Novel Peptide Analogues of Valorphin-Conjugated 1,8-Naphthalimide as Photodynamic Antimicrobial Agent in Solution and on Cotton Fabric
Abstract
1. Introduction
2. Results and Discussion
2.1. Design and Synthesis of New Peptides
2.2. NMR Characterization of the Peptides
2.3. Modification of Cotton Fabric with the Peptides
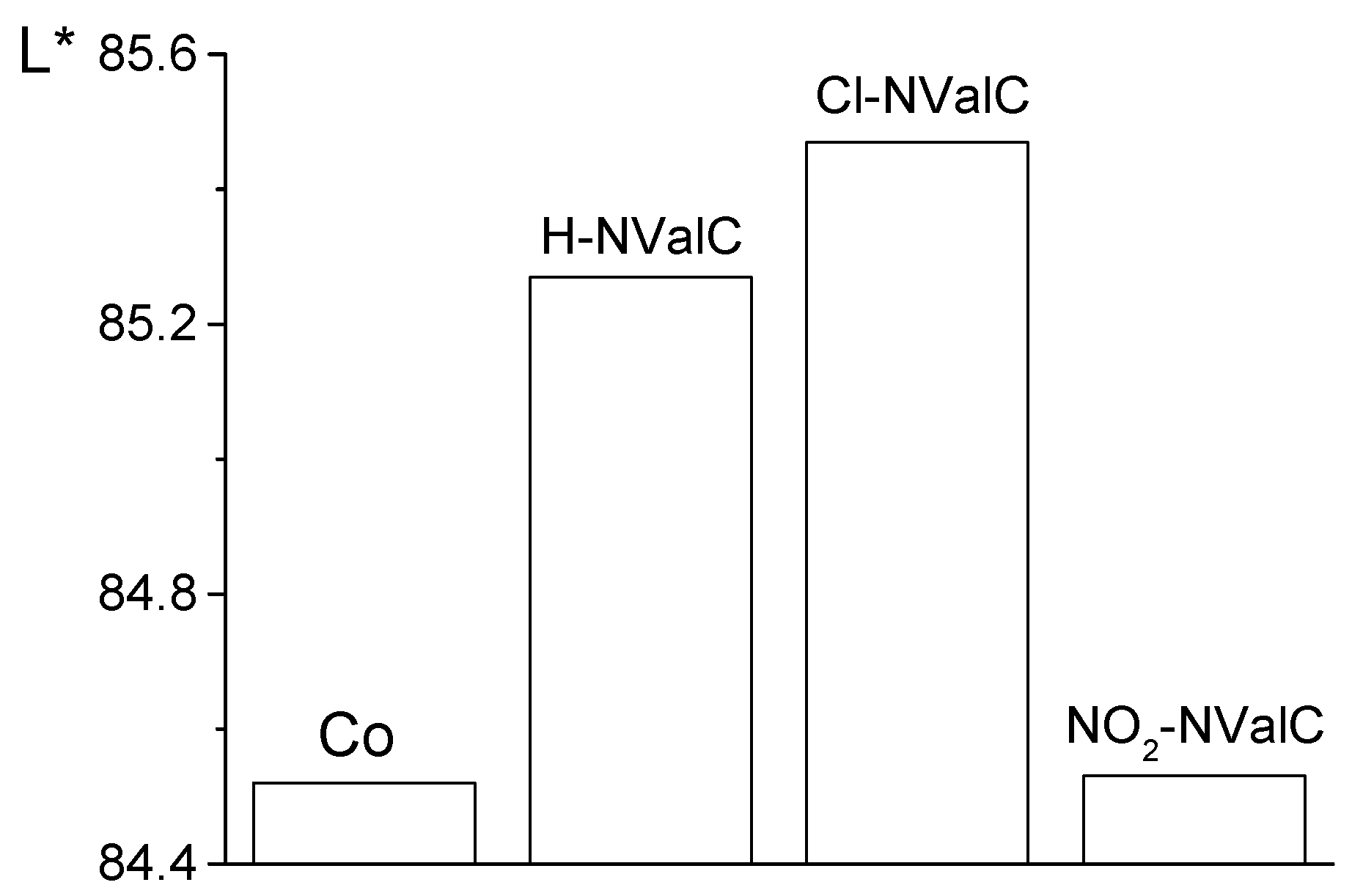
2.4. Colourimetric Analysis of the Studied Fabrics
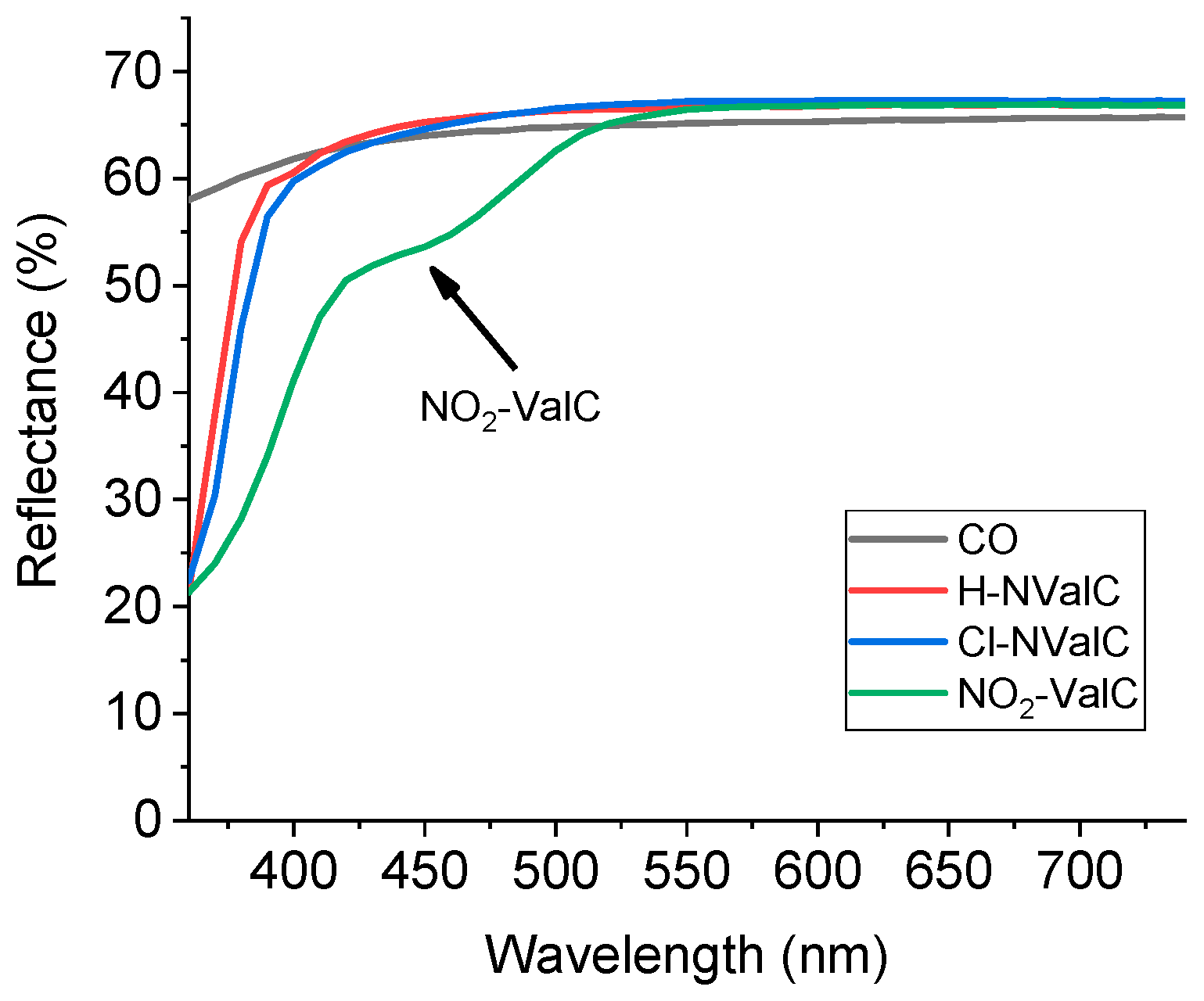
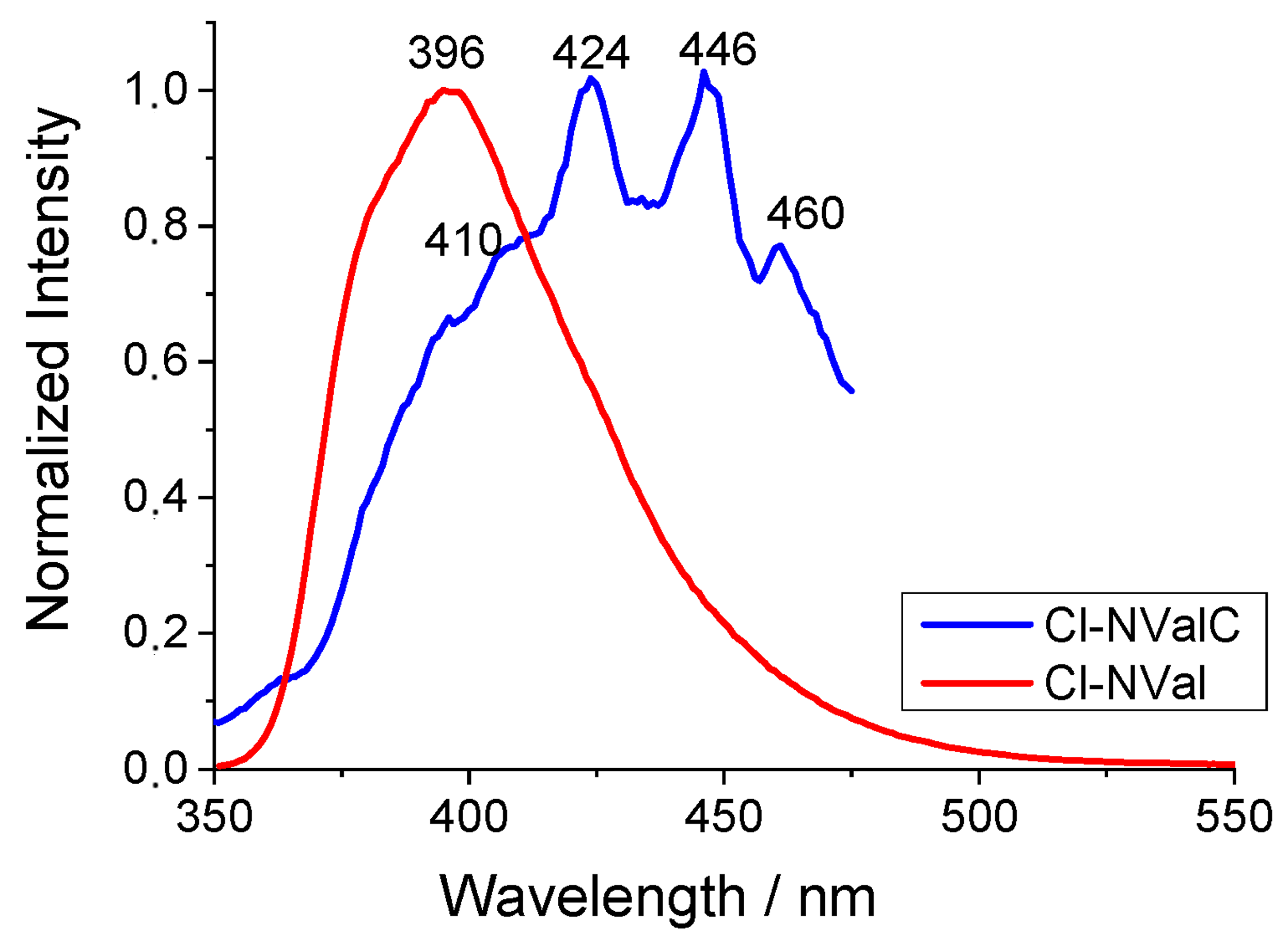
2.5. Spectral Analysis of the H-NVal, Cl-NVal, and NO2-NVal Peptides in Ethanol Solution and After Their Deposition on Cotton Fabric
2.6. Determination of Released Singlet Oxygen by Studying the Photooxidation of Potassium Iodide
2.7. Antibacterial Assay of New Peptides in Solution and on the Cotton Fabric and Their Photoactivity
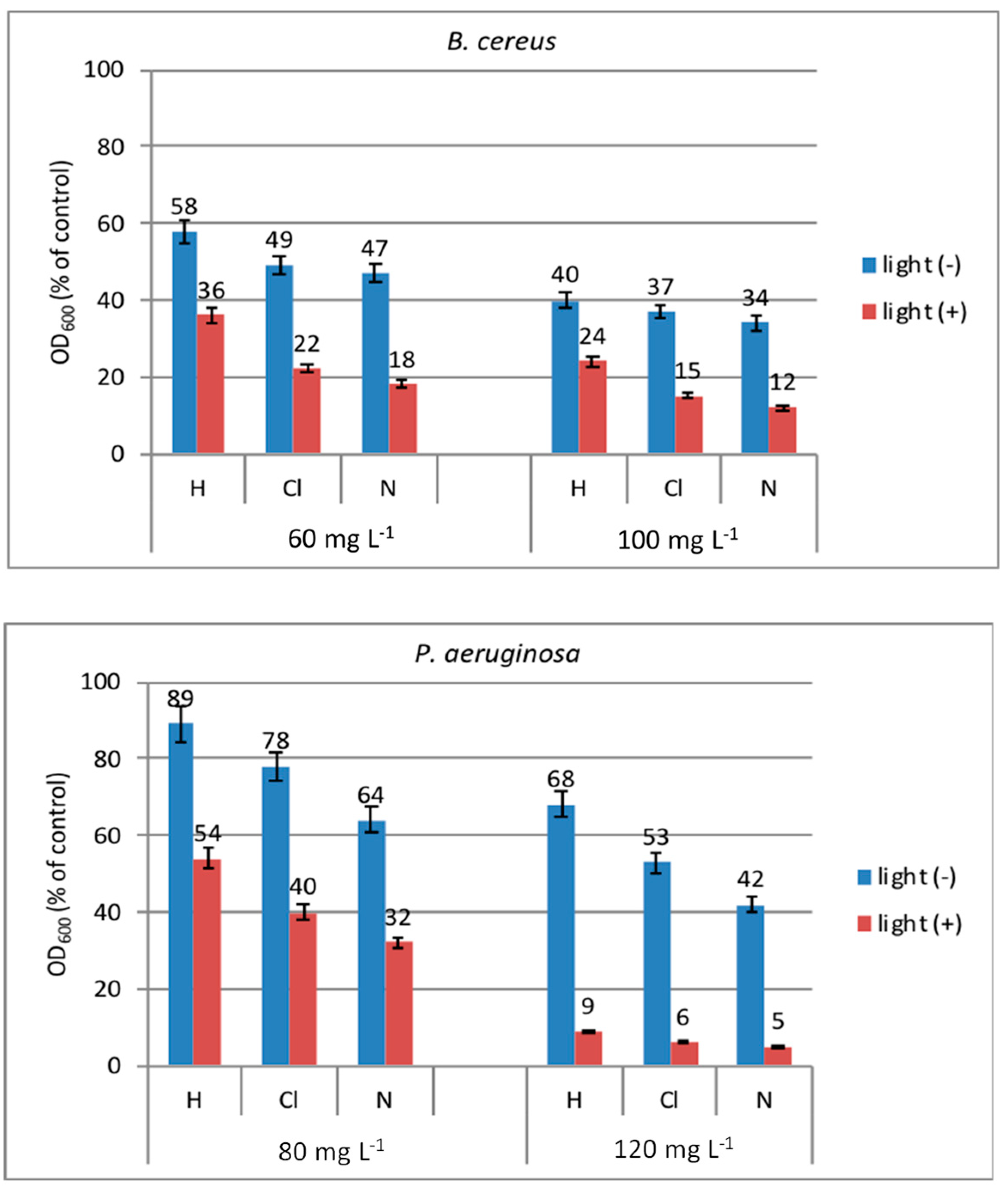
2.7.1. Antibacterial Photoactivity of New Peptides in Solution
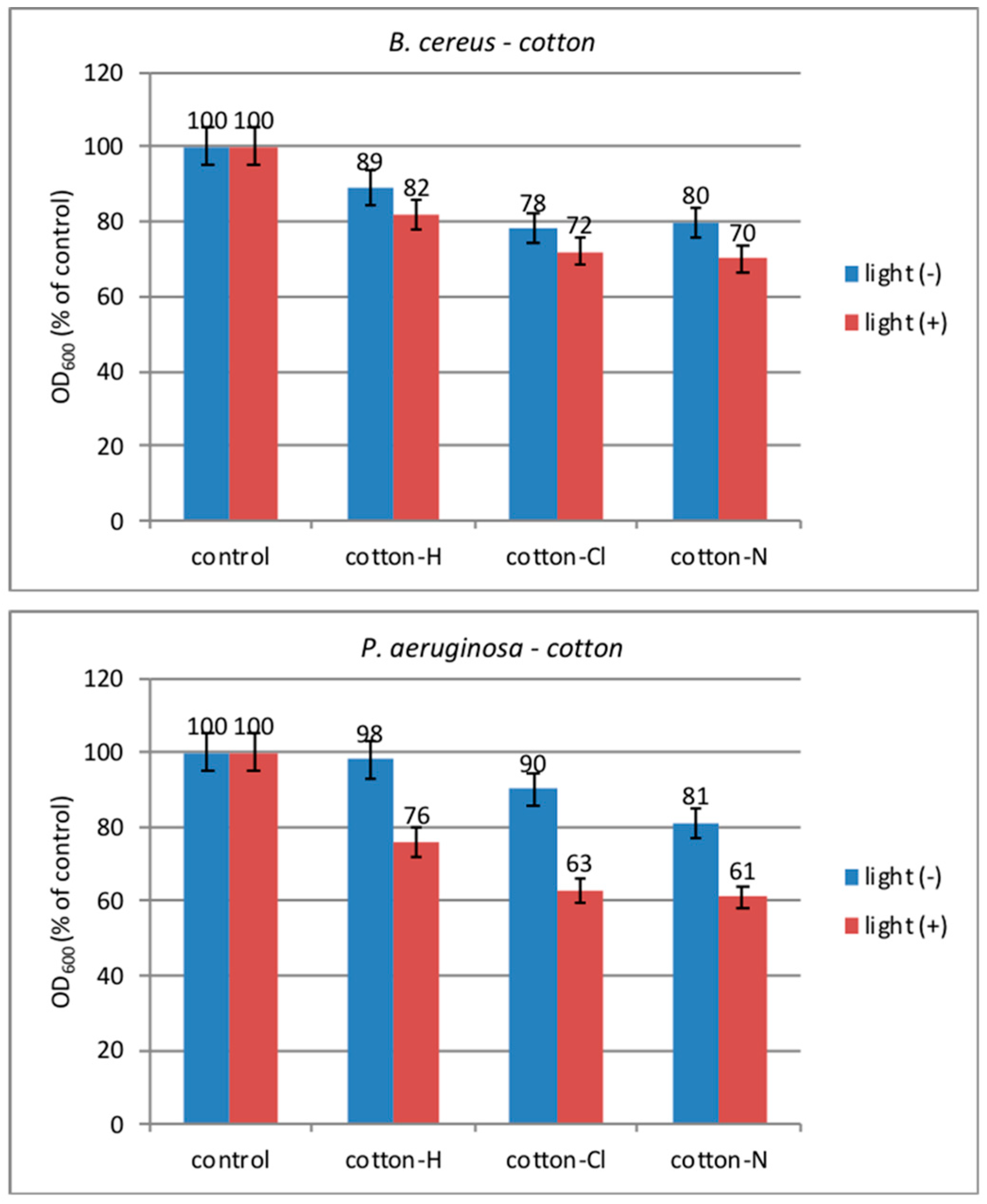
2.7.2. Antibacterial Photoactivity of New Peptides on Cotton Fabric
3. Experimental Part
3.1. Synthesis of the 1,8-Naphtalimide-β-alanine (1), 4-Chloro-1,8-naphtalimide-β-alanine (2), and 4-Nitro-1,8-naphtalimide-β-alanine (3)
3.2. The Analytical Data for the Synthesized Peptides Prepared Were as Follows
3.2.1. Compound H-NVal: (S)-5-Amino-4-((2S,3R)-2-((S)-2-((S)-1-((3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoyl)-L-valyl-L-valyl-L-tyrosyl)pyrrolidine-2-carboxamido)-3-(1H-indol-3-yl)propanamido)-3-hydroxybutanamido)-5-oxopentanoic Acid (Figure 11)
3.2.2. Compound Cl-NVal: (S)-5-Amino-4-((2S,3R)-2-((S)-2-((S)-1-((3-(6-chloro-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoyl)-L-valyl-L-valyl-L-tyrosyl)pyrrolidine-2-carboxamido)-3-(1H-indol-3-yl)propanamido)-3-hydroxybutanamido)-5-oxopentanoic Acid (Figure 12)
3.2.3. Compound NO2-NVal: (S)-5-Amino-4-((2S,3R)-2-((S)-2-((S)-1-((3-(6-nitro-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoyl)-L-valyl-L-valyl-Ltyrosyl) pyrrolidine-2-carboxamido)-3-(1H-indol-3-yl)propanamido)-3-hydroxybutanamido)-5-oxopentanoic Acid (Figure 13)
3.3. Cotton Fabric Modification with New Peptides H-NVal, Cl-NVal, and NO2-NVal
3.4. Color Measurements of the Fabrics Modified with Peptides
3.5. Fluorescent Properties of Peptides and Cotton Fabrics
3.6. Determination of the Released Singlet Oxygen from the Fabrics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antimicrobial Textiles Market Report by Fabric (Polyester, Polyamide, Cotton, and Others), Active Agents (Metal and Metallic Salts, Synthetic Organic Compounds, Biobased Agents), Application (Medical Textiles, Home Textiles, Apparels, and Others), and Region 2024–2032. Imarc. 2023. Report ID: SR112024A5363. Available online: https://www.imarcgroup.com/antimicrobial-textiles-market (accessed on 19 October 2024).
- Santiago, D.; Cabral, I.; Cunha, J. Children’s Functional Clothing: Design Challenges and Opportunities. Appl. Sci. 2024, 14, 4472. [Google Scholar] [CrossRef]
- Rangelova, R. Development tendencies of children fashion since the beginning of XXI century–contemporary fashion. Online Mag. Text. Cloth. Leather Technol. 2019, 12, 5–8. [Google Scholar]
- Marinova, P.; Tamahkyarova, K. Synthesis and Biological Activities of Some Metal Complexes of Peptides: A Review. BioTech 2024, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef] [PubMed]
- Kapil, S.; Sharma, V. d-Amino acids in antimicrobial peptides: A potential approach to treat and combat antimicrobial resistance. Can. J. Microbiol. 2021, 67, 119–137. [Google Scholar] [CrossRef]
- Hoelscher, M.P.; Forner, J.; Calderone, S.; Krämer, C.; Taylor, Z.; Loiacono, F.V.; Agrawal, S.; Karcher, D.; Moratti, F.; Kroop, X.; et al. Expression strategies for the efficient synthesis of antimicrobial peptides in plastids. Nat. Commun. 2022, 13, 5856. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Brinton, S.L.; Cavagnero, K.J.; O’Neill, A.M.; Chen, Y.; Dokoshi, T.; Butcher, A.M.; Osuoji, O.C.; Shafiq, F.; Espinoza, J.L.; et al. Competition between skin antimicrobial peptides and commensal bacteria in type 2 inflammation enables survival of S. aureus. Cell Rep. 2023, 42, 112494. [Google Scholar] [CrossRef]
- Nedjar-Arroume, N.; Dubois-Delval, V.; Adje, E.Y.; Traisnel, J.; Krier, F.; Mary, P.; Kouach, M.; Briand, G.; Guillochon, D. Bovine hemoglobin: An attractive source of antibacterial peptides. Peptides 2008, 29, 969–977. [Google Scholar] [CrossRef]
- Giardina, B. Hemoglobin: Multiple molecular interactions and multiple functions. An example of energy optimization and global molecular organization. Mol. Asp. Med. 2022, 84, 101040. [Google Scholar] [CrossRef]
- Outman, A.; Deracinois, B.; Flahaut, C.; Diab, M.A.; Gressier, B.; Eto, B.; Nedjar, N. Potential of Human Hemoglobin as a Source of Bioactive Peptides: Comparative Study of Enzymatic Hydrolysis with Bovine Hemoglobin and the Production of Active Peptide α137–141. Int. J. Mol. Sci. 2023, 24, 11921. [Google Scholar] [CrossRef]
- Todorov, P.; Georgieva, S.; Tchekalarova, J. Recent Synthesis, Characterization, and Pharmacological Evaluation of Multifunctional Hemorphins Containing Non-Natural Amino Acids with Potential Biological Importance. Pharmaceuticals 2022, 15, 1425. [Google Scholar] [CrossRef] [PubMed]
- Mak, P.; Wójcik, K.; Silberring, J.; Dubin, A. Antimicrobial peptides derived from heme-containing proteins: Hemocidins. Antonie Van. Leeuwenhoek 2000, 77, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Todorov, P.; Georgieva, S.; Staneva, D.; Peneva, P.; Grozdanov, P.; Nikolova, I.; Grabchev, I. Synthesis of new modified with Rhodamine B peptides for antiviral protection of textile materials. Molecules 2021, 26, 6608. [Google Scholar] [CrossRef] [PubMed]
- Todorov, P.; Georgieva, S.; Staneva, D.; Peneva, P.; Grozdanov, P.; Nikolova, I.; Vasileva-Tonkova, E.; Grabchev, I. Study of Novel Peptides for Antimicrobial Protection in Solution and on Cotton Fabric. Molecules 2022, 27, 4770. [Google Scholar] [CrossRef]
- Efimov, A.; Mordon, S. Photoantimicrobial and Photoantiviral Textiles: Underestimated Potential. Pharmaceuticals 2024, 17, 1164. [Google Scholar] [CrossRef]
- Bartwal, G.; Manivannan, R.; Son, Y.-A. Synergistic integration of a rhodamine-labelled tripeptide into AIE-active fluorogenic probe: Enabling nanomolar detection of Al3+ ions through test strips, thin films, and Arduino-assisted optosensing platform. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 323, 124874. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, J.; Jing, Q.; Chen, Z.; Ullah, A.; Jiang, L.; Zheng, K.; Yuan, C.; Huang, M. Development of a Potent Antimicrobial Peptide With Photodynamic Activity. Front. Microbiol. 2021, 12, 624465. [Google Scholar] [CrossRef]
- Antunes, L.; Faustino, G.; Mouro, C.; Vaz, J.; Gouveia, I.C. Bioactive microsphere-based coating for biomedical-textiles with encapsulated antimicrobial peptides (AMPs). Cienc. Tecnol. Dos Mater. 2014, 26, 118–125. [Google Scholar] [CrossRef]
- Tang, A.Y.L.; Kan, C.-W. Non-aqueous dyeing of cotton fibre with reactive dyes: A review. Color. Technol. 2020, 136, 214–223. [Google Scholar] [CrossRef]
- Yuan, D.; Brown, R.G.; Hepworth, J.D.; Alexiou, M.S.; Tyman, J.H.P. The Synthesis and Fluorescence of Novel N-Substituted-1,8-naphthylimides. J. Heterocyclic. Chem. 2008, 45, 397. [Google Scholar] [CrossRef]
- Grabchev, I.; Dumas, S.; Chovelon, J.-M.; Nedelcheva, A. First generation poly(propyleneimine) dendrimers functionalised with 1,8-naphthalimide units as fluorescence sensors for metal cations and protons. Tetrahedron 2008, 64, 2113–2119. [Google Scholar] [CrossRef]
- Chanh, T.C.; Lewis, D.E.; Judy, M.M.; Bernal, F.S.; Michalek, G.R.; Utecht, R.E.; Skiles, H.; Chang, S.-C.; Matthews, J.L. Inhibition of retrovirusinduced syncytium formation by photoproducts of a brominated 1,8-naphthalimide compound. Antivir. Res. 1994, 25, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Mutovska, M.; Skabeev, A.; Konstantinov, K.; Cabanetos, C.; Stoyanov, S.; Zagranyarski, Y. One-pot synthesis of fused-rings heterocyclic systems based on symmetrically benzofuran annulated 1,8-naphthalimides. Dyes. Pigm. 2023, 220, 111701. [Google Scholar] [CrossRef]
- Dodangeh, M.; Grabchev, I.; Staneva, D.; Gharanjig, K. 1,8-Naphthalimide Derivatives as Dyes for Textile and Polymeric Materials: A Review. Fiber. Polym. 2021, 22, 2368–2379. [Google Scholar] [CrossRef]
- Kaiser, E.; Colescott, R.L.; Bossinger, C.D.; Cook, P.I. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal. Biochem. 1970, 34, 595–598. [Google Scholar] [CrossRef]
- Sarin, V.K.; Kent, S.B.H.; Tam, J.P.; Merrifield, R.B. Quantitative monitoring of solid-phase peptide synthesis by the ninhydrin reaction. Anal. Biochem. 1981, 117, 147–157. [Google Scholar] [CrossRef]
- Kamal, A.; Bolla, N.R.; Srikanth, P.S.; Srivastava, A.K. Naphthalimide derivatives with therapeutic characteristics: A patent review. Expert. Opin. Ther. Pat. 2013, 23, 299–317. [Google Scholar] [CrossRef]
- Mata, M.A.; Satterly, N.; Versteeg, G.A.; Frantz, D.; Wei, S.; Williams, N.; Schmolke, M.; Peña-Llopis, S.; Brugarolas, J.; Forst, C.V.; et al. Chemical inhibition of RNA viruses reveals REDD1 as a host defense factor. Nat. Chem. Biol. 2011, 7, 712–719. [Google Scholar] [CrossRef]
- Zerbe, O.; Jurt, S. Applied NMR Spectroscopy for Chemists and Life Scientists, 1st ed.; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Kumar, A.; Ernst, R.R.; Wuthrich, K. A two-dimensional nuclear Overhauser enhancement (2D NOE) experiment for the elucidation of complete proton-proton cross-relaxation networks in biological macromolecules. Biochem. Biophys. Res. Commun. 1980, 64, 2229–2246. [Google Scholar] [CrossRef]
- Bax, A. Two-Dimensional NMR and Protein Structure. Annu. Rev. Biochem. 1989, 58, 223–256. [Google Scholar] [CrossRef]
- Mosinger, J.; Mosinger, B. Photodynamic sensitizers assay: Rapid and sensitive iodometric measurement. Experientia 1995, 51, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Yiming, X. Iodine-sensitized oxidation of ferrous ions under UV and visible light: The influencing factors and reaction mechanism. Photochem. Photobiol. Sci. 2013, 12, 2084–2089. [Google Scholar] [CrossRef] [PubMed]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, F.F.; Huang, Y.-Y.; Hamblin, M.R. Antimicrobial photodynamic therapy to kill Gram-negative bacteria. Recent Pat. Antiinfect. Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Faustino, M.A.F.; Tomé, J.P.C. Photodynamic inactivation of bacteria: Finding the effective targets. Future Med. Chem. 2015, 7, 1221–1224. [Google Scholar] [CrossRef]
- Mahanta, U.; Khandelwal, M.; Deshpande, A.S. Antimicrobial surfaces: A review of synthetic approaches, applicability and outlook. J. Mater. Sci. 2021, 56, 17915–17941. [Google Scholar] [CrossRef]
- Rapacka-Zdonczyk, A.; Wozniak, A.; Michalska, K.; Pieranski, M.; Ogonowska, P.; Grinholc, M.; Nakonieczna, J. Factors Determining the Susceptibility of Bacteria to Antibacterial Photodynamic Inactivation. Front. Med. 2021, 8, 642609. [Google Scholar] [CrossRef]
- Dahl, T.; Midden, R.; Hartman, P. Comparison of Killing of Gram-Negative and Gram-Positive Bacteria by Pure Singlet Oxygen. J. Bacteriol. 1989, 171, 2188–2194. [Google Scholar] [CrossRef]



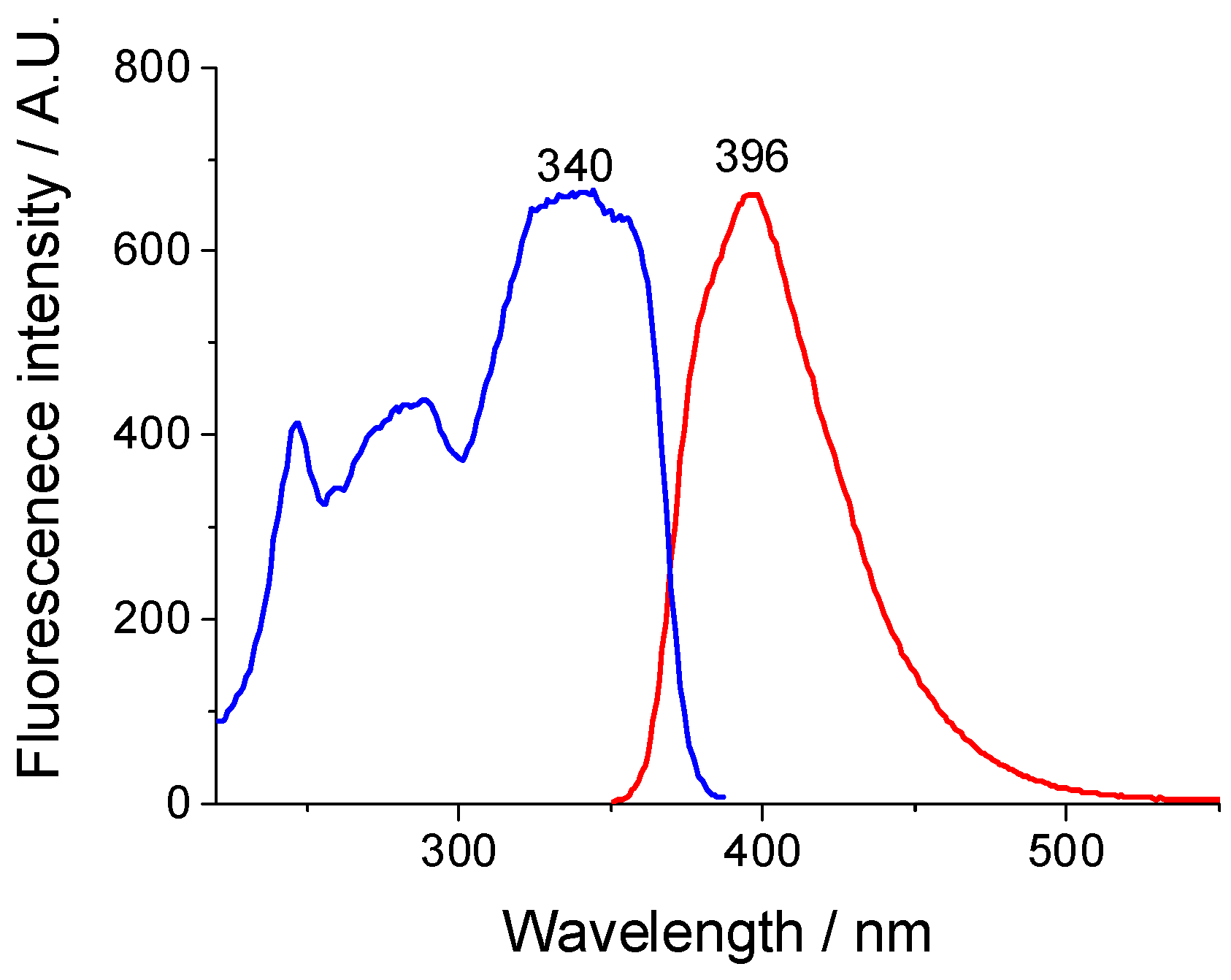
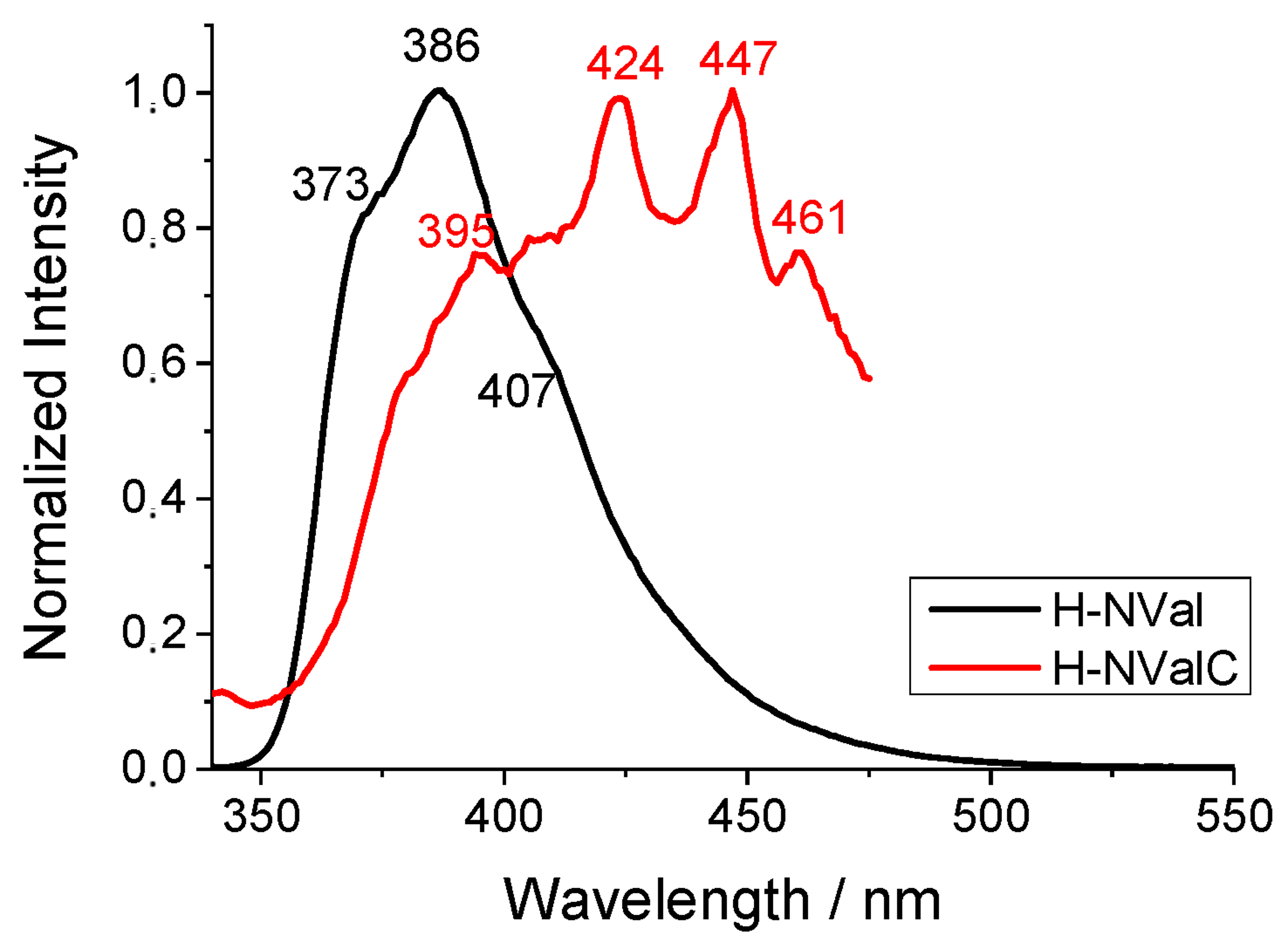

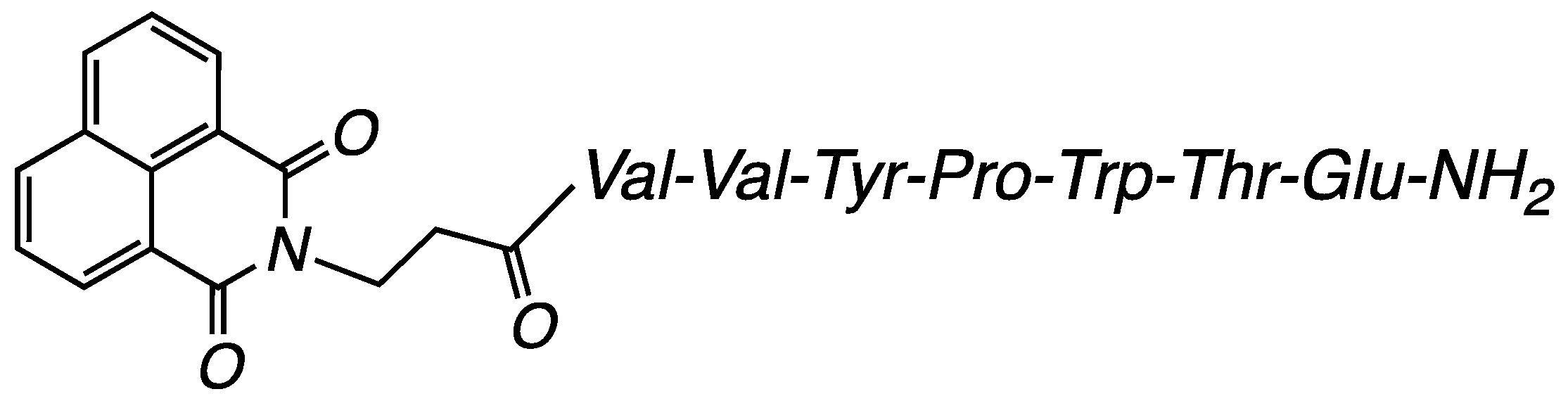

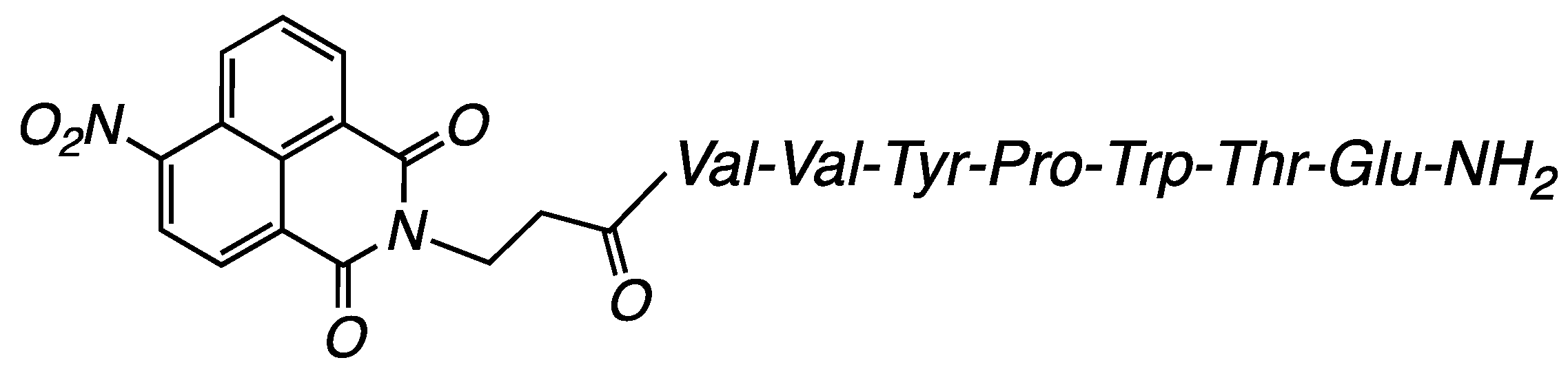
| Peptides | λA (nm) | ε (mol L−1 cm−1) | λF (nm) | νA-νF (cm−1) | ΦF |
|---|---|---|---|---|---|
| H-NVal | 335,343 | 11,850 | 386 | 3944 | 0.0038 |
| Cl-NVal | 343,355 | 12,510 | 396 | 3902 | 0.0021 |
| NO2-NVal | 350 | 8810 | 390 | 2930 | 0.0009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staneva, D.; Todorov, P.; Georgieva, S.; Peneva, P.; Grabchev, I. Novel Peptide Analogues of Valorphin-Conjugated 1,8-Naphthalimide as Photodynamic Antimicrobial Agent in Solution and on Cotton Fabric. Molecules 2024, 29, 5421. https://doi.org/10.3390/molecules29225421
Staneva D, Todorov P, Georgieva S, Peneva P, Grabchev I. Novel Peptide Analogues of Valorphin-Conjugated 1,8-Naphthalimide as Photodynamic Antimicrobial Agent in Solution and on Cotton Fabric. Molecules. 2024; 29(22):5421. https://doi.org/10.3390/molecules29225421
Chicago/Turabian StyleStaneva, Desislava, Petar Todorov, Stela Georgieva, Petia Peneva, and Ivo Grabchev. 2024. "Novel Peptide Analogues of Valorphin-Conjugated 1,8-Naphthalimide as Photodynamic Antimicrobial Agent in Solution and on Cotton Fabric" Molecules 29, no. 22: 5421. https://doi.org/10.3390/molecules29225421
APA StyleStaneva, D., Todorov, P., Georgieva, S., Peneva, P., & Grabchev, I. (2024). Novel Peptide Analogues of Valorphin-Conjugated 1,8-Naphthalimide as Photodynamic Antimicrobial Agent in Solution and on Cotton Fabric. Molecules, 29(22), 5421. https://doi.org/10.3390/molecules29225421











