Synthesis of Hybrid Molecules with Imidazole-1,3,4-thiadiazole Core and Evaluation of Biological Activity on Trypanosoma cruzi and Leishmania donovani
Abstract
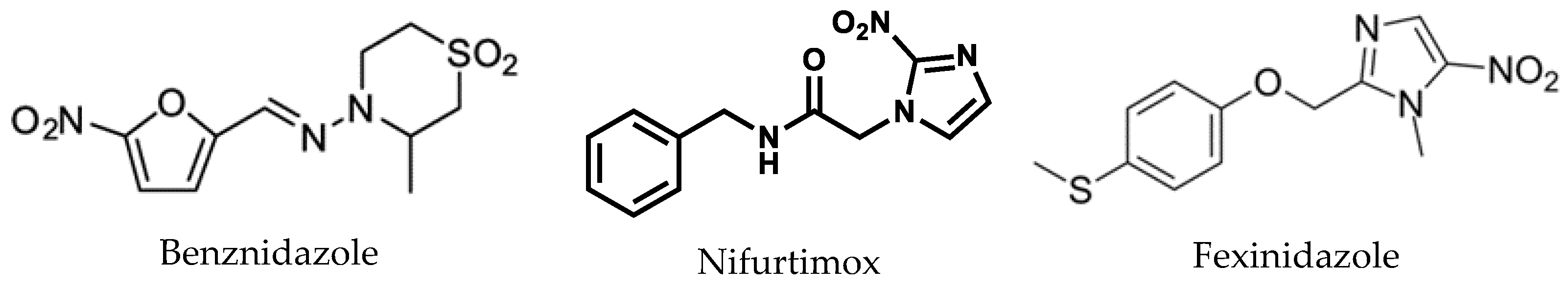
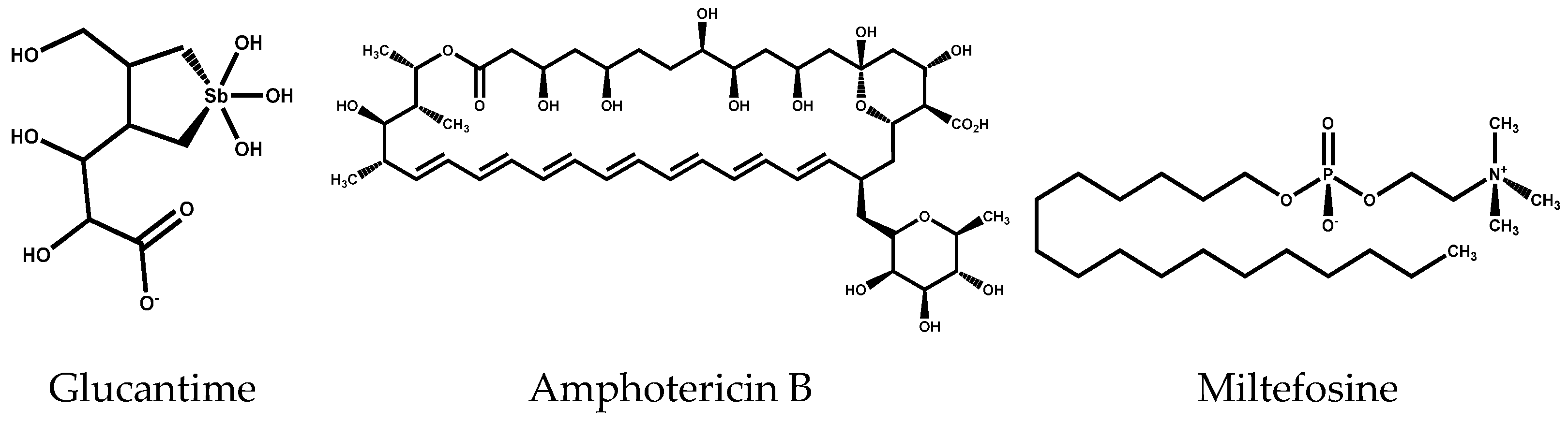
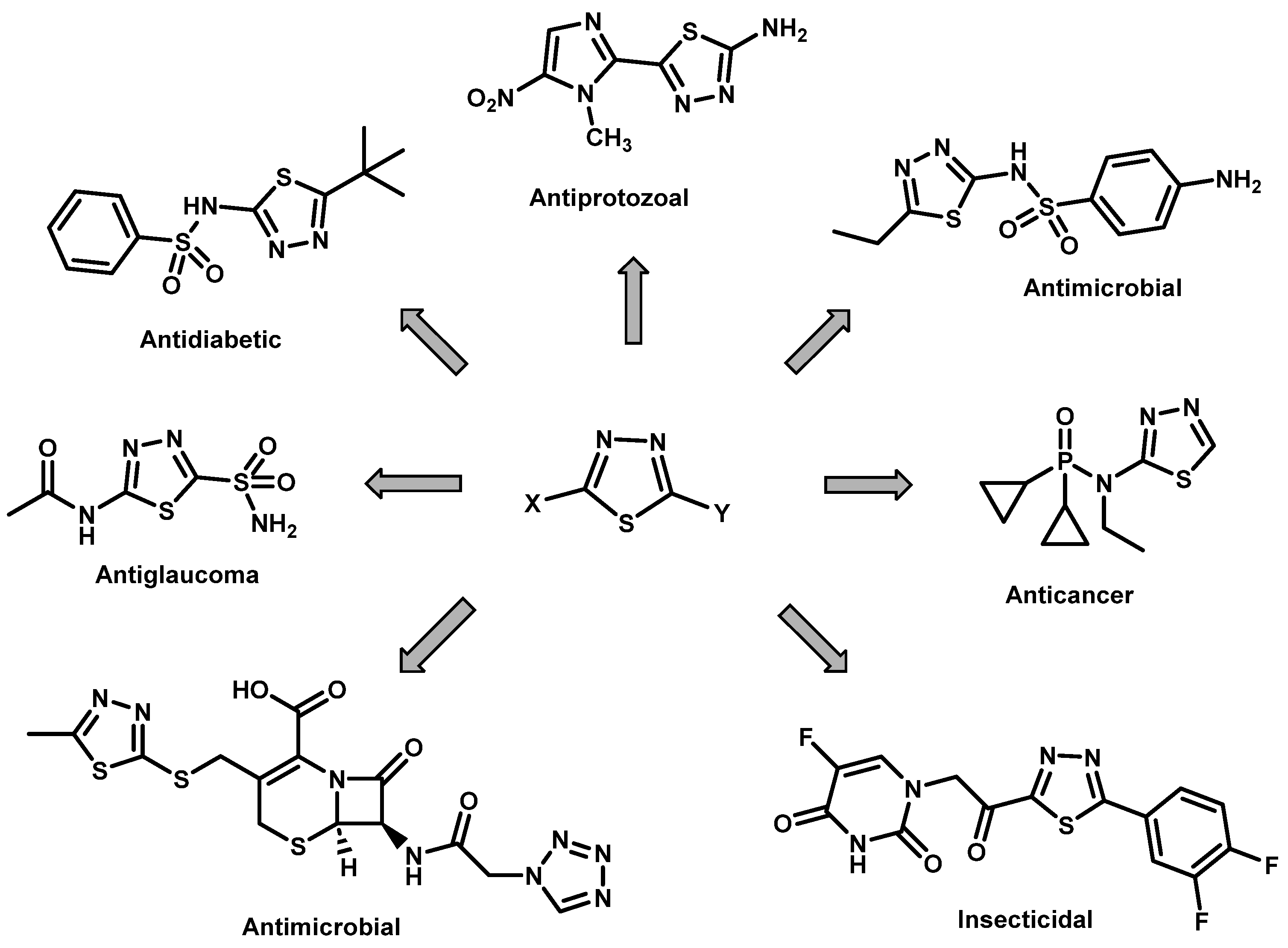
1. Introduction
2. Results and Discussion
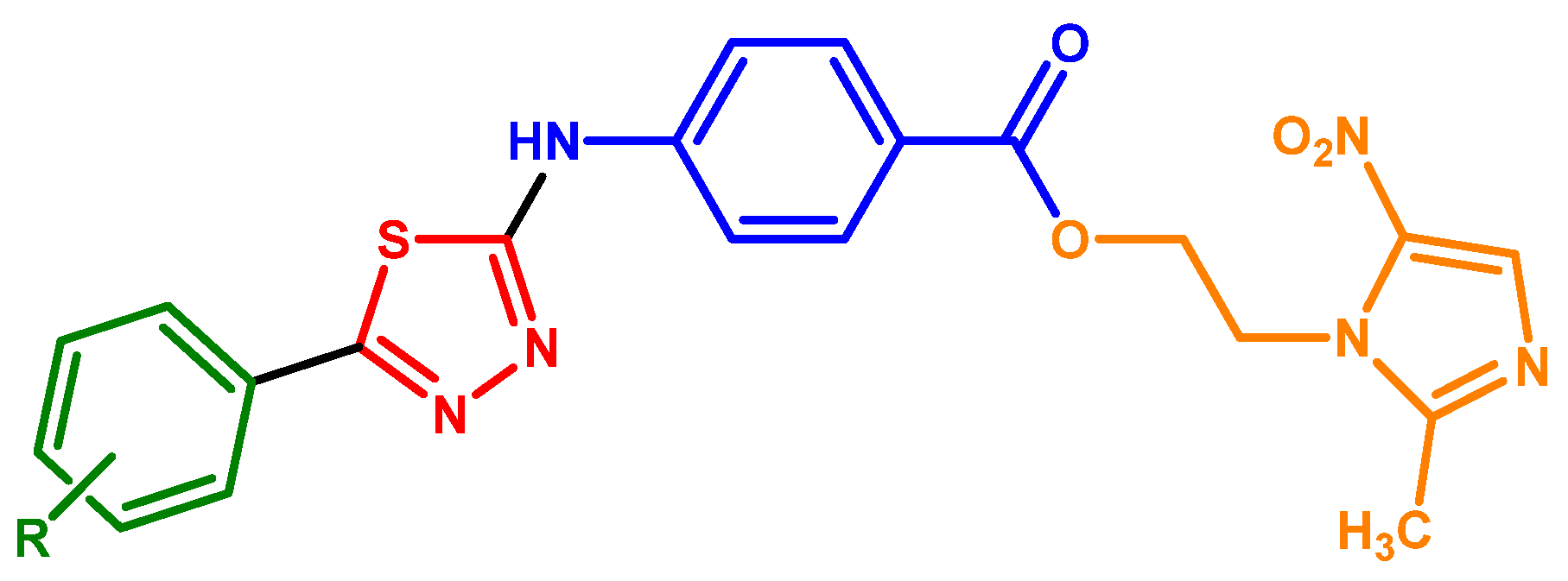
2.1. Synthesis
| No | R2″ | X | R4″ | R5″ | R6″ |
| 18 | H | N | H | H | H |
| 19 | H | CCl | H | H | H |
| 20 | H | CH | Cl | H | H |
| 21 | H | CH | H | H | H |
| 22 | H | CH | Ph | H | H |
| 23 | H | COCH3 | OCH3 | OCH3 | H |
2.2. Antiprotozoal Activity
3. Materials and Methods
3.1. Synthesis of 4-Ethoxycarbonylphenylphenyl Isothiocyanate 3
3.2. General Procedure for the Synthesis of Thiosemicarbazides 5–10
- Ethyl 4-(2-nicotinoylhydrazine-1-carbothioamido)benzoate (5). Solid beige, yield: 74%, mp: 200–202 °C. IR (cm−1): 3361 (NH), 3181 (CHarom), 2979 (CHali), 1710 (C=O), 1656 (C=O), 1601 (C=C), 1366 (NCS), 1273 (C-O). 1H NMR (100 MHz, DMSO d6) δ ppm: 1.35 (t, 3H, CH3, J = 7 Hz), 4.34 (q, 2H, CH2O, J = 7 Hz), 7.58 (dd, 1H, H5″, J = 7 Hz), 7.75 (d, 2H, H2′,6′, J = 8 Hz), 7.97 (d, 2H, H3′,5′, J = 8 Hz), 8.32 (d, 1H, H4″, J = 7 Hz), 8.79 (d, 1H, H6″, J = 5 Hz), 9.15 (s, 1H, H2″), 10.07 (brs, 2H, NH), 10.80 (brs, 1H, NH). 13C NMR (25.8 MHz, DMSO d6) δ ppm: 14.7, 61.1, 123.9, 124.8, 126.4, 128.7, 129.7, 136.0, 144.2, 149.4, 152.9, 165.2, 165.9, 181.8. Calcd for C16H16N4O3S: C, 55.80; H, 4.68; N, 16.27; S, 9.31. Found: C, 55.82; H, 4.67; N, 16.35; S, 9.43.
- Ethyl 4-[2-(3-chlorobenzoyl)hydrazine-1-carbothioamido]benzoate (6). White solid, yield: 62%, mp: 188–190 °C. IR (cm−1): 3317 (NH), 3160 (CHarom), 2981 (CHali), 1718 (C=O), 1634 (C=O), 1610 (C=C), 1352 (NCS), 1282 (C-O). 1H NMR (100 MHz, DMSO d6) δ ppm: 1.36 (t, 3H, CH3, J = 7 Hz), 4.38 (q, 2H, CH2O, J = 7 Hz), 7.50–8.05 (m, 8H, Ar), 9.95 (brs, 1H, NH), 10.06 (brs, 1H, NH), 10.72 (brs, 1H, NH). 13C NMR (25.8 MHz, DMSO d6) δ ppm: 14.7, 61.0, 124.6, 127.1, 128.2, 129.7, 130.8, 132.2, 133.6, 135.0, 144.3, 165.2, 181.8. Calcd for C17H16ClN3O3S: C, 54.04; H, 4.27; N, 11.12; S, 8.49. Found: C, 53.97, H, 4.32; N, 11.29; S, 8.73.
- Ethyl 4-[2-(4-chlorobenzoyl)hydrazine-1-carbothioamido]benzoate (7). White solid, yield: 59%, mp: 185–186 °C. IR (cm−1): 3300, 3211 (NH), 3174 (CHarom), 2975 (CHali), 1715 (C=O), 1612 (C=C), 1338 (NCS), 1277 (C-O). 1H NMR (100 MHz, DMSO d6) δ ppm: 1.28 (t, 3H, CH3, J = 7 Hz), 4.27 (q, 2H, CH2O, J = 7 Hz), 7.57 (d, 2H, H2′,6′, J = 8 Hz), 7.68 (brs, 2H, H3″,5″), 7.89 (d, 2H, H3′,5′, J = 8 Hz), 7.94 (d, 2H, H2″,6″, J = 8 Hz), 9.93 (brs, 1H, NH), 10.02 (brs, 1H, NH), 10.67 (brs, 1H, NH). 13C NMR(125 MHz, DMSO d6) δ ppm: 14.7, 61.1, 125.6, 126.6, 128.9, 129.6, 130.3, 131.7, 137.3, 144.2, 165.9, 180.2. Calcd for C17H16ClN3O3S: C, 54.04; H, 4.27; N, 11.12; S, 8.49. Found: C, 54.11, H, 4.29; N, 11.35; S, 8.61.
- Ethyl 4-(2-benzoylhydrazine-1-carbothioamido)benzoate (8). White solid, yield: 81%, mp: 176–178 °C. IR (cm−1): 3300 (NH), 3211 (NH), 3174 (CHarom), 2975 (CHali), 1698 (C=O), 1604 (C=C), 1338 (CNS), 1280 (C-O). 1H NMR (500 MHz, DMSO d6) δ ppm: 1.39 (t, 3H, CH3, J = 7 Hz), 4.36 (q, 2H, CH2O, J = 7 Hz), 7.53 (t, 1H, H4″, J = 8 Hz), 7.62–7.64 (m, 4H, Ar), 7.86 (d, 2H, H3′,5′, J = 8 Hz), 8.00–8.02 (m, 2H, Ar), 9.21 (brs, 1H, NH), 10.24 (brs, 1H, NH), 10.67 (brs, 1H, NH). 13C NMR (125 MHz, DMSO d6) δ ppm: 14.7, 61.0, 125.5, 126.6, 128.4, 128.8, 129.5, 132.4, 144.3, 165.9, 180.7. Calcd for C17H17N3O3S: C, 59.46; H, 4.99; N, 12.24; S, 9.34. Found: C, 59.53, H, 5.10; N, 12.41; S, 9.57.
- Ethyl 4-{2-[(1,1′-biphenyl)-4-carbonyl]hydrazine-1-carbothioamido}benzoate (9). White solid, yield: 78%, mp: 190–192 °C. IR (cm−1): 3307 (NH), 3254 (NH), 3152 (CHarom), 2981 (CHali), 1693 (C=O), 1659 (C=O), 1603 (C=C), 1284 (C-O). 1H NMR (500 MHz, DMSO d6) δ ppm: 1.25 (t, 3H, CH3, J = 7 Hz), 4.23 (q, 2H, CH2O, J = 7 Hz), 7.37 (t, 1H, 4′′′, J = 8 Hz), 7.45 (t, 2H, H3′′′, 5′′′, J = 8 Hz), 7.66 (d, 2H, H2′′′, 6′′′), 7.74 (d, 4H, H2″,6″,2′,6′, J = 8 Hz), 7.87 (d, 2H, Ar), 7.97 (d, 2H, H3′,5′, J = 8 Hz), 9.84 (brs, 1H, NH), 9.99 (brs, 1H, NH), 10.62 (brs, 1H, NH). 13C NMR (125 MHz, DMSO d6) δ ppm: 14.5, 61.4, 127.0, 128.8, 129.6, 139.3, 144.1, 166.2, 180.1. Calcd for C23H21N3O3S: C, 65.85; H, 5.05; N, 10.02; S, 7.64. Found: C, 65.91, H, 5.07; N, 9.87; S, 7.85.
- Ethyl 4-[2-(3,4,5-trimethoxybenzoyl)hydrazine-1-carbothioamido]benzoate (10). White solid, yield: 86%, mp: 196–198 °C. IR (cm−1): 3314 (NH), 3277 (NH), 3161 (CHarom), 2984 (CHali), 1728 (C=O), 1663 (C=C), 1610 (C=C), 1281 (C-O). 1H NMR (100 MHz, DMSO d6) δ ppm: 1.35 (t, 3H, CH3, J = 7 Hz), 3.77 (s, 3H, OCH3), 3.88 (s, 6H, OCH3), 4.33 (c, 2H, CH2O, J = 7 Hz), 7.33 (s, 2H, H2″,6″,), 7.74 (d, 2H, H2′,6′, J = 8 Hz), 7.95 (d, 2H, H3′,5′, J = 8 Hz), 10.02 (brs, 2H, NH), 10.54 (brs, 1H, NH). 13C NMR (25.8 MHz, DMSO d6) δ ppm: 14.7, 56.7, 60.8, 61.0, 106.2, 124.5, 127.9, 129.7, 144.3, 153.1, 165.8, 180.7. Calcd for C20H23N3O6S: C, 55.42; H, 5.35; N, 9.69; S, 7.40. Found: C, 55.29, H, 5.37; N, 9.93; S, 7.61.
3.3. General Procedure for the Synthesis of 4-[(1,3,4-Thiadiazol-2-yl)amino]benzoic Acid Derivatives 11–16
- 4-{[5-(Pyridin-3-yl)-1,3,4-thiadiazol-2-yl]amino}benzoic Acid (11). White solid, purification by recrystallization in ethanol–water, yield: 87%, mp: >300 °C. IR (cm−1): 3252 (NH), 3187 (NH), 3056 (CHarom), 2741 (OH), 1681 (C=O), 1601 (C=C), 1557 (C=N), 1418 (OH), 1268 (C-O). 1H NMR (500 MHz, DMSO d6) δ ppm: 7.55 (t, 1H, H5″, J = 5 Hz), 7.72 (d, 2H, H3′,5′, J = 8 Hz), 7.92 (d, 2H, H2′,6′, J = 8 Hz), 8.23 (d, 1H, H4″, J = 7 Hz), 8.65 (brs, 1H, H6″), 9.02 (brs, 1H, H2″), 10.99 (brs, 1H, COOH). 13C NMR (125 MHz, DMSO d6) δ ppm: 117.3, 124.9, 131.3, 133.6, 134.8, 139.1, 147.7, 151.5, 156.5, 161.1, 167.4. Calcd for C14H10N4O2S: C, 56.37; H, 3.38; N, 18.78; S, 10.75. Found: C, 56.31, H, 3.39; N, 18.95; S, 10.67.
- 4-{[5-(3-Chlorophenyl)-1,3,4-thiadiazol-2-yl]amino}benzoic Acid (12). Light yellow solid, purification by recrystallization in ethanol–water, yield: 75%, mp: 232–234 °C. IR (cm−1): 3255 (NH), 3187 (NH), 3056 (CHarom), 2981 (OH), 1674 (C=O), 1605 (C=C), 1553 (C=N), 1422 (OH), 1293 (C-O). 1H NMR (500 MHz, DMSO d6) δ ppm: 7.50–755 (m, 2H, H4″,6″), 7.75 (d, 2H, H3′,5′, J = 8 Hz), 7.79 (t, 1H, H5″, J = 8 Hz), 7.88 (s, 1H, H2″), 7.93 (d, 2H, H2′,6′, J = 8 Hz), 11.11 (brs, 1H, COOH). 13C NMR (125 MHz, DMSO d6) δ ppm: 117.3, 124.4, 126.2, 126.5, 130.6, 131.3, 131.7, 132.5, 134.4, 144.5, 157.6, 164.4, 167.4. Calcd for C15H10ClN3O2S: C, 54.30; H, 3.04; N, 12.67; S, 9.66. Found: C, 54.38, H, 2.98; N, 12.63; S, 9.78.
- 4-{[5-(4-Chlorophenyl)-1,3,4-thiadiazol-2-yl]amino}benzoic Acid (13). White solid, purification by recrystallization in ethanol–water, yield: 71%, mp: > 300 °C. IR (cm−1): 3248 (NH), 3187 (NH), 3174 (CHarom), 2920 (OH), 1640 (C=O), 1560 (C=N), 1480 (C=C), 1240 (C-O). 1H NMR (270 MHz, DMSO d6) δ ppm: 7.59 (d, 2H, Ar), 7.76 (d, 2H, H3′,5′, J = 8 Hz), 7.90 (d, 2H, Ar, J = 8 Hz), 7.97 (d, 2H, H2′,6′, J = 8 Hz), 10.98 (brs, 1H, NH), 12.70 (brs, 1H, COOH). 13C NMR (67.9 MHz, DMSO d6) δ ppm: 117.3, 124.4, 129.1, 129.5, 129.9, 131.4, 135.5, 144.6, 158.2, 164.2, 167.5. Calcd for C15H10ClN3O2S: C, 54.30; H, 3.04; N, 12.67; S, 9.66. Found: C, 54.27, H, 3.09; N, 12.81; S, 9.93.
- 4-[(5-Phenyl-1,3,4-thiadiazol-2-yl)amino]benzoic Acid (14). White crystalline solid, purification by recrystallization in ethanol–water, yield: 95%, mp: >300 °C. IR (cm−1): 3281 (NH), 3207 (NH), 3089 (CHarom), 2981 (OH), 1689 (C=O), 1611 (C=C), 1501 (OH), 1249 (C-O). 1H NMR (270 MHz, DMSO d6) δ ppm: 7.50–7.52 (m, 3H, Ar), 7.78 (d, 2H, H2′,6′, J = 8 Hz), 7.88–7.89 (m, 2H, Ar), 7.96 (d, 2H, H3′,5′, J = 8 Hz), 10.98 (brs, 1H, NH), 12.29 (brs, 1H, COOH). 13C NMR (67.9 MHz, DMSO d6) δ ppm: 117.3, 124.5, 127.5, 129.9, 130.7, 131.0, 131.4, 144.8, 159.2, 163.9, 167.5. Calcd for C15H11N3O2S: C, 60.59; H, 3.73; N, 14.13; S, 10.78. Found: C, 60.64, H, 3.73; N, 14.47; S, 10.87.
- 4-{[5-((1,1’-Biphenyl)-4-yl)-1,3,4-thiadiazol-2-yl]amino}benzoic Acid (15). White crystalline solid, purification by recrystallization in ethanol–water, yield: 84%, mp: >300 °C. IR (cm−1): 3281 (NH), 3207 (NH), 3089 (CHarom), 2981 (OH), 1689 (C=O), 1611 (C=C), 1501 (OH), 1249 (C-O). 1H NMR (500 MHz, DMSO d6) δ ppm: 7.68–7.72 (m, 5H, Ar), 7.75 (d, 2H, H3′,5′, J = 8 Hz), 7.82 (d, 2H, H3″,5″, J = 8 Hz), 7.93–7.95 (m, 4H, Ar), 10.90 (brs, 1H, COOH). 13C NMR (125 MHz, DMSO d6) δ ppm: 117.2, 126.5, 126.8, 127.9, 128.0, 129.7, 131.3, 139.4, 141.9, 148.5, 158.7, 163.9, 167.6. Calcd for C21H15N3O2S: C, 67.54; H, 4.05; N, 11.25; S, 8.59. Found: C, 67.43, H, 3.97; N, 11.43; S, 8.71.
- 4-{[5-(3,4,5-Trimethoxyphenyl)-1,3,4-thiadiazol-2-yl]amino}benzoic Acid (16). Brown solid, purification by recrystallization in ethanol–water, yield: 91%, mp: >300 °C. IR (cm−1): 3249 (NH), 3199 (NH), 3048 (CHarom), 2982 (OH), 1687 (C=O), 1601 (C=C), 1415 (OH), 1286 (C-O). 1H NMR (500 MHz, DMSO d6) δ ppm: 3.67 (s, 3H, OCH3), 3.79 (s, 3H, OCH3), 3.81 (s, 3H, OCH3), 6.09–7.06 (m, 3H, Ar), 7.75 (d, 2H, H2′,6′, J = 8 Hz), 11.54 (brs, 1H, COOH). 13C NMR (125 MHz, DMSO d6) δ ppm: 56.5, 56.6, 60.9, 104.8, 117.1, 120.8, 123.8, 126.1, 131.2, 138.4, 144.8, 148.6, 153.7, 159.7, 163.7, 167.6. Calcd for C18H17N3O5S, C, 55.81; H, 4.42; N, 10.85; S, 8.28. Found: C, 55.78, H, 4.49; N, 11.09; S, 8.53.
3.4. General Procedure for the Synthesis of 2-(2-Methyl-5-nitro-1H-imidazol-1-yl)ethyl 4-[(5-phenyl-1,3,4-thiadiazol-2-yl)amino]benzoate Derivatives 18–23
- 2-(2-Methyl-5-nitro-1H-imidazol-1-yl)ethyl-4-[(5-(pyridin-3-yl)-1,3,4-thiadiazol-2-yl]amino)benzoate (18). Beige solid, recrystallization from ethanol–water, yield: 55%, mp: 232–234 °C. IR (cm−1): 3273 (NH), 3199 (NH), 3057 (CHarom), 3000–2800 (CHali), 1715 (C=O), 1610 (C=C), 1491 (C=C), 1270 (C-O). 1H NMR (500 MHz, DMSO d6) δ ppm: 2.45 (s, 3H, CH3), 4.59 (t, 2H, CH2O, J = 5 Hz), 4.70 (t, 2H, CH2N, J = 5 Hz), 7.54 (m, 1H, H5”), 7.74 (d, 2H, H3′,5′, J = 9 Hz), 7.84 (d, 2H, H2′,6′, J = 9 Hz), 8.03 (s, 1H, H3), 8.25 (m, 1H, H4”), 8.67 (s, 1H, H2”), 9.05 (m, 1H, H6″), 11.06 (brs, 1H, NH). 13C NMR (125 MHz, DMSO d6) δ ppm: 14.4, 45.3, 62.9, 117.4, 122.4, 131.1, 133.6, 134.7, 145.1, 147.9, 151.6, 156.5, 164.3, 165.3. Calcd for C20H17N7O4S: C, 53.21; H, 3.80; N, 21.72; S, 7.10. Found: C, 53.32, H, 3.86; N, 21.84; S, 6.91.
- 2-(2-Methyl-5-nitro-1H-imidazol-1-yl)ethyl-4-{[5-(3-chlorophenyl)-1,3,4-thiadiazol-2-yl]amino}benzoate (19). White solid, recrystallization from ethanol–water, yield: 40%, mp: 202–204 °C. IR (cm−1): 3327 (NH), 3199 (NH), 3140–3070 (CHarom), 3000–2800 (CHali), 1690 (C=O), 1604 (C=C), 1496 (C=C), 1276 (C-O). 1H NMR (500 MHz, DMSO d6) δ ppm: 2.48 (s, 3H, CH3), 4.62 (t, 2H, CH2O, J = 5 Hz), 4.73 (t, 2H, CH2N, J = 5 Hz), 7.43 (m, 1H, H5”), 7.47 (d, 2H, H3′,5′, J = 8 Hz), 7.48 (m, 1H, H4″), 7.80 (d, 2H, H2′,6′, J = 8 Hz), 7.91 (m, 2H, H4, H6″), 7.95 (s, 1H, H2″), 11.01 (brs, 1H, NH). 13C NMR (125 MHz, DMSO d6) δ ppm: 14.7, 43.7, 62.3, 116.7, 120.1, 134.9, 129.0, 131.7, 132.9, 134.7, 134.8, 138.4, 144.0, 151.7, 152.7, 165.9, 174.1. Calcd for C21H17ClN6O4S: C, 52.02; H, 3.53; N, 17.33; S, 6.61. Found: C, 51.93, H, 3.61; N, 17.62; S, 6.87.
- 2-(2-Methyl-5-nitro-1H-imidazol-1-yl)ethyl-4-{[5-(4-chlorophenyl)-1,3,4-thiadiazol-yl]amino}benzoate (20). Beige solid, recrystallization from ethanol–water, yield: 54%, mp: 242–244 °C. IR (cm−1): 3273 (NH), 3199 (NH), 3114–3070 (CHarom), 3000–2800 (CHali), 1711 (C=O), 1613 (C=C), 1480 (C=C), 1271 (C-O). 1H NMR (500 MHz, DMSO d6) δ ppm: 2.45 (s, 3H, CH3), 4.58 (t, 2H, CH2O, J = 5 Hz), 4.69 (t, 2H, CH2N, J = 5 Hz), 7.54 (d, 2H, H3”,5”, J = 8,5 Hz), 7.72 (d, 2H, H3′,5′, J = 8.5 Hz), 7.80 (d, 2H, H2′,6′, J = 8.5 Hz), 7.85 (d, 2H, H2”,6”, J = 8.5 Hz), 8.02 (s, 1H, H4), 11.1 (brs, 1H, NH). 13C NMR (125 MHz, DMSO d6) δ ppm: 14.4, 45.3, 62.9, 117.3, 122.3, 128.9, 129.4, 129.8, 131.1, 133.6, 135.5, 139.1, 145.1, 151.9, 158.2, 163.9, 165.3. Calcd for C21H17ClN6O4S: C, 52.02; H, 3.53; N, 17.33; S, 6.61. Found: C, 52.17, H, 3.54; N, 17.29; S, 6.94.
- 2-(2-Methyl-5-nitro-1H-imidazol-1-yl)ethyl-4-[(5-phenyl-1,3,4-thiadiazol-2-yl)amino]benzoate (21). Beige solid, recrystallization from ethanol–water, yield: 60%, mp: 240–242 °C. IR (cm−1): 3277 (NH), 3200 (NH), 3120–3072 (CHarom), 2970 (CHali), 1702 (C=O), 1611 (C=C), 1492 (C=C), 1286 (C-O). 1H NMR (500 MHz, DMSO d6) δ ppm: 2.46 (s, 3H, CH3), 4.60 (t, 2H, CH2O, J = 5 Hz), 4.71 (t, 2H, CH2N, J = 5 Hz), 7.49–7.58 (m, 2H, Ar), 7.74 (d, 2H, H3′,5′, J = 8.5 Hz), 7.83 (d, 2H, H2′,6′, J = 8.5 Hz), 7.85–7.87 (m, 3H, Ar), 10.92 (brs, 1H, NH). 13C NMR (125 MHz, DMSO d6) δ ppm: 14.4, 45.3, 62.9, 117.3, 122.3, 127.4, 129.8, 129.9, 130.6, 130.9, 131.1, 133.6, 145.3, 159.4, 163.8, 165.4. Calcd for C21H18N6O4S: C, 55.99; H, 4.03; N, 18.66; S, 7.12. Found: C, 56.075, H, 4.01; N, 18.83; S, 7.37.
- 2-(2-Methyl-5-nitro-1H-imidazol-1-yl)ethyl-4-{[5-((1,1’-biphenyl)-4-yl)-1,3,4-thiadiazol-2-yl]amino}benzoate (22). Light yellow solid, recrystallization from ethanol–water, yield: 60%, mp: >300 °C. IR (cm−1): 3327 (NH), 3199 (NH), 3140 (CHarom), 3000–2800 (CHali), 1690 (C=O), 1604 (C=C), 1496 (C=C), 1276 (C-O). 1H NMR (500 MHz, DMSO d6) δ ppm: 2.49 (s, 3H, CH3), 4.61 (t, 2H, CH2O, J = 4.5 Hz), 4.74 (t, 2H, CH2N, J = 4.5 Hz), 7.70 (m, 5H, H Ar), 7.84 (d, 2H, H3′,5′, J = 8.5 Hz), 7.88 (2d, 4H, H Ar), 7.93 (d, 2H, H2’,6’, J = 8.5 Hz), 8.05 (s, 1H, H4), 11.04 (brs, 1H, NH). 13C NMR (125 MHz, DMSO d6) δ ppm: 14.3, 44.7, 62.4, 117.5, 121.8, 127.6, 127.9, 129.2, 129.9, 130.7, 132.9, 138.4, 140.6, 145.3, 151.4, 152.1, 163.4, 165.8. Calcd for C27H22N6O4S: C, 61.59; H, 4.21; N, 15.96; S, 6.09. Found: C, 61.75, H, 3.97; N, 15.83; S, 7.28.
- 2-(2-Methyl-5-nitro-1H-imidazol-1-yl)ethyl-4-{[5-(3,4,5-trimethoxyphenyl)-1,3,4-thiadiazol-2-yl]amino}benzoate (23). Yellow solid, recrystallization from ethanol–water, yield: 66%, mp: 252 °C. IR (cm−1): 3278 (NH), 3192 (NH), 3061 (CHarom), 3000–2820 (CHali), 1731 (C=O), 1604 (C=C), 1496 (C=C), 1267 (C-O). 1H NMR (500 MHz, DMSO d6) δ ppm: 2.48 (s, 3H, CH3), 3.70 (s, 3H, OCH3), 3.84 (s, 6H, OCH3), 4.59 (t, 2H, CH2O, J = 5 Hz), 4.70 (t, 2H, CH2N, J = 5 Hz), 7.12 (s, 2H, H6”), 7.39 (d, 2H, H3’,5’, J = 8 Hz), 7.74 (d, 2H, H2’,6’, J = 8 Hz), 8.11 (s, 1H, H4), 11.06 (brs, 1H, NH). 13C NMR (125 MHz, DMSO d6) δ ppm: 14.3, 45.2, 56.1, 60.8, 62.3, 105.3, 117.8, 122.1, 127.3, 130.7, 132.2, 138.4, 139.2, 144.8, 147.4, 152.7, 153.4, 164.5, 165.7. Calcd for C24H24N6O7S: C, 53.33; H, 4.48; N, 15.55; S, 5.93. Found: C, 53.37, H, 4.52; N, 15.71; S, 6.25.
3.5. Biology
3.5.1. Biological Material
3.5.2. In Vitro Evaluation of the Effect of Derivatives 17–22 on the Proliferation of Epimastigotes of Trypanosoma cruzi: MTT Test
3.5.3. In Vitro Evaluation of the Effect of Derivatives 17–22 on the Proliferation of Promastigotes of Leishmania donovani: MTT Test
3.5.4. Estimation of Half Maximal Inhibitory Concentration (IC50) of Derivatives 19, 20, and 21 on Epimastigotes of T. cruzi and Promastigotes of L. donovani: MTT Test
3.5.5. Host Cell Toxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/9789240010352 (accessed on 7 March 2024).
- World Health Organization. Available online: http://www.who.int/chagas/en/ (accessed on 5 March 2024).
- Castillo-Riquelme, M. Chagas disease in non-endemic countries. Lancet Glob. Health 2017, 5, e379–e380. [Google Scholar] [CrossRef]
- World Health Organization|Epidemiology, WHO. 2021. Available online: http://www.who.int/chagas/epidemiology/en/ (accessed on 5 March 2024).
- Coura, J.R.; Borges-Pereira, J. Chagas disease: 100 years after its discovery. A systemic review. Acta Tropica. 2010, 115, 5–13. [Google Scholar] [CrossRef]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Santos-Cruz, L.F.; Ramírez-Cruz, B.G.; García-Salomé, M.; Olvera-Romero, Z.Y.; Hernández-Luis, F.; Hernández-Portilla, L.B.; Durán-Díaz, Á.; Dueñas-García, I.E.; Castañeda-Partida, L.; Piedra-Ibarra, E.; et al. Genotoxicity assessment of four novel quinazoline-derived trypanocidal agents in the Drosophila wing somatic mutation and recombination test. Mutagenesis 2020, 35, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Symptoms, Transmission, and Current Treatments for Chagas Disease DNDi. 2020. Available online: https://dndi.org/diseases/chagas/facts/ (accessed on 7 March 2024).
- Ribeiro, V.; Dias, N.; Paiva, T.; Hagström-Bex, L.; Nitz, N.; Pratesi, R.; Hecht, M. Current trends in the pharmacological management of Chagas disease. Int. J. Parasitol. Drugs Drug Resi. 2020, 12, 7–17. [Google Scholar] [CrossRef]
- Fexinidazole for Chagas DNDi. 2020. Available online: https://dndi.org/research-development/portfolio/fexinidazole-chagas/ (accessed on 7 March 2024).
- Torrico, F.; Gascón, J.; Ortiz, L.; Pinto, J.; Rojas, G.; Palacios, A.; Barreira, F.; Blum, B.; Schijman, A.G.; Vaillant, M.; et al. A Phase 2, randomized, multicenter, placebo-controlled, proof-of-concept trial of oral fexinidazole in adults with chronic indeterminate Chagas Disease. Clin. Infect. Dis. 2023, 76, e1186–e1194. [Google Scholar] [CrossRef]
- Do Vale Chaves e Mello, F.; Castro Salomão Quaresma, B.M.; Resende Pitombeira, M.C.; Araújo de Brito, M.; Farias, P.P.; Lisboa de Castro, S.; Salomão, K.; Silva de Carvalho, A.; Oliveira de Paula, J.I.; de Brito Nascimento, S.; et al. Novel nitroimidazole derivatives evaluated for their trypanocidal, cytotoxic, and genotoxic activities. Eur. J. Med. Chem. 2020, 186, 111887. [Google Scholar] [CrossRef]
- Patterson, S.; Wyllie, S. Nitro drugs for the treatment of trypanosomatid diseases: Past, present, and future prospects. Trends Parasitol. 2014, 30, 289–298. [Google Scholar] [CrossRef]
- Boechat, N.; Carvalho, A.S.; Salomão, K.; de Castro, S.L.; Araujo-Lima, C.F.; Mello, F.V.; Felzenszwalb, I.; Aiub, C.A.; Conde, T.R.; Zamith, H.P.; et al. Studies of genotoxicity and mutagenicity of nitroimidazoles: Demystifying this critical relationship with the nitro group. Mem. Inst. Oswaldo Cruz. 2015, 110, 492–499. [Google Scholar] [CrossRef]
- Mello, F.V.C.; Carvalho, A.S.; Bastos, M.M.; Boechat, N.; Aiub, C.A.F.; Felzenszwalb, I. Evaluation of genotoxic effects of new molecules with possible trypanocidal activity for Chagas disease treatment. Sci. World J. 2013, 2013, 287319. [Google Scholar] [CrossRef]
- De Carvalho, A.S.; Salomão, K.; de Castro, S.L.; Conde, T.R.; da Silva Zamith, H.P.; Caffarena, E.R.; Hall, B.S.; Wilkinson, S.R.; Boechat, N. Megazol and its bioisostere 4H-1,2,4-triazole: Comparing the trypanocidal, cytotoxic and genotoxic activities and their in vitro and in silico interactions with the Trypanosoma brucei nitroreductase enzyme. Mem. Inst. Oswaldo Cruz 2014, 109, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E.J. A Química Medicinal e o paradigma do composto-protótipo. Rev. Virtual Quim. 2009, 1, 26–34. [Google Scholar] [CrossRef]
- Zauli-Nascimento, R.C.; Miguel, D.C.; Yokoyama-Yasunaka, J.K.; Pereira, L.I.; Pelli de Oliveira, M.A.; Ribeiro-Dias, F.; Dorta, M.L.; Uliana, S.R. In vitro sensitivity of Leishmania (Viannia) braziliensis and Leishmania (Leishmania) amazonensis Brazilian isolates to meglumine antimoniate and amphotericin B. Trop. Med. Int. Health 2010, 15, 68–76. [Google Scholar] [CrossRef]
- Chakravarty, J.; Sundar, S. Drug resistance in leishmaniasis. J. Glob. Infect. dis. 2010, 2, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Savoia, D. Recent updates and perspectives on leishmaniasis. J. Infect. Dev. Ctries 2015, 9, 588–596. [Google Scholar] [CrossRef]
- Fortin, S.; Bérubé, G. Advances in the development of hybrid anticancer drugs. Expert Opin. Drug Discov. 2013, 8, 1029–1047. [Google Scholar] [CrossRef]
- Morphy, R.; Rankovic, Z. Designed multiple ligands. An emerging drug discovery paradigm. J. Med. Chem. 2005, 48, 6523–6543. [Google Scholar] [CrossRef] [PubMed]
- Pawełczyk, A.; Sowa-Kasprzak, K.; Olender, D.; Zaprutko, L. Molecular consortia—Various structural and synthetic concepts for more effective therapeutics synthesis. Int. J. Mol. Sci. 2018, 19, 1104. [Google Scholar] [CrossRef]
- Sampath, H.M.; Herrmann, L.; Tsogoeva, S.B. Structural hybridization as a facile approach to new drug candidates. Bioorg. Med. Chem. Lett. 2020, 30, 127514. [Google Scholar] [CrossRef]
- Soltan, O.M.; Shoman, M.F.; Abdel-Aziz, S.A.; Narumi, A.; Konno, H.; Abdel-Aziz, M. Molecular hybrids: A five-year survey on structures of multiple targeted hybrids of protein kinase inhibitors for cancer therapy. Eur. J. Med. Chem. 2021, 225, 113768. [Google Scholar] [CrossRef]
- Leitsch, D. A review on metronidazole: An old warhorse in antimicrobial chemotherapy. Parasitology 2019, 146, 1167–1168. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Kumar, H.; Kumar, V.; Deep, A.; Sharma, A.; Marwaha, M.G.; Marwaha, R.K. Mechanism-based approaches of 1,3,4-thiadiazole scaffolds as potent enzyme inhibitors for cytotoxicity and antiviral activity. Med. Drug Dis. 2023, 17, 100150. [Google Scholar] [CrossRef]
- Rossi, R.; Ciofalo, M. An updated review on the synthesis and antibacterial activity of molecular hybrids and conjugates bearing imidazole moiety. Molecules 2020, 25, 5133. [Google Scholar] [CrossRef]
- Hu, Y.; Li, C.-Y.; Wang, X.-M.; Yang, Y.-H.; Zhu, H.-L. 1,3,4-Thiadiazole: Synthesis, reactions, and applications in medicinal, agricultural, and materials chemistry. Chem. Rev. 2014, 114, 5572–5610. [Google Scholar] [CrossRef]
- Atmaram, U.A.; Roopan, S.M. Biological activity of oxadiazole and thiadiazole derivatives. Appl. Microbiol. Biotechnol. 2022, 106, 3489–3505. [Google Scholar] [CrossRef] [PubMed]
- Segawa, J.; Kitano, M.; Kazuno, K.; Matsuoka, M.; Shirahase, I.; Ozaki, M.; Matsuda, M.; Tomii, Y.; Kise, M. Studies on pyridonecarboxylic acids. 1. Synthesis and antibacterial evaluation of 7-substituted-6-halo-4-oxo-4H[1,3]thiazeto[3,2-a]quinoline-3-carboxylic acids. J. Med. Chem. 1992, 35, 4727–4738. [Google Scholar] [CrossRef]
- Vinkler, E.; Klinényi, F.; Stájer, G.; Ferenczy, L. Contibutions to the synthesis of mustard oil carbonic acid esters of antimicronial action. Acta Pharm. Hung. 1967, 37, 250–253. [Google Scholar] [PubMed]
- Rollas, S.; Karakuş, S.; Barlas–Durgun, B.; Kiraz, M.; Erdeniz, H. Synthesis and antimicrobial activity of some 1,4-disubstituted thiosemicarbazide and 2,5-disubstituted-1,3,4-thiadiazole derivatives. Farmaco 1996, 51, 811–814. [Google Scholar] [CrossRef]
- Hoggarth, E. Compounds related to thiosemicarbazide. Part II. 1-Benzoylthiosemicarbazides. J. Chem. Soc. 1949, 1163–1167. [Google Scholar] [CrossRef]
- Neises, B.; Steglich, W. Simple method for the esterification of carboxylic acids. Angew. Chem. Int. Engl. 1978, 17, 522–524. [Google Scholar] [CrossRef]
- Zingales, B.; Andrade, S.G.; Briones, M.R.S.; Campbell, D.A.; Chiari, E.; Fernandes, O.; Guhl, F.; Lages-Silva, E.; Macedo, A.M.; Machado, C.R.; et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: Second revision meeting recommends TcI to TcVI. Mem. Inst. Oswaldo Cruz 2009, 104, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Mijoba, A.; Fernandez-Moreira, E.; Parra-Giménez, N.; Espinosa-Tapia, S.; Blanco, Z.; Ramírez, H.; Charris, J.E. Synthesis of Benzocycloalkanone-based Michael Acceptors and Biological Activities as Antimalarial and Antitrypanosomal Agents. Molecules 2023, 28, 5569. [Google Scholar] [CrossRef] [PubMed]
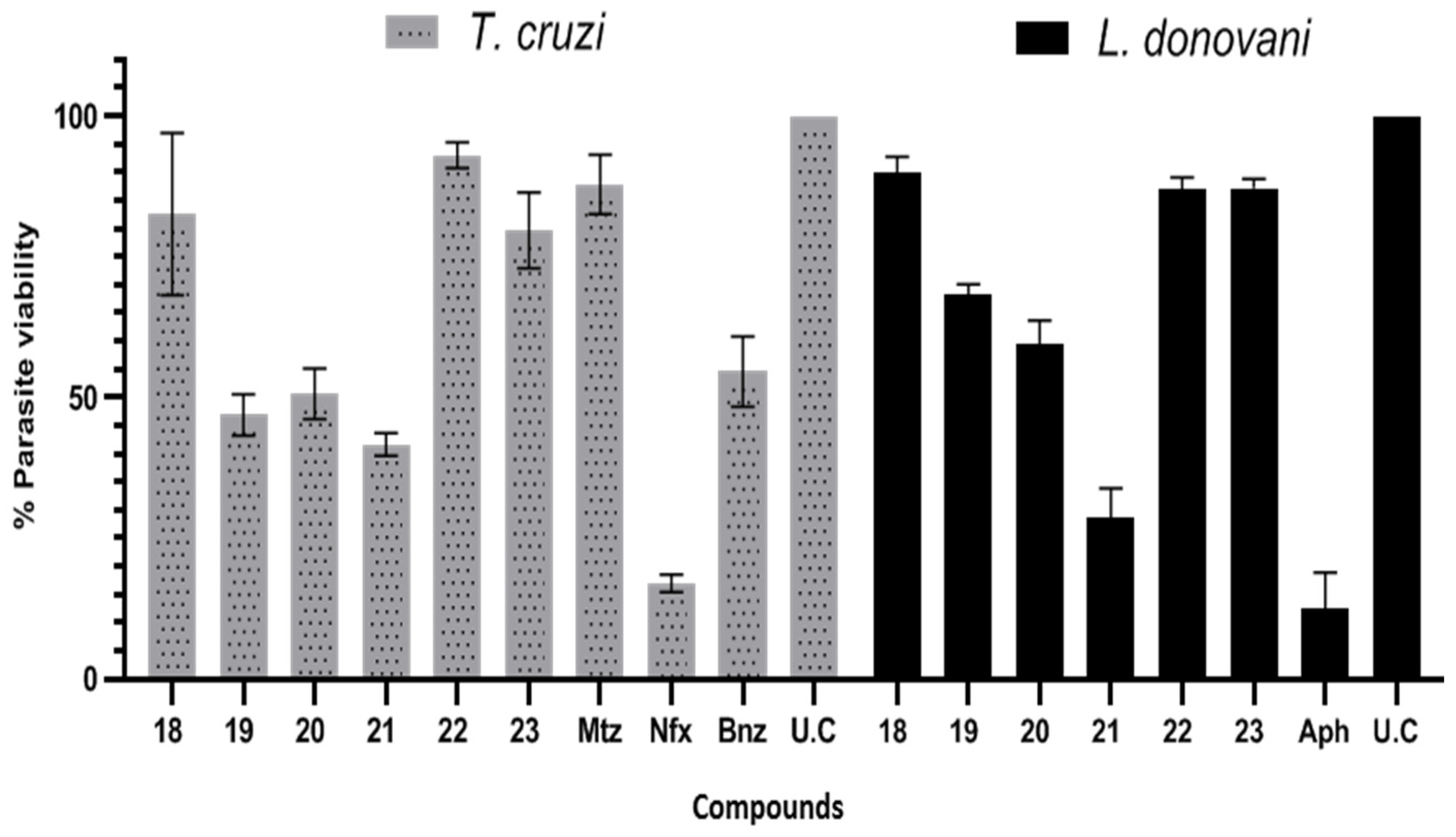
| T. cruzi | L. donovani | IC50 μM T. cruzi | IC50 μM L. donovani | |
|---|---|---|---|---|
| No | % Inhibition ± SD | |||
| 18 | 17.44 ± 14.42 | 10.08 ± 2.92 | nd | nd |
| 19 | 53.10 ± 3.68 | 31.77 ± 1.95 | 56.34 ± 6.83 | 59.72 ± 8.82 |
| 20 | 49.37 ± 4.45 | 40.38 ± 4.18 | 51.70 ± 6.59 | 54.48 ± 8.51 |
| 21 | 58.35 ± 2.05 | 71.42 ± 5.28 | 55.48 ± 7.20 | 10.07 ± 2.21 |
| 22 | 7.00 ± 2.28 | 12.95 ± 2.08 | nd | nd |
| 23 | 20.30 ± 6.73 | 12.74 ± 2.31 | nd | nd |
| Mtz | 12.18 ± 5.30 | -- | 180.20 ± 18.9 | 50.31 ± 11.9 |
| Bnz | 45.39 ± 6.21 | -- | nd | -- |
| Nfx | 83.00 ± 1.60 | -- | 1.81 ± 0.45 | -- |
| Aph | -- | 62.44 ± 6.60 | -- | 0.33 ± 0.02 |
| No | CC50 (μM) | T. cruzi (SI) | L. donovani (SI) |
|---|---|---|---|
| 19 | >500 | >8.87 | >8.37 |
| 20 | >500 | >9.67 | >9.17 |
| 21 | >500 | >9.01 | >49.65 |
| Nfx | >90 | 48.65 | -- |
| Aph | >250 | -- | 757.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mijoba, A.; Parra-Giménez, N.; Fernandez-Moreira, E.; Ramírez, H.; Serrano, X.; Blanco, Z.; Espinosa, S.; Charris, J.E. Synthesis of Hybrid Molecules with Imidazole-1,3,4-thiadiazole Core and Evaluation of Biological Activity on Trypanosoma cruzi and Leishmania donovani. Molecules 2024, 29, 4125. https://doi.org/10.3390/molecules29174125
Mijoba A, Parra-Giménez N, Fernandez-Moreira E, Ramírez H, Serrano X, Blanco Z, Espinosa S, Charris JE. Synthesis of Hybrid Molecules with Imidazole-1,3,4-thiadiazole Core and Evaluation of Biological Activity on Trypanosoma cruzi and Leishmania donovani. Molecules. 2024; 29(17):4125. https://doi.org/10.3390/molecules29174125
Chicago/Turabian StyleMijoba, Ali, Nereida Parra-Giménez, Esteban Fernandez-Moreira, Hegira Ramírez, Xenón Serrano, Zuleima Blanco, Sandra Espinosa, and Jaime E. Charris. 2024. "Synthesis of Hybrid Molecules with Imidazole-1,3,4-thiadiazole Core and Evaluation of Biological Activity on Trypanosoma cruzi and Leishmania donovani" Molecules 29, no. 17: 4125. https://doi.org/10.3390/molecules29174125
APA StyleMijoba, A., Parra-Giménez, N., Fernandez-Moreira, E., Ramírez, H., Serrano, X., Blanco, Z., Espinosa, S., & Charris, J. E. (2024). Synthesis of Hybrid Molecules with Imidazole-1,3,4-thiadiazole Core and Evaluation of Biological Activity on Trypanosoma cruzi and Leishmania donovani. Molecules, 29(17), 4125. https://doi.org/10.3390/molecules29174125








