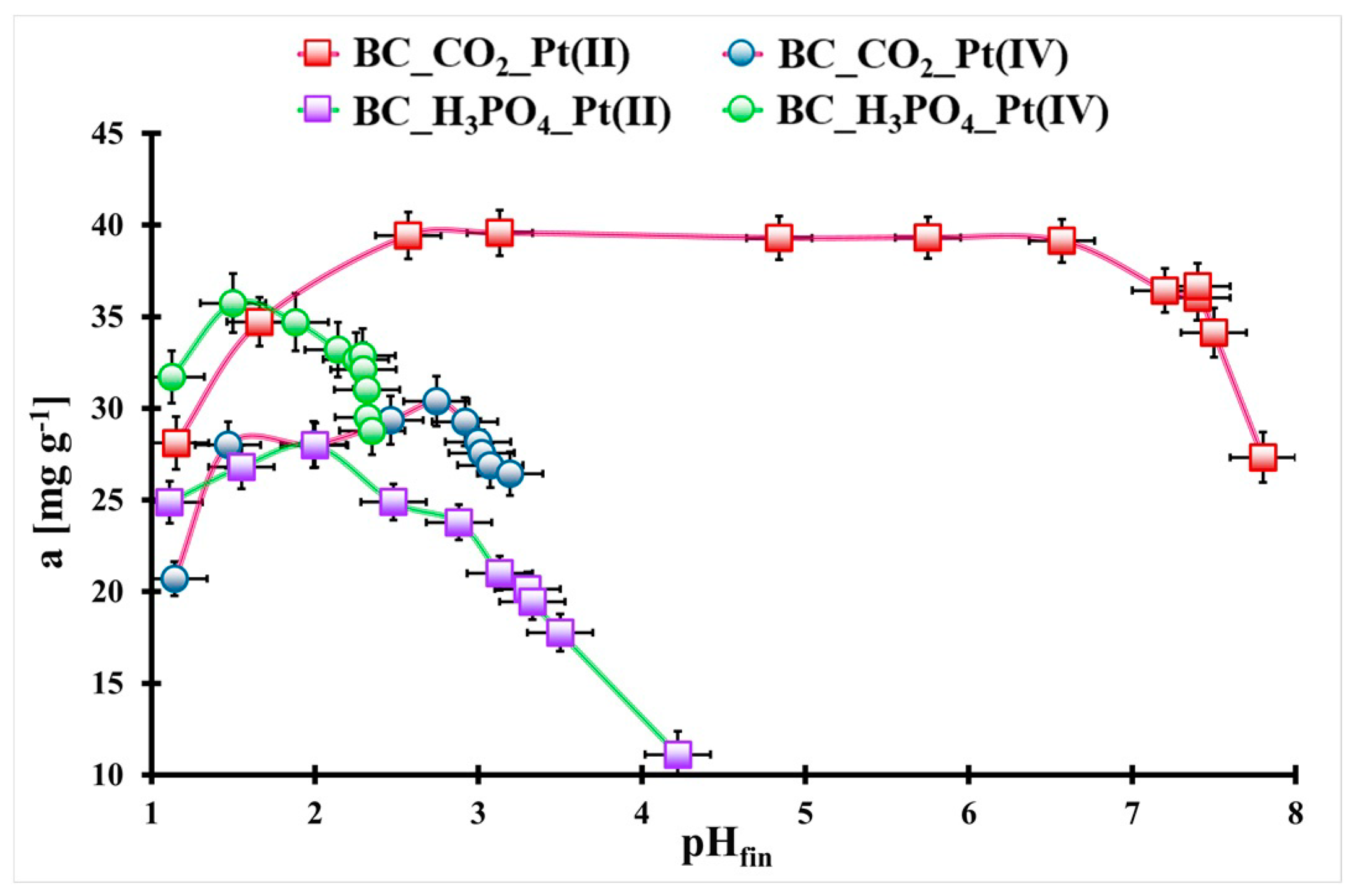
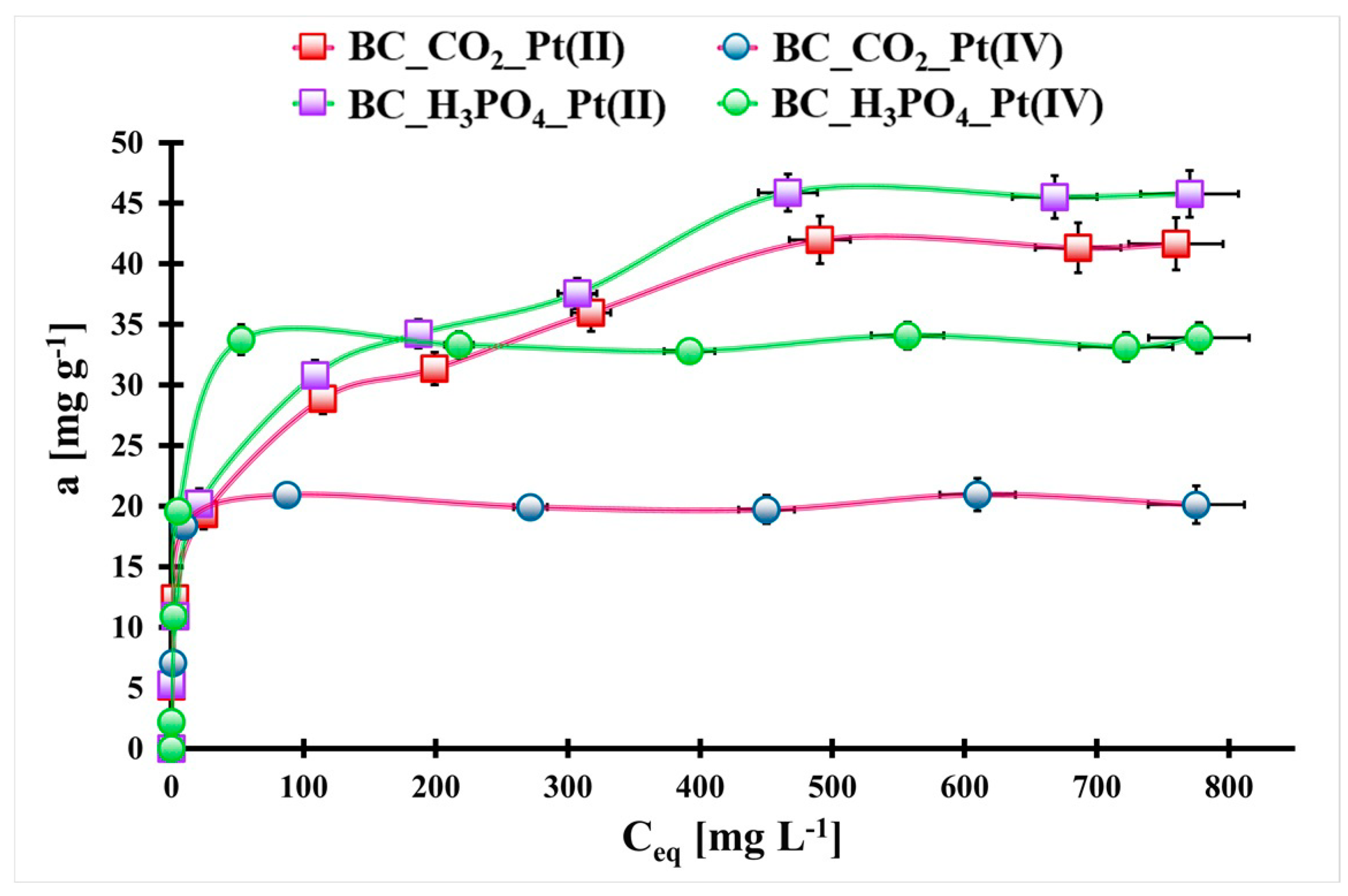
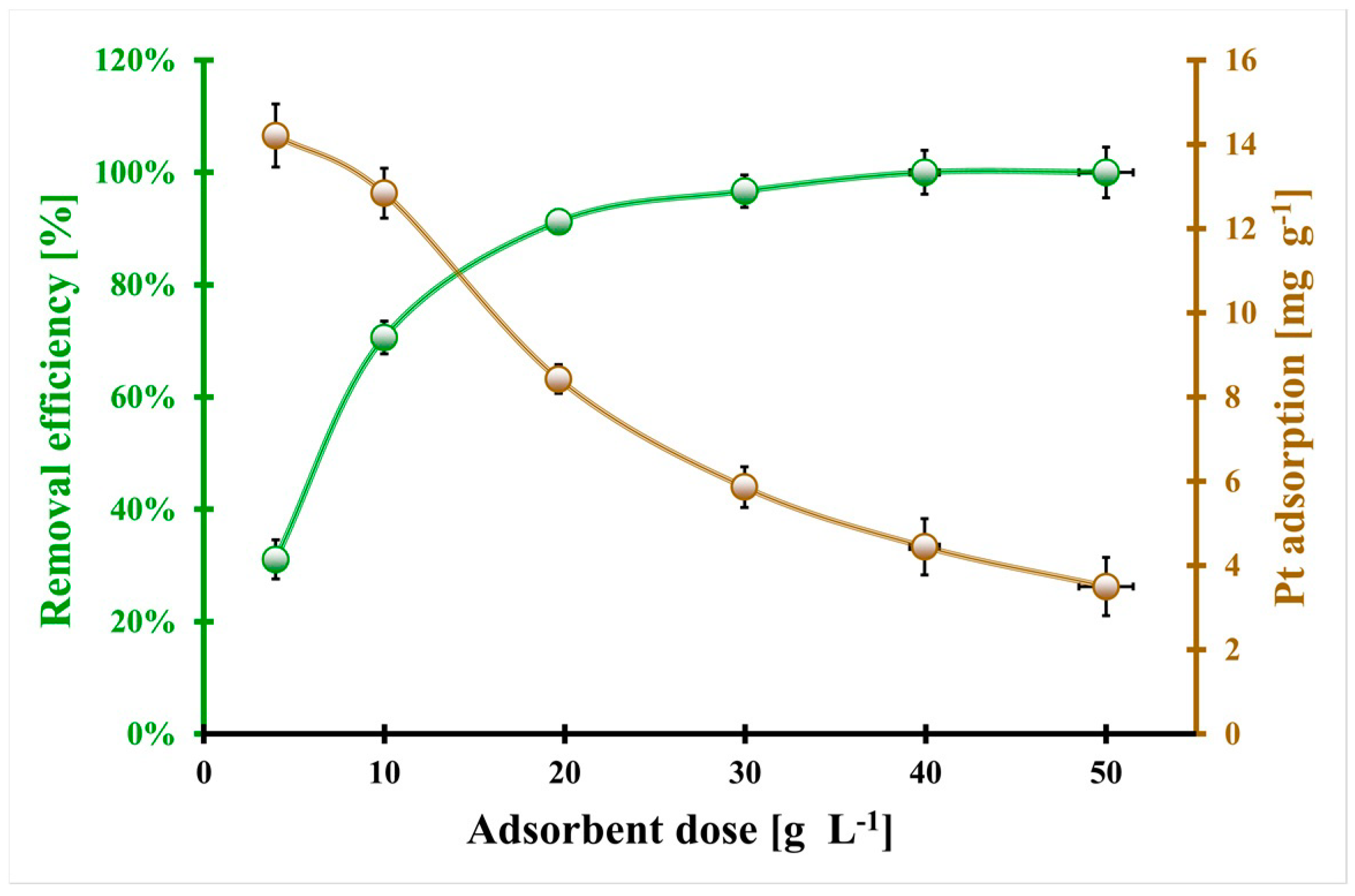
2.2. Pt(II) and Pt(IV) Adsorption Optimization
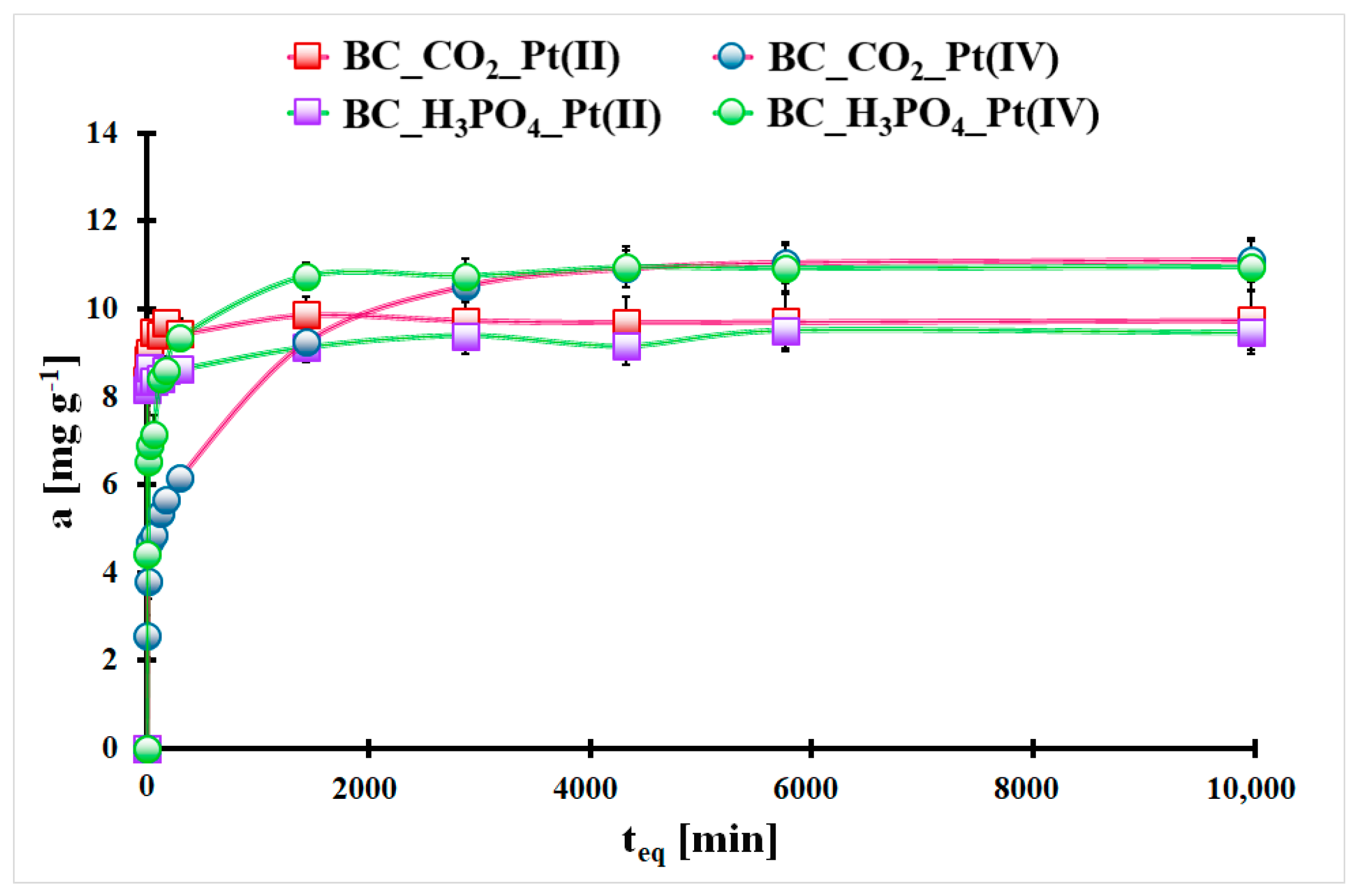
2.3. Pt Desorption Studies
2.4. Adsorption Mechanism
2.5. Pt(IV) Removal from Catalyst-Originated Extract
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Slumgum Carbonization Activation
3.3. Instrumentation
3.4. Pt(II) and Pt(IV) Adsorption Studies
3.5. Adsorption of Pt(IV) by Biochar from Spent Automotive Catalyst Leaching Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trucillo, P.; Lancia, A.; Di Natale, F. Recovery of platinum from diesel catalysts by combined use of H2O2/HCl leaching and adsorption. J. Environ. Chem. Eng. 2022, 10, 107730. [Google Scholar] [CrossRef]
- Limjuco, L.A.; Burnea, F.K. Evaluation of dithiadiamide-based molecular ion imprinted polymer (MIIP) for selective recovery of platinum from acid-digested spent automobile catalytic converter (ACC) solution. MRS Communic. 2022, 12, 175–182. [Google Scholar] [CrossRef]
- Dong, H.; Zhao, J.; Chen, J.; Wu, Y.; Li, B. Recovery of platinum group metals from spent catalysts: A review. Intern. J. Mineral Proc. 2015, 145, 108–113. [Google Scholar] [CrossRef]
- Grilli, M.L.; Slobozeanu, A.E.; Larosa, C.; Paneva, D.; Yakoumis, I.; Cherkezova-Zheleva, Z. Platinum Group Metals: Green Recovery from Spent Auto-Catalysts and Reuse in New Catalysts—A Review. Crystals 2023, 13, 550. [Google Scholar] [CrossRef]
- Chen, M.; Li, S.; Jin, C.; Shao, M.; Huang, Z.; Xie, X. Removal of metal-cyanide complexes and recovery of Pt(II) and Pd(II) from wastewater using an alkali–tolerant metal-organic resin. J. Hazard. Mater. 2021, 406, 124315. [Google Scholar] [CrossRef]
- Iftekhar, S.; Heidari, G.; Amanat, N.; Zare, E.N.; Asif, M.B.; Hassanpour, M.; Lehto, V.P.; Sillanpaa, M. Porous materials for the recovery of rare earth elements, platinum group metals, and other valuable metals: A review. Environ. Chem. Lett. 2022, 20, 3697–3746. [Google Scholar] [CrossRef]
- Lo, S.-Y.; Dianbudiyanto, W.; Liu, S.-H. Selective recovery of platinum from spent autocatalyst solution by thiourea modified magnetic biocarbons. Sci. Rep. 2021, 11, 19281. [Google Scholar] [CrossRef]
- Kancharla, S.; Sasaki, K. Selective extraction of precious metals from simulated automotive catalyst waste and their conversion to carbon supported PdPt nanoparticle catalyst. Coll. Surf. A Phys. Eng. Asp. 2023, 665, 131179. [Google Scholar] [CrossRef]
- Eissa, M.E.; Sakr, A.K.; Hanfi, M.Y.; Sayyed, M.; Al-Otaibi, J.S.; Abdel-Lateef, A.M.; Cheira, M.F.; Abdelmonem, H.A. Physicochemical investigation of mercury sorption on mesoporous thioacetamide/chitosan from wastewater. Chemosphere 2023, 341, 140062. [Google Scholar] [CrossRef]
- Zakusilova, V.; Tereshatov, E.E.; Boltoeva, M.; Folden, C.M., III. Characterization and application of alkanethiolate self-assembled monolayers on Au-coated chips for Ir (IV) and Rh (III) sorption. Appl. Surf. Sci. 2024, 642, 158356. [Google Scholar] [CrossRef]
- Morales-Corts, R.; Gomez-Sanchez, M.A.; Perez-Sanchez, R.; Prieto-Calvo, C. Characterization of beekeeping wastes for using in seedling production. Span. J. Agric. Res. 2010, 8, 493–500. [Google Scholar] [CrossRef]
- Morales-Corts, R.; Gómez-Sánchez, M.A.; Pérez-Sánchez, R. Evaluation of green/pruning wastes compost and vermicompost, slumgum compost and their mixes as growing media for horticultural production. Sci. Hortic. 2014, 172, 155–160. [Google Scholar] [CrossRef]
- Calatayud-Vernich, P.; Calatayud, F.; Simó, E.; Picó, Y. Pesticide residues in honey bees, pollen and beeswax: Assessing beehive exposure. Environ. Pollut. 2018, 241, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Oh, J.-I.; Vithanage, M.; Park, Y.-K.; Lee, J.; Kwon, E.E. Modification of biochar properties using CO2. Chem. Eng. J. 2019, 372, 383–389. [Google Scholar] [CrossRef]
- Peng, H.; Gao, P.; Chu, G.; Pan, B.; Peng, J.; Xing, B. Enhanced adsorption of Cu(II) and Cd(II) by phosphoric acid-modified biochars. Environ. Pollut. 2017, 229, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Zeng, X.-Y.; Wang, Y.; Li, R.-X.; Cao, H.-L.; Li, Y.-F.; Lü, J. Impacts of temperatures and phosphoric-acid modification to the physicochemical properties of biochar for excellent sulfadiazine adsorption. Biochar 2022, 4, 14. [Google Scholar] [CrossRef]
- Joshi, M.; Bhatt, D.; Srivastava, A. Enhanced Adsorption Efficiency through Biochar Modification: A Comprehensive Review. Ind. Eng. Chem. Res. 2023, 62, 13748–13761. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Chen, S.S.; Tsang, D.C.; Zhang, M.; Vithanage, M.; Mandal, S.; Gao, B.; Bolan, N.S.; Ok, Y.S. Engineered/designer biochar for contaminant removal/immobilization from soil and water: Potential and implication of biochar modification. Chemosphere 2016, 148, 276–291. [Google Scholar] [CrossRef]
- Olchowski, R.; Podkościelna, B.; Zawisza, B.; Morlo, K.; Dobrowolski, R. U(VI) removal from water by novel P-modified activated carbon derived from polymeric microspheres. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100788. [Google Scholar] [CrossRef]
- Tagliaferro, A.; Rovere, M.; Padovano, E.; Bartoli, M.; Giorcelli, M. Introducing the Novel Mixed Gaussian-Lorentzian Lineshape in the Analysis of the Raman Signal of Biochar. Nanomaterials 2020, 10, 1748. [Google Scholar] [CrossRef]
- Hepburn, H.R.; Uangphakdee, O.D.; Pirk, C.W.W. Physical properties of honeybee silk: A review. Apidologie 2013, 44, 600–610. [Google Scholar] [CrossRef]
- Sutherland, T.D.; Weisman, S.; Walker, A.A.; Mudie, S.T. The Coiled Coil Silk of Bees, Ants, and Hornets. Biopolymers 2011, 97, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Raicopol, M.; Andronescu, C.; Atasiei, R.; Hanganu, A.; Pilan, L. Post-polymerization electrochemical functionalization of a conducting polymer: Diazonium salt electroreduction at polypyrrole electrodes. J. Electrochem. Soc. 2014, 161, G103–G113. [Google Scholar] [CrossRef]
- Arrigo, R.; Hävecker, M.; Wrabetz, S.; Blume, R.; Lerch, M.; McGregor, J.; Parrott, E.P.J.; Zeitler, J.A.; Gladden, L.F.; Knop-Gericke, A.; et al. Tuning the acid/base properties of nanocarbons by functionalization via amination. J. Am. Chem. Soc. 2010, 132, 9616–9630. [Google Scholar] [CrossRef]
- Gupta, N.K.; Peng, B.; Haller, G.L.; Ember, E.E.; Lercher, J.A. Nitrogen modified carbon nano-materials as stable catalysts for phosgene synthesis. ACS Catal. 2016, 6, 5843–5855. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, J.; Yue, Y.; Sheng, G.; Lai, H.; Rui, J.; He, H.; Hu, Z.; Feng, F.; Zhang, Q.; et al. Carbon with surface-enriched nitrogen and sulfur supported Au catalysts for acetylene hydrochlorination. Chem. Cat. Chem. 2019, 11, 1002–1009. [Google Scholar] [CrossRef]
- Dobrzyńska, J.; Dąbrowska, M.; Olchowski, R.; Zięba, E.; Dobrowolski, R. Development of a method for removal of platinum from hospital wastewater by novel ion-imprinted mesoporous organosilica. J. Environ. Chem. Eng. 2021, 9, 105302. [Google Scholar] [CrossRef]
- Dobrzyńska, J.; Mróz, A.; Olchowski, R.; Zięba, E.; Dobrowolski, R. Modified multi-walled carbon nanotubes as effective Pt(IV) ions adsorbent with respect to analytical application. Appl. Surf. Sci. 2022, 602, 154388. [Google Scholar] [CrossRef]
- Kalam, S.; Abu-Khamsin, S.A.; Kamal, M.S.; Patil, S. Surfactant adsorption isotherms: A review. ACS Omega 2021, 6, 32342–32348. [Google Scholar] [CrossRef]
- Dembowski, J.; Marosi, L.; Essig, M. Platinum sulfide by XPS. Surf. Sci. Spectra 1993, 2, 104–108. [Google Scholar] [CrossRef]
- Stadnichenko, A.; Svintsitskiy, D.; Kibis, L.; Fedorova, E.; Stonkus, O.; Slavinskaya, E.; Lapin, I.; Fakhrutdinova, E.; Svetlichnyi, V.; Romanenko, A.; et al. Influence of titania synthesized by pulsed laser ablation on the state of platinum during ammonia oxidation. Appl. Sci. 2020, 10, 4699. [Google Scholar] [CrossRef]
- Folens, K.; Abebe, A.; Tang, J.; Ronsse, F.; Du Laing, G. Biosorption of residual cisplatin, carboplatin and oxaliplatin antineoplastic drugs in urine after chemotherapy treatment. Environ. Chem. 2018, 15, 506–512. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A.; Inamuddin; Asiri, A.M. Exploring the reusability of synthetically contaminated wastewater containing crystal violet dye using tectona grandis sawdust as a very low-cost adsorbent. Sci. Rep. 2018, 8, 8314. [Google Scholar] [CrossRef]
- Chęć, M.; Olszewski, K.; Dziechciarz, P.; Skowronek, P.; Pietrow, M.; Borsuk, G.; Bednarczyk, M.; Jasina, G.; Jasina, J.; Gagoś, M. Effect of stearin and paraffin adulteration of beeswax on brood survival. Apidologie 2021, 52, 432–446. [Google Scholar] [CrossRef]
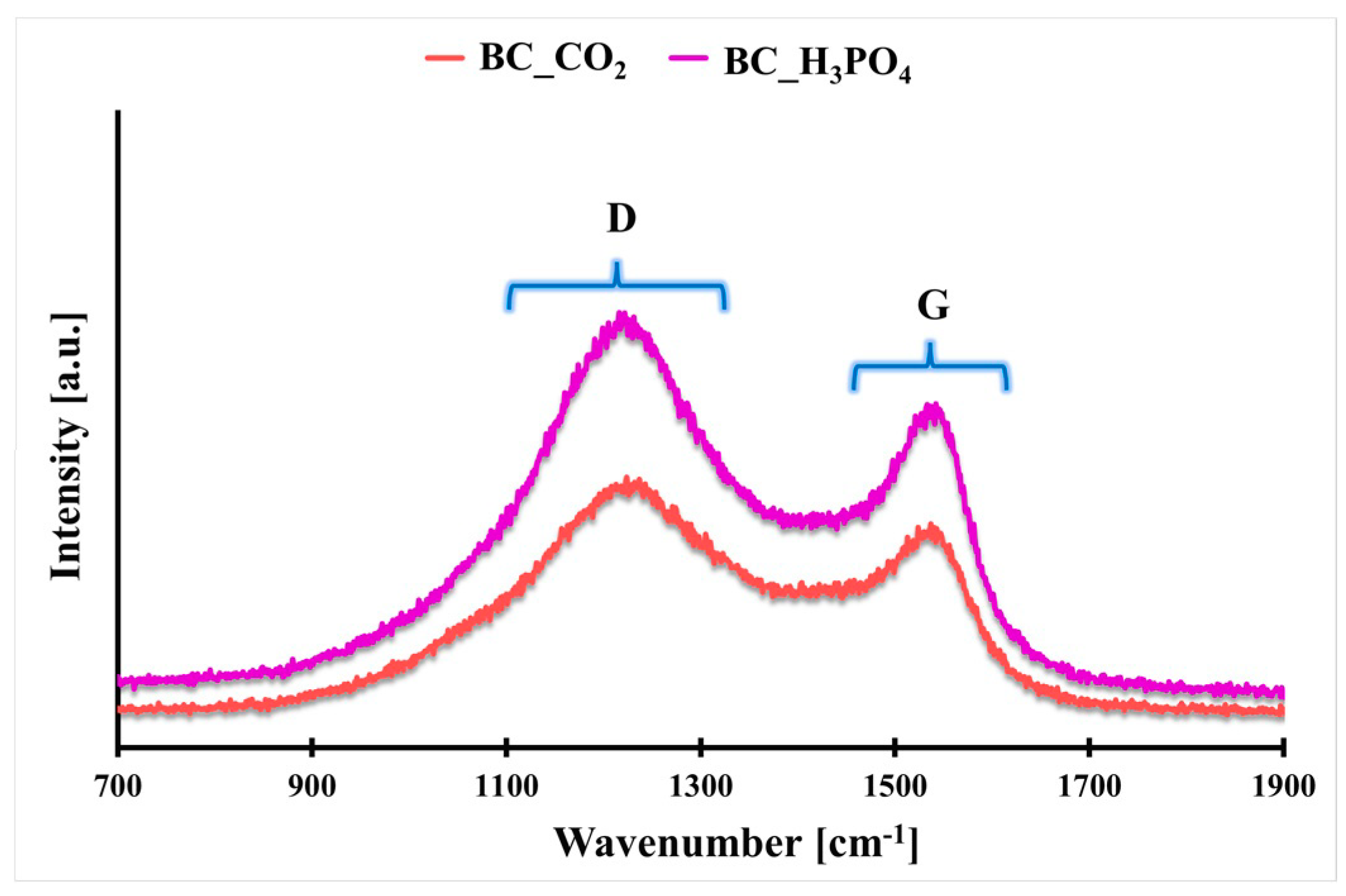



| Biochar Symbol | SBET [m2 g−1] | VT [cm3 g−1] | dBJH [nm] | ID/IG [a. u.] | ζ [mV] |
|---|---|---|---|---|---|
| BC_CO2 | 237 # ± 11 $ | 0.13 # ± 0.01 $ | 3.7 # ± 0.1 $ | 1.25 # ± 0.03 $ | −4.22 # ± 0.01 $ |
| BC_H3PO4 | 608 # ± 25 $ | 0.37 # ± 0.02 $ | 3.9 # ± 0.1 $ | 1.32 # ± 0.04 $ | −28.6 # ± 0.9 $ |
| Biochar Symbol | XPS | XRF | |||||||||
| C [wt. %] | O [wt. %] | N [wt. %] | P [wt. %] | K [wt. %] | P [wt. %] | Si [wt. %] | S [wt. %] | Cl [wt. %] | K [wt. %] | Fe [wt. %] | |
| BC_CO2 | 70.0 # ± 2.1 $ | 23.8 # ± 1.2 $ | 2.5 # ± 0.2 $ | 1.4 # ± 0.1 $ | 2.3 # ± 0.1 $ | 0.85 # ± 0.01 $ | 2.87 # ± 0.07 $ | 0.73 # ± 0.01 $ | 5.58 # ± 0.12 $ | 1.61 # ± 0.03 $ | 6.91 # ± 0.27 $ |
| BC_H3PO4 | 57.3 # ± 2.4 $ | 27.2 # ± 3.3 $ | 4.1 # ± 0.5 $ | 11.4 # ± 1.8 $ | nr | 13.6 # ± 1.8 $ | 1.85 # ± 0.02 $ | nr | nr | nr | 1.25 # ± 0.07 $ |
| Biochar Symbol | SEM-EDS | ||||||||||
| C [wt. %] | O [wt. %] | N [wt. %] | P [wt. %] | K [wt. %] | Cl [wt. %] | Mg [wt. %] | Ca [wt. %] | ||||
| BC_CO2 | 83.0 # ± 3.4 $ | 9.06 # ± 1.48 $ | 3.97 # ± 0.41 $ | 0.29 # ± 0.45 $ | 0.43 # ± 0.61 $ | 1.04 # ± 0.38 $ | 0.18 # ± 0.25 $ | 0.22 # ± 0.27 $ | |||
| BC_H3PO4 | 55.6 # ± 3.2 $ | 26.7 # ± 3.3 $ | 4.25 # ± 0.27 $ | 10.3 # ± 0.6 $ | 0.22 # ± 0.09 $ | nr | 0.12 # ± 0.05 $ | 0.25 # ± 0.13 $ | |||
| Material Symbol | Pt(II) | ||||||
| PSO | Elovich | ||||||
| qeq,t. [mg g−1] | qeq,exp. [mg g−1] | k2 [g mg−1 min−1] | R2 | a [mg g−1 min−1] | b [g mg−1] | R2 | |
| BC_CO2 | 9.63 # ± 0.06 $ | 9.86 # ± 0.37 $ | 0.12 # ± 0.02 $ | 0.995 | 3.3 × 1023 #,* | 6.56 # ± 0.26 $ | 0.993 |
| BC_H3PO4 | 8.96 # ± 0.13 $ | 9.51 # ± 0.21 $ | 0.17 # ± 0.08 $ | 0.976 | 1.2 × 1018 #,* | 5.57 # ± 0.13 $ | 0.996 |
| Material Symbol | Pt(IV) | ||||||
| PSO | Elovich | ||||||
| qeq,t. [mg g−1] | qeq,exp. [mg g−1] | k2 [g mg−1 min−1] | R2 | a [mg g−1 min−1] | b [g mg−1] | R2 | |
| BC_CO2 | 10.4 # ± 0.7 $ | 11.1 # ± 0.5 $ | 0.001 * | 0.850 | 1.30 # ± 0.01 $ | 0.81 # ± 0.04 $ | 0.976 |
| BC_H3PO4 | 10.3 # ± 0.3 $ | 10.9 # ± 0.3 $ | 0.009 * | 0.921 | 92.9 # ± 6.4 $ | 1.19 # ± 0.08 $ | 0.976 |
| Material Symbol | Pt(II) | ||||||
| Langmuir | Freundlich | ||||||
| qmax,t. [mg g−1] | qmax,exp. [mg g−1] | kL [L mg−1] | R2 | nF [a. u.] | kF [mg1−nF LnF g−1] | R2 | |
| BC_CO2 | 41.1 # ± 2.8 $ | 42.0 # ± 1.8 $ | 0.03 # ± 0.01 $ | 0.917 | 0.22 # ± 0.01 $ | 9.99 # ± 0.68 $ | 0.994 |
| BC_H3PO4 | 45.0 # ± 2.7 $ | 45.9 # ± 2.2 $ | 0.03 # ± 0.01 $ | 0.939 | 0.25 # ± 0.01 $ | 9.36 # ± 0.83 $ | 0.991 |
| Material symbol | Pt(IV) | ||||||
| Langmuir | Freundlich | ||||||
| qmax,t. [mg g−1] | qmax,exp. [mg g−1] | kL [L mg−1] | R2 | nF [a. u.] | kF [mg1−nF LnF g−1] | R2 | |
| BC_CO2 | 24.2 # ± 2.9 $ | 20.9 # ± 0.3 $ | 0.31 # ± 0.07 $ | 0.666 | 0.12 # ± 0.03 $ | 9.81 # ± 1.89 $ | 0.859 |
| BC_H3PO4 | 34.0 # ± 0.4 $ | 34.1 # ± 1.5 $ | 0.28 # ± 0.02 $ | 0.996 | 0.15 # ± 0.03 $ | 13.2 # ± 2.7 $ | 0.878 |
| Parameter | Value |
|---|---|
| Lamp current [mA] | 10.0 |
| Wavelength [nm] | 265.9 |
| Slit width [nm] | 0.5 |
| Sample volume [µL] | 15 |
| Atomization temperature [°C] | 2700 |
| Pyrolysis temperature [°C] | 1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morlo, K.; Olchowski, R.; Dobrowolski, R. Optimization of Pt(II) and Pt(IV) Adsorption from a Water Solution on Biochar Originating from Honeycomb Biomass. Molecules 2024, 29, 547. https://doi.org/10.3390/molecules29020547
Morlo K, Olchowski R, Dobrowolski R. Optimization of Pt(II) and Pt(IV) Adsorption from a Water Solution on Biochar Originating from Honeycomb Biomass. Molecules. 2024; 29(2):547. https://doi.org/10.3390/molecules29020547
Chicago/Turabian StyleMorlo, Kinga, Rafał Olchowski, and Ryszard Dobrowolski. 2024. "Optimization of Pt(II) and Pt(IV) Adsorption from a Water Solution on Biochar Originating from Honeycomb Biomass" Molecules 29, no. 2: 547. https://doi.org/10.3390/molecules29020547
APA StyleMorlo, K., Olchowski, R., & Dobrowolski, R. (2024). Optimization of Pt(II) and Pt(IV) Adsorption from a Water Solution on Biochar Originating from Honeycomb Biomass. Molecules, 29(2), 547. https://doi.org/10.3390/molecules29020547







