CuAAC-Based Synthesis, Copper-Catalyzed Aldehyde-Forming Hydrolytic Fission and Antiproliferative Evaluation of Novel Ferrocenoylamino-Substituted Triazole-Tethered Quinine–Chalcone Hybrids
Abstract
1. Introduction
2. Results
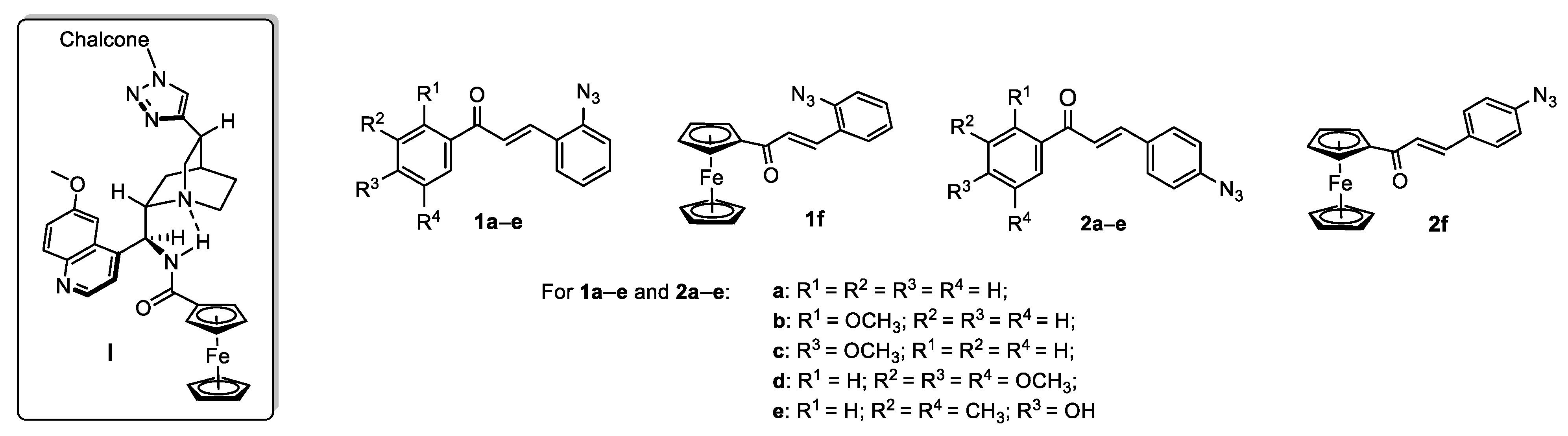
2.1. Synthesis of the Targeted Hybrids
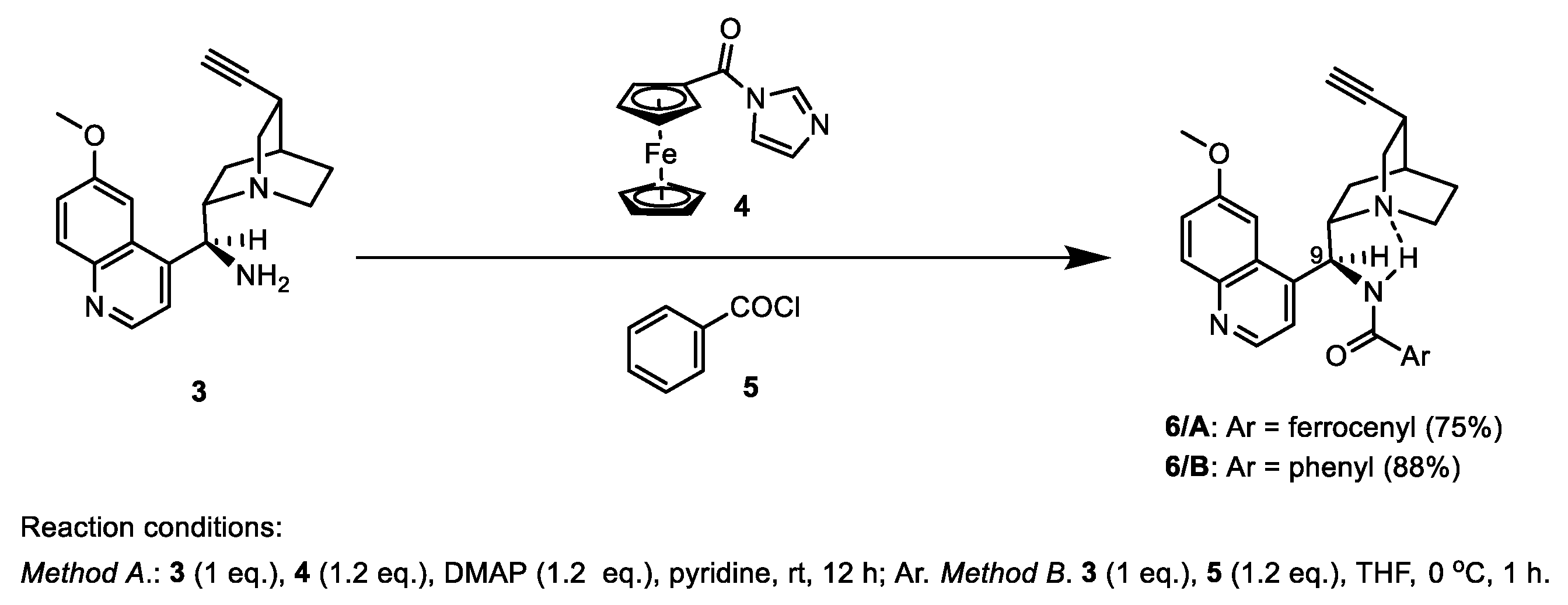
2.1.1. Synthesis of the Azidochalcone and Aroylaminoquinine-Based Alkyne Components Used for the Click Reactions
2.1.2. Attempted CuAAC Reactions to Construct the Targeted Hybrid Molecules
2.2. Structural Elucidation of the Novel Hybrid Compounds
2.3. In Vitro Antiproliferative Activities of the Novel Hybrid Compounds
3. Materials and Methods
3.1. Synthesis of N-((S)-((1S,2S,4S,5S)-5-Ethynylquinuclidin-2-yl)(6-methoxy-quinolin-4-yl)methyl)ferrocenecarboxamide 6/A

3.2. Synthesis of N-((S)-((1S,2S,4S,5S)-5-Ethynylquinuclidin-2-yl)(6-methoxyquinolin-4-yl)methyl)benzamide 6/B
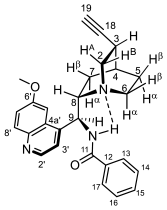
3.3. General Procedures for the Synthesis of Quinine–Chalcone Hybrids with 1,4-Disubstituted Triazole Linkers
3.3.1. Method C
3.3.2. Method D
3.3.3. Method E
3.4. Characterization of the Products
3.4.1. N-((6-Methoxyquinolin-4-yl)((2R,5S)-5-(1-(2-((E)-3-oxo-3-phenylprop-1-en-1-yl)phenyl)-1H-1,2,3-triazol-4-yl)quinuclidin-2-yl)methyl)ferrocenecarboxamide (7a/A)

3.4.2. N-(((2S)-5-(1-(2-((E)-3-(2-Methoxyphenyl)-3-oxoprop-1-en-1-yl)phenyl)-1H-1,2,3-triazol-4-yl)-quinuclidin-2-yl)(6-methoxyquinolin-4-yl)methyl)ferrocenecarboxamide (7b/A)

3.4.3. N-(((2S)-5-(1-(2-((E)-3-(4-Methoxyphenyl)-3-oxoprop-1-en-1-yl)phenyl)-1H-1,2,3-triazol-4-yl)-quinuclidin-2-yl)(6-methoxyquinolin-4-yl)methyl)ferrocenecarboxamide (7c/A)
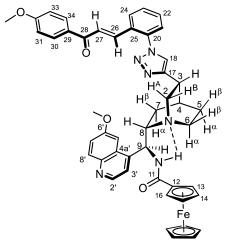
3.4.4. N-(((2S)-5-(1-(2-((E)-3-(4-Hydroxy-3,5-dimethylphenyl)-3-oxoprop-1-en-1-yl)phenyl)-1H-1,2,3-triazol-4-yl)quinuclidin-2-yl)(6-methoxyquinolin-4-yl)methyl)ferrocenecarboxamide (7e/A)
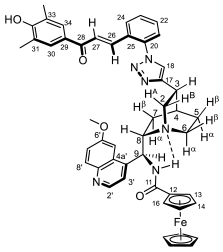
3.4.5. N-((6-Methoxyquinolin-4-yl)((2R,5S)-5-(1-(2-((E)-3-oxo-3-ferrocenylprop-1-en-1-yl)phenyl)-1H-1,2,3-triazol-4-yl)quinuclidin-2-yl)methyl)ferrocenecarboxamide (7f/A)

3.4.6. N-(((2S)-5-(1-(4-((E)-3-(2-Methoxyphenyl)-3-oxoprop-1-en-1-yl)phenyl)-1H-1,2,3-triazol-4-yl)quinuc-lidin-2-yl)(6-methoxyquinolin-4-yl)methyl)ferrocenecarboxamide (9b/A)

3.4.7. N-(((2S)-5-(1-(4-((E)-3-(4-Methoxyphenyl)-3-oxoprop-1-en-1-yl)phenyl)-1H-1,2,3-triazol-4-yl)quinuc-lidin-2-yl)(6-methoxyquinolin-4-yl)methyl)ferrocenecarboxamide (9c/A)
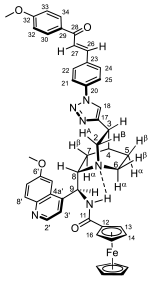
3.4.8. N-(((2S)-5-(1-(4-((E)-3-(3.4,5-Trimethoxyphenyl)-3-oxoprop-1-en-1-yl)phenyl)-1H-1,2,3-triazol-4-yl)-quinuclidin-2-yl)(6-methoxyquinolin-4-yl)methyl)ferrocenecarboxamide (9d/A)

3.4.9. N-(((2S)-5-(1-(2-((E)-3-(4-Hydroxy-3,5-dimethylphenyl)-3-oxoprop-1-en-1-yl)phenyl)-1H-1,2,3-triazol-4-yl)quinuclidin-2-yl)(6-methoxyquinolin-4-yl)methyl)benzamide (7e/B)

3.4.10. N-(((2S)-5-(1-(4-((E)-3-(3.4,5-Trimethoxyphenyl)-3-oxoprop-1-en-1-yl)phenyl)-1H-1,2,3-triazol-4-yl)-quinuclidin-2-yl)(6-methoxyquinolin-4-yl)methyl)benzamide (9d/B)

3.4.11. N-(((2R,5S)-5-(1-(4-Formylphenyl)-1H-1,2,3-triazol-4-yl)quinuclidin-2-yl)(6-methoxyquinolin-4-yl)-methyl)ferrocenecarboxamide (8/A)
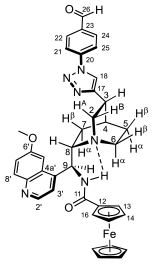
3.4.12. N-(((2R,5S)-5-(1-(4-Formylphenyl)-1H-1,2,3-triazol-4-yl)quinuclidin-2-yl)(6-methoxyquinolin-4-yl)-methyl)benzamide (8/B)
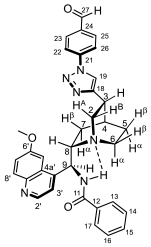
3.5. Determination of Antiproliferative Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Hu, J.; Lu, C.; Liu, Y. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020, 11, 797. [Google Scholar] [CrossRef]
- Garcia-Mayea, Y.; Mir, C.; Masson, F.; Paciucci, R.; LLeonart, M.E. Insights into new mechanisms and models of cancer stem cell multidrug resistance. Semin. Cancer Biol. 2020, 60, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Kucuksayan, E.; Ozben, T. Hybrid Compounds as Multitarget Directed Anticancer Agents. Curr. Top. Med. Chem. 2017, 17, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Fortin, S.; Bérubé, G. Advances in the development of hybrid anticancer drugs. Expert Opin. Drug Disc. 2013, 8, 1029–1047. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, Y.; Luo, Q.; Zhang, Y.; Wu, K.; Wang, F. Multi-Targeted Anticancer Agents. Curr. Top. Med. Chem. 2017, 17, 3084–3098. [Google Scholar] [CrossRef]
- Ferguson, P.J.; Phillips, J.R.; Seiner, M.; Cass, C.E. Differential Activity of Vincristine and Vinblastine against Cultured Cells. Cancer Res. 1984, 44, 3307–3312. [Google Scholar]
- Isah, T. Anticancer Alkaloids from Trees: Development into Drugs. Pharmacogn. Rev. 2016, 10, 90–99. [Google Scholar] [CrossRef]
- Noble, C.O.; Guo, Z.; Hayes, M.E.; Marks, J.D.; Park, J.W.; Benz, C.C.; Kirpotin, D.B.; Drummond, D.C. Characterization of Highly Stable Liposomal and Immunoliposomal Formulations of Vincristine and Vinblastine. Cancer Chemother. Pharmacol. 2009, 64, 741–751. [Google Scholar] [CrossRef]
- Binet, S.; Chaineau, E.; Fellous, A.; Lataste, H.; Krikorian, A.; Couzinier, J.P.; Meininger, V. Immunofluorescence study of the action of navelbine, vincristine and vinblastine on mitotic and axonal microtubules. Int. J. Cancer 1990, 46, 262–266. [Google Scholar] [CrossRef]
- Wattel, E.; Solary, E.; Hecquet, B.; Caillot; Ifrah; Brion; MahÉ; Milpied; Janvier; Guerci; et al. Quinine improves the results of intensive chemotherapy in myelodysplastic syndromes expressing P glycoprotein: Results of a randomized study. Br. J. Haematol. 1998, 102, 1015–1024. [Google Scholar] [CrossRef]
- Miller, T.P.; Chase, E.M.; Dorr, R.; Dalton, W.S.; Lam, K.S.; Salmon, S.E. A phase I/II trial of paclitaxel for non-Hodgkin’s lymphoma followed by paclitaxel plus quinine in drug-resistant disease. Anti-Cancer Drugs 1998, 9, 135–140. [Google Scholar] [CrossRef]
- Károlyi, B.I.; Bősze, S.; Orbán, E.; Sohár, P.; Drahos, L.; Gál, E.; Csámpai, A. Acylated mono-, bis- and tris-Cinchona-Based Amines Containing Ferrocene or Organic Residues: Synthesis, Structure and in Vitro Antitumor Activity on Selected Human Cancer Cell Lines. Molecules 2010, 17, 2316–2329. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, R.; Deepthi, S.B.; Giribabu, L.; Sridhar, B.; Sujitha, P.; Ganesh Kumar, C.; Ramakrishna, K.V. Synthesis, crystal structure, electronic spectroscopy, electrochemistry and biological studies of carbohydrate containing ferrocene amides. Appl. Organomet. Chem. 2012, 26, 369–376. [Google Scholar] [CrossRef]
- Skoupilova, H.; Bartosik, M.; Sommerova, L.; Pinkas, J.; Vaculovic, T.; Kanicky, V.; Karban, J.; Hrstka, R. Ferrocenes as new anticancer drug candidates: Determination of the mechanism of action. Eur. J. Pharm. 2020, 867, 172825. [Google Scholar] [CrossRef]
- Věžník, J.; Konhefr, M.; Fohlerová, Z.; Lacina, K. Redox-dependent cytotoxicity of ferrocene derivatives and ROS-activated prodrugs based on ferrocenyliminoboronates. J. Inorg. Biochem. 2020, 224, 111561. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, J.; Das, R.; Kamble, S.; Chowdhury, M.G.; Kapoor, S.; Gupta, A.; Vyas, H.; Shard, A. Ferrocene-based modulators of cancer-associated tumor pyruvate kinase M2. J. Organomet. Chem. 2022, 968–969, 122338. [Google Scholar] [CrossRef]
- Yan, J.; Yue, K.; Fan, X.; Xu, X.; Wang, J.; Qin, M.; Zhang, Q.; Hou, X.; Li, X.; Wang, Y. Synthesis and bioactivity evaluation of ferrocene-based hydroxamic acids as selective histone deacetylase 6 inhibitors. Eur. J. Med. Chem. 2023, 246, 115004. [Google Scholar] [CrossRef]
- Wang, R.; Chen, H.; Weitao, W.; Zheng, M.; Zhang, T.; Zhang, Y. Ferrocene-containing hybrids as potential anticancer agents: Current developments, mechanisms of action and structure-activity relationships. Eur. J. Med. Chem. 2020, 190, 112109. [Google Scholar] [CrossRef]
- Resnier, P.; Galopin, N.; Yann Sibiril, Y.; Clavreul, A.; Cayon, J.; Briganti, A.; Legras, P.; Vessières, A.; Montier, T.; Jaouen, G.; et al. Efficient ferrocifen anticancer drug and Bcl-2 gene therapy using lipid nanocapsules on human melanoma xenograft in mouse. Pharmacol. Res. 2017, 126, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Ornelas, C. Application of ferrocene and its derivatives in cancer research. New J. Chem. 2011, 35, 1973–1985. [Google Scholar] [CrossRef]
- Braga, S.S.; Silva, A.M.S. A New Age for Iron: Antitumoral Ferrocenes. Organometallics 2013, 32, 5626–5639. [Google Scholar] [CrossRef]
- Top, S.; Vessières, A.; Leclercq, G.; Quivy, J.; Tang, J.; Vaissermann, J.; Huché, M.; Jaouen, G. Synthesis, biochemical properties and molecular modelling studies of organometallic specific estrogen receptor modulators (SERMs), the ferrocifens and hydroxyferrocifens: Evidence for an antiproliferative effect of hydroxyferrocifens on both hormone-dependent and hormone-independent breast cancer cell lines. Chemistry 2003, 9, 5223–5236. [Google Scholar] [CrossRef]
- Csókás, D.; Károlyi, B.I.; Bősze, S.; Szabó, I.; Báti, G.; Drahos, L.; Csámpai, A. 2,3-Dihydroimidazo[1,2-b]ferroceno[d]pyridazines and a 3,4-dihydro-2H-pyrimido[1,2-b]ferroceno-[d]pyridazine: Synthesis, structure and in vitro antiproliferation activity on selected human cancer cell lines. J. Organomet. Chem. 2013, 750, 41–48. [Google Scholar] [CrossRef]
- Jernei, T.; Bősze, S.; Szabó, R.; Hudecz, F.; Majrik, K.; Csámpai, A. N-ferrocenylpyridazinones and new organic analogues: Synthesis, cyclic voltammetry, DFT analysis and in vitro antiproliferative activity associated with ROS-generation. Tetrahedron 2017, 73, 6181–6192. [Google Scholar] [CrossRef]
- McCluskey, A.; Russell, C. Chalcones: Potential Anticancer Agents. In Translational Research in Cancer; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, J.; Chen, X.; Fu, X.; Sun, S.; Wu, Q. Chalcone Derivatives: Role in Anticancer Therapy. Biomolecules 2021, 11, 894. [Google Scholar] [CrossRef]
- Shukla, S.; Sood, A.K.; Goyal, K.; Singh, A.; Sharma, V.; Guliya, N.; Gulati, S.; Kumar, S. Chalcone Scaffolds as Anticancer Drugs: A Review on Molecular Insight in Action of Mechanisms and Anticancer Properties. Anti-Cancer Agents Med. Chem. 2021, 21, 1650–1670. [Google Scholar] [CrossRef]
- Gao, F.; Huang, G.; Xiao, J. Chalcone hybrids as potential anticancer agents: Current development, mechanism of action, and structure-activity relationship. Med. Res. Rev. 2020, 40, 2049–2084. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, T.; Lungu, C.N. Anticancer Activity of Natural and Synthetic Chalcones. Int. J. Mol. Sci. 2021, 22, 11306. [Google Scholar] [CrossRef]
- Jernei, T.; Duró, C.; Dembo, A.; Lajkó, E.; Takács, A.; Kőhidai, L.; Schlosser, G.; Csámpai, V. Synthesis, Structure and in Vitro Cytotoxic Activity of Novel Cinchona-Chalcone Hybrids with 1,4-Disubstituted- and 1,5-Disubstituted 1,2,3-Triazole Linkers. Molecules 2019, 24, 4077. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Johnson, T.E.; Lad, R.; Xing, C. Structure-activity relationship studies of chalcone leading to 3-hydroxy-4,3′,4′,5′-tetramethoxychalcone and its analogues as potent nuclear factor kappaB inhibitors and their anticancer activities. J. Med. Chem. 2009, 52, 7228–7235. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Tan, S.; Zhou, Y.; Lin, J.; Wang, H.; Oyang, L.; Tian, Y.; Liu, L.; Su, M.; Wang, H.; et al. Role of the NFκB-signaling pathway in cancer. OncoTargets Ther. 2018, 11, 2063–2073. [Google Scholar] [CrossRef]
- Riaz, S.; Iqbal, M.; Ullah, R.; Zahra, R.; Chotana, G.A.; Faisal, A.; Saleem, R.S.Z. Synthesis and evaluation of novel α-substituted chalcones with potent anti-cancer activities and ability to overcome multidrug resistance. Bioorg. Chem. 2019, 87, 123–135. [Google Scholar] [CrossRef]
- Xiao, J.; Gao, M.; Diao, Q.; Gao, F. Chalcone Derivatives and their Activities against Drug-resistant Cancers: An Overview. Curr. Top. Med. Chem. 2021, 21, 348–362. [Google Scholar] [CrossRef]
- De Souza, P.S.; Bibá, G.C.C.; Melo, E.D.D.N.; Muzitano, M.F. Chalcones against the hallmarks of cancer: A mini-review. Nat. Prod. Res. 2022, 36, 4809–4826. [Google Scholar] [CrossRef] [PubMed]
- Bhukal, A.; Kumar, V.; Kumar, L.; Lal, K. Recent advances in chalcone-triazole hybrids as potential pharmacological agents. Results Chem. 2023, 6, 101173. [Google Scholar] [CrossRef]
- Kocsis, L.; Szabó, I.; Bősze, S.; Jernei, T.; Hudecz, F.; Csámpai, A. Synthesis, structure and in vitro cytostatic activity of ferrocene—Cinchona hybrids. Bioorg. Med. Chem. Lett. 2015, 26, 946–949. [Google Scholar] [CrossRef]
- Podolski-Renić, A.; Bősze, S.; Dinić, J.; Kocsis, L.; Hudecz, F.; Csámpai, A.; Pešić, M. Ferrocene–cinchona hybrids with triazolyl-chalcone linkers act as pro-oxidants and sensitize human cancer cell lines to paclitaxel. Metallomics 2017, 9, 1132–1141. [Google Scholar] [CrossRef]
- Kacprzak, K.; Ruszkowski, P.; Valentini, L.; Huczyński, A.; Steverding, D. Cytotoxic and trypanocidal activities of cinchona alkaloid derivatives. Chem. Biol. Drug Des. 2018, 92, 1778–1787. [Google Scholar] [CrossRef]
- Imrie, C.; Cook, L.; Levendis, D.C. An investigation of the chemistry of ferrocenoyl derivatives. The synthesis and reactions of ferrocenoyl imidazolide and its derivatives. J. Organomet. Chem. 2001, 637–639, 266–275. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Chan, T.R.; Hilgraf, R.; Sharpless, K.B.; Fokin, V.V. Polytriazoles as Copper(I)-Stabilizing Ligands in Catalysis. Org. Lett. 2004, 6, 2853–2855. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Godbout, N.; Salahub, D.R.; Andzelm, J.; Wimmer, E. Optimization of Gaussian-type basis sets for local spin density functional calculations. Part I. Boron through neon, optimization technique and validation. Can. J. Chem. 1992, 70, 560–571. [Google Scholar] [CrossRef]
- Paier, J.; Marsman, M.; Kresse, G. Why does the B3LYP hybrid functional fail for metals? J. Chem. Phys. 2007, 127, 024103. [Google Scholar] [CrossRef] [PubMed]
- Meldal, M.; Tornøe, C.W. Cu-Catalyzed Azide−Alkyne Cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef] [PubMed]
- Rodionov, V.O.; Fokin, V.V.; Finn, M.G. Mechanism of the ligand-free CuI-catalyzed azide-alkyne cycloaddition reaction. Angew. Chem. Int. Ed. 2005, 44, 2210–2215. [Google Scholar] [CrossRef]
- Bock, V.D.; Hiemstra, H.; van Maarseveen, J.H. CuI-Catalyzed Alkyne–Azide “Click” Cycloadditions from a Mechanistic and Synthetic Perspective. Eur. J. Org. Chem. 2006, 2006, 51–68. [Google Scholar] [CrossRef]
- Worell, B.T.; Malik, J.A.; Fokin, V.V. Direct Evidence of a Dinuclear Copper Intermediate in Cu(I)-Catalyzed Azide-Alkyne Cycloadditions. Science 2013, 340, 457–460. [Google Scholar] [CrossRef]
- Neumann, S.; Biewend, M.; Rana, S.; Binder, W. The CuAAC: Principles, Homogeneous and Heterogeneous Catalysts, and Novel Developments and Applications. Macromol. Rapid Commun. 2019, 41, 1900359. [Google Scholar] [CrossRef] [PubMed]
- Hyejeong Lee, H.; Ju, C.-H. Microwave-assisted, tetrabutylammonium hydroxide catalysed 1,4-addition of water to α,β-unsaturated ketones and α,β-ynones in aqueous solution. RSC Adv. 2014, 4, 48331–48335. [Google Scholar] [CrossRef]
- Enders, D.; Nguyen, T.V. Secondary amine catalyzed retro-aldol reactions of enals and enones: One-pot conversion of enals to α-substituted derivatives. Tetrahedron Lett. 2012, 53, 2091–2095. [Google Scholar] [CrossRef]
- Sodhi, R.K.; Satya Paul, S.; Gupta, V.K.; Kant, R. Conversion of a,b-unsaturated ketones to 1,5-diones via tandem retro-Aldol and Michael addition using Co(acac)2 covalently anchored onto amine functionalized silica. Tetrahedron Lett. 2015, 56, 1944–1948. [Google Scholar] [CrossRef]
- Zhou, Y.; Rao, C.; Mai, S.; Song, Q. Substituent-Controlled Chemoselective Cleavage of C=C or Csp2− C(CO) Bond in α,β-Unsaturated Carbonyl Compounds with H-Phosphonates Leading to β-Ketophosphonates. J. Org. Chem. 2016, 81, 2027–2034. [Google Scholar] [CrossRef]
- Parr, R.G.; von Szentpaly, L.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Parr, R.G.; Donnelly, R.A.; Levy, M.; Palke, W.E. Electronegativity—The Density Functional Viewpoint. J. Chem. Phys. 1978, 68, 3801–3807. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute Hardness: Companion Parameter to Absolute Electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Dávid, C.Z.; Kúsz, N.; Pinke, G.; Kulmány, Á.; Zupkó, I.; Hohmann, J.; Vasas, A. Jacaranone derivatives with antiproliferative activity from Crepis pulchra and relevance of this group of plant metabolites. Plants 2022, 11, 782. [Google Scholar] [CrossRef]
- Schei, H.; Shen, Q.; Hilderbrandt, R.L. The structure of quinuclidine (1-azabicyclo[2.2.2.] octane) as determined by gas phase electron diffraction. J. Mol. Struct. 1980, 65, 297. [Google Scholar] [CrossRef]
- Wann, D.A.; Blockhuys, F.; Van Alsenoy, C.; Robertson, H.E.; Himmel, H.-J.; Tang, C.Y.; Cowley, A.R.; Downs, A.J.; Rankin, D.W.H. Molecular structures of free quinuclidine and its adducts with metal trihydrides, MH3 (M = B, Al or Ga), studied by gas-phase electron diffraction, X-ray diffraction and quantum chemical calculations. Dalton Trans. 2007, 17, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Malz, F. Chapter 2—Quantitative NMR in the Solution State NMR. NMR Spect. Pharm. Anal. 2008, 43–62. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09; Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]

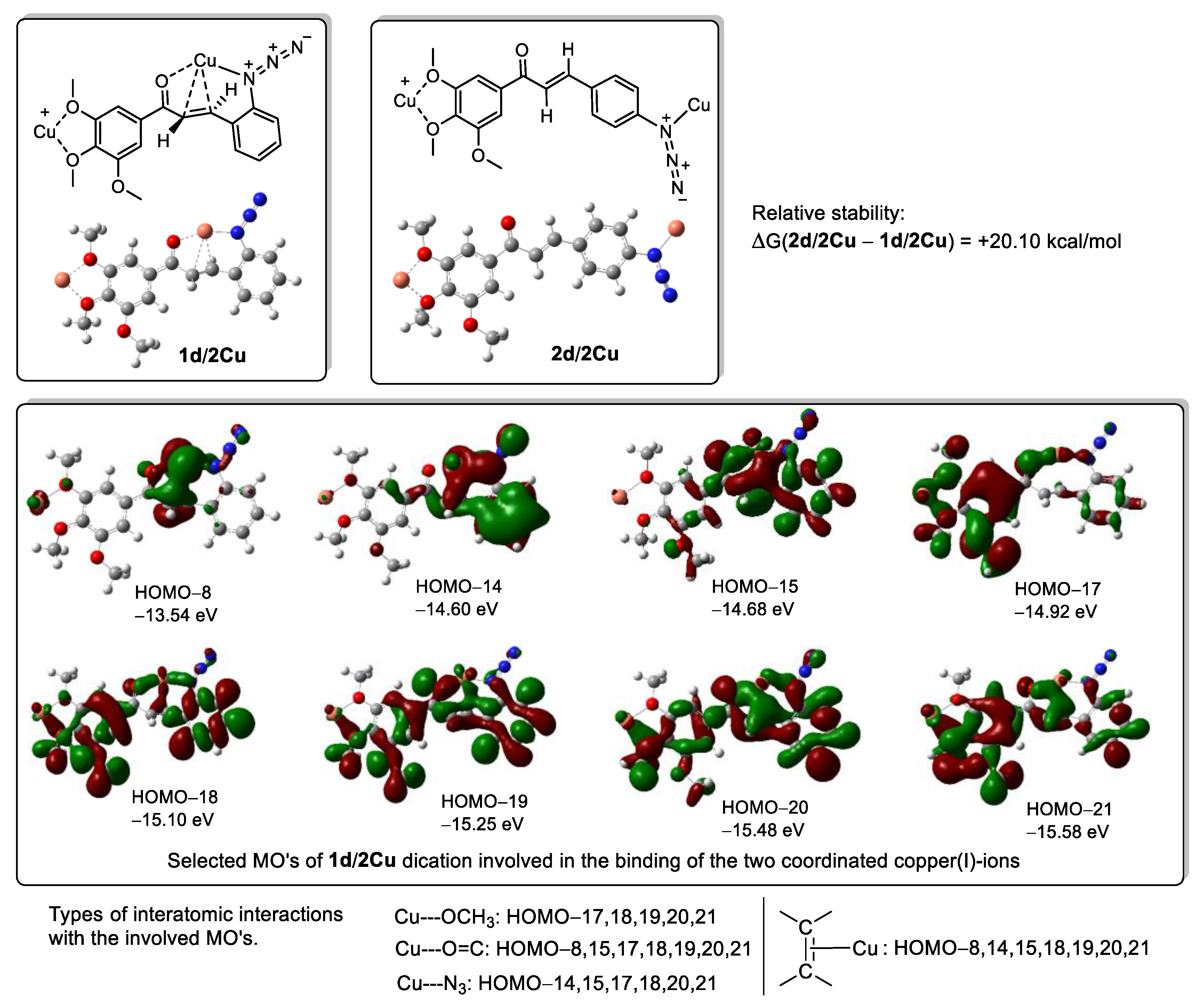
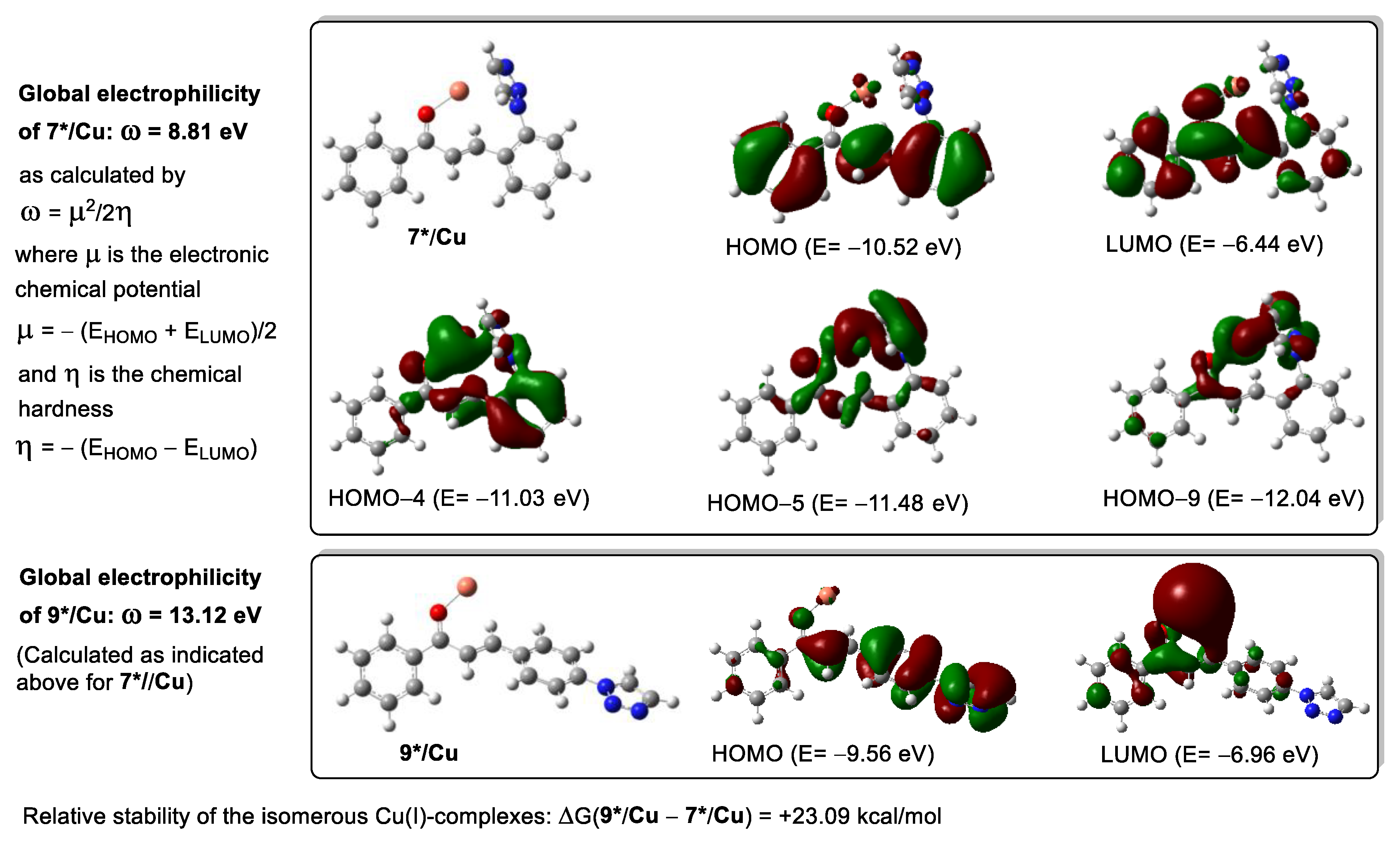
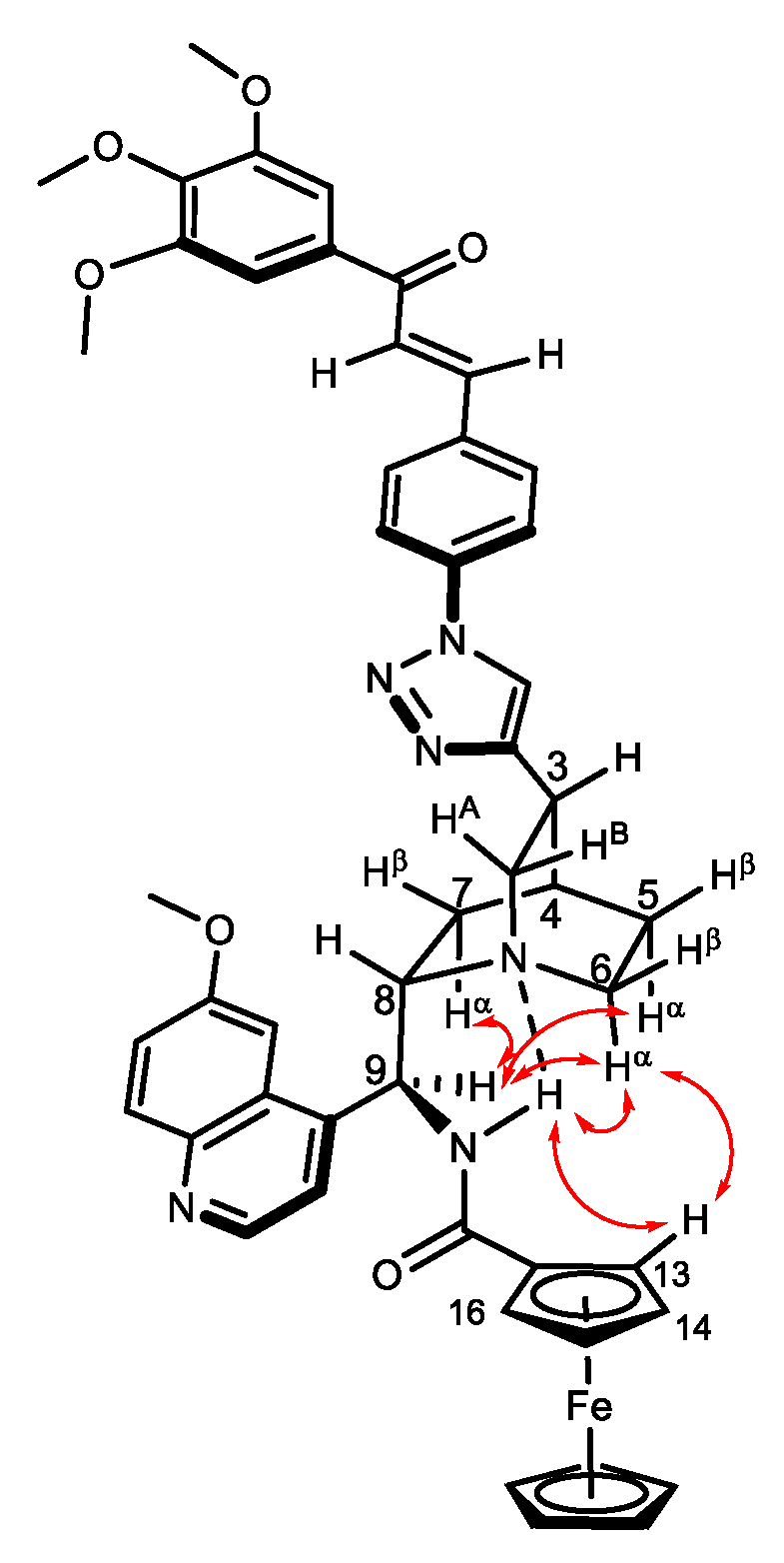
| Product | Method C | Method D | Method E | Method F |
|---|---|---|---|---|
| 7a/A | 43 | 52 | 37 | - |
| 7b/A | 56 | 52 | 45 | - |
| 7c/A | 30 | 52 | 56 | - |
| 7e/A | 31 | 36 | 33 | - |
| 7f/A | 21 | 30 | 36 | - |
| 8/A | 71 (from 6/A+2a) 28 (from 6/A+2b) 31 (from 6/A+2c) 34 (from 6/A+2d) 63 (from 6/A+2e) 57 (from 6/A+2f) | 68 (from 6/A+2a) 22 (from 6/A+2b) 19 (from 6/A+2c) 32 (from 6/A+2d) 52 (from 6/A+2e) 42 (from 6/A+2f) | 59 (from 6/A+2a) 20 (from 6/A+2b) 24 (from 6/A+2c) 20 (from 6/A+2d) 57 (from 6/A+2e) 45 (from 6/A+2f) | 80 (from 9b/A) 74 (from 9c/A) 88 (from 6d/A) - |
| 9b/A | 47 | 51 | 63 | - |
| 9c/A | 42 | 55 | 60 | - |
| 9d/A | 49 | 62 | 67 | - |
| 7e/B | - | - | 56 | - |
| 8b/B | - | - | 77 (from 6/B+2a) 23 (from 6/B+2d) 69 (from 6/B+2e) 47 (from 6/B+2f) | 84 |
| 9d/B | - | - | 64 | - |
| IC50 (µM) [95% Confidence Interval] | |||
|---|---|---|---|
| HeLa | MDA-MB-231 | A2780 | |
| 7a/A | 2.58 [2.26–2.94] | 1.55 [1.39–1.71] | 0.944 [0.866–1.03] |
| 7b/A | 2.17 [1.90–2.47] | 1.80 [1.58–2.05] | 1.19 [1.05–1.34] |
| 7c/A | 4.18 [3.69–4.75] | 2.07 [1.58–2.72] | 1.51 [1.34–1.71] |
| 7e/A | 4.87 [4.34–5.42] | 2.69 [2.46–2.95] | 2.56 [2.31–2.84] |
| 8/A | 18.51 [13.70–24.99] | 17.80 [12.50–25.37] | 11.28 [9.28–13.72] |
| 9b/A | 4.86 [4.20–5.61] | 3.44 [2.69–4.39] | 1.10 [0.949–1.27] |
| 9d/A | 2.97 [2.56–3.44] | 0.854 [0.752–0.950] | 0.438 [0.401–0.579] |
| 7e/B | 4.79 [4.18–5.49] | 2.22 [1.97–2.50] | 1.04 [0.945–1.14] |
| 8/B | 17.60 [15.03–20.61] | 16.14 [14.09–18.48] | 12.75 [11.09–14.65] |
| 9d/B | 2.12 [1.71–2.62] | 1.71 [1.53–1.91] | 0.776 [0.652–0.924] |
| Cisplatin * | 14.02 [12.65–15.56] | 18.65 [16.67–20.85] | 5.27 [4.37–6.35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dembo, A.; Ferenczi, E.; Jernei, T.; Bor, A.; Schelz, Z.; Zupkó, I.; Varga, S.; Csámpai, A. CuAAC-Based Synthesis, Copper-Catalyzed Aldehyde-Forming Hydrolytic Fission and Antiproliferative Evaluation of Novel Ferrocenoylamino-Substituted Triazole-Tethered Quinine–Chalcone Hybrids. Molecules 2024, 29, 375. https://doi.org/10.3390/molecules29020375
Dembo A, Ferenczi E, Jernei T, Bor A, Schelz Z, Zupkó I, Varga S, Csámpai A. CuAAC-Based Synthesis, Copper-Catalyzed Aldehyde-Forming Hydrolytic Fission and Antiproliferative Evaluation of Novel Ferrocenoylamino-Substituted Triazole-Tethered Quinine–Chalcone Hybrids. Molecules. 2024; 29(2):375. https://doi.org/10.3390/molecules29020375
Chicago/Turabian StyleDembo, António, Etelka Ferenczi, Tamás Jernei, Andrea Bor, Zsuzsanna Schelz, István Zupkó, Szilárd Varga, and Antal Csámpai. 2024. "CuAAC-Based Synthesis, Copper-Catalyzed Aldehyde-Forming Hydrolytic Fission and Antiproliferative Evaluation of Novel Ferrocenoylamino-Substituted Triazole-Tethered Quinine–Chalcone Hybrids" Molecules 29, no. 2: 375. https://doi.org/10.3390/molecules29020375
APA StyleDembo, A., Ferenczi, E., Jernei, T., Bor, A., Schelz, Z., Zupkó, I., Varga, S., & Csámpai, A. (2024). CuAAC-Based Synthesis, Copper-Catalyzed Aldehyde-Forming Hydrolytic Fission and Antiproliferative Evaluation of Novel Ferrocenoylamino-Substituted Triazole-Tethered Quinine–Chalcone Hybrids. Molecules, 29(2), 375. https://doi.org/10.3390/molecules29020375








