Advanced Photodegradation of Azo Dye Methyl Orange Using H2O2-Activated Fe3O4@SiO2@ZnO Composite under UV Treatment
Abstract
1. Introduction
2. Results and Discussion
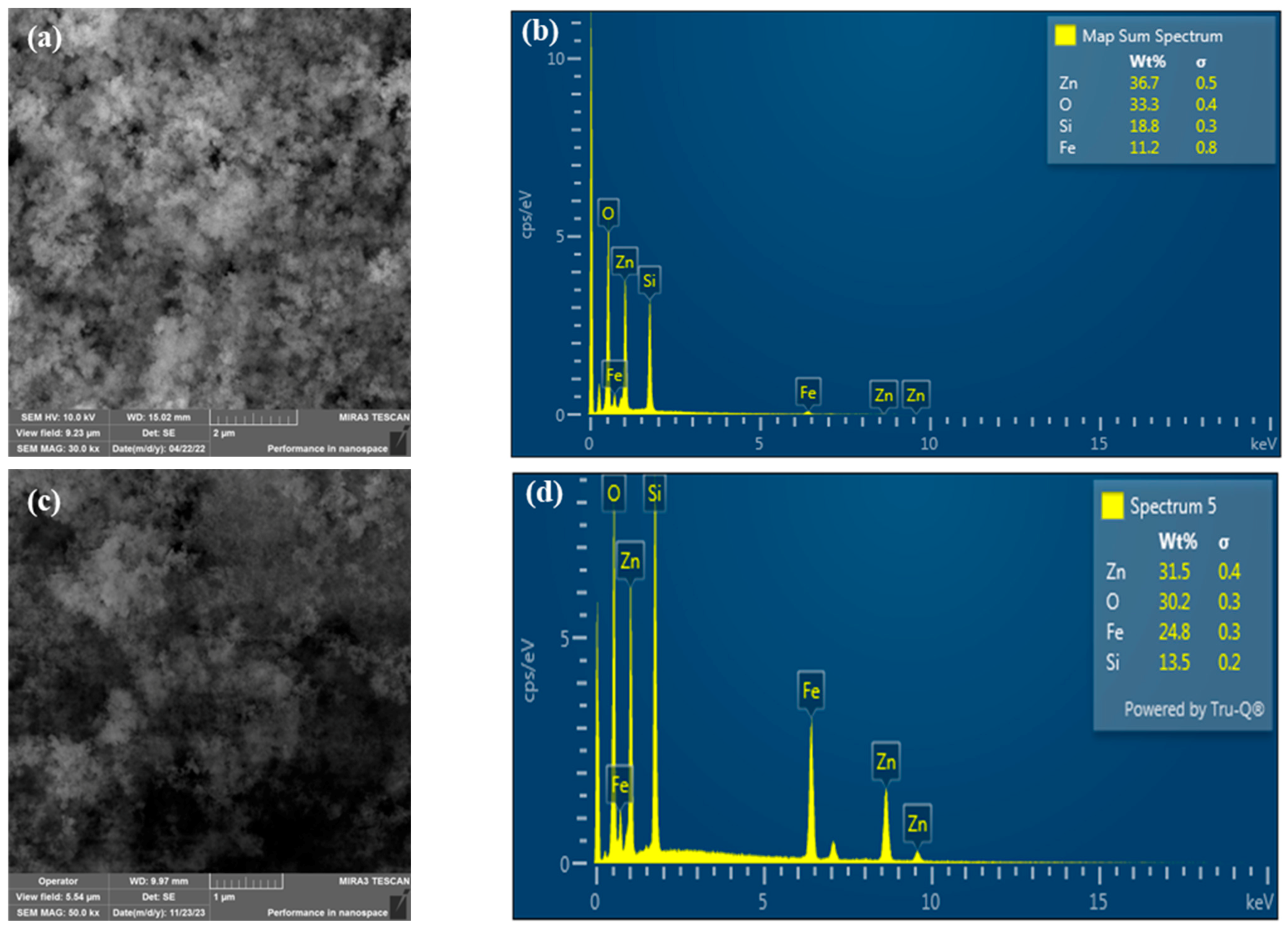
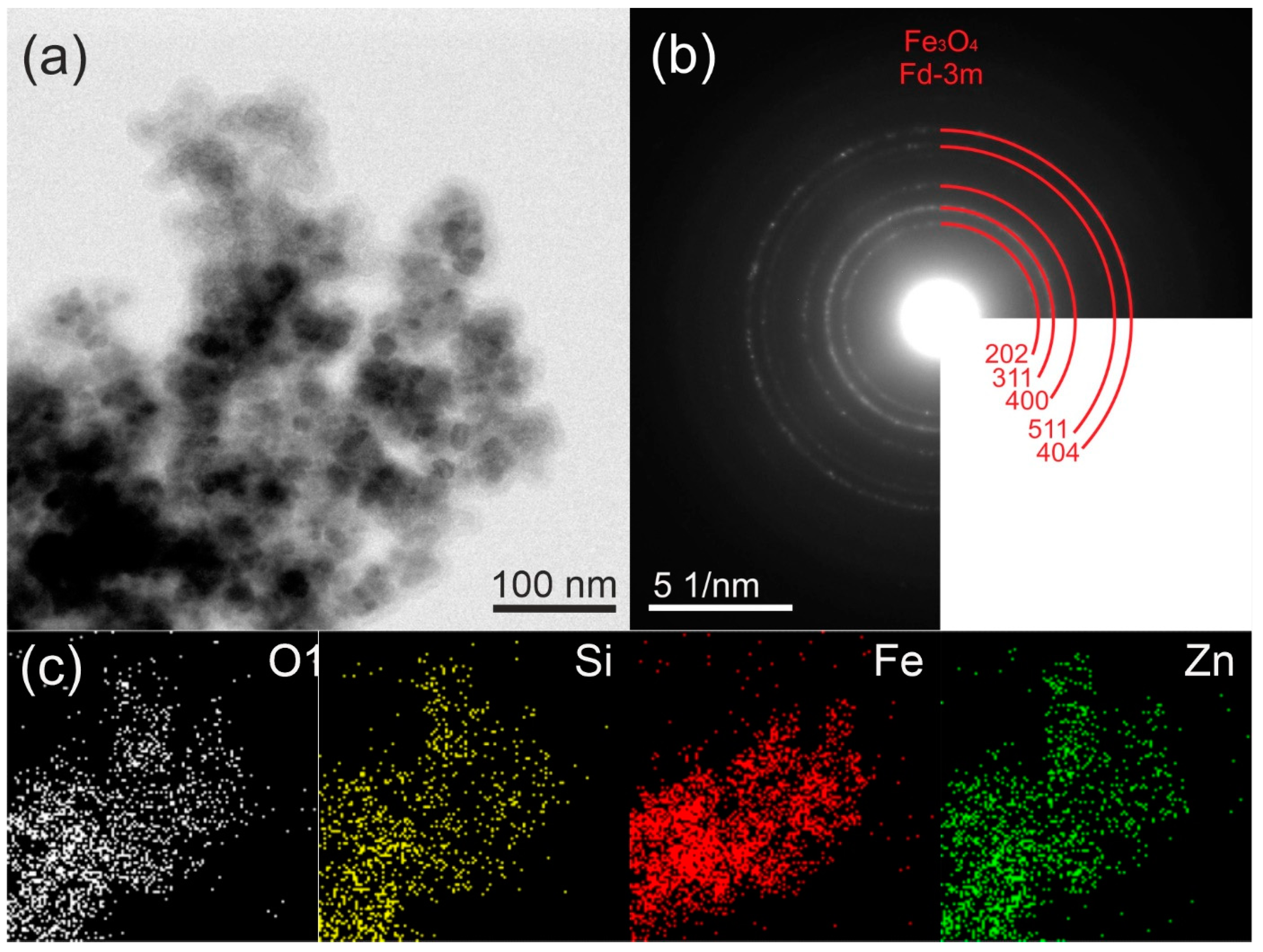
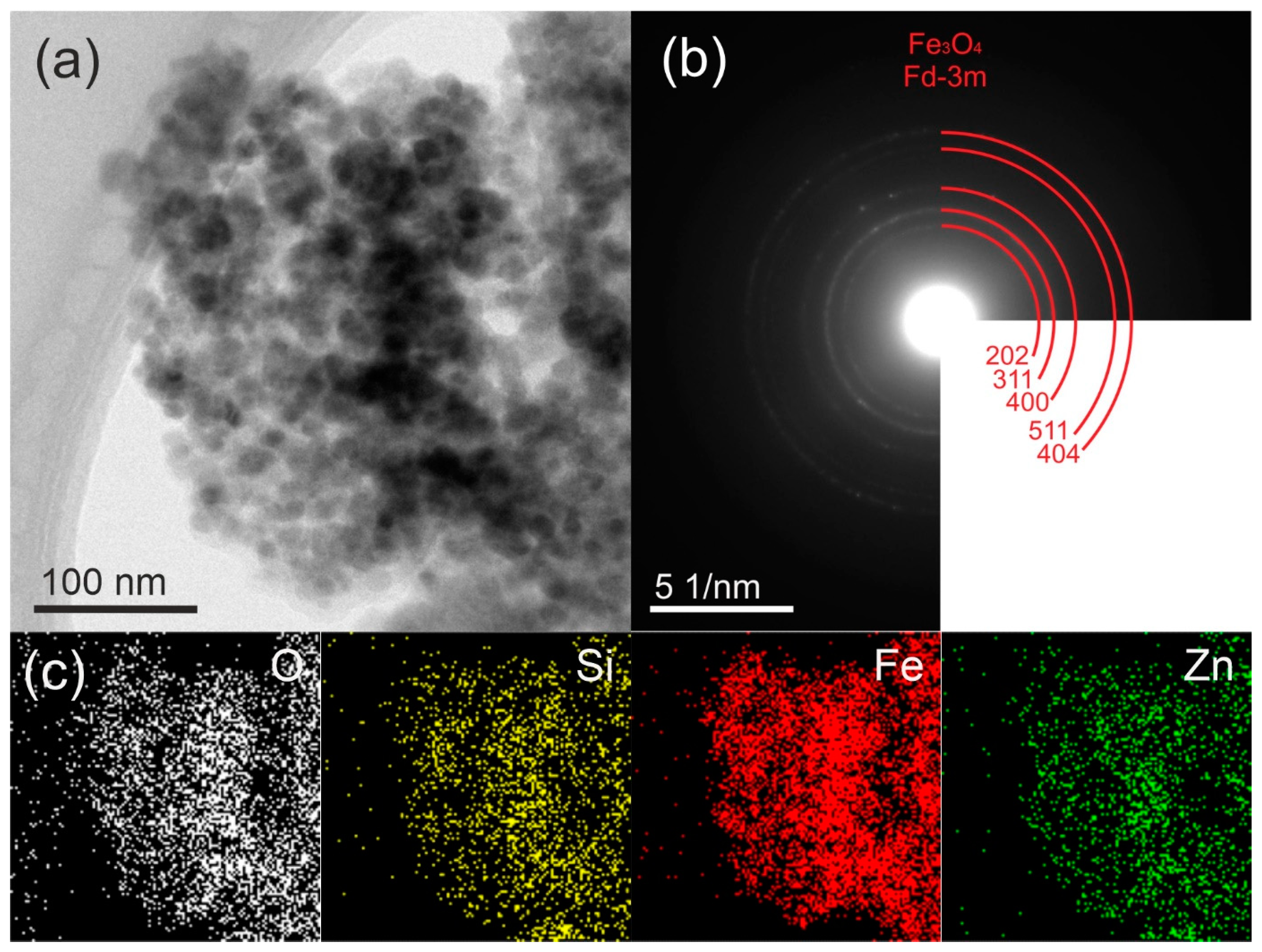
2.1. Morphology
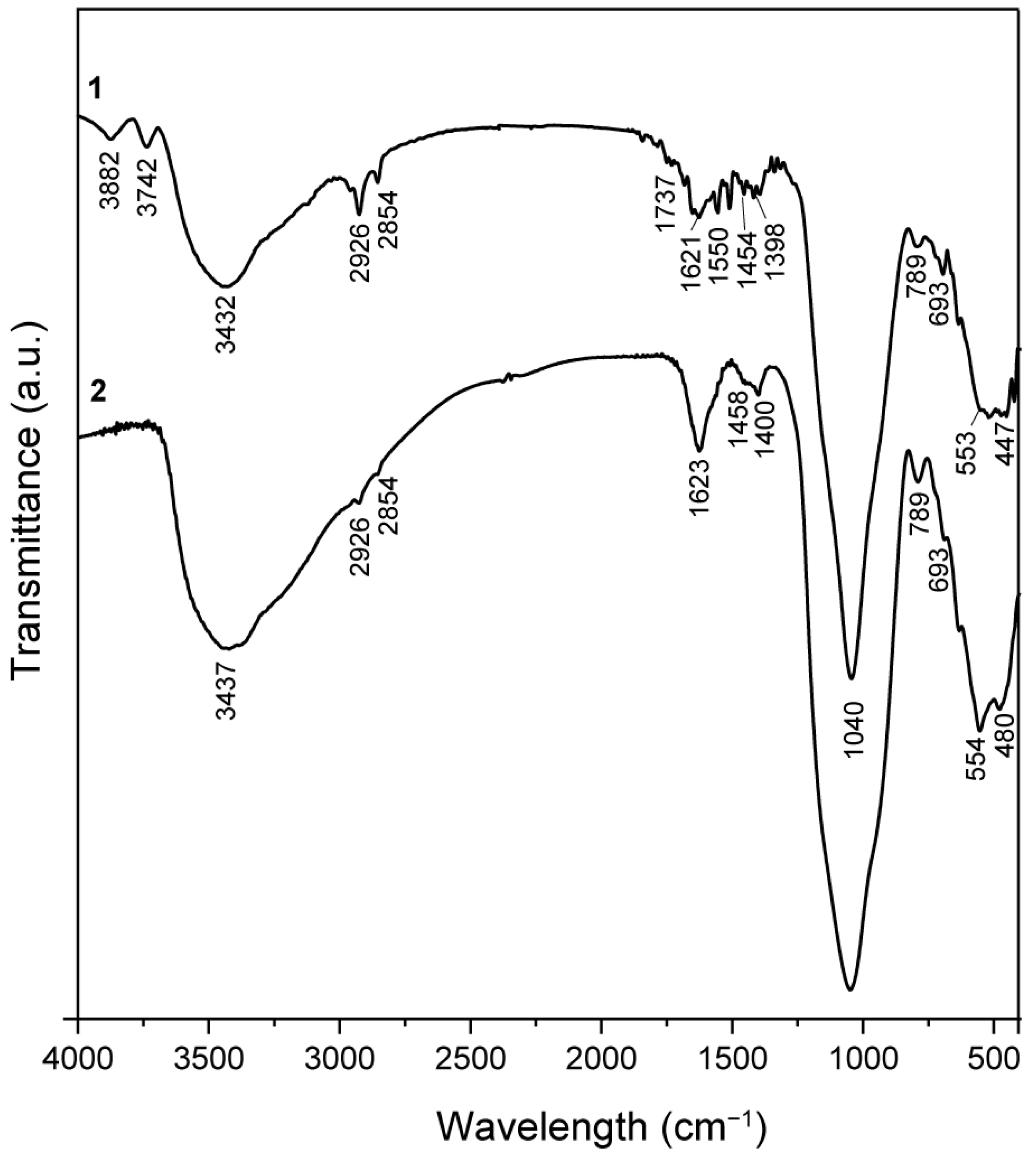
2.2. FT-IR Spectroscopy
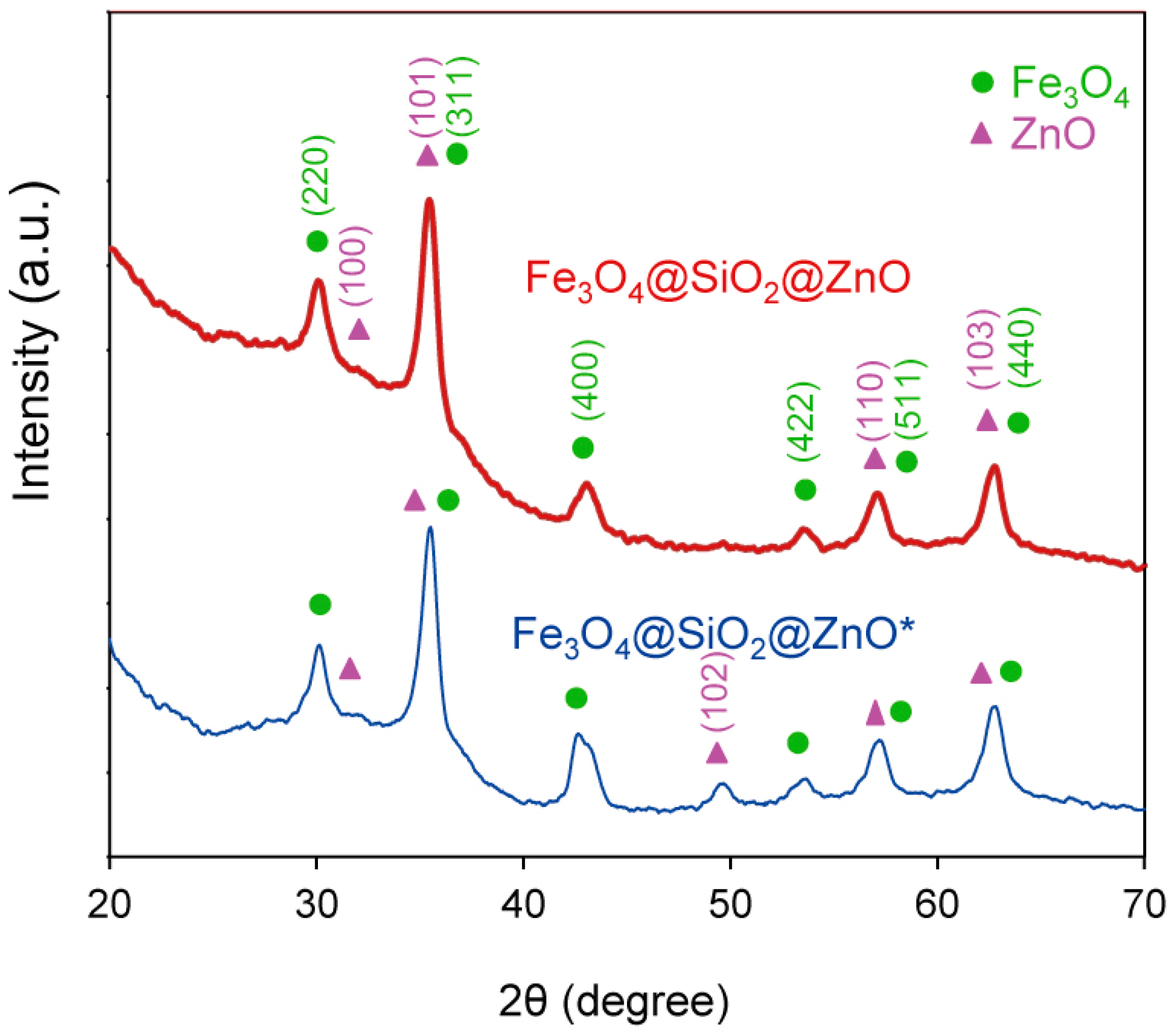
2.3. The Phase Analysis Using XRD
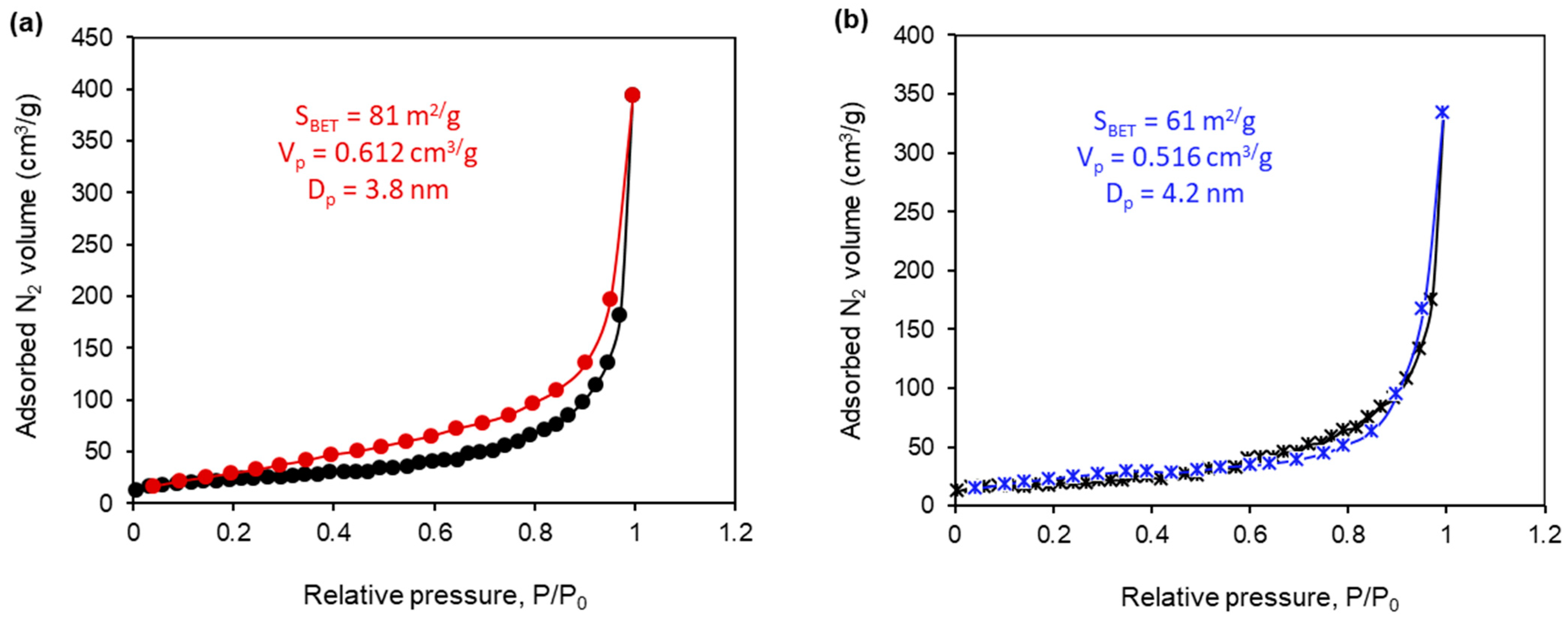
2.4. The Textural Properties of the Synthesized Composites
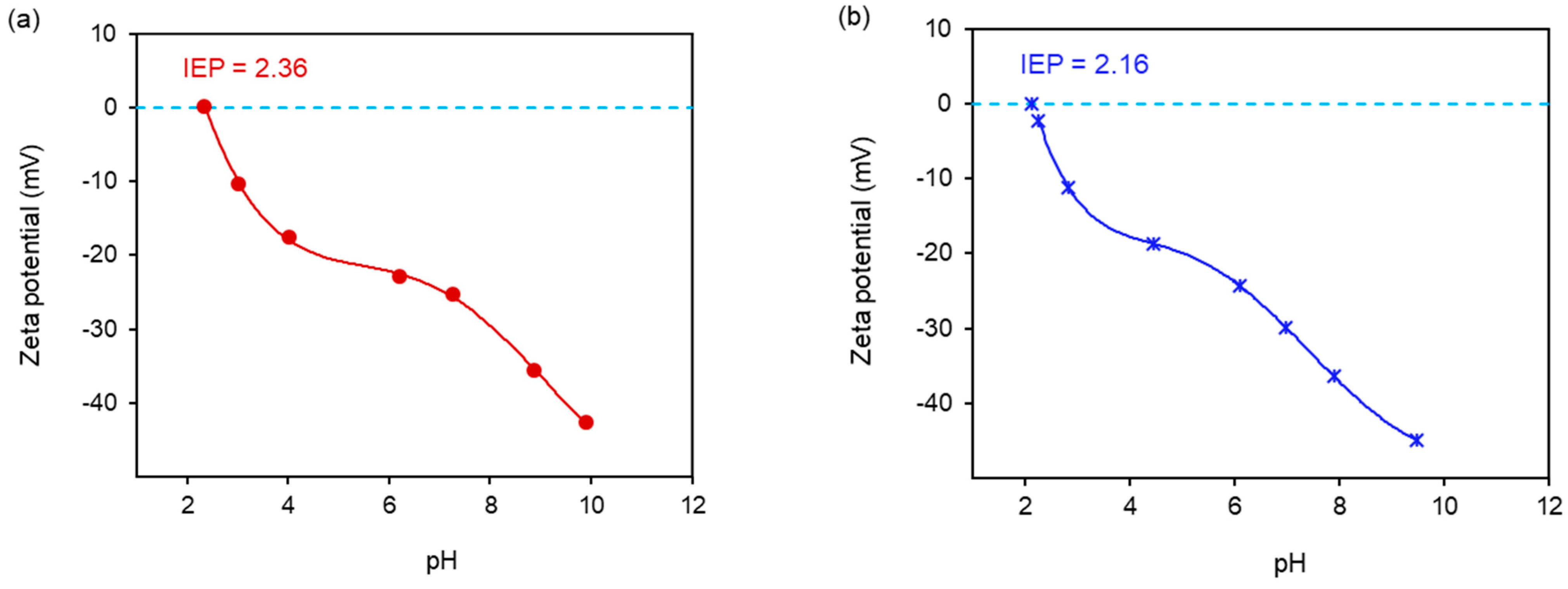
2.5. Zeta Potential Measurements
2.6. Photoluminescence (PL) Properties
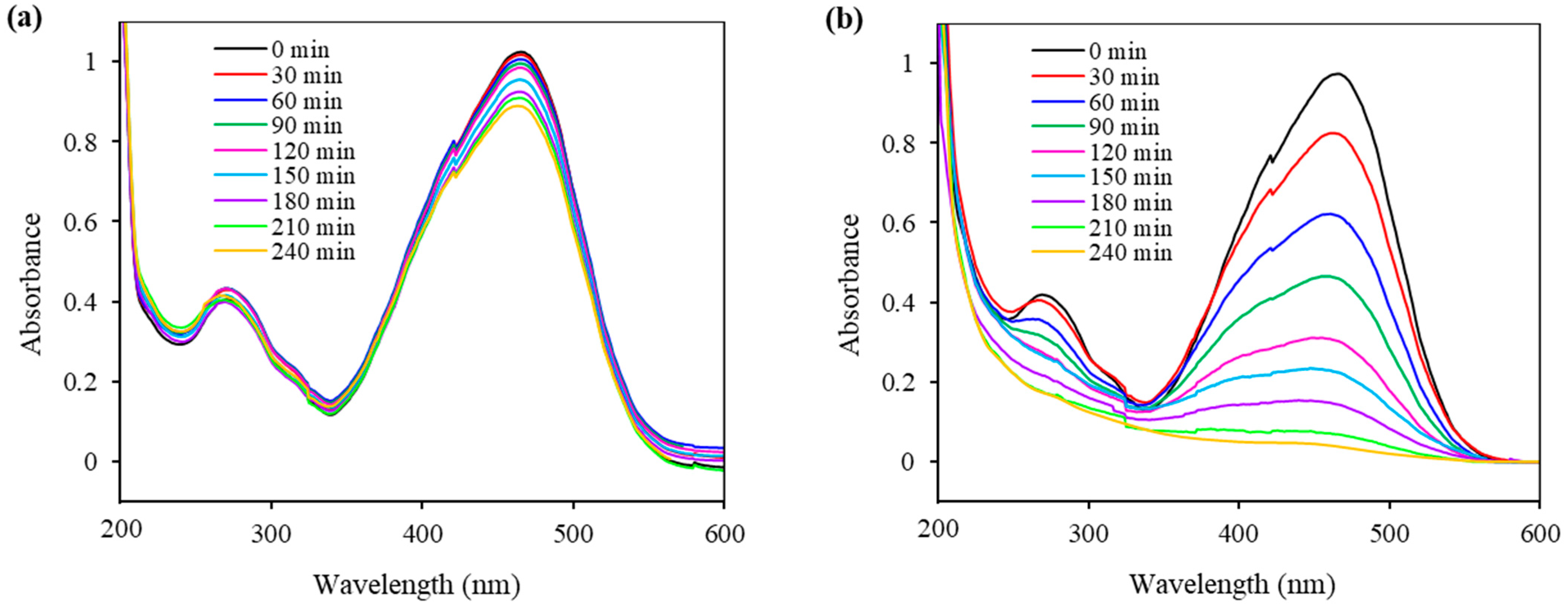
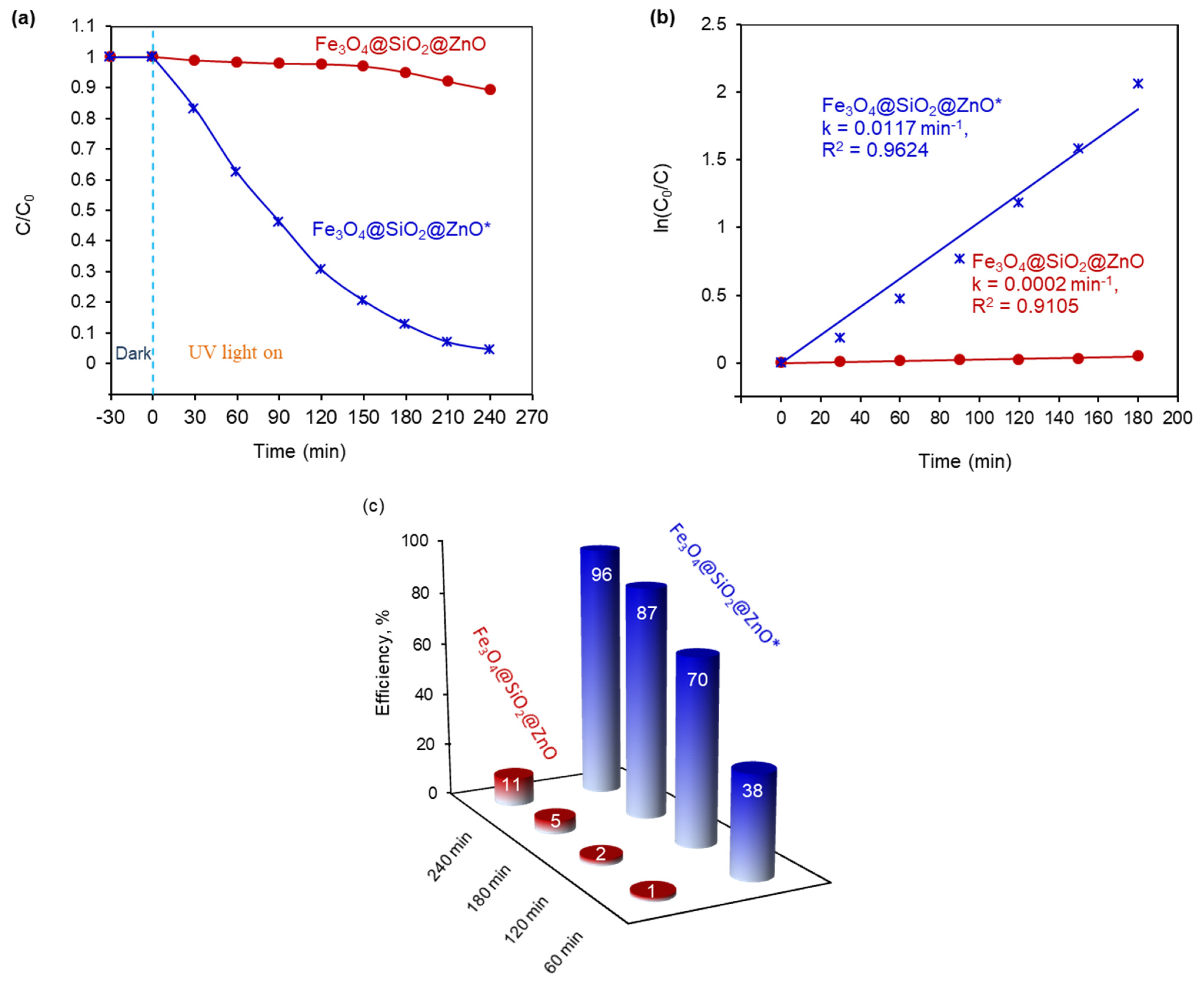
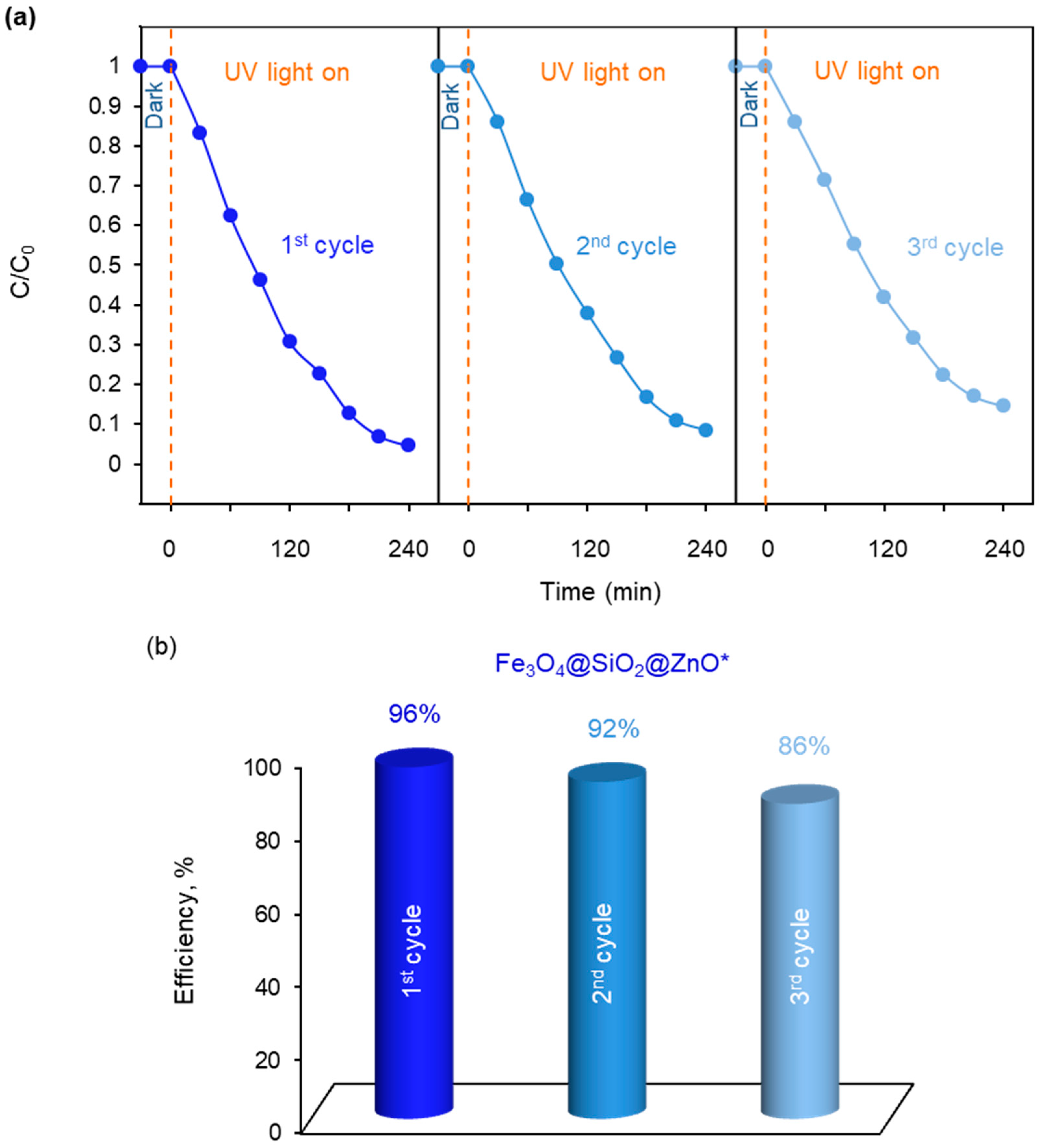
2.7. Photocatalytic Ability of the Synthesized Fe3O4@SiO2@ZnO and Fe3O4@SiO2@ZnO* Composites
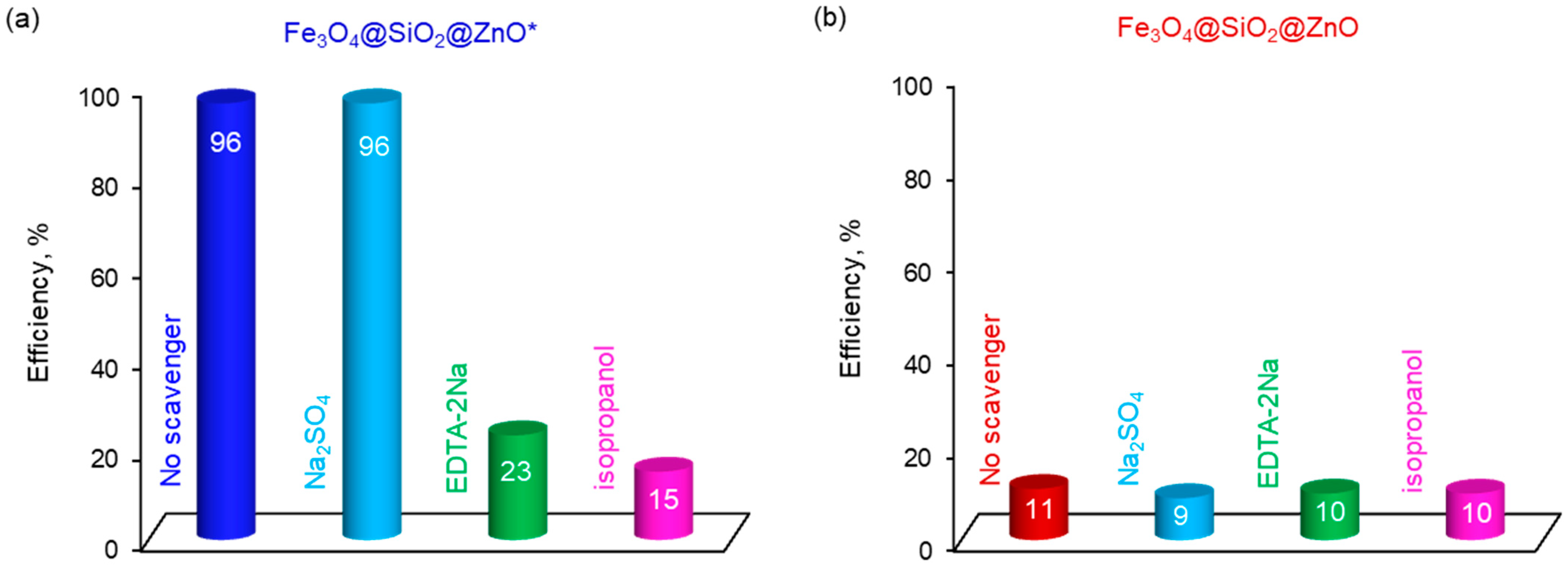
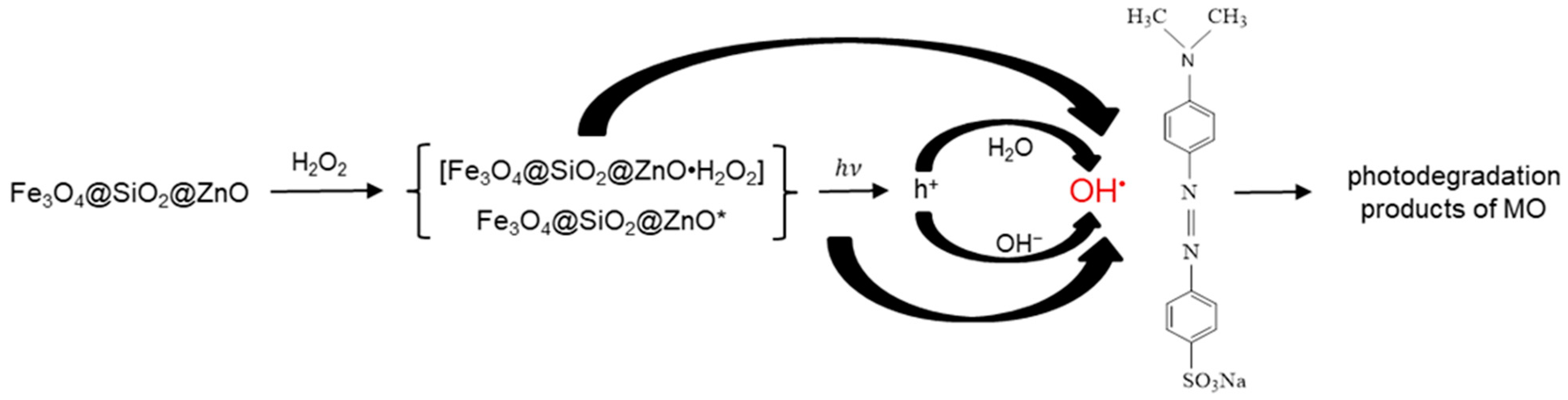
2.8. Proposed Mechanism of the Photodegradation of MO in the Presence of Fe3O4@SiO2@ZnO* Composite under UV Irradiation
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Magnetite Fe3O4 Nanoparticles
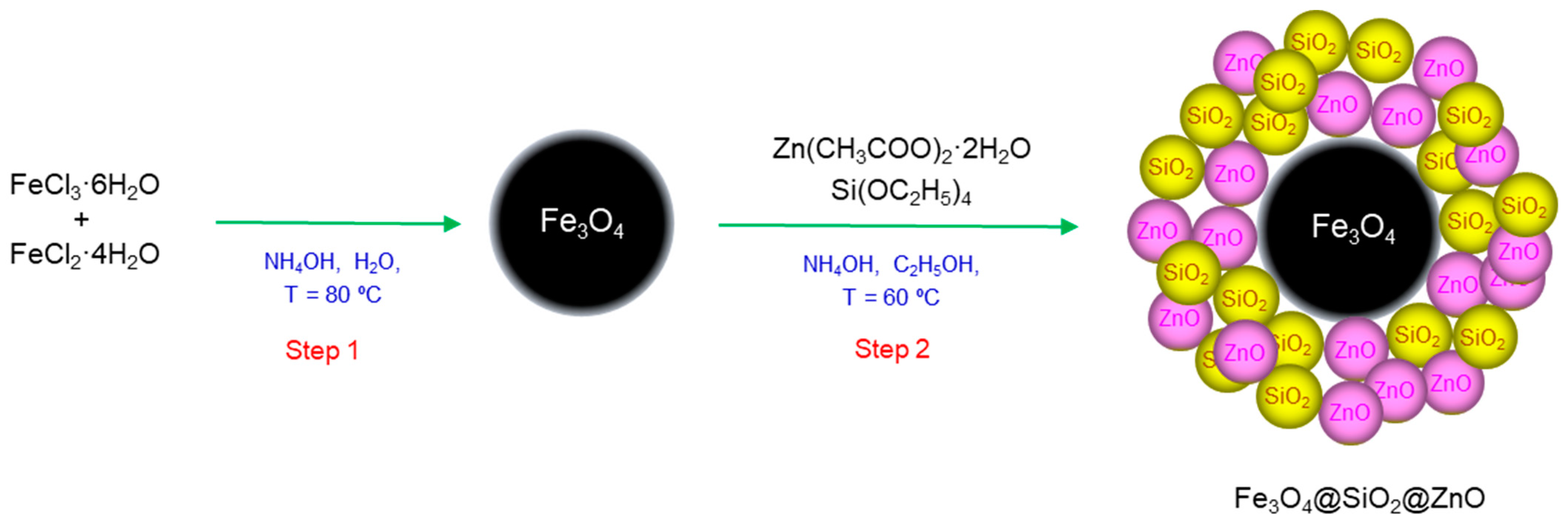
3.3. Synthesis of Fe3O4@SiO2@ZnO Composite
3.4. Synthesis of Fe3O4@SiO2@ZnO* Sample
3.5. Methods
3.6. Photocatalytic Degradation Activities of Fe3O4@SiO2@ZnO Composite and Its Activated Form
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaplin, M.F. Water: Its importance to life. Biochem. Mol. Biol. Educ. 2001, 29, 54–59. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, Q.; Wu, Q.; Han, C.; Tao, J. An integrated diagnostic framework for water resource spatial equilibrium considering water-economy-ecology nexus. J. Clean. Prod. 2023, 414, 137592. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, W.; Cao, R.; Zhuo, Y.; Fu, J.; Wang, J. Overloading risk assessment of water environment-water resources carrying capacity based on a novel Bayesian methodology. J. Hydrol. 2023, 622, 129697. [Google Scholar] [CrossRef]
- Waghchaure, R.H.; Adole, V.A.; Jagdale, B.S. Photocatalytic degradation of methylene blue, rhodamine B, methyl orange and Eriochrome black T dyes by modified ZnO nanocatalysts: A concise review. Inorg. Chem. Commun. 2022, 143, 109764. [Google Scholar] [CrossRef]
- Thambiliyagodage, C. Activity enhanced TiO2 nanomaterials for photodegradation of dyes—A review. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100592. [Google Scholar] [CrossRef]
- Sutar, S.; Patil, P.; Jadhav, J. Recent advances in biochar technology for textile dyes wastewater remediation: A review. Environ. Res. 2022, 209, 112841. [Google Scholar] [CrossRef]
- Chen, F.; Yu, C.; Wei, L.; Fan, Q.; Ma, F.; Zeng, J.; Yi, J.; Yang, K.; Ji, H. Fabrication and characterization of ZnTiO3/Zn2Ti3O8/ZnO ternary photocatalyst for synergetic removal of aqueous organic pollutants and Cr(VI) ions. Sci. Total. Environ. 2020, 706, 136026. [Google Scholar] [CrossRef]
- Zeng, D.; Yu, C.; Fan, Q.; Zeng, J.; Wei, L.; Li, Z.; Yang, K.; Ji, H. Theoretical and experimental research of novel fluorine doped hierarchical Sn3O4 microspheres with excellent photocatalytic performance for removal of Cr(VI) and organic pollutants. Chem. Eng. J. 2020, 391, 123607. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Ali, N.; Khan, I.; Zhang, B.; Sadiq, M. Heterogeneous photodegradation of industrial dyes: An insight to different mechanisms and rate affecting parameters. J. Environ. Chem. Eng. 2020, 8, 104364. [Google Scholar] [CrossRef]
- Mutalib, A.A.A.; Jaafar, N.F.; Tajuddin, S.H.; Torlaema, T.A.M. Efficient photodegradation of methyl orange dye using electrogenerated copper-zinc oxide hybrid. Mater. Today Proc. 2023, 88, 1–5. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Rokon, M.Z.I.; Rahim, M.A.; Hossain, M.I.; Islam, M.S.; Ali, M.R.; Bacchu, M.S.; Waizumi, H.; Komeda, T.; Khan, M.Z.H. Enhanced photocatalytic activity of Cu and Ni-doped ZnO nanostructures: A comparative study of methyl orange dye degradation in aqueous solution. Heliyon 2023, 9, e16506. [Google Scholar] [CrossRef]
- Bhosale, A.; Kadam, J.; Gade, T.; Sonawane, K.; Garadkar, K. Efficient photodegradation of methyl orange and bactericidal activity of Ag doped ZnO nanoparticles. J. Indian Chem. Soc. 2023, 100, 100920. [Google Scholar] [CrossRef]
- Abo Zeid, E.F.; Obiedallah, F.M.; Abu-Sehly, A.-H.; Mohamed, W.A.; El-Aal, M.A. A comparative study of single and bi-doped Co3O4 nanocatalysts for the photodegradation of methyl orange dye. J. Mol. Struct. 2023, 1293, 136203. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, W.; Meng, Y.; Xie, B.; Ni, Z.; Xia, S. Investigation onto the performance and mechanism of visible light photodegradation of methyl orange catalyzed by M/CeO2 (M=Pt, Ag, Au). Mater. Res. Bull. 2021, 144, 111497. [Google Scholar] [CrossRef]
- Liu, H.; Wang, K.; Zhang, D.; Zhao, D.; Zhai, J.; Cui, W. Adsorption and catalytic removal of methyl orange from water by PIL-GO/TiO2/Fe3O4 composites. Mater. Sci. Semicond. Process. 2023, 154, 107215. [Google Scholar] [CrossRef]
- Gerawork, M. Photodegradation of methyl orange dye by using Zinc Oxide–Copper Oxide nanocomposite. Optik 2020, 216, 164864. [Google Scholar] [CrossRef]
- Quevedo-Robles, R.V.; Vilchis-Nestor, A.R.; Luque, P.A. Study of optical and morphological properties of nanoparticles semiconductors of zinc oxide synthesized using Mimosa tenuiflora extract for photodegradation of methyl orange. Opt. Mater. 2022, 128, 112450. [Google Scholar] [CrossRef]
- Ashiegbu, D.C.; Potgieter, H.J. ZnO-based heterojunction catalysts for the photocatalytic degradation of methyl orange dye. Heliyon 2023, 9, e20674. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.H.; Lee, S.Y.; Gwon, J.G.; Vijayakumar, E.; Lee, H.G.; Lee, W.H. Effects of hydrothermal treatment of cellulose nanocrystal templated TiO2 films on their photodegradation activity of methylene blue, methyl orange, and rhodamine B. Ceram. Int. 2023, 49, 2911–2922. [Google Scholar] [CrossRef]
- Sun, F.; He, J.; Wu, P.; Zeng, Q.; Liu, C.; Jiang, W. Magnetic photocatalyst CoFe2O4-Ag2O with magnetic aggregation bed photocatalytic reactor for continuous photodegradation of methyl orange. Chem. Eng. J. 2020, 397, 125397. [Google Scholar] [CrossRef]
- Pourshirband, N.; Nezamzadeh-Ejhieh, A. An efficient Z-scheme CdS/g-C3N4 nano catalyst in methyl orange photodegradation: Focus on the scavenging agent and mechanism. J. Mol. Liq. 2021, 335, 116543. [Google Scholar] [CrossRef]
- Chenab, K.K.; Sohrabi, B.; Jafari, A.; Ramakrishna, S. Water treatment: Functional nanomaterials and applications from adsorption to photodegradation. Mater. Today Chem. 2020, 16, 100262. [Google Scholar] [CrossRef]
- Bhosale, A.; Gophane, A.; Kadam, J.; Sabale, S.; Sonawane, K.; Garadkar, K. Fabrication of visible-active ZnO-gC3N4 nanocomposites for photodegradation and cytotoxicity of methyl orange and antibacterial activity towards drug resistance pathogens. Opt. Mater. 2023, 136, 113392. [Google Scholar] [CrossRef]
- Gayathri, R.C.; Elakkiya, V.; Sumathi, S. Synthesis of cerium and bismuth doped nickel aluminate for the photodegradation of methylene blue, methyl orange and rhodamine B dyes. Chemosphere 2022, 303, 135056. [Google Scholar] [CrossRef]
- Rao, A.V.; Narsimha, K.; Swarupa, G.; Anuradha, N.; Kumar, B.K.; Reddy, D.R.; Upender, G.; Kumar, B.V. Sn doped CdWO4 nanorods for augmented photodegradation of methyl orange. Mater. Lett. 2023, 353, 135304. [Google Scholar] [CrossRef]
- Bi, T.; Du, Z.; Chen, S.; He, H.; Shen, X.; Fu, Y. Preparation of flower-like ZnO photocatalyst with oxygen vacancy to enhance the photocatalytic degradation of methyl orange. Appl. Surf. Sci. 2023, 614, 156240. [Google Scholar] [CrossRef]
- Karajz, D.A.; Szilágyi, I.M. Review of photocatalytic ZnO nanomaterials made by atomic layer deposition. Surf. Interfaces 2023, 40, 103094. [Google Scholar] [CrossRef]
- Xia, J.; Wang, A.; Liu, X.; Su, Z. Preparation and characterization of bifunctional, Fe3O4/ZnO nanocomposites and their use as photocatalysts. Appl. Surf. Sci. 2011, 257, 9724–9732. [Google Scholar] [CrossRef]
- Wu, J.; Ke, K.; Qin, N.; Lin, E.; Kang, Z.; Bao, D. Magnetically retrievable Fe3O4@SiO2@ZnO piezo-photocatalyst: Synthesis and multiple catalytic properties. J. Colloid Interface Sci. 2023, 636, 167–175. [Google Scholar] [CrossRef]
- Lv, X.; Huang, W.; Ding, X.; He, J.; Huang, Q.; Tan, J.; Cheng, H.; Feng, J.; Li, L. Preparation and photocatalytic activity of Fe3O4@SiO2@ZnO:La. J. Rare Earths 2020, 38, 1288–1296. [Google Scholar] [CrossRef]
- Kiziltaş, H.; Tekin, T.; Tekin, D. Synthesis, characterization of Fe3O4@SiO2@ZnO composite with a core-shell structure and evaluation of its photocatalytic activity. J. Environ. Chem. Eng. 2020, 8, 104160. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Li, X.; Wei, B.; Wang, D.; Song, H.; Zhai, H.; Li, X. Synthesis of Fe3O4@SiO2@ZnO–Ag core–shell microspheres for the repeated photocatalytic degradation of rhodamine B under UV irradiation. J. Mol. Catal. A Chem. 2015, 406, 97–105. [Google Scholar] [CrossRef]
- Wang, D.; Han, D.; Yang, J.; Wang, J.; Li, X.; Song, H. Controlled preparation of superparamagnetic Fe3O4@SiO2@ZnO-Au core-shell photocatalyst with superior activity: RhB degradation and working mechanism. Powder Technol. 2018, 327, 489–499. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Mortazavi-Derazkola, S.; Zazouli, M.A. Eco-friendly green synthesis of novel magnetic Fe3O4/SiO2/ZnO-Pr6O11 nanocomposites for photocatalytic degradation of organic pollutant. J. Rare Earths 2020, 38, 13–20. [Google Scholar] [CrossRef]
- Wang, D.; Han, D.; Shi, Z.; Wang, J.; Yang, J.; Li, X.; Song, H. Optimized design of three-dimensional multi-shell Fe3O4/SiO2/ZnO/ZnSe microspheres with type II heterostructure for photocatalytic applications. Appl. Catal. B Environ. 2018, 227, 61–69. [Google Scholar] [CrossRef]
- Xu, T.; Wang, P.; Wang, D.; Zhao, K.; Wei, M.; Liu, X.; Liu, H.; Cao, J.; Chen, Y.; Fan, H.; et al. Ultrasound-assisted synthesis of hyper-dispersed type-II tubular Fe3O4@SiO2@ZnO/ZnS core/shell heterostructure for improved visible-light photocatalysis. J. Alloy. Compd. 2020, 838, 155689. [Google Scholar] [CrossRef]
- Ammar, S.H.; Abdulnabi, W.A.; Kader, H.D.A. Synthesis, characterization and environmental remediation applications of polyoxometalates-based magnetic zinc oxide nanocomposites (Fe3O4@ZnO/PMOs). Environ. Nanotechnol. Monit. Manag. 2020, 13, 100289. [Google Scholar] [CrossRef]
- Areerob, Y.; Cho, J.Y.; Jang, W.K.; Oh, W.-C. Enhanced sonocatalytic degradation of organic dyes from aqueous solutions by novel synthesis of mesoporous Fe3O4-graphene/ZnO@SiO2 nanocomposites. Ultrason. Sonochemistry 2018, 41, 267–278. [Google Scholar] [CrossRef]
- Barros, M.M.P.; Almeida, K.J.C.; Conceição, M.V.S.; Pereira, D.H.; Botelho, G. Photodegradation of bisphenol A by ZnS combined with H2O2: Evaluation of photocatalytic activity, reaction parameters, and DFT calculations. J. Mol. Liq. 2023, 371, 121096. [Google Scholar] [CrossRef]
- Zou, J.; Gao, J. H2O2-sensitized TiO2/SiO2 composites with high photocatalytic activity under visible irradiation. J. Hazard. Mater. 2011, 185, 710–716. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, L.; Watanabe, S. Facile modification of TiO2 nanoparticles with H2O2 + NH4F for enhanced visible light photodegradation of rhodamine B and methylene blue. Mater. Today Commun. 2022, 33, 104213. [Google Scholar] [CrossRef]
- Yu, Z.; Zhu, S.; Zhang, L.; Watanabe, S. Mesoporous single crystal titanium oxide microparticles for enhanced visible light photodegradation. Opt. Mater. 2022, 127, 112297. [Google Scholar] [CrossRef]
- Prabhu, M.; Mayandi, J.; Mariammal, R.N.; Vishnukanthan, V.; Pearce, J.M.; Soundararajan, N.; Ramachandran, K. Peanut shaped ZnO microstructures: Controlled synthesis and nucleation growth toward low-cost dye sensitized solar cells. Mater. Res. Express 2015, 2, 066202. [Google Scholar] [CrossRef]
- Ha, L.P.P.; Vinh, T.H.T.; Thuy, N.T.B.; Thi, C.M.; Van Viet, P. Visible-light-driven photocatalysis of anisotropic silver nanoparticles decorated on ZnO nanorods: Synthesis and characterizations. J. Environ. Chem. Eng. 2021, 9, 105103. [Google Scholar] [CrossRef]
- Wu, S.-H.; Wu, J.-L.; Jia, S.-Y.; Chang, Q.-W.; Ren, H.-T.; Liu, Y. Cobalt(II) phthalocyanine-sensitized hollow Fe3O4@SiO2@TiO2 hierarchical nanostructures: Fabrication and enhanced photocatalytic properties. Appl. Surf. Sci. 2013, 287, 389–396. [Google Scholar] [CrossRef]
- Liu, K.; Qin, Y.; Muhammad, Y.; Zhu, Y.; Tang, R.; Chen, N.; Shi, H.; Zhang, H.; Tong, Z.; Yu, B. Effect of Fe3O4 content and microwave reaction time on the properties of Fe3O4/ZnO magnetic nanoparticles. J. Alloys Compd. 2019, 781, 790–799. [Google Scholar] [CrossRef]
- Madhubala, V.; Kalaivani, T. Phyto and hydrothermal synthesis of Fe3O4@ZnO core-shell nanoparticles using Azadirachta indica and its cytotoxicity studies. Appl. Surf. Sci. 2018, 449, 584–590. [Google Scholar] [CrossRef]
- Nayebi, P.; Babamoradi, M. Synthesis of ZnO nanorods/Fe3O4/polypyrrole nanocomposites for photocatalytic activity under the visible light irradiation. Optik 2021, 244, 167497. [Google Scholar] [CrossRef]
- Maddalena, R.; Hall, C.; Hamilton, A. Effect of silica particle size on the formation of calcium silicate hydrate [C-S-H] using thermal analysis. Thermochim. Acta 2019, 672, 142–149. [Google Scholar] [CrossRef]
- Mel’nik, I.V.; Zub, Y.L.; Alonso, B.; Abramov, N.V.; Gorbik, P.P. Creation of a functional polysiloxane layer on the surface of magnetic nanoparticles using the sol-gel method. Glas. Phys. Chem. 2012, 38, 96–104. [Google Scholar] [CrossRef]
- Melnyk, I.V.; Pogorilyi, R.P.; Zub, Y.L.; Vaclavikova, M.; Gdula, K.; Dąbrowski, A.; Seisenbaeva, G.A.; Kessler, V.G. Protection of Thiol Groups on the Surface of Magnetic Adsorbents and Their Application for Wastewater Treatment. Sci. Rep. 2018, 8, 8592. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, F.; Li, Y.; Xu, M.; Li, L.; Wu, C.; Miyoshi, H.; Liu, Y. VCAM-1-targeted core/shell nanoparticles for selective adhesion and delivery to endothelial cells with lipopolysaccharide-induced inflammation under shear flow and cellular magnetic resonance imaging in vitro. Int. J. Nanomed. 2013, 8, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Sun, T.; Xing, J.; Fan, X. Efficient removal of ethidium bromide from aqueous solution by using DNA-loaded Fe3O4 nanoparticles. Environ. Sci. Pollut. Res. 2019, 26, 2387–2396. [Google Scholar] [CrossRef] [PubMed]
- Degen, A.; Kosec, M. Effect of pH and impurities on the surface charge of zinc oxide in aqueous solution. J. Eur. Ceram. Soc. 2000, 20, 667–673. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, J.; Chen, M. Novel Z-scheme LaVO4/Bi3O4Cl heterojunctions for highly efficient degradation of ofloxacin under visible light irradiation. J. Alloys Compd. 2022, 925, 166653. [Google Scholar] [CrossRef]
- Sansenya, T.; Masri, N.; Chankhanittha, T.; Senasu, T.; Piriyanon, J.; Mukdasai, S.; Nanan, S. Hydrothermal synthesis of ZnO photocatalyst for detoxification of anionic azo dyes and antibiotic. J. Phys. Chem. Solids 2021, 160, 110353. [Google Scholar] [CrossRef]
- Chankhanittha, T.; Nanan, S. Visible-light-driven photocatalytic degradation of ofloxacin (OFL) antibiotic and Rhodamine B (RhB) dye by solvothermally grown ZnO/Bi2MoO6 heterojunction. J. Colloid Interface Sci. 2021, 582, 412–427. [Google Scholar] [CrossRef]
- Susanti, Y.D.; Afifah, N.; Saleh, R. Multifunctional Photocatalytic Degradation of Methylene Blue Using LaMnO3/Fe3O4 Nanocomposite on Different Types of Graphene. J. Phys. Conf. Ser. 2017, 820, 012021. [Google Scholar] [CrossRef]
- Bahramian, H.; Fattah-Alhosseini, A.; Karbasi, M. Development of porous ceramic coatings via the PEO process: The key role of CuO nanoparticles in methylene blue photodegradation under visible light illumination. Appl. Surf. Sci. Adv. 2023, 18, 100511. [Google Scholar] [CrossRef]
- Lousada, C.M.; Johansson, A.J.; Brinck, T.; Jonsson, M. Mechanism of H2O2 Decomposition on Transition Metal Oxide Surfaces. J. Phys. Chem. C 2012, 116, 9533–9543. [Google Scholar] [CrossRef]
- Feng, Q.; Li, S.; Ma, W.; Fan, H.-J.; Wan, X.; Lei, Y.; Chen, Z.; Yang, J.; Qin, B. Synthesis and characterization of Fe3O4/ZnO-GO nanocomposites with improved photocatalytic degradation methyl orange under visible light irradiation. J. Alloys Compd. 2017, 737, 197–206. [Google Scholar] [CrossRef]

| Photocatalyst | Photocatalyst Dosage, mg | MO Concentration | MO Volume, mL | Light Source | Time, min | Efficiency, % | Rate Constant, k1, min−1 | Ref. |
|---|---|---|---|---|---|---|---|---|
| Fe3O4/ZnO-GO | 20 | 1 × 10−5 M | 100 | 300 W, Xe lamp | 150 | 92.8 | 0.05558 | [57] |
| Fe3O4@ZnO/PW | 40 | 4 × 10−5 mol/L | 50 | 2 × 20 W, white LED lamps | 180 | 92.3 | 0.0138 | [35] |
| Fe3O4@SiO2@ZnO | 100 | 5 mg/L | 100 | 100 mW cm−2, mercury lamp | 60 | – | 0.004 | [27] |
| Fe3O4@SiO2@ZnO@La | 800 | 3 mg/L | 50 | 300 W, mercury lamp | 100 | 94 | – | [28] |
| Fe3O4@SiO2@ZnO | 800 | 3 mg/L | 50 | 300 W, mercury lamp | 100 | 88 | – | [28] |
| Fe3O4@SiO2@ZnO* | 50 | 13.5 mg/L | 50 | 9 W, UV | 240 | 96 | 0.0117 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makota, O.; Dutková, E.; Briančin, J.; Bednarcik, J.; Lisnichuk, M.; Yevchuk, I.; Melnyk, I. Advanced Photodegradation of Azo Dye Methyl Orange Using H2O2-Activated Fe3O4@SiO2@ZnO Composite under UV Treatment. Molecules 2024, 29, 1190. https://doi.org/10.3390/molecules29061190
Makota O, Dutková E, Briančin J, Bednarcik J, Lisnichuk M, Yevchuk I, Melnyk I. Advanced Photodegradation of Azo Dye Methyl Orange Using H2O2-Activated Fe3O4@SiO2@ZnO Composite under UV Treatment. Molecules. 2024; 29(6):1190. https://doi.org/10.3390/molecules29061190
Chicago/Turabian StyleMakota, Oksana, Erika Dutková, Jaroslav Briančin, Jozef Bednarcik, Maksym Lisnichuk, Iryna Yevchuk, and Inna Melnyk. 2024. "Advanced Photodegradation of Azo Dye Methyl Orange Using H2O2-Activated Fe3O4@SiO2@ZnO Composite under UV Treatment" Molecules 29, no. 6: 1190. https://doi.org/10.3390/molecules29061190
APA StyleMakota, O., Dutková, E., Briančin, J., Bednarcik, J., Lisnichuk, M., Yevchuk, I., & Melnyk, I. (2024). Advanced Photodegradation of Azo Dye Methyl Orange Using H2O2-Activated Fe3O4@SiO2@ZnO Composite under UV Treatment. Molecules, 29(6), 1190. https://doi.org/10.3390/molecules29061190







