Improved Alkaline Hydrogen Evolution Performance of Dealloying Fe75−xCoxSi12.5B12.5 Electrocatalyst
Abstract
1. Introduction
2. Results and Discussion
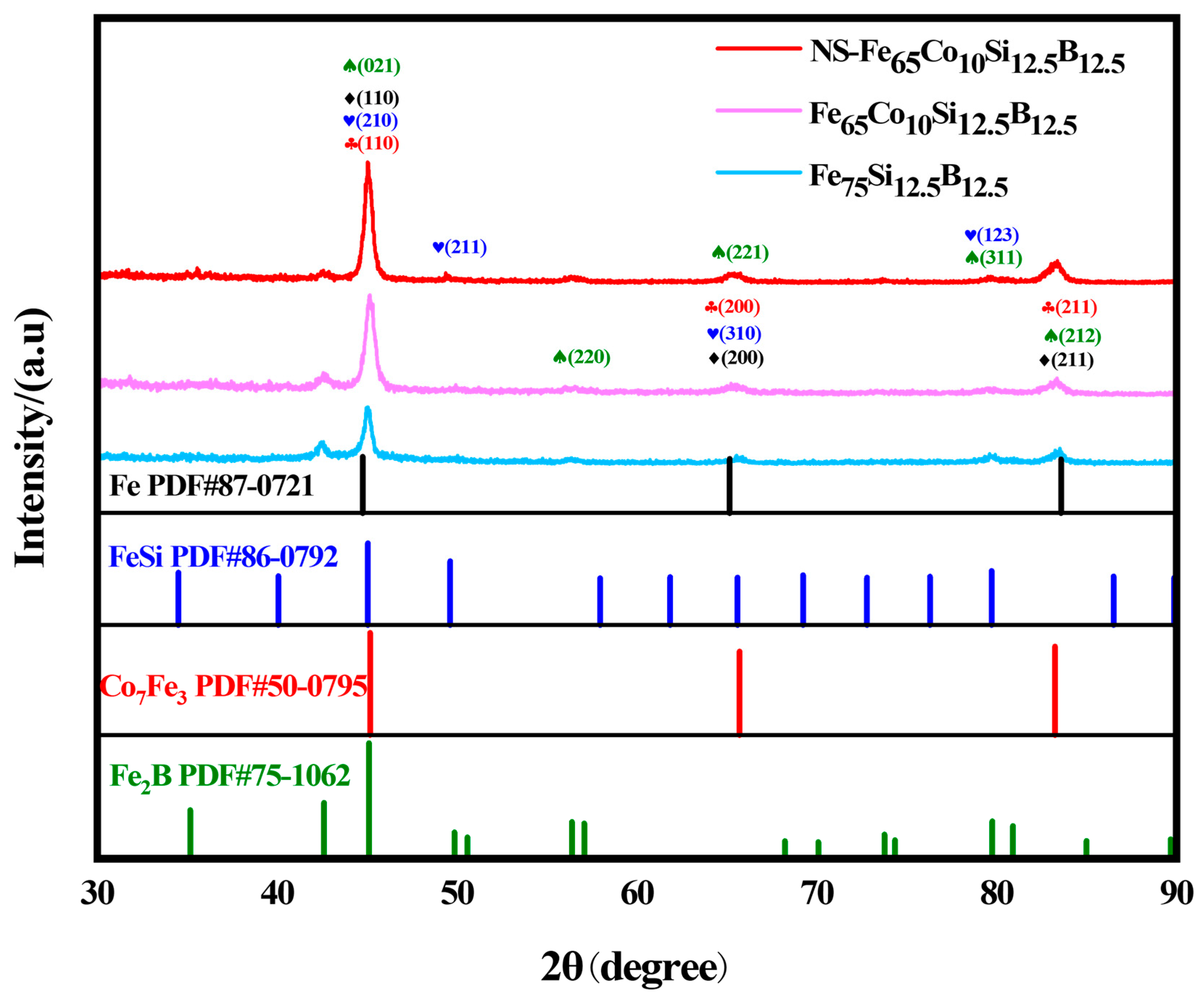
2.1. Structure Characterization
2.2. Morphology Characterization
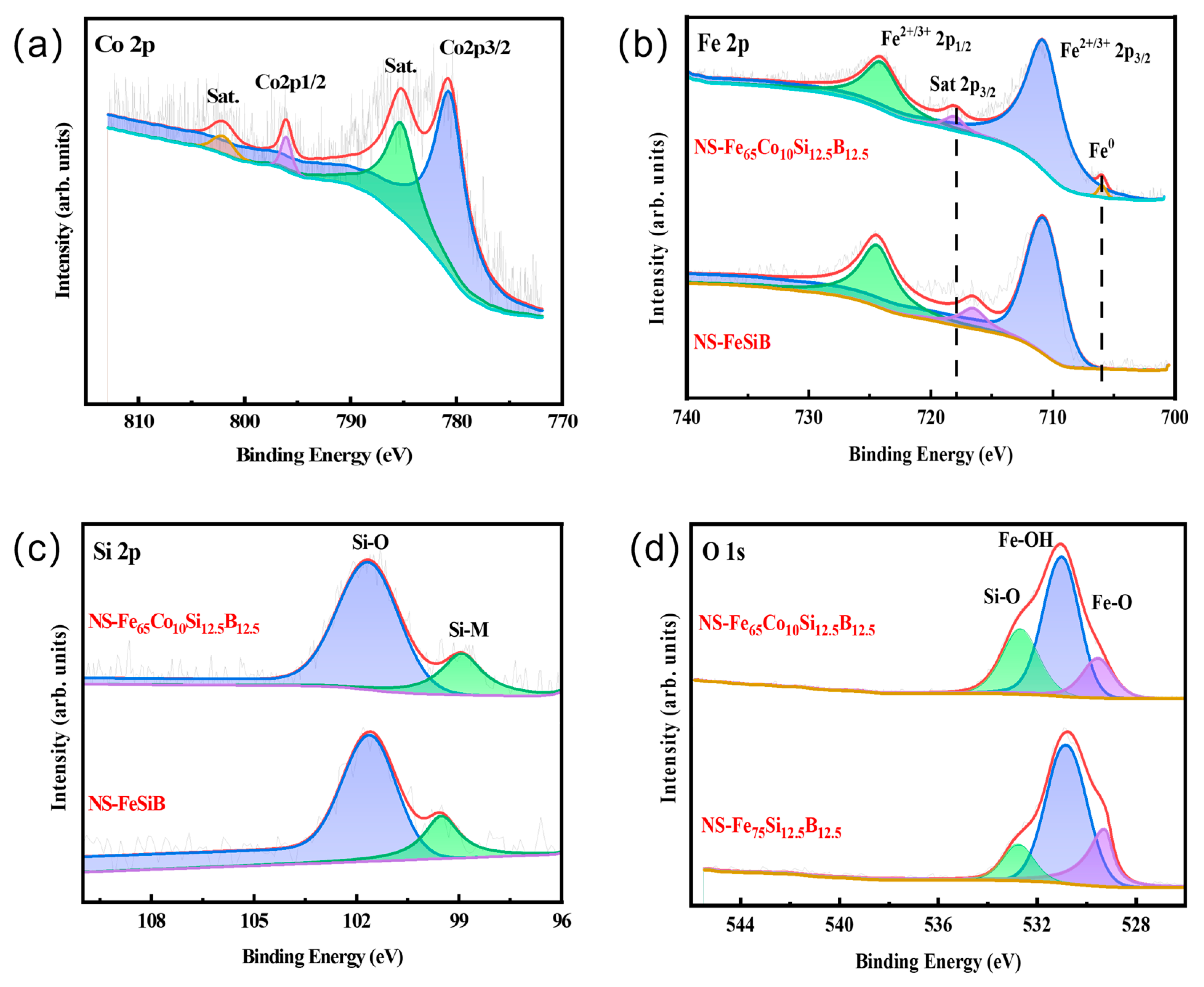
2.3. Chemical State Analysis
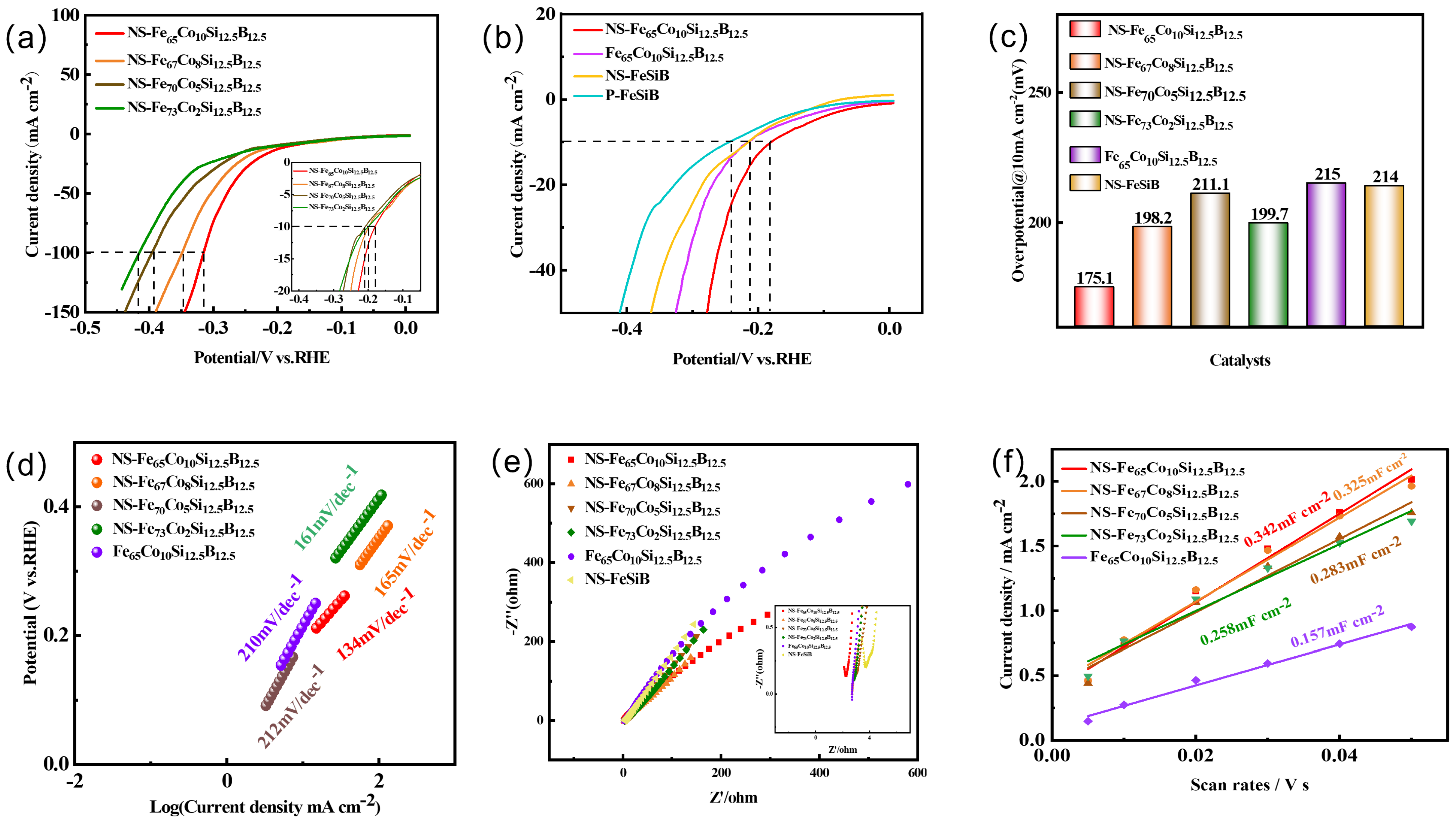
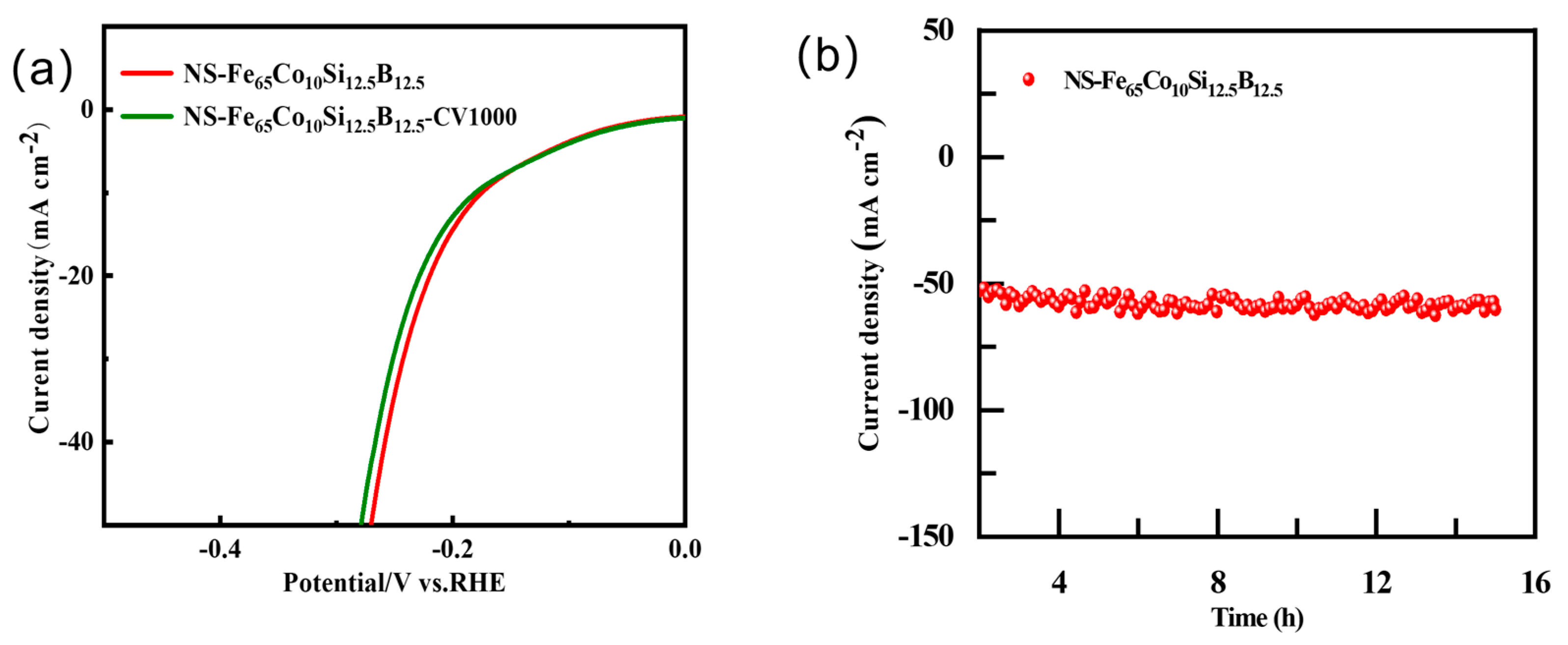
2.4. Electrochemical Parameters
3. Experimental Methods
3.1. Materials
3.2. Preparation of Bulk Alloy Electrodes
3.3. Electrochemical Tests
3.4. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, D.; Tong, R.; Qu, Y.; Zhu, Q.; Zhong, X.; Fang, M.; Ho Lo, K.; Zhang, F.; Ye, Y.; Tang, Y.; et al. Highly improved electrocatalytic activity of NiSx: Effects of Cr-doping and phase transition. Appl. Catal. B 2020, 267, 118721. [Google Scholar] [CrossRef]
- Xu, X.; Zhong, Y.; Wajrak, M.; Bhatelia, T.; Jiang, S.P.; Shao, Z. Grain boundary engineering: An emerging pathway toward efficient electrocatalysis. InfoMat 2024, 6, e12608. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, C.; Zhang, J.; Zhang, C.; Zhang, Z. Hierarchically porous Mo-doped Ni–Fe oxide nanowires efficiently catalyzing oxygen/hydrogen evolution reactions. J. Mater. Chem. A 2018, 6, 8430–8440. [Google Scholar] [CrossRef]
- Xu, X.; Shao, Z.; Jiang, S.P. High-Entropy Materials for Water Electrolysis. Energy Technol. 2022, 10, 2200573. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, Y.; Chen, Z.; Li, Z.; Ma, T.; Wu, Z.; Wang, L. Trifle Pt coupled with NiFe hydroxide synthesized via corrosion engineering to boost the cleavage of water molecule for alkaline water-splitting. Appl. Catal. B 2021, 297, 120395. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, N.; Liu, H.; Ma, W.; Hippalgaonkar, K.; Liu, Z.; Huang, Y. Ordering-Dependent Hydrogen Evolution and Oxygen Reduction Electrocatalysis of High-Entropy Intermetallic Pt4FeCoCuNi. Adv. Mater. 2023, 35, 2302067. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, L.; Wu, G.; Su, D.; Lv, H.; Zhang, S.; Zhu, W.; Casimir, A.; Zhu, H.; Mendoza-Garcia, A.; et al. New Approach to Fully Ordered fct-FePt Nanoparticles for Much Enhanced Electrocatalysis in Acid. Nano Lett. 2015, 15, 2468–2473. [Google Scholar] [CrossRef]
- Li, J.; Luo, J.; Chen, H.; Qin, B.; Yuan, C.; Wu, N.; Liu, G.; Liu, X. Strong electronic metal–support interaction between Pt and stainless mesh for enhancing the hydrogen evolution reaction. Chem. Commun. 2022, 58, 9918–9921. [Google Scholar] [CrossRef]
- Cui, Z.; Chen, H.; Zhao, M.; Marshall, D.; Yu, Y.; Abruña, H.; DiSalvo, F.J. Synthesis of Structurally Ordered Pt3Ti and Pt3V Nanoparticles as Methanol Oxidation Catalysts. J. Am. Chem. Soc. 2014, 136, 10206–10209. [Google Scholar] [CrossRef]
- Chung, D.Y.; Jun, S.W.; Yoon, G.; Kwon, S.G.; Shin, D.Y.; Seo, P.; Yoo, J.M.; Shin, H.; Chung, Y.-H.; Kim, H.; et al. Highly Durable and Active PtFe Nanocatalyst for Electrochemical Oxygen Reduction Reaction. J. Am. Chem. Soc. 2015, 137, 15478–15485. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Jiang, S.P.; Shao, Z. Designing single-atom catalysts toward improved alkaline hydrogen evolution reaction. Mater. Rep. Energy 2022, 2, 100144. [Google Scholar] [CrossRef]
- Sun, H.; Yan, Z.; Liu, F.; Xu, W.; Cheng, F.; Chen, J. Self-Supported Transition-Metal-Based Electrocatalysts for Hydrogen and Oxygen Evolution. Adv. Mater. 2020, 32, e1806326. [Google Scholar] [CrossRef]
- Wei, Y.; Shin, C.-H.; Gyan-Barimah, C.; Tetteh, E.B.; Park, G.; Yu, J.-S. Positive self-reconstruction in an FeNiMo phosphide electrocatalyst for enhanced overall water splitting. Sustain. Energy Fuels 2021, 5, 5789–5797. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, H.; Bao, J.; Song, Y.; She, X.; Lv, G.; Deng, J.; Li, H.; Xu, H. Amorphized core–shell NiFeMo electrode for efficient bifunctional water splitting. Appl. Surf. Sci. 2023, 607, 154803. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Huang, C.-L.; Chen, Y.-A.; Lu, S.-Y. NiFeMo alloy inverse-opals on Ni foam as outstanding bifunctional catalysts for electrolytic water splitting of ultra-low cell voltages at high current densities. Appl. Catal. B 2020, 267, 118376. [Google Scholar] [CrossRef]
- Sun, Z.; Yuan, M.; Yang, H.; Lin, L.; Jiang, H.; Ge, S.; Li, H.; Sun, G.; Ma, S.; Yang, X. 3D Porous Amorphous gamma-CrOOH on Ni Foam as Bifunctional Electrocatalyst for Overall Water Splitting. Inorg. Chem. 2019, 58, 4014–4018. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, Y.; Ma, T.; Yang, C.; Peng, Y.; Zhou, Z.; Zhou, M.; Li, S.; Wang, Y.; Cheng, C. Designing MOF Nanoarchitectures for Electrochemical Water Splitting. Adv. Mater. 2021, 33, 2006042. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, X.; Liu, J.; Li, X.; Xing, C.; Lyu, X.; Cai, W.; Wang, W.; Li, Y. Cr-Dopant Induced Breaking of Scaling Relations in CoFe Layered Double Hydroxides for Improvement of Oxygen Evolution Reaction. Small 2019, 15, e1902373. [Google Scholar] [CrossRef]
- Maurya, A.; Yadav, M. Sphere shaped Mo- doped transition metal oxide as electrocatalyst for oxygen evolution reaction in alkaline medium. J. Alloys Compd. 2023, 956, 170208. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, H.C. Adsorption and On-Site Transformation of Transition Metal Cations on Ni-Doped AlOOH Nanoflowers for OER Electrocatalysis. ACS Sustain. Chem. Eng. 2019, 7, 5953–5962. [Google Scholar] [CrossRef]
- Hou, J.; Tang, Z.; Wei, K.; Lai, Q.; Liang, Y. Surface reconstruction of Ni doped Co–Fe Prussian blue analogues for enhanced oxygen evolution. Catal. Sci. Technol. 2021, 11, 1110–1115. [Google Scholar] [CrossRef]
- Tamboli, A.M.; Jung, Y.; Sim, J.; Kim, B.; Kim, W.S.; Kim, M.; Lee, C.; Kim, K.; Lim, C.; Kim, K.; et al. Boosting oxygen evolution reaction activity with Mo incorporated NiFe-LDH electrocatalyst for efficient water electrolysis. Chemosphere 2023, 344, 140314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shao, Q.; Pi, Y.; Guo, J.; Huang, X. A Cost-Efficient Bifunctional Ultrathin Nanosheets Array for Electrochemical Overall Water Splitting. Small 2017, 13, 1700355. [Google Scholar] [CrossRef]
- Yu, Y.X.; Xu, J.L.; Zhang, L.W.; Ma, Y.C.; Luo, J.M. Electrochemically treated AlCoCrFeNi high entropy alloy as a self-supporting electrode for overall water splitting. Int. J. Hydrogen Energy 2024, 72, 209–219. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, J.; Xie, H.; Yin, H.; Zhang, Z.; Liu, E.; Ma, L.; Chen, B.; Sha, J.; Qian, L.; et al. Boosting the oxygen evolution of high-entropy (oxy)hydroxide epitaxially grown on high entropy alloy by lattice oxygen activation. Appl. Catal. B 2024, 341, 123331. [Google Scholar] [CrossRef]
- Zhao, Y.; Luo, M.; Chu, S.; Peng, M.; Liu, B.; Wu, Q.; Liu, P.; de Groot, F.M.F.; Tan, Y. 3D nanoporous iridium-based alloy microwires for efficient oxygen evolution in acidic media. Nano Energy 2019, 59, 146–153. [Google Scholar] [CrossRef]
- Wu, K.; Meng, Y.; Li, X.; Ma, J.; Zhang, P.; Li, W.; Huo, L.; Lin, H.-J. Improved alkaline hydrogen evolution performance of a Fe78Si9B13 metallic glass electrocatalyst by ultrasonic vibrations. Intermetallics 2020, 125, 106820. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.; Zhang, J.; Wu, N.; Liu, G.; Chen, H.; Yuan, C.; Liu, X. Optimizing electronic structure of porous Ni/MoO2 heterostructure to boost alkaline hydrogen evolution reaction. J. Colloid. Interface Sci. 2022, 627, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cao, X.; Huo, J.; Qu, J.; Chen, W.; Liu, C.; Zhao, Y.; Liu, H.; Wang, G. High valence metals engineering strategies of Fe/Co/Ni-based catalysts for boosted OER electrocatalysis. J. Energy Chem. 2023, 76, 195–213. [Google Scholar] [CrossRef]
- Zhu, Q.; Shao, M.; Yu, S.H.; Wang, X.; Tang, Z.; Chen, B.; Cheng, H.; Lu, Z.; Chua, D.; Pan, H. One-Pot Synthesis of Co-Doped VSe2 Nanosheets for Enhanced Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2018, 2, 644–653. [Google Scholar] [CrossRef]
- Lontio Fomekong, R.; Kammi Yontchoum, P.; Matemb Ma Ntep, T.J.; Tedjieukeng Kamta, H.M.; Kenfack Tsobnang, P.; Krüger, S.; Šturala, J.; Sofer, Z.; Janiak, C.; Saruhan, B.; et al. From Doped Coordination Polymer Precursor to Cobalt-Doped ZnO Electrocatalyst for Alkaline Hydrogen Evolution Reaction. Adv. Energy Sustain. Res. 2023, 5, 2300232. [Google Scholar] [CrossRef]
- Kim, D.; Park, S.; Yan, B.; Hong, H.; Cho, Y.; Kang, J.; Qin, X.; Yoo, T.; Piao, Y. Synthesis of Remarkably Thin Co–Fe Phosphide/Carbon Nanosheet for Enhanced Oxygen Evolution Reaction Electrocatalysis Driven by Readily Generated Active Oxyhydroxide. ACS Appl. Energy Mater. 2022, 5, 2400–2411. [Google Scholar] [CrossRef]
- Zhong, X.; Zhu, Y.a.; Dai, W.; Yu, J.; Lu, T.; Pan, Y. Electrochemically reconstructed high-entropy amorphous FeCoNiCrVB as a highly active oxygen evolution catalyst. New J. Chem. 2022, 46, 8398–8406. [Google Scholar] [CrossRef]
- Jiang, J.; Sun, F.; Zhou, S.; Hu, W.; Zhang, H.; Dong, J.; Jiang, Z.; Zhao, J.; Li, J.; Yan, W.; et al. Atomic-level insight into super-efficient electrocatalytic oxygen evolution on iron and vanadium co-doped nickel (oxy)hydroxide. Nat. Commun. 2018, 9, 2885. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Ke, W.; Xie, M.; Shen, X.; Yan, Z.; Ji, Z.; Zhu, G.; Xu, K.; Zhou, H. Amorphous CoFe(OH)x hollow hierarchical structure: An efficient and durable electrocatalyst for oxygen evolution reaction. Catal. Sci. Technol. 2020, 10, 215–221. [Google Scholar] [CrossRef]
- He, Y.; Yu, T.; Wen, H.; Guo, R. Boosting the charge transfer of FeOOH/Ni(OH)2 for excellent oxygen evolution reaction via Cr modification. Dalton Trans. 2021, 50, 9746–9753. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, L.; Xu, W.; Xiong, C.; Chen, W.; Xiang, X.; Zhang, B.; Shang, H. Carbon-supported high-entropy Co-Zn-Cd-Cu-Mn sulfide nanoarrays promise high-performance overall water splitting. Nano Res. 2022, 15, 6054–6061. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, S.-C.; Cui, Z.; Li, J.; Tian, G.-R.; Zhou, Z.-H.; Jiao, H.-F.; Xiong, J.-F.; Wang, L.-C.; Xiang, J.; Wu, F.-F.; et al. Improved Alkaline Hydrogen Evolution Performance of Dealloying Fe75−xCoxSi12.5B12.5 Electrocatalyst. Molecules 2024, 29, 4130. https://doi.org/10.3390/molecules29174130
Zhong S-C, Cui Z, Li J, Tian G-R, Zhou Z-H, Jiao H-F, Xiong J-F, Wang L-C, Xiang J, Wu F-F, et al. Improved Alkaline Hydrogen Evolution Performance of Dealloying Fe75−xCoxSi12.5B12.5 Electrocatalyst. Molecules. 2024; 29(17):4130. https://doi.org/10.3390/molecules29174130
Chicago/Turabian StyleZhong, Si-Cheng, Zhe Cui, Jia Li, Guang-Run Tian, Zhong-Hong Zhou, Hong-Fei Jiao, Jie-Fu Xiong, Li-Chen Wang, Jun Xiang, Fu-Fa Wu, and et al. 2024. "Improved Alkaline Hydrogen Evolution Performance of Dealloying Fe75−xCoxSi12.5B12.5 Electrocatalyst" Molecules 29, no. 17: 4130. https://doi.org/10.3390/molecules29174130
APA StyleZhong, S.-C., Cui, Z., Li, J., Tian, G.-R., Zhou, Z.-H., Jiao, H.-F., Xiong, J.-F., Wang, L.-C., Xiang, J., Wu, F.-F., & Zhao, R.-D. (2024). Improved Alkaline Hydrogen Evolution Performance of Dealloying Fe75−xCoxSi12.5B12.5 Electrocatalyst. Molecules, 29(17), 4130. https://doi.org/10.3390/molecules29174130





