Abstract
There are scientific studies indicating that the attachment of an indole moiety to the triterpene scaffold can lead to increased anticancer potential. Lipophilicity is one of the factors that may influence biological properties and is therefore an important parameter to determine for newly obtained compounds as drug candidates. In the present study, previously synthesized 3 and/or 28-indole-betulin derivatives were evaluated for lipophilicity by reversed-phase thin-layer chromatography. The experimental values of lipophilicity (logPTLC) were then subjected to correlation analysis with theoretical values of logP, as well as for selected physicochemical and pharmacokinetic parameters and anticancer activity. A toxicity test using zebrafish embryos and larvae was also conducted. High correlation was observed between the experimental and theoretical values of lipophilicity. We presented correlation equations and statistical parameters describing the relationships between logPTLC and several physicochemical and ADME parameters. We also revealed the lack of correlation between the experimental values of lipophilicity and anticancer activity. Moreover, experiments on zebrafish have confirmed no toxicity of the tested compounds, which was consistent with the results of the in silico toxicity analysis. The results demonstrated, using the example of indole derivatives of betulin, the utility of lipophilicity values in the context of predicting the biological activity of new compounds.
1. Introduction
Natural and synthetic heterocyclic compounds constitute a promising group of substances with anticancer activity. The presence of heteroatoms (nitrogen, sulfur and oxygen) in the structure means that heterocyclic compounds can act as acceptors and donors of hydrogen bonds. Thanks to this, these compounds have the ability to bind to many receptors through intermolecular hydrogen bonds, which leads to an appropriate pharmacological effect. The current trend in the search for new, more effective and selective chemotherapeutics is the combination of pharmacophore systems with different mechanisms of action. In the synthesis of such conjugates, nitrogen heterocyclic systems such as pyrazole, piperazine, quinolines, triazole and indole are often used. It has been shown that molecules containing the indole moiety can exert anticancer effects through various molecular targets, such as kinases, tubulin, aromatase or DNA topoisomerase [1,2,3,4,5,6,7,8,9].
The indole derivatives of triterpenoids have shown significant anticancer activity against hepatocarcinoma (SMMC-7721, HepG2), melanoma (A5375), leukemia (U937, K562, Jurkat, SR), lung cancer (NCI-H460) and glioma (U251, C6) cell lines. Attachment of the indole moiety to the triterpene scaffold may lead to increased anticancer activity in relation to individual components and reduced drug resistance [9,10,11,12,13,14].
The search for new drugs with the desired biological properties is the subject of the work of many teams of scientists. They pay special attention to lipophilicity, as it is a property that plays a significant role when designing new drugs [15]. Lipophilicity, generally speaking, determines the ability of a compound to dissolve in oil, fats, lipids and other nonpolar solvents. Lipophilicity is expressed as a logP value. Studies on this property are very often based on calculations of the partition coefficient between n-octanol and water [16]. Computer methods are also used for this purpose, sometimes created by leading pharmaceutical companies. Lipophilicity is closely related to ADME properties: solubility, permeability, clearance, metabolism or bioavailability, and distribution. Lipophilicity is also one of the factors that may influence biological properties [17], particularly the solubility, reactivity or even degradation of drugs. It also determines transport through biological membranes. In the laboratory, lipophilicity can be determined classically using the shake-flask method, as well as solid-phase microextraction (SPME) or potentiometric titration. One of the indirect methods using chromatography, both RP-TLC (reversed-phase thin-layer chromatography) and HPLC (high-performance liquid chromatography), has many practical applications. LogP values are often predicted on the basis of various computational models [18]. These calculations can be based on the structure of compounds, using a whole range of topological indices, E-state descriptors or even neural networks.
The zebrafish is an important biomedical model that provides an opportunity to understand the pathogenesis of many human diseases, such as osteoporosis, inflammation, autism spectrum disorders, obesity, Type 2 diabetes, heart failure and cancers. The advantages of this model include the ability to obtain a large number of embryos in a single clutch and the optical transparency of the developing embryo, which illustrates the course of changes induced by various factors in a living organism. Zebrafish embryos are often used in toxicity studies because this species is as sensitive to teratogenic compounds as mammals. The zebrafish model also allows for the rapid assessment of in vivo toxicity mechanisms induced by newly discovered chemical compounds considered as drug candidates [19,20,21,22,23].
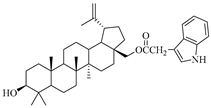
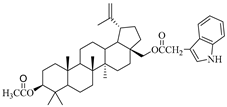
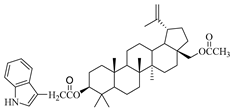
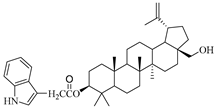
In this study, previously synthesized indole derivatives of botulin, shown in Table 1, were assessed for lipophilicity by the reversed-phase thin-layer chromatography method (RP-TLC).

Table 1.
The chemical structures of indole-functionalized derivatives of betulin.
Additionally, the experimental values of lipophilicity (logPTLC) were subjected to correlation analysis for theoretical values of logP, selected physicochemical and ADME parameters and anticancer activity. Indole derivatives that were the most active against MCF-7 cells were subjected to a toxicity test using zebrafish (Danio rerio) embryos and larvae.
2. Results and Discussion
2.1. Lipophilicity—Experimental and Theoretical Studies
Lipophilicity is a physicochemical property of a compound describing its behavior in a two-phase system consisting of a nonpolar organic phase and a polar phase, most often aqueous. This parameter is one of the key features of compounds required to assess the processes of absorption, distribution and transport in biological systems, next to solubility, stability and acid–base characteristics. Moreover, lipophilicity is a factor determining the affinity of the compound to the target proteins, which is responsible for the final effect of biological action [24,25,26].
The studies we conducted involved the use of reversed-phase thin-layer chromatography (RP-TLC) to determine the experimental logPTLC values of previously synthesized 3 and/or 28-indole-betulin derivatives (Table 1) [27,28].
The initial stage of the studies aimed at obtaining the logPTLC values of the tested compounds concerned the determination of the standard curve. For this purpose, the lipophilicity values were determined experimentally (RM0), and values from the literature (logPlit) [29,30] were used for the following standard substances: acetanilide, prednisone, 4-bromoacetophenone, benzophenone, anthracene, dibenzyl and DDT (dichlorodiphenyltrichloroethane). Determination of the RM0 values for reference compounds was performed under the same chromatographic conditions as for the indole derivatives EB355A, EB365, EB366 and EB367. The standard curve of the relationship between logPlit and RM0 is described by the following equation:
LogPTLC = 1.1332 RM0 + 0.6683 (r = 0.992)
Table 2 presents the literature-based (logPlit) and experimental values of lipophilicity (RM0 and logPTLC) for the reference substances.

Table 2.
The experimental (RM0 and logPTLC) and literature-based (logPlit) lipophilicity values for reference compounds (mobile phase: acetone–Tris buffer).
Using the equations RM = RM0 + bC and logPTLC = 1.1332RM0 + 0.6683 and the RM values determined during the experiment for indole derivatives of betulin, logPTLC values were calculated. These values are presented in Table 3.

Table 3.
The experimental values of lipophilicity for the tested derivatives in the mobile phase (acetone–Tris buffer).
The experimentally determined logPTLC values for the indole derivatives of betulin ranged from 7.52 to 8.28. The highest lipophilicity among the compounds tested was characterized by the diester derivative EB365, which had an acetyl substituent at the C-3 position, and the lowest was characterized by the monoester derivative EB355A containing a free hydroxyl group at the C-3 carbon atom. Both of these derivatives have an indolyl moiety at the C-28 position. The indole derivatives EB355A and EB367, containing a free hydroxyl group at positions C-3 and C-28, respectively, are characterized by reduced lipophilicity compared with the disubstituted derivatives EB365 and EB366. In the conducted study, betulin was used as a reference compound (logPTLC = 6.11).
The results obtained by us confirmed previous studies on the lipophilicity parameters of betulin’s mono- and diester derivatives. The lower values of the lipophilicity parameter of betulin’s monoester derivatives resulted from the presence of a hydrophilic hydroxyl group attached to the hydrophobic triterpene scaffold [31,32].
Except for the lipophilicity values obtained from the thin-layer chromatography experiment, the lipophilicity values obtained using computer programs or Internet databases (iLOGP, XLOPG2, XLOPG3, WLOGP, MLOGP, SILICOS-IT, milogP, ACD/logP, KOWWIN and ALOGPs) were considered as well. These values of the theoretical lipophilicity for the analyzed triterpenoids are presented in the Table 4.

Table 4.
The theoretical values of lipophilicity for betulin and its indole derivatives.
The theoretical values of the logP parameter of the indole derivatives of betulin ranged from 4.89 to 12.52. The graph below (Figure 1) shows a comparison of the experimentally determined values of lipophilicity with the theoretical values obtained using various commercially available computer programs.
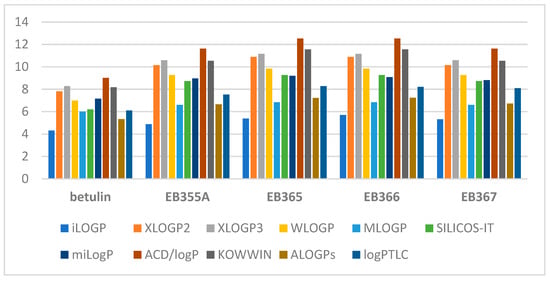
Figure 1.
A comparison of the lipophilicity values obtained for the tested triterpenoids.
By analyzing the above graph, it can be concluded that the lowest lipophilicity values are characteristic of betulin (logP = 4.31–8.28). The modification of the betulin molecule caused all its derivatives to have higher lipophilicity values. iLOGP predicted the lowest values for all compounds, and ACD/logP had the highest values. The experimental logPTLC value of betulin’s indole derivatives was closest to the theoretical value obtained using ALOGP.
2.2. Correlation Analysis
Table 5 presents the results of the correlation analysis between all values considered in the studies. These include the lipophilicity value determined experimentally (logPTLC) and 10 lipophilicity values determined theoretically using generally available online calculators. The correlations between logPTLC and other values were specific for the analysis. High correlation values (all above 0.9) allowed us to set the correlation equations to calculate logPTLC based only on the theoretical value.

Table 5.
Values of the coefficients for the correlation analysis between the experimental and theoretical values of lipophilicity for compounds investigated (mobile phase: acetone–Tris buffer).
A correlation analysis was also performed between the logPTLC parameters and the values of the physicochemical (M, molar weight; TPSA, topological polar surface; nROTB, rotational bonds; nHBD, hydrogen bond donors; nHBA, hydrogen bond acceptors; MR, molar refractivity) and ADME properties (logPapp, Caco-2 permeability; logKp, skin permeability) (Table 6).

Table 6.
Selected physicochemical and ADME properties of the tested triterpenoids.
And in this case (Table 6), all correlation coefficients, except nHBD, were also high. In the case of this parameter, it was impossible to find a correlation equation that would describe the linear relationship between logPTLC and nHBD. In this case, the correlation coefficient was 0.608 and the significance level was p = 0.28. As can be seen in Table 6, the number of donors for betulin and the EB355A and EB367 derivatives was the same, while the compounds differed in lipophilicity (logPTLC value). This situation resulted from introducing a lipophilic indole system and replacing one of the donors (OH in Position C-3 or C-28) with an NH group of similar acidity. In the case of excluding betulin from the calculations, a higher correlation coefficient was obtained from the dependence of logPTLC on nHBD. Another parameter of hydrogen bonds assumed in the calculations was the number of hydrogen bond acceptors (nHBA), which were different for betulin and its indole derivatives, for which a high correlation was obtained. Table 7 presents all statistically significant correlation equations and the parameters describing them. Due to the high values of the correlation coefficients, all equations presented here could be of use for determining the lipophilicity of the tested compounds without the need to conduct an experiment.

Table 7.
Correlation equations and statistical parameters describing the relationships between logPTLC and other data obtained.
2.3. Cluster Analysis (CA)
Cluster analysis for the results obtained, including the experimental lipophilicity (RM0 and logPTLC) and those calculated using computer programs, was performed. The analysis is presented in Figure 2. Figure 2 shows three visible clusters that grouped the results obtained. The first one included RM0, MLOGP and ALOGPs; the second included logPTLC, WLOGP, SILICOS-IT and milogP; and the third one consisted of XLOGP2, XLOGP3 and KOWWIN. The grouping arose from the similarity in the individual values of lipophilicity. The values from WLOGP, SILICOS-IT and milogP were the most similar to the experimental logPTLC value. ACD/logP and iLOGP were the ones that deviated the most from the rest, which was obviously due to their values. The ACD/logP values were the highest, and iLOGP was the lowest among the others.
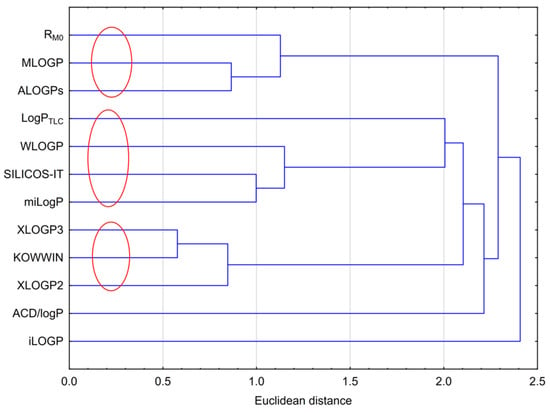
Figure 2.
Analysis of similarities between the experimental and theoretical logP values.
To compare the compounds analyzed, another similarity analysis was performed. This analysis showed (Figure 3) that the compounds formed two pairs, i.e., EB355A and EB367, and EB365 and EB366, which resulted directly from their structure. The first two had a free OH group in the C-3 and C-28 positions, and the remaining two had an acetyl group in analogous positions. Betulin, because its structure differed the most from other newly synthesized compounds, did not belong to any group.
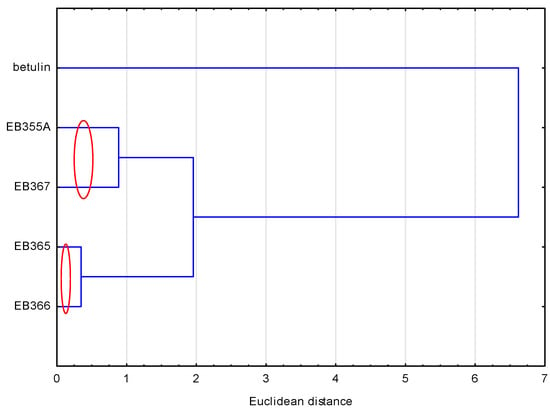
Figure 3.
Analysis of similarities of the analyzed compounds, based on their lipophilicity values.
Similar conclusions as in the case of lipophilicity were obtained by analyzing all the values of the physicochemical properties and ADME values (Figure 4). The compounds were grouped in the same way as in the case of the analysis based on the lipophilicity values. Because the values of the analyzed data were calculated according to the SMILE code, which resulted directly from the structure of the compound, compounds with similar structures formed one cluster.
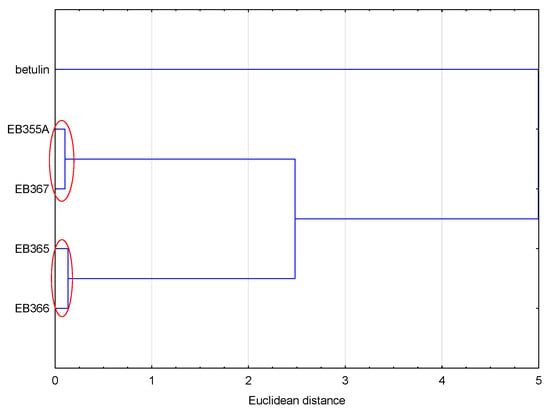
Figure 4.
Analysis of similarities of the analyzed compounds, based on the values of their physicochemical and ADME properties.
2.4. Principal Component Analysis (PCA)
PCA analysis was performed for the logP values (experimental and theoretically calculated), physicochemical properties and ADME properties. The analyzed data were standardized. Four eigenvalues, which described 100% of the variability of the system, were obtained. To select the number of eigenvalues, a scree plot was used (Figure 5). The first eigenvalue had a very high percentage of the total variance (94.21%). The remaining eigenvalues complemented it. When we analyzed the contributions of individual data values to the main components, it was visible that the first component contained the highest shares of almost all data. Only the nHBD value made the highest contribution to the second eigenvalue. A graph of the cases projected onto the factor plane was prepared using the obtained eigenvalues (Figure 6). The analyzed compounds were grouped in pairs, i.e., EB355A and EB367, and EB365 and EB366. The same pairs of compounds formed clusters when analyzing the lipophilicity values using CA. The compounds that formed pairs on the plot had very similar structures, and betulin, which differed from the others, was located in a completely different part of the chart. The relationships discussed here are presented in the figures below.
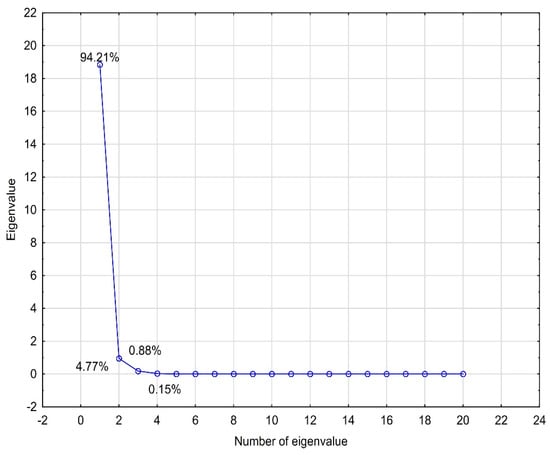
Figure 5.
Scree plot obtained for the lipophilicity, ADME and physicochemical values of the compounds investigated.

Figure 6.
Projection of cases on the plane of factors for the PCA of the logP, physicochemical and ADME values of the analyzed compounds.
2.5. Correlation with IC50 Values
The cytotoxic activity of indole-functionalized betulin derivatives was tested in vitro against amelanotic melanoma (A375, C32), triple-negative breast cancer (MDA-MB-231), estrogen receptor-positive breast cancer (MCF-7), lung cancer (A549) and colon adenocarcinoma (DLD-1, HT-29) cells [27,28].
A correlation analysis of all the values of lipophilicity, ADME, physicochemical properties and IC50 values, describing the cytotoxic activity of indole derivatives of betulin, was also performed. Due to the fact that only three of the tested compounds (EB355A, EB366 and EB367) had cytotoxic activity, such a correlation was performed only for them. Another limitation was the cell lines against which the abovementioned compounds showed cytotoxicity; therefore, the correlation applied only to the MCF-7 cell line (estrogen-receptor-positive breast cancer). The IC50 values of the EB355A, EB366 and EB367 derivatives against MCF-7 cells were 67, 156 and 35 µM, respectively. The correlation analysis between the IC50 values for MCF-7 and the other analyzed values for the compounds EB355A, EB366 and EB367 is presented in Table 8.

Table 8.
Correlation analysis between the IC50 values for MCF-7 and the other values analyzed for the compounds EB355A, EB366 and EB367.
As Table 8 shows, the correlation coefficients had low values. The highest value was for the relationship between the IC50 for MCF-7 and milogP. Most of the analyzed data were determined on the basis of the structure. Hence, we could conclude that the structure of the compound is not so significant for the IC50 value. However, the lack of a correlation with the experimental values of lipophilicity (RM0 and logPTLC) indicated no dependence between these two properties, i.e., logP does not depend on IC50 nor does IC50 depend on logP.
2.6. In Silico Analysis of Toxicity 3- and 28-Indolobetulin Derivatives
The development of new drugs is a costly and lengthy process, often burdened with failure due to the occurrence of toxicity of the bioactive substance. The toxic effects are most often observed at late stages of the development of the drug. Therefore, there is a need for a rapid assessment of the toxicity of a chemical compound in the early stages of research. For this purpose, various in silico predictions are used, which allow us, among other matters, to determine toxicity at the organ level (hepatotoxicity, nephrotoxicity, neurotoxicity, cardiotoxicity and skin toxicity) [33,34]. Predictions of toxicity for indole derivatives of betulin were performed using the web tool admetSAR 3.0 [35,36] (Table 9).

Table 9.
Selected toxicity parameters of the indole derivatives of betulin performed using the admetSAR 3.0 web tool.
On the basis of the data obtained, a compound is classified as nontoxic when the label is 0 or as toxic when the label is 1. All compounds were characterized by the absence of hepatotoxic, nephrotoxic, neurotoxic and skin-sensitizing effects. In silico prediction showed that only the 28-indolosubstituted derivative EB355A may exhibit cardiotoxic activity.
2.7. The Effects of EB355A or EB367A on the Development of Zebrafish Embryos/Larvae
2.7.1. Embryos of 0–2 hpf
Zebrafish embryos and larvae are good models for assessing the embryotoxic and teratogenic toxicity of biologically active substances. This model allows us to determine whether the tested compound is a teratogen, i.e., a substance that causes the development of a congenital anomaly or increases the occurrence of a specific hereditary deficiency. Analysis of the teratogenic effects of tested compounds is easy to perform due to the transparency of zebrafish embryos, including tail and notochord malformations, pericardial and yolk edema, scoliosis and growth retardation [37].
The effects of the compounds EB355A or EB367 on survival and early embryonic development were studied at 4, 8, 12, 24, 48, 72 and 96 h after exposure. No significant differences were noted between untreated embryos and embryos incubated with tested compounds. Treatment of the embryos with EB355A or EB367 did not affect the heart rate of the striped danio, reflected as the HR (heart rate), compared with the control embryos (Figure 7).
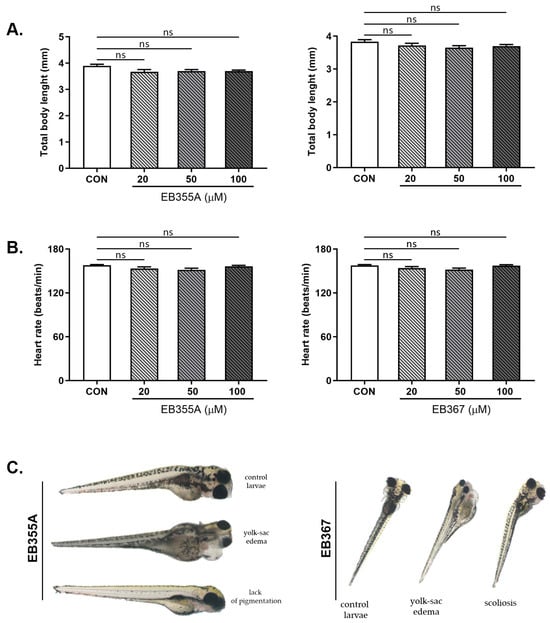
Figure 7.
Total body length (A), heart rate (B) and sublethal alternations (C) of zebrafish embryos at 0–2 hpf exposed to EB355A, EB367 or the control (CON) for 96 h. Data are shown as the mean ± SD; n = 60 for each concentration. Abbreviations: hpf, hours post-fertilization; ns, not significant.
2.7.2. Larvae of 72 hpf
A similar effect to the embryo study was obtained when both EB355A and EB367 were tested in 72 hpf larvae. Both compounds, at all concentrations used, did not cause changes in the tested larvae’s parameters with respect to the control larvae. No statistically significant growth defects or developmental abnormalities were observed under exposure to the tested compounds during the assessment to the endpoint (Figure 8).
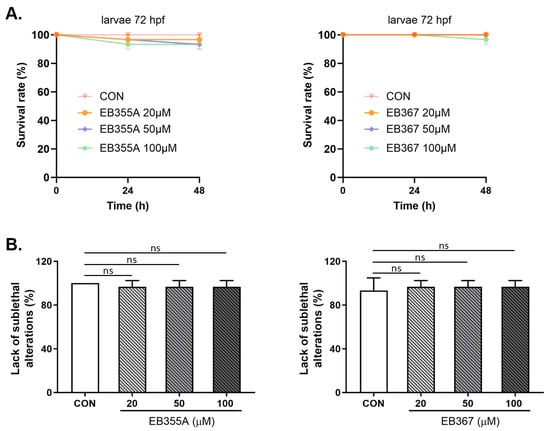
Figure 8.
Survival rate (A) and lack of sublethal alternations (B) for larvae at 72 hpf exposed to EB355A, EB367A, or the control (CON) for 24 h and 48 h. Data are shown as the mean ± SEM; n = 60 for each concentration. Abbreviations: hpf, hours post-fertilization; ns, not significant.
3. Materials and Methods
3.1. Chemicals
The reagents and solvents applied in the synthesis of the tested compounds (EB355A, EB365, EB366 and EB367) and the chromatography materials were purchased from Merck (Darmstadt, Germany). Indole derivatives were obtained under the conditions of the Steglich method using betulin, 3-acetylbetulin, 28-acetylbetulin and 28-tetrahydropyranyl ether of betulin as substrates, which were reacted with 3-indoleacetic acid in the presence of DCC (N,N′-dicyclohexylcarbodiimide) and DMAP (4-dimethylaminopyridine). The 3- and 28-indolobetulin derivatives were obtained with yields ranging from 48% to 58%. The synthesis and chemical structure of the studied indole betulin derivatives have been described previously in the literature [27,28].
3.2. Thin-Layer Chromatography
An experiment for determining the lipophilicity with use of RP-TLC was carried out on plates precoated with silica gel RP-18F254 (#1.05559, Merck, Darmstadt, Germany). The horizontal chamber in which the plates were developed was previously saturated with vapor of the mobile phase. The mobile phase was mixture of acetone (Merck, Darmstadt, Germany) and a 0.2 M Tris buffer (Merck, Darmstadt, Germany) characterized by pH = 7.4. The volume ratio was 90:10, 85:15, 80:20, 75:25, 70:30, 65:35 and 60:40 for the reference compounds, and 90:10, 85:15, 80:20, 75:25, 70:30 and 65:35 for the betulin derivatives. The average values of the retardation factor (RF) were calculated and converted to RM values according to the equation:
The RM values were linearly dependent on the acetone content in the mobile phase. The RM0 was also calculated according to the equation
by extrapolating the concentration of acetone to zero. In the Equation (3), C is the concentration of acetone (% v/v) in the mobile phase whilst b is the regression term.
RM = RM0 + bC
3.3. In Silico Studies
Many values of theoretical lipophilicity were used for the analysis. These were as follows.
- -
- iLOGP, XLOGP3, WLOGP, MLOGP and SILICOS-IT were obtained on the basis of the SMILE code for compounds investigated using the web tool operated by the Molecular Modelling Group of the University of Lausanne and the SIB Swiss Institute of Bioinformatics [38,39];
- -
- XLOPG2, ALOGPs data were obtained with the use of the Virtual Computational Chemistry Laboratory [40,41];
- -
- MilogP data were obtained by using Molinspiration Free Services, based on the structural formula [42];
- -
- KOWWIN data were obtained by using the freely available computer program EPIWEB 4.1;
- -
- ACD/logP data were based on the structural formula using the program ChemSketch ACD/Chem Sketch(Freeware) 2021.2.1.
Other data (i.e., selected physicochemical and ADME properties of betulin and its derivatives) were obtained in the same way as some values of lipophilicity with the use of web tools [38,39]. They were as follows: molar weight (M), topological surface area (TPSA), the number of rotatable bonds (nROTB), the number of the hydrogen bond donors (nHBD), the number of hydrogen bond acceptors (nHBA), molar refractivity (MR), Caco-2 permeability (logPapp) and skin permeability (logKp).
3.4. Chemometric Data Analysis
In order to analyze and evaluate the results obtained for the lipophilicity values, the physicochemical and ADME properties of the tested compounds, a correlation analysis, a similarity analysis (CA) and a principal component analysis (PCA) were performed. On the basis of the correlation analysis, correlation equations and their statistical parameters were determined. Similarity analysis allowed us to group the received data in terms of their similarity. During the analysis, the calculations were based on Euclidean distances and single linkage distances [43]. Principal component analysis, on the other hand, allowed the reduction in the number of variables to the smallest possible number necessary to describe the system [44]. The number of main components was determined on the basis of scree plots. All data used for the PCA were standardized. The analyses were performed using Statistica 13.3 software.
3.5. Zebrafish Husbandry
The zebrafish embryos were maintained at a temperature of 28.0 ± 1.0 °C and a light/dark cycle, which was in accordance with the guidelines of the Department of Research Animals of the esteemed RSPCA (Royal Society for the Prevention of Cruelty to Animals). According to EU Directive 2010/63/EU, the earliest life stages of the striped danios (embryo and eleuteropod embryo cultures) are considered to be equivalent to in vitro cell cultures; therefore, they are not subject to the regulatory framework for animal experimentation. The embryos and larvae of striped danios were used before 120 hpf (hours after fertilization); therefore, ethical approval was not required. The embryos of striped danios were obtained by mating adult striped danios.
3.5.1. Zebrafish Toxicity Assay
The fish embryo toxicity (FET) test was performed with modifications. Fertilized wild-type (WT) zebrafish embryos (0–2 hpf) or 72 hpf larvae were transferred to 6-well plates with a standard E3 medium and a series of EB355A or EB367 test compounds at concentrations of 20, 50 and 100 μM. Dimethylsulfoxide (DMSO, Sigma-Aldrich) was used as a solvent. The final concentration of DMSO in the solutions did not reach toxic concentrations above 1%. Control embryos were incubated in an embryo medium in the presence of 1% DMSO. Control and treated embryos were examined after 4, 8, 12, 24, 48, 72 and 96 h of treatment under a stereomicroscope with a camera. Experiments were conducted in triplicate, and 20 embryos were used in each group. Up to four apical observations were recorded every 24 h as indicators of mortality: coagulation of fertilized eggs, lack of somite formation, lack of detachment of the tail bud from the yolk sac and lack of a heartbeat. Pigmentation at 48 h was also observed. Additional developmental changes (heart rate, total body length) and embryonic malformations, such as pericardial edema, yolk sac edema, tail curvature, somite formation and scoliosis, were checked after 96 h.
For the toxicity test with 72 hpf larvae, three replicates were performed. For each replicate, 20 objects were used for each concentration and 20 larvae were used as controls (1% DMSO). The larvae were monitored at 24 and 48 h after treatment with different concentrations of EB355A or EB367 compounds. The survival rate and morphological deformities were examined and documented using a stereomicroscope equipped with a camera. After completing the observations, all remaining embryos/larvae were euthanized using a buffered tricaine methanesulphonate solution as per the OECD test guideline 236 (Organization for Economic Co-operation and Development 2013).
3.5.2. Statistical Analysis
Shapiro–Wilk’s W test of normality was used for the analysis of data distribution. The normally distributed data were analyzed using a one-way analysis of variance (ANOVA) and shown as the mean ± SEM. The statistical analysis was conducted using GraphPad Prism 9.4 software (version 9.4.1). The differences were deemed statistically significant at p < 0.05.
4. Conclusions
In the present study, we revealed high correlations between the experimental and theoretical values of lipophilicity obtained for indole derivatives of betulin. We also presented the relationships between lipophilicity and the physicochemical and ADME parameters, as well as the lack of a correlation between the experimental values of the lipophilicity of the derivatives and their anticancer activity against MCF-7 cells. Moreover, our experiments on zebrafish confirmed that the tested compounds are not toxic, which was consistent with the results of in silico toxicity analysis. In summary, the study demonstrated, using the example of indole derivatives of betulin, the utility of lipophilicity values in the context of predicting the biological activity of new compounds.
Author Contributions
Conceptualization, K.B.-M., E.B., Z.R., E.C. and D.W.; methodology, K.B.-M., E.B., E.C., Z.R., J.M.H. and A.S.; software, K.B.-M., E.B., Z.R. and A.S.; validation, D.W. and E.B.; formal analysis, Z.R., K.B.-M., E.B., E.C. and J.M.H.; investigation, K.B.-M., E.B., Z.R., E.C., D.W., A.S. and J.M.H.; resources, D.W. and J.M.H.; data curation, Z.R. and E.B.; writing—original draft preparation, E.B., Z.R., K.B.-M. and E.C.; visualization, Z.R., E.B. and K.B.-M.; supervision, D.W. and E.B.; funding acquisition, D.W., J.M.H. and Z.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Medical University of Bialystok, Poland (grant number: B.SUB.24.334/01.S) and the Medical University of Silesia in Katowice, Poland (grant number: BNW-1-037/N/4/F, BNW-2-038/K/4/F).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dhiman, A.; Sharma, R.; Singh, R. K Target-based anticancer indole derivatives and insight into structure–activity relationship: A mechanistic review update (2018–2021). Acta Pharm. Sin. B 2022, 12, 3006–3027. [Google Scholar] [CrossRef] [PubMed]
- Dhuguru, J.; Skouta, R. Role of indole scaffolds as pharmacophores in the development of anti-lung cancer agents. Molecules 2020, 25, 1615. [Google Scholar] [CrossRef] [PubMed]
- Mehra, A.; Sharma, V.; Verma, A.; Venugopal, S.; Mittal, A.; Singh, G.; Kaur, B. Indole derived anticancer agent. Chem. Sel. 2022, 7, e202202361. [Google Scholar] [CrossRef]
- Wan, Y.; Li, Y.; Yan, C.; Yan, M.; Tang, Z. Indole: A privileged scaffold for the design of anti-cancer agents. Eur. J. Med. Chem. 2019, 183, 111691. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, K.M.; Patel, U.P.; Dabhi, R.C.; Maru, J.J. Synthesis, computational insights, and anticancer activity of novel indole–Schiff base derivatives. Russ. J. Bioorg Chem. 2022, 48, 601–608. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, C.; Zhang, N.; Fan, R.; Ye, Y.; Xu, J. Recent advances in the development of pyrazole derivatives as anticancer agents. Int. J. Mol. Sci. 2023, 24, 12724. [Google Scholar] [CrossRef]
- Singh, D.; Patel, R.; Aggarwal, A.; Das, A.; Sharma, S.; Behera, B.; Panigrahy, R.; Kirane, A.R.; Kharkwal, H.; Kumar, P.; et al. Phenylthiazolidin-4-one piperazine conjugates: Design, synthesis, anticancer and antimicrobial studies. Results Chem. 2024, 7, 101237. [Google Scholar] [CrossRef]
- Govindarao, K.; Srinivasan, N.; Suresh, R.; Raheja, R.K.; Annadurai, S.; Bhandare, R.R.; Shaik, A.B. Quinoline conjugated 2-azetidinone derivatives as prospective anti-breast cancer agents: In vitro antiproliferative and anti-EGFR activities, molecular docking and in-silico drug likeliness studies. J. Saudi Chem. Soc. 2022, 26, 101471. [Google Scholar] [CrossRef]
- Khwaza, V.; Mlala, S.; Oyedeji, O.O.; Aderibigbe, B.A. Pentacyclic triterpenoids with nitrogen-containing heterocyclic moiety, privileged hybrids in anticancer drug discovery. Molecules 2021, 26, 2401. [Google Scholar] [CrossRef]
- Li, A.L.; Hao, Y.; Wang, W.Y.; Liu, Q.S.; Sun, Y.; Gu, W. Design, synthesis, and anticancer evaluation of novel indole derivatives of ursolic acid as potential topoisomerase II inhibitors. Int. J. Mol. Sci. 2020, 21, 2876. [Google Scholar] [CrossRef]
- Lombrea, A.; Semenescu, A.-D.; Magyari-Pavel, I.Z.; Turks, M.; Lugiņina, J.; Peipiņš, U.; Muntean, D.; Dehelean, C.A.; Dinu, S.; Danciu, C. Comparison of in vitro antimelanoma and antimicrobial activity of 2,3-indolo-betulinic acid and its glycine conjugates. Plants 2023, 12, 1253. [Google Scholar] [CrossRef] [PubMed]
- Spivak, A.Y.; Nedopekina, D.A.; Gubaidullin, R.R.; Davletshin, E.V.; Tukhbatullin, A.A.; D’yakonov, V.A.; Yunusbaeva, M.M.; Dzhemileva, L.U.; Dzhemilev, U.M. Pentacyclic triterpene acid conjugated with mitochondria-targeting cation F16: Synthesis and evaluation of cytotoxic activities. Med. Chem. Res. 2021, 30, 940–951. [Google Scholar] [CrossRef]
- Khusnutdinova, E.F.; Petrova, A.V.; Kukovinets, O.S.; Kazakova, O.B. Synthesis and cytotoxicity of 28-N-propargylaminoalkylated 2,3-indolotriterpenic acids. Nat. Prod. Commun. 2018, 13, 1934578X180130060. [Google Scholar] [CrossRef]
- Fan, H.; Geng, L.; Yang, F.; Dong, X.; He, D.; Zhang, Y. Ursolic acid derivative induces apoptosis in glioma cells through down-regulation of cAMP. Eur. J. Med. Chem. 2019, 176, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Arnott, J.A.; Planey, S.L. The influence of lipophilicity in drug discovery and design. Expert. Opin. Drug Dis. 2012, 7, 863–875. [Google Scholar] [CrossRef]
- Waring, M.J. Lipophilicity in drug discovery. Expert. Opin. Drug Dis. 2010, 5, 235–248. [Google Scholar] [CrossRef]
- Rutkowska, E.; Pająk, K.; Jóźwiak, K. Lipophilicity and methods of determination and its role in medicinal chemistry. Acta Pol. Pharm. Drug Res. 2013, 70, 3–18. [Google Scholar]
- Ulrich, N.; Goss, K.-U.; Ebert, A. Exploring the octanol–water partition coefficient dataset using deep learning techniques and data augmentation. Commun. Chem. 2021, 4, 90. [Google Scholar] [CrossRef]
- Choi, T.Y.; Choi, T.I.; Lee, Y.R.; Choe, S.K.; Kim, C.H. Zebrafish as an animal model for biomedical research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [CrossRef]
- Adhish, M.; Manjubala, I. Effectiveness of zebrafish models in understanding human diseases- a review of models. Heliyon 2023, 9, e14557. [Google Scholar] [CrossRef]
- Zang, L.; Torraca, V.; Shimada, Y.; Nishimura, N. Editorial: Zebrafish models for human disease studies. Front. Cell Dev. Biol. 2022, 10, 861941. [Google Scholar] [CrossRef] [PubMed]
- Teame, T.; Zhang, Z.; Ran, C.; Zhang, H.; Yang, Y.; Ding, Q.; Xie, M.; Gao, C.; Ye, Y.; Duan, M.; et al. The use of zebrafish (Danio rerio) as biomedical models. Animal Front. 2019, 9, 68–77. [Google Scholar] [CrossRef]
- McGrath, P.; Li, C.Q. Zebrafish: A predictive model for assessing drug-induced toxicity. Drug Discov. Today 2008, 13, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, T.; Mieszkowska, A.; Kempińska-Kupczyk, D.; Kot-Wasik, A.; Namieśnik, J.; Mazerska, Z. The impact of lipophilicity on environmental processes, drug delivery and bioavailability of food components. Microchem. J. 2019, 146, 393–406. [Google Scholar] [CrossRef]
- Liu, X.; Testa, B.; Fahr, A. Lipophilicity and its relationship with passive drug permeation. Pharm. Res. 2011, 28, 962–977. [Google Scholar] [CrossRef] [PubMed]
- Valko, K.L. Lipophilicity and biomimetic properties measured by HPLC to support drug discovery. J. Pharm. Biomed. Anal. 2016, 130, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Bębenek, E.; Chrobak, E.; Rzepka, Z.; Wrześniok, D. New betulin derivatives with nitrogen heterocyclic moiety -synthesis and anticancer activity in vitro. Biomolecules 2022, 12, 1540. [Google Scholar] [CrossRef]
- Rzepka, Z.; Bębenek, E.; Chrobak, E.; Wrześniok, D. Synthesis and anticancer activity of indole-functionalized derivatives of betulin. Pharmaceutics 2022, 14, 2372. [Google Scholar] [CrossRef]
- Pęcak, P.; Świtalska, M.; Chrobak, E.; Boryczka, G.; Bębenek, E. Betulin acid ester derivatives inhibit cancer cell growth by inducing apoptosis through caspase cascade activation: A comprehensive in vitro and in silico study. Int. J. Mol. Sci. 2023, 24, 196. [Google Scholar] [CrossRef]
- Mannhold, R.; Cruciani, G.; Dross, K.; Rekker, R. Multivariate analysis of experimental and computational descriptors of molecular lipophilicity. J. Comput. Aided Mol. Des. 1998, 12, 573–581. [Google Scholar] [CrossRef]
- Achrem-Achremowicz, J.; Kępczyńska, E.; Żylewski, M.; Janeczko, Z. Synthesis of betulin derivatives and the determination of their relative lipophilicities using reversed-phase thin-layer chromatography. Biomed. Chromatogr. 2010, 24, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Grymel, M.; Pastuch-Gawołek, G.; Lalik, A.; Zawojak, M.; Boczek, S.; Krawczyk, M.; Erfurt, K. Glycoconjugation of betulin derivatives using copper-catalyzed 1,3-dipolar azido-alkyne cycloaddition reaction and a preliminary assay of cytotoxicity of the obtained compounds. Molecules 2020, 25, 6019. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ren, Q.; Liu, X.; Gao, L.; Xiao, L.; Yu, W. In Silico prediction of human organ toxicity via artificial intelligence methods. Chem. Res. Toxicol. 2023, 36, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S. In silico ADME-Tox modeling: Progress and prospects. Expert. Opin. Drug Metab. Toxicol. 2017, 13, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Yu, Z.; Wang, Y.; Chen, L.; Lou, C.; Yang, C.; Li, W.; Liu, G.; Tang, Y. admetSAR3.0: A comprehensive platform for exploration, prediction and optimization of chemical ADMET properties. Nucleic Acids Res. 2024, 52, W432–W438. [Google Scholar] [CrossRef]
- admetSAR 3.0. Available online: http://lmmd.ecust.edu.cn/admetsar3/about.php (accessed on 1st July 2024).
- Modarresi Chahardehi, A.; Arsad, H.; Lim, V. Zebrafish as a successful animal model for screening toxicity of medicinal plants. Plants 2020, 9, 1345. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- SwissADME. Available online: http://www.swissadme.ch (accessed on 3 March 2024).
- Tetko, I.V.; Gasteiger, J.; Todeschini, R.; Mauri, A.; Livingstone, D.; Ertl, P.; Palyulin, V.A.; Radchenko, E.V.; Zefirov, N.S.; Makarenko, A.S.; et al. Virtual computational chemistry laboratory—Design and description. J. Comput. Aid Mol. Des. 2005, 19, 453–463. [Google Scholar] [CrossRef]
- VCCLAB, Virtual Computational Chemistry Laboratory. 2005. Available online: https://vcclab.org (accessed on 3 March 2024).
- Available online: https://www.molinspiration.com/cgi/properties (accessed on 3 March 2024).
- Stanisz, A. Przystępny Kurs Statystyki z Zastosowaniem STATISTICA PL na Przykładach Medycyny; Analizy Wielowymiarowe, 3rd ed.; StatSoft Polska: Kraków, Poland, 2007; Volume 3. [Google Scholar]
- Jolliffe, J.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. 2016, 374, 20150202. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).