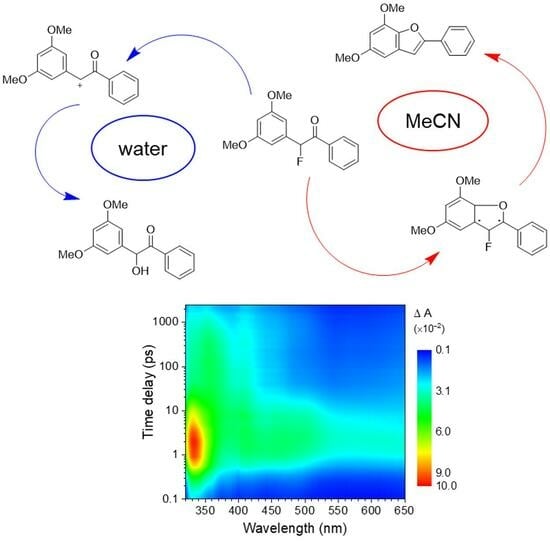
Transient Absorption Spectroscopic Investigation of the Photocyclization–Deprotection Reaction of 3′,5′-Dimethoxybenzoin Fluoride
Abstract
1. Introduction
2. Results and Discussion
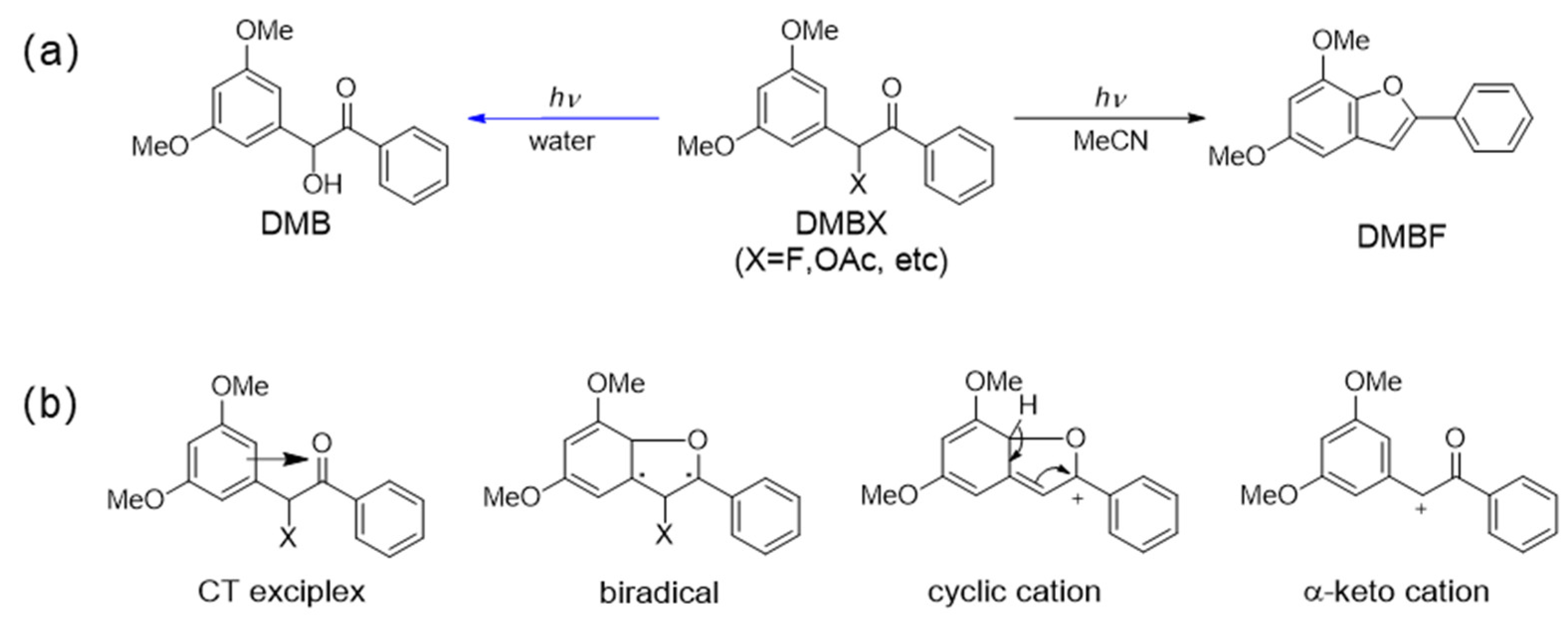
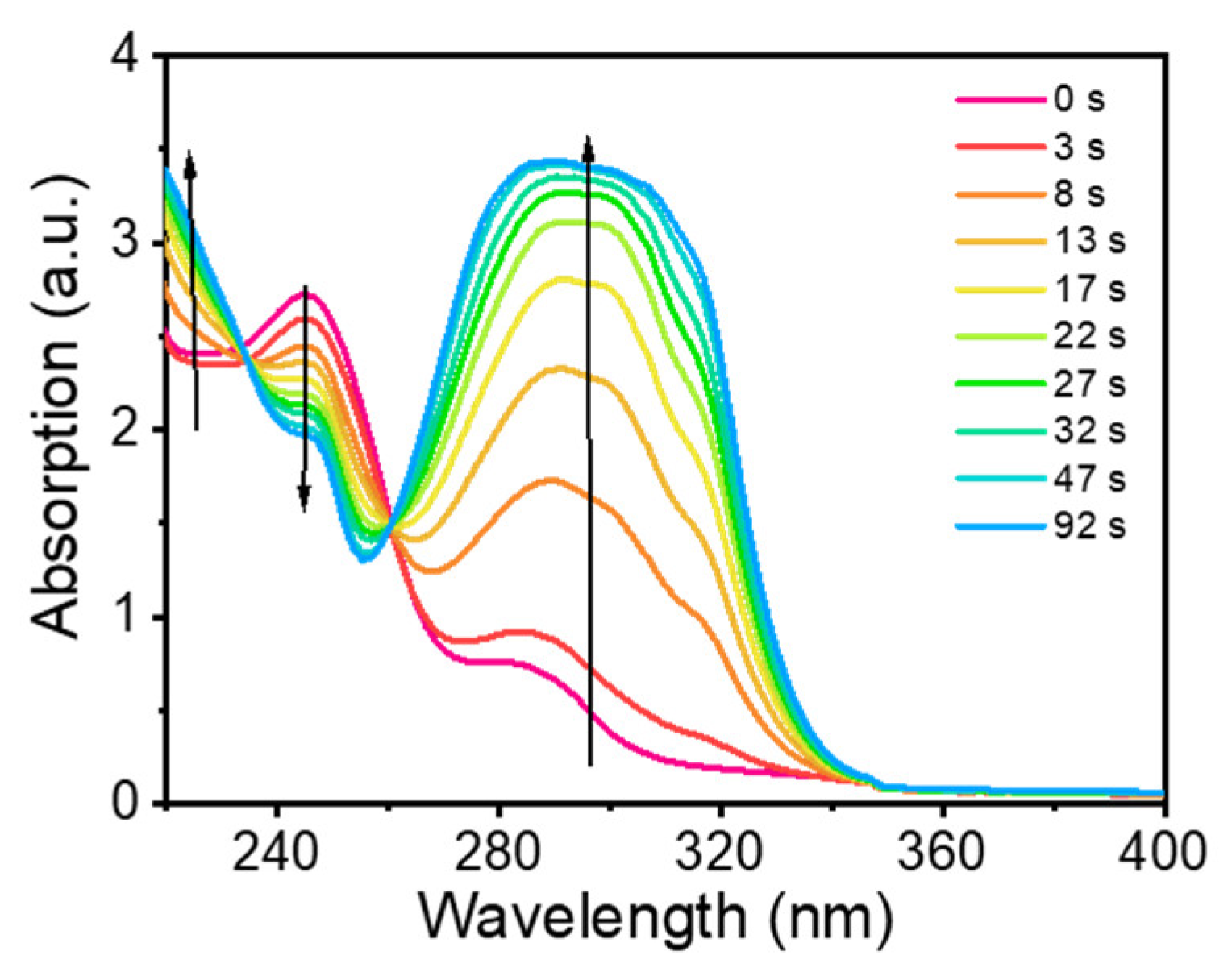
2.1. Product Analysis of DMB Fluoride in MeCN and Aqueous Solutions after Photoexcitation
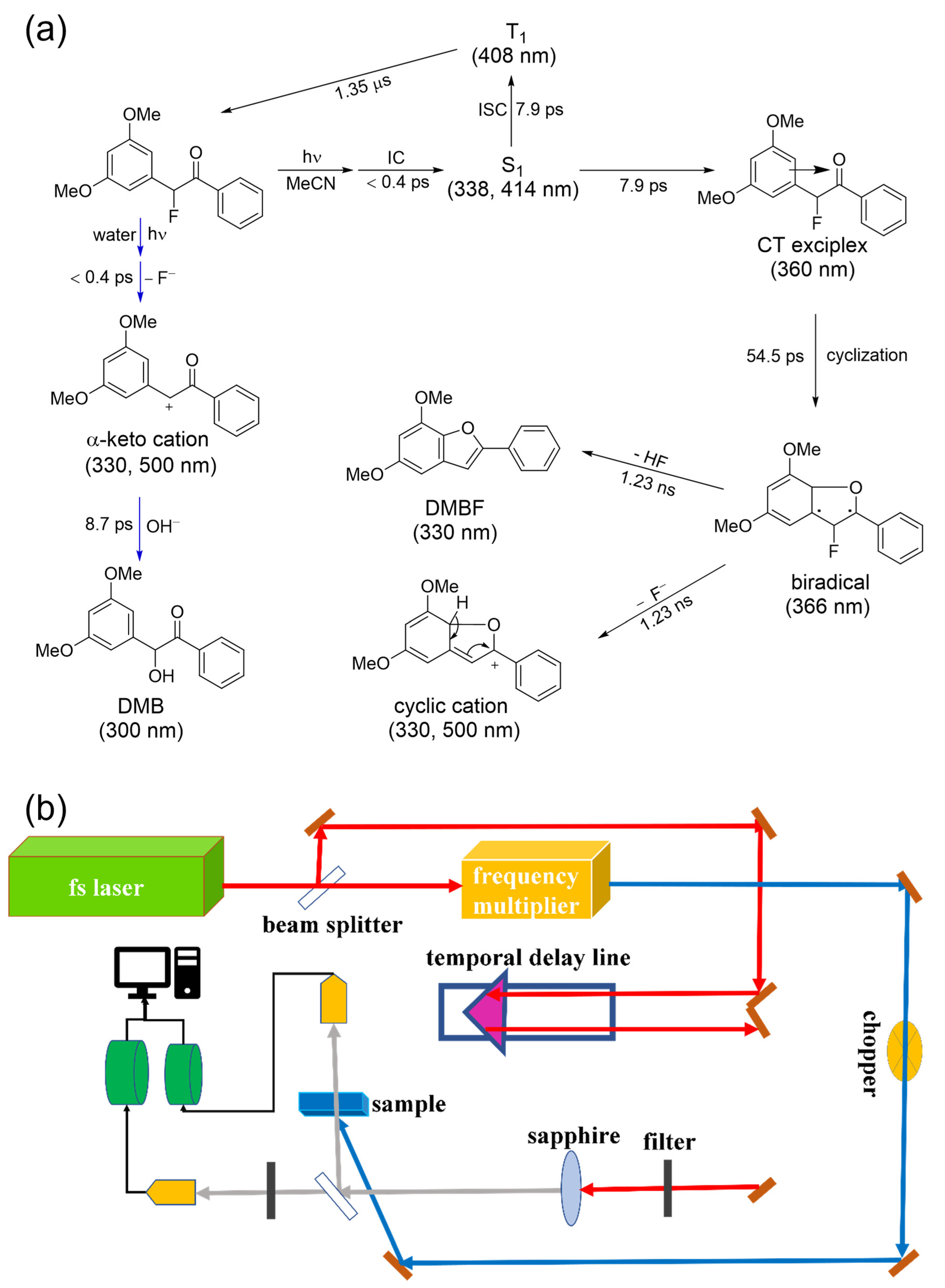
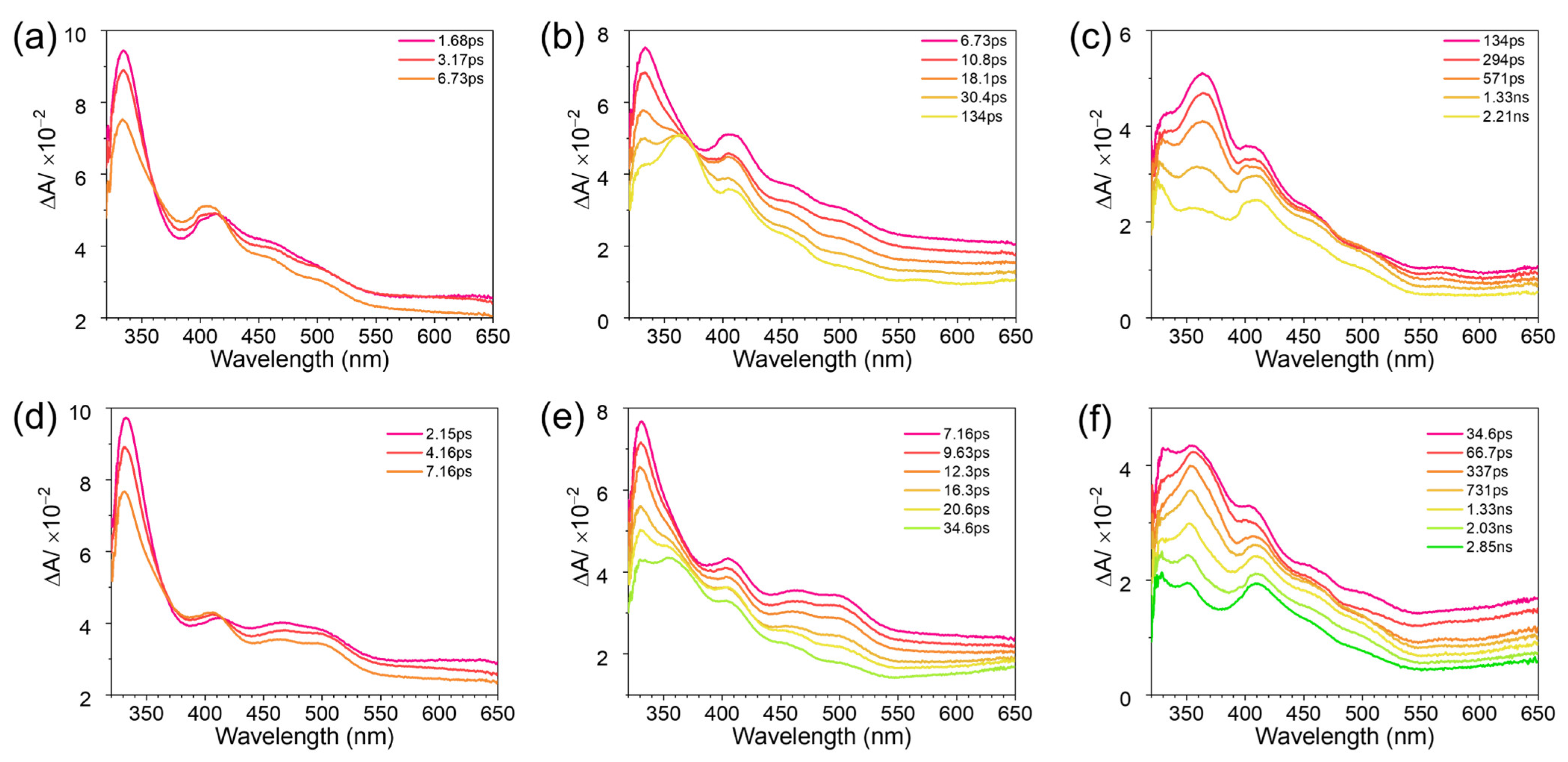
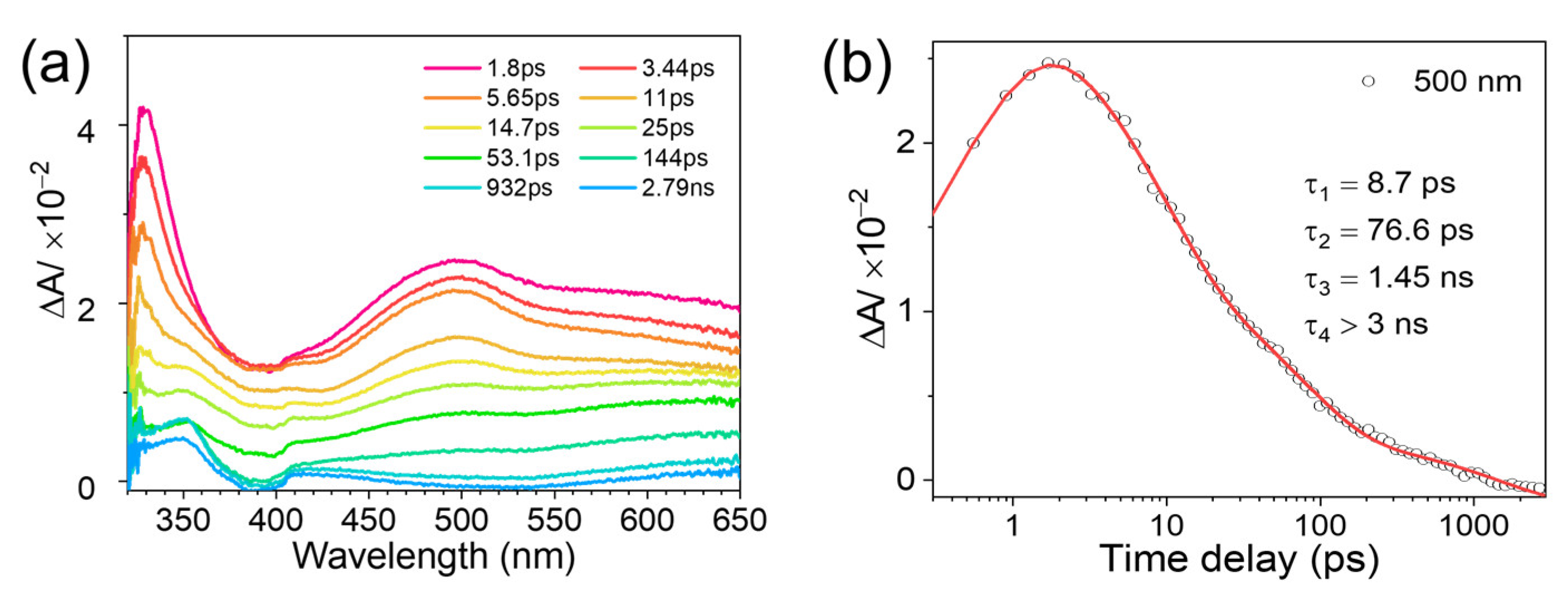
2.2. Transient Absorption Spectroscopic Investigation of DMB Fluoride in Neat MeCN and Aqueous Solutions
3. Materials and Methods
3.1. Synthesis and Characterization
3.2. Experimental
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Givens, R.S.; Athey, P.S.; Kueper, L.W.; Matuszewski, B.; Xue, J.Y. Photochemistry of .alpha.-keto phosphate esters: Photorelease of a caged cAMP. J. Am. Chem. Soc. 1992, 114, 8708–8710. [Google Scholar] [CrossRef]
- Givens, R.S.; Kueper, L.W. Photochemistry of phosphate esters. Chem. Rev. 1993, 93, 55–66. [Google Scholar] [CrossRef]
- Rock, R.S.; Chan, S.I. Preparation of a Water-Soluble “Cage” Based on 3′,5′-Dimethoxybenzoin. J. Am. Chem. Soc. 1998, 120, 10766–10767. [Google Scholar] [CrossRef]
- Sheehan, J.C.; Wilson, R.M.; Oxford, A.W. The Photolysis of Methoxy-Substituted Benzoin Esters. A Photosensitive Protecting Group for Carboxylic Acids. J. Am. Chem. Soc. 1971, 93, 7222–7228. [Google Scholar] [CrossRef]
- Pirrung, M.C.; Fallon, L.; Lever, D.C.; Shuey, S.W. Inverse Phosphotriester DNA Synthesis Using Photochemically-Removable Dimethoxybenzoin Phosphate Protecting Groups. J. Org. Chem. 1996, 61, 2129–2136. [Google Scholar] [CrossRef]
- Pirrung, M.C.; Bradley, J.-C. Dimethoxybenzoin Carbonates: Photochemically-Removable Alcohol Protecting Groups Suitable for Phosphoramidite-Based DNA Synthesis. J. Org. Chem. 1995, 60, 1116–1117. [Google Scholar] [CrossRef]
- Peach, J.M.; Pratt, A.J.; Snaith, J.S. Photolabile benzoin and furoin esters of a biologically active peptide. Tetrahedron 1995, 51, 10013–10024. [Google Scholar] [CrossRef]
- McCoy, C.P.; Rooney, C.; Edwards, C.R.; Jones, D.S.; Gorman, S.P. Light-triggered molecule-scale drug dosing devices. J. Am. Chem. Soc. 2007, 129, 9572–9573. [Google Scholar] [CrossRef] [PubMed]
- Pirrung, M.C.; Bradley, J.-C. Comparison of Methods for Photochemical Phosphoramidite-Based DNA Synthesis. J. Org. Chem. 1995, 60, 6270–6276. [Google Scholar] [CrossRef]
- Rock, R.S.; Hansen, K.C.; Larsen, R.W.; Chan, S.I. Rapid photochemical triggering of protein unfolding in a nondenaturing environment. Chem. Phys. 2004, 307, 201–208. [Google Scholar] [CrossRef]
- Corrie, J.E.T.; Trentham, D.R. Synthetic, mechanistic and photochemical studies of phosphate esters of substituted benzoins. J. Chem. Soc. Perkin Trans. 1 1992, 18, 2409–2417. [Google Scholar] [CrossRef]
- Sheehan, J.C.; Wilson, R.M. Photolysis of Desyl Compounds. A New Photolytic Cyclization. J. Am. Chem. Soc. 1964, 86, 5277–5281. [Google Scholar] [CrossRef]
- Ma, C.; Du, Y.; Kwok, W.M.; Phillips, D.L. Femtosecond transient absorption and nanosecond time-resolved resonance Raman study of the solvent-dependent photo-deprotection reaction of benzoin diethyl phosphate. Chem. A Eur. J. 2007, 13, 2290–2305. [Google Scholar] [CrossRef]
- Ma, C.; Kwok, W.M.; An, H.Y.; Guan, X.; Fu, M.Y.; Toy, P.H.; Phillips, D.L. A time-resolved spectroscopic study of the bichromophoric phototrigger 3′,5′-dimethoxybenzoin diethyl phosphate: Interaction between the two chromophores determines the reaction pathway. Chem. A Eur. J. 2010, 16, 5102–5118. [Google Scholar] [CrossRef]
- Pirrung, M.C.; Shuey, S.W. Photoremovable Protecting Groups for Phosphorylation of Chiral Alcohols. Asymmetric Synthesis of Phosphotriesters of (-)-3′,5′-Dimethoxybenzoin. J. Org. Chem. 1994, 59, 3890–3897. [Google Scholar] [CrossRef]
- Boudebous, H.; Kosmrlj, B.; Sket, B.; Wirz, J. Primary photoreactions of the 3′,5′-dimethoxybenzoin cage and determination of the release rate in polar media. J. Phys. Chem. A 2007, 111, 2811–2813. [Google Scholar] [CrossRef]
- Shi, Y.; Corrie, J.E.; Wan, P. Mechanism of 3′,5′-Dimethoxybenzoin Ester Photochemistry: Heterolytic Cleavage Intramolecularly Assisted by the Dimethoxybenzene Ring Is the Primary Photochemical Step. J. Org. Chem. 1997, 62, 8278–8279. [Google Scholar] [CrossRef]
- Chen, X.; Ma, C.; Phillips, D.L.; Fang, W.H. A case of fast photocyclization: The model of a downhill ladder reaction pathway for the bichromophoric phototrigger 3′,5′-dimethoxybenzoin acetate. Org. Lett. 2010, 12, 5108–5111. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Q.; Lo, K.C.; Yan, Z.; Phillips, D.L.; Tang, W.; Du, L. Time-Resolved Spectroscopic Study of a Photoinduced Intramolecular Chloride Exchange Reaction of 3′, 5′-Dimethoxybenzoin Chloride. J. Phys. Chem. B 2023, 127, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, C.S.; Givens, R.S.; Wirz, J. Kinetics and Mechanism of Phosphate Photorelease from Benzoin Diethyl Phosphate: Evidence for Adiabatic Fission to an α-Keto Cation in the Triplet State. J. Am. Chem. Soc. 2000, 122, 611–618. [Google Scholar] [CrossRef]
- Han, C.; Kundu, B.K.; Liang, Y.; Sun, Y. Near-Infrared Light-Driven Photocatalysis with an Emphasis on Two-Photon Excitation: Concepts, Materials, and Applications. Adv. Mater. 2023, 36, 2307759. [Google Scholar] [CrossRef]
- Kundu, B.K.; Han, G.; Sun, Y. Derivatized benzothiazoles as two-photon-absorbing organic photosensitizers active under near infrared light irradiation. J. Am. Chem. Soc. 2023, 145, 3535–3542. [Google Scholar] [CrossRef]
- Kundu, B.K.; Han, C.; Srivastava, P.; Nagar, S.; White, K.E.; Krause, J.A.; Elles, C.G.; Sun, Y. Trifluoromethylative Bifunctionalization of Alkenes via a Bibenzothiazole-Derived Photocatalyst under Both Visible-and Near-Infrared-Light Irradiation. ACS Catal. 2023, 13, 8119–8127. [Google Scholar] [CrossRef]
- Miyata, K.; Kurashige, Y.; Watanabe, K.; Sugimoto, T.; Takahashi, S.; Tanaka, S.; Takeya, J.; Yanai, T.; Matsumoto, Y. Coherent singlet fission activated by symmetry breaking. Nat. Chem. 2017, 9, 983–989. [Google Scholar] [CrossRef]
- Fang, J.; Debnath, T.; Bhattacharyya, S.; Döblinger, M.; Feldmann, J.; Stolarczyk, J.K. Photobase effect for just-in-time delivery in photocatalytic hydrogen generation. Nat. Commun. 2020, 11, 5179. [Google Scholar] [CrossRef]
- Hammer, C.A.; Falahati, K.; Jakob, A.; Klimek, R.; Burghardt, I.; Heckel, A.; Wachtveitl, J. Sensitized two-photon activation of coumarin photocages. J. Phys. Chem. Lett. 2018, 9, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.F.; Willson, C.G.; Fréchet, J.M.J. Photogeneration of Amines from α-Keto Carbamates: Photochemical Studies. J. Am. Chem. Soc. 1996, 118, 12925–12937. [Google Scholar] [CrossRef]
- Newcomb, M.; Horner, J.H.; Whitted, P.O.; Crich, D.; Huang, X.; Yao, Q.; Zipse, H. β-Phosphatoxyalkyl Radical Reactions: Competing Phosphate Migration and Phosphoric Acid Elimination from a Radical Cation−Phosphate Anion Pair Formed by Heterolytic Fragmentation. J. Am. Chem. Soc. 1999, 121, 10685–10694. [Google Scholar] [CrossRef]
- Liang, R.; Xiong, W.; Bai, X.; Du, L.; Phillips, D.L. Direct Observation of the Triplet Excited States and Dynamics of Platinum(II)-Tetraphenylethylene Complexes by Time-Resolved Transient Absorption Spectroscopy. J. Phys. Chem. C 2021, 125, 11432–11439. [Google Scholar] [CrossRef]
- Du, L.; Zhu, R.; Xue, J.; Du, Y.; Phillips, D.L. Time-resolved spectroscopic and density functional theory investigation of the photochemistry of suprofen. J. Raman Spectrosc. 2015, 46, 117–125. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Tenderholt, A.L.; Langner, K.M. cclib: A library for package-independent computational chemistry algorithms. J. Comput. Chem. 2008, 29, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Schlegel, G.W.T.H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; Li, X.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, R.; Li, Y.; Lo, K.C.; Yan, Z.; Tang, W.; Du, L.; Phillips, D.L. Transient Absorption Spectroscopic Investigation of the Photocyclization–Deprotection Reaction of 3′,5′-Dimethoxybenzoin Fluoride. Molecules 2024, 29, 842. https://doi.org/10.3390/molecules29040842
Liang R, Li Y, Lo KC, Yan Z, Tang W, Du L, Phillips DL. Transient Absorption Spectroscopic Investigation of the Photocyclization–Deprotection Reaction of 3′,5′-Dimethoxybenzoin Fluoride. Molecules. 2024; 29(4):842. https://doi.org/10.3390/molecules29040842
Chicago/Turabian StyleLiang, Runhui, Yuanchun Li, Kin Cheung Lo, Zhiping Yan, Wenjian Tang, Lili Du, and David Lee Phillips. 2024. "Transient Absorption Spectroscopic Investigation of the Photocyclization–Deprotection Reaction of 3′,5′-Dimethoxybenzoin Fluoride" Molecules 29, no. 4: 842. https://doi.org/10.3390/molecules29040842
APA StyleLiang, R., Li, Y., Lo, K. C., Yan, Z., Tang, W., Du, L., & Phillips, D. L. (2024). Transient Absorption Spectroscopic Investigation of the Photocyclization–Deprotection Reaction of 3′,5′-Dimethoxybenzoin Fluoride. Molecules, 29(4), 842. https://doi.org/10.3390/molecules29040842