Controlling and Tuning the Dispersion Properties of Calcined Kaolinite Particles in Various Organic Solvents via the Modification Method Using Triethoxyvinylsilane and 3-Mercaptopropionic Acid
Abstract
1. Introduction
2. Results and Discussion
2.1. FT-IR Spectra of Kaolinite and Modified Kaolinite (Kg)
2.2. Thermal Analysis
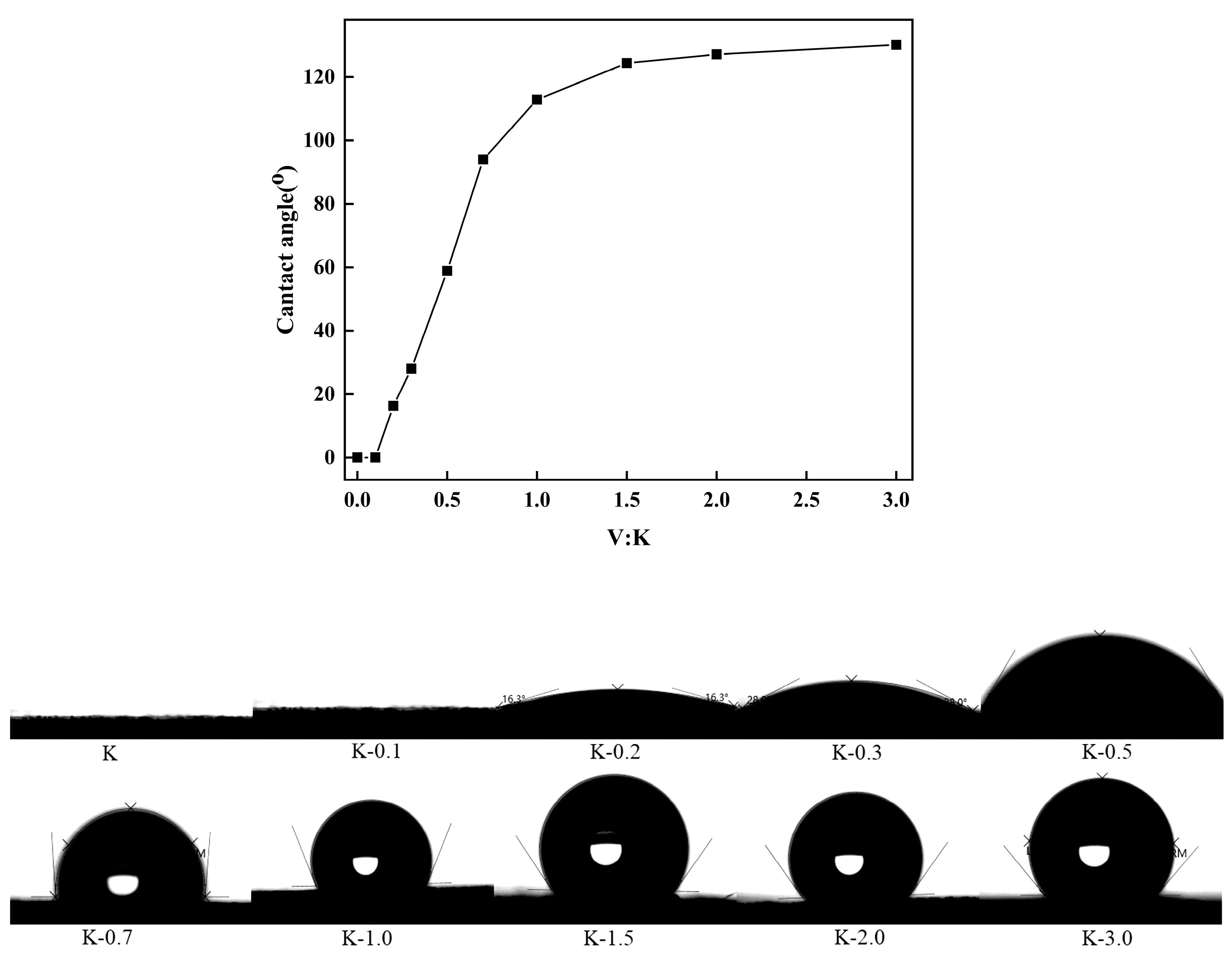
2.3. Water Contact Angle Analysis
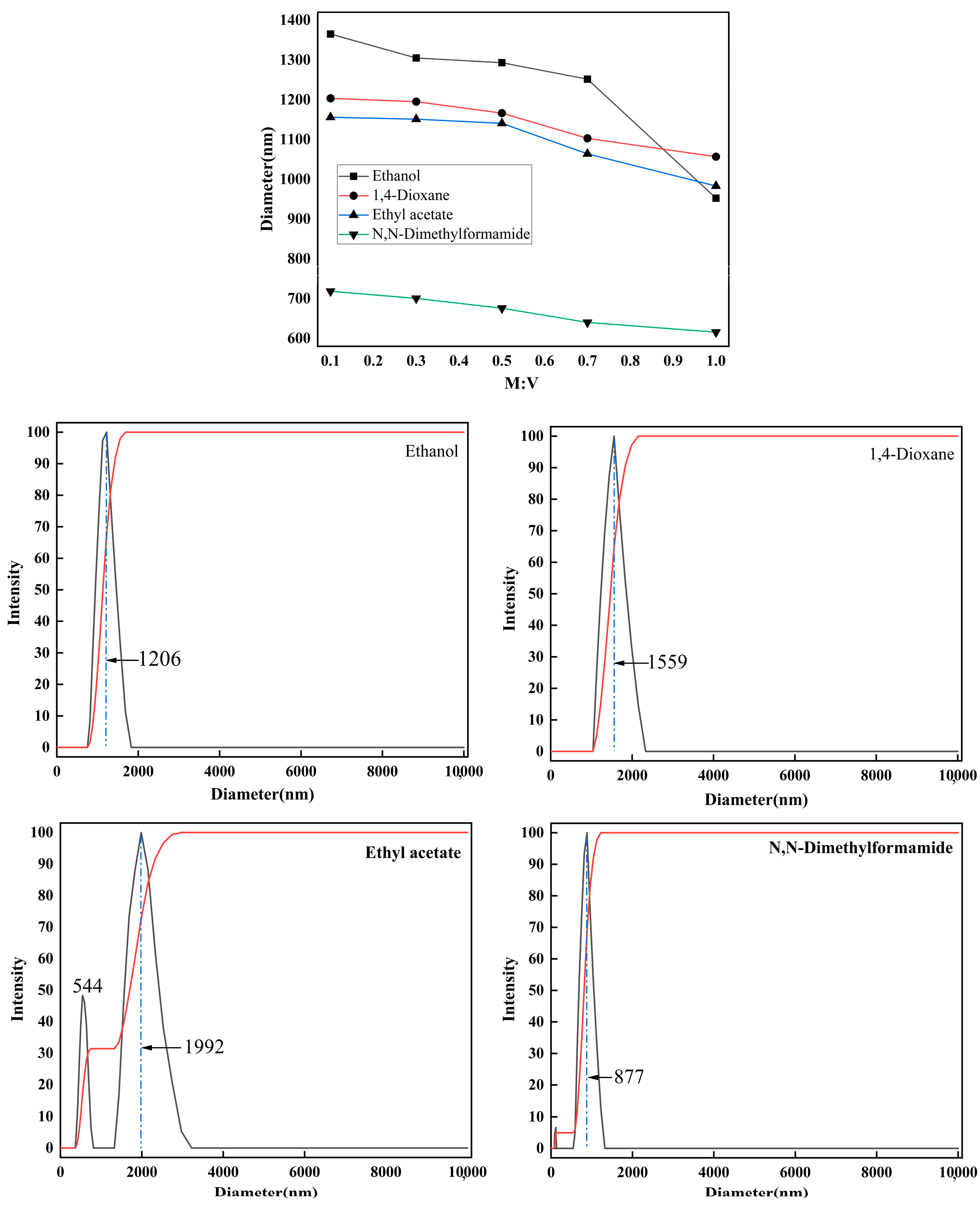
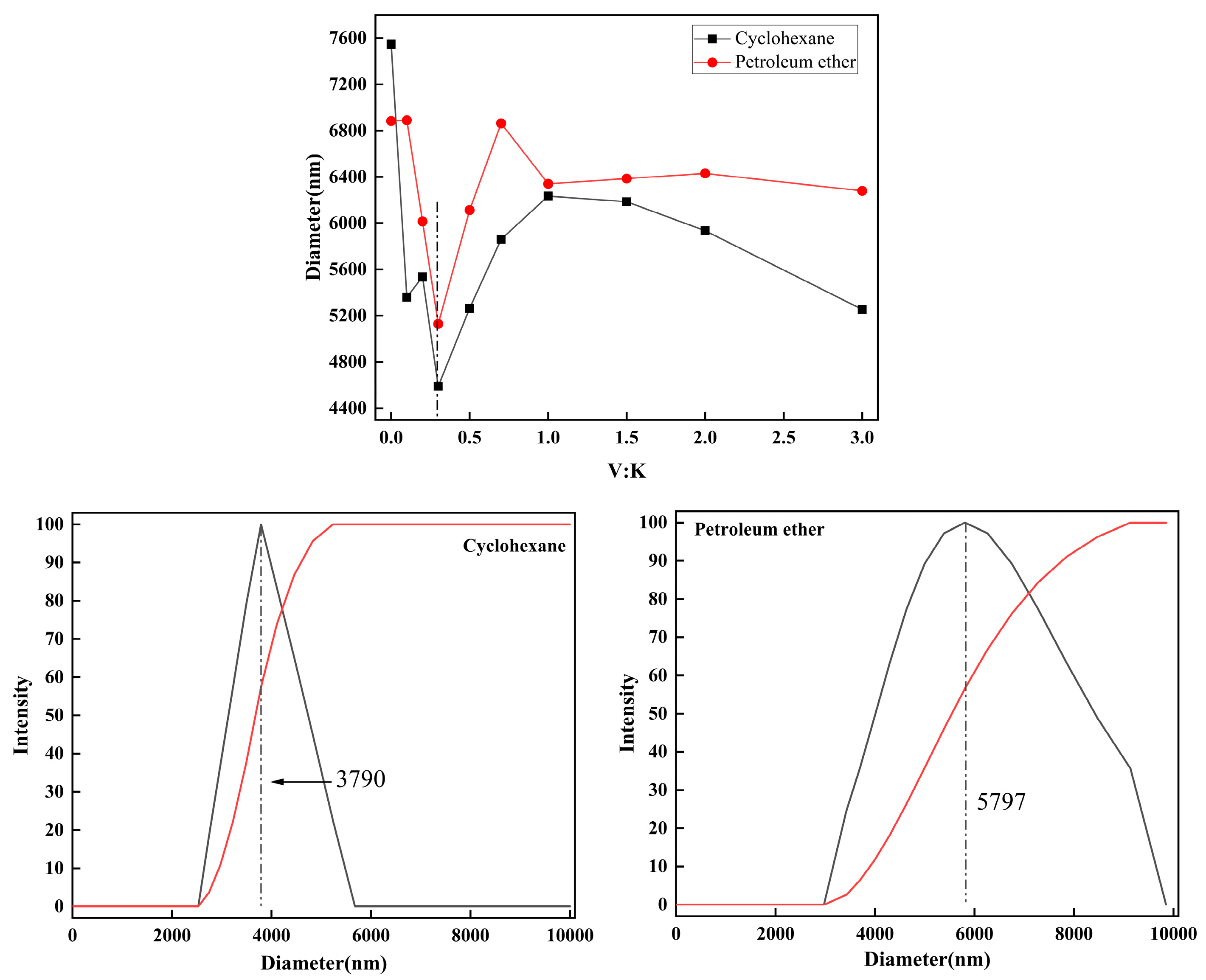
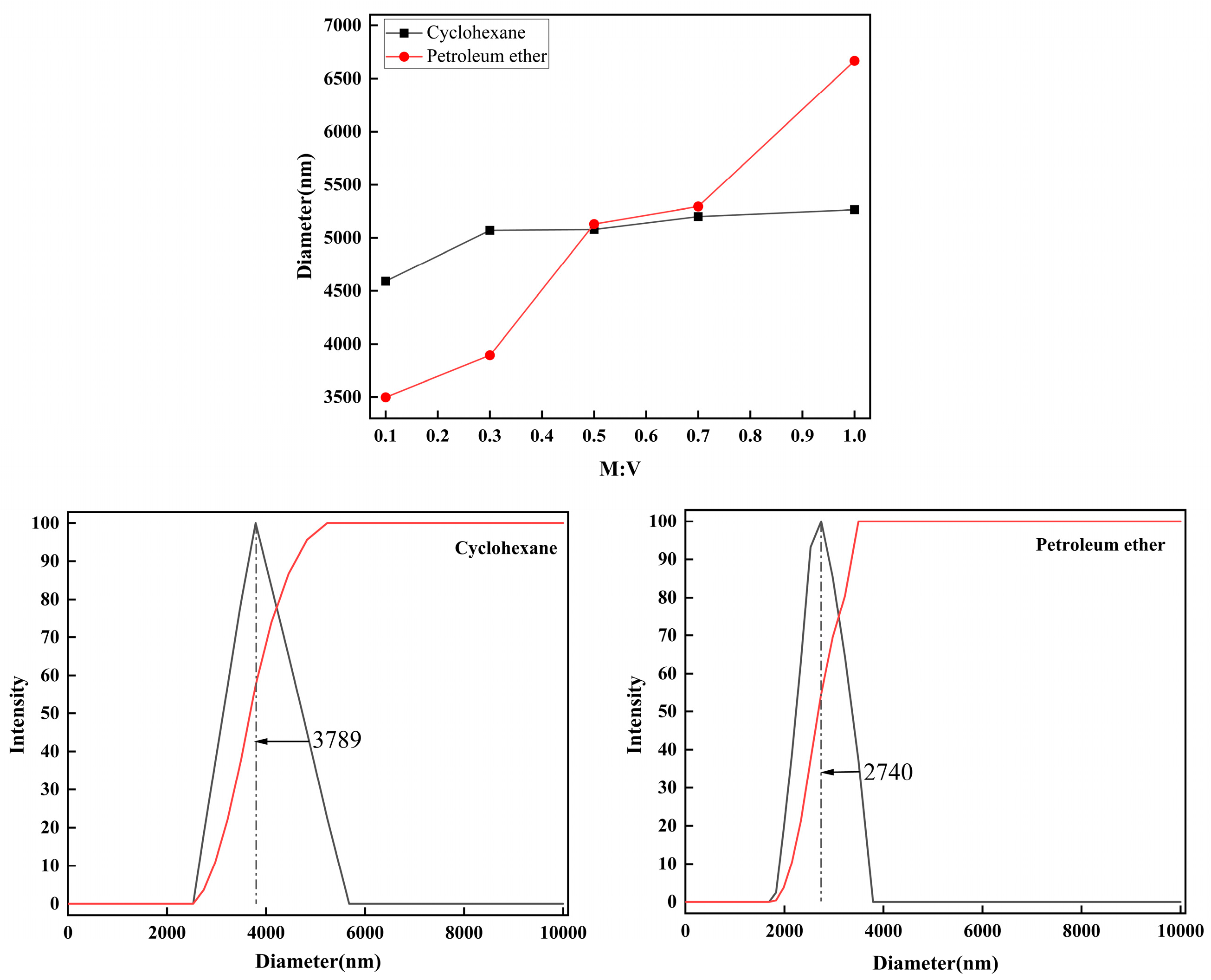
2.4. Dispersion Properties of Kg Particles in Various Organic Solvents
2.4.1. Kg Dispersibility in Polar Solvents
2.4.2. Dispersion of Kg in Non-Polar Solvents
2.5. Scanning Electron Microscopy
3. Experimental
3.1. Materials
3.2. Preparation of Modified Kaolin Particles by One-Pot Method
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yar, A.; Okbaz, A.; Parlayıcı, S. A biocompatible, eco-friendly, and high-performance triboelectric nanogenerator based on sepiolite, bentonite, and kaolin decorated chitosan composite film. Nano Energy 2023, 110, 108354. [Google Scholar] [CrossRef]
- Peter, R.; Sreelekshmi, R.V.; Menon, A.R.R. Cetyltrimethyl ammonium bromide modified kaolin as a reinforcing filler for natural rubber. J. Polym. Environ. 2018, 26, 39–47. [Google Scholar] [CrossRef]
- Wen, X.; Du, C.; Wan, J.; Zeng, G.; Huang, D.; Yin, L.; Deng, R.; Tan, S.; Zhang, J. Immobilizing laccase on kaolinite and its application in treatment of malachite green effluent with the coexistence of Cd (П). Chemosphere 2019, 217, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.; Pan, T.; Wu, F.; Zeng, M.; Huang, D.; Zhang, L.; Jia, L.; Chen, Y.; Cheng, Z. Facile one-step microwave-assisted modification of kaolinite and performance evaluation of pickering emulsion stabilization for oil recovery application. J. Environ. Manag. 2019, 238, 257–262. [Google Scholar] [CrossRef]
- Joshi, P.; Raturi, A.; Srivastava, M.; Khatri, O.P. Graphene oxide, kaolinite clay and PVA-derived nanocomposite aerogel as a regenerative adsorbent for wastewater treatment applications. J. Environ. Chem. Eng. 2022, 10, 108597. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Y.; Zhang, Y.; Gong, Y.; Hao, Z. Preparation and characterization of Kaolinite supported lanthanum-hydroxide and its improvements for natural rubber composites. Appl. Clay Sci. 2022, 216, 106342. [Google Scholar] [CrossRef]
- Méité, N.; Konan, L.K.; Tognonvi, M.T.; Doubi, B.I.H.G.; Gomina, M.; Oyetola, S. Properties of hydric and biodegradability of cassava starch-based bioplastics reinforced with thermally modified kaolin. Carbohydr. Polym. 2020, 254, 117322. [Google Scholar] [CrossRef]
- Rammak, T.; Boonsuk, P.; Kaewtatip, K. Mechanical and barrier properties of starch blend films enhanced with kaolin for application in food packaging. Int. J. Biol. Macromol. 2021, 192, 1013–1020. [Google Scholar] [CrossRef]
- dos Santos, A.J.; Ribeiro, M.M.; Corrêa, A.d.C.; Rodrigues, J.d.S.; Silva, D.S.; Junio, R.F.; Monteiro, S.N. Morphological, chemical and mechanical properties of hybrid polyester composites reinforced with bamboo fibers and kaolin waste. J. Mater. Res. Technol. 2024, 30, 1–15. [Google Scholar] [CrossRef]
- Li, C.; Fu, L.; Ouyang, J.; Tang, A.; Yang, H. Kaolinite stabilized paraffin composite phase change materials for thermal energy storage. Appl. Clay Sci. 2015, 115, 212–220. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, Z.Z.; Yang, M.M.; Yuan, J.; Li, P.; Liu, M.; He, Y. Ag nanoparticles homogeneously anchored on kaolin synergistically improve the tribological performance of PBO/phenolic resin liner composites. Tribol. Int. 2021, 168, 107424. [Google Scholar] [CrossRef]
- Qu, H.; He, S.; Su, H. Efficient preparation of kaolinite/methanol intercalation composite by using a Soxhlet extractor. Sci. Rep. 2019, 9, 8351. [Google Scholar] [CrossRef]
- Sun, Z.; Zhu, R.; Ding, T.; Zhang, X.; Li, C. Induced morphology orientation of α-FeOOH by kaolinite for enhancing peroxymonosulfate activation. J. Colloid Interface Sci. 2022, 626, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Jana, A.; Bathula, C.; Park, Y.; Kadam, A.; Sree, V.G.; Ansar, S.; Kim, H.-S.; Im, H. Facile synthesis and optical study of organic-inorganic lead bromide perovskite-clay (kaolinite, montmorillonite, and halloysite) composites. Surfaces Interfaces 2022, 29, 101785. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Zhang, K.; Liu, L.; Ji, L.; Liu, Q. Vulcanization, interfacial interaction, and dynamic mechanical properties of in-situ organic amino modified kaolinite/SBR nanocomposites based on latex compounding method. Appl. Clay Sci. 2020, 185, 105366. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, H.; Wang, C.; Zhou, Y. Kaolinite nanotube-stearic acid composite as a form-stable phase change material for thermal energy storage. Appl. Clay Sci. 2021, 201, 105930. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, Z.Z.; Liu, M.; He, Y.; Li, P.; Yuan, J.; Yang, M. Effects of rod-like attapulgite and lamellar kaolin reinforcement on the tribological behavior of PBO textile-resin composite liner. Tribol. Int. 2022, 174, 107689. [Google Scholar] [CrossRef]
- Li, M.; Chen, Y.; Wu, L.; Zhang, Z.; Mai, K. A novel polypropylene composite filled by kaolin particles with β-nucleation. J. Therm. Anal. Calorim. 2019, 135, 2137–2145. [Google Scholar] [CrossRef]
- Yan, D.; Ming, W.; Liu, S.; Yin, G.; Zhang, Y.; Tang, B.; Zhang, S. Polyethylene glycol (PEG)/silicon dioxide grafted aminopropyl group and carboxylic multi-walled carbon nanotubes (SAM) composite as phase change material for light-to-heat energy conversion and storage. J. Energy Storage 2021, 36, 102428. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, J.; Guo, L.; Zhang, J.; Feng, S.; Zhang, X. Disproportionated SiOx/C composite anode materials for lithium-ion batteries. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129386. [Google Scholar] [CrossRef]
- Molatlhegi, O.; Alagha, L. Adsorption characteristics of chitosan grafted copolymer on kaolin. Appl. Clay Sci. 2017, 150, 342–353. [Google Scholar] [CrossRef]
- Qu, Y.X.; Fan, D.K.; Li, F.J.; Ouyang, P.; Fu, L.; Yang, H. Exfoliating kaolin to ultrathin nanosheets with high aspect ratio and pore volume: A comparative study of three kaolin clays in China. Appl. Surf. Sci. 2023, 635, 157778. [Google Scholar] [CrossRef]
- Tatang, H.B.; Mbey, J.A.; Sabouang, C.J.N.; Mache, J.R.; Gley, R.; Kong, S. Kaolinites structural defects related to urea and dimethyl sulfoxide intercalation. Appl. Clay Sci. 2024, 255, 107415. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, H. Controlling and tuning the dispersion properties of calcined kaolinite particles in various organic solvents via stepwise modification method using 3-glycidoxypropyltrimethoxysilane and dodecylamine. Appl. Surf. Sci. 2013, 277, 281–287. [Google Scholar] [CrossRef]
- Plueddemann, E.P.; Clark, H.A.; Nelson, L.E.; Hoffmann, K.R. Silane coupling agents for reinforced plastics. Mod. Plast. 1962, 39, 135. [Google Scholar]
- Huang, Z.; Bu, J.; Wang, H. Application of two modified kaolin materials in removing micro-plastics from water. J. Mater. Cycles Waste Manag. 2022, 24, 1460–1475. [Google Scholar] [CrossRef]
- Özcan, A.; Özcan, A.A.; Demirci, Y.; Şener, E. Preparation of Fe2O3 modified kaolin and application in heterogeneous electro-catalytic oxidation of enoxacin. Appl. Catal. B Environ. 2017, 200, 361–371. [Google Scholar] [CrossRef]
- Konduri, M.K.R.; Fatehi, P. Dispersion of kaolin particles with carboxymethylated xylan. Appl. Clay Sci. 2017, 137, 183–191. [Google Scholar] [CrossRef]
- Ge, X.; Chang, M.; Jiang, W.; Zhang, B.; Xing, R.; Bulin, C. Selective location of kaolin and effects of maleic anhydride in kaolin/poly (ε-caprolactone)/poly (lactic acid) composites. Appl. Clay Sci. 2020, 189, 105524. [Google Scholar] [CrossRef]
- Hasan, A.; Fatehi, P. Stability of kaolin dispersion in the presence of lignin-acrylamide polymer. Appl. Clay Sci. 2018, 158, 72–82. [Google Scholar] [CrossRef]
- Iijima, M.; Kamiya, H. Layer-by-layer surface modification of functional nanoparticles for dispersion in organic solvents. Langmuir 2010, 26, 17943–17948. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y. How titanium dioxide cleans itself. Science 2018, 361, 753. [Google Scholar] [PubMed]
- Sun, N.; Wang, J.; Yao, J.; Chen, H.; Deng, C. Magnetite nanoparticles coated with mercaptosuccinic acid-modified mesoporous titania as a hydrophilic sorbent for glycopeptides and phosphopeptides prior to their quantitation by LC-MS/MS. Microchim. Acta 2019, 186, 159. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Li, C.; Dai, L.; Tang, Q.; Cai, X.; Zhen, B.; Xie, X.; Wang, L. Selective modification of kaolinite with vinyltrimethoxysilane for stabilization of Pickering emulsions. Appl. Clay Sci. 2018, 161, 282–289. [Google Scholar] [CrossRef]
- Krainoi, A.; Sripornsawat, B.; Toh-Ae, P.; Kitisavetjit, W.; Pittayavinai, P.; Tangchirapat, W.; Kalkornsurapranee, E.; Johns, J.; Nakaramontri, Y. Utilization of high and low calcium oxide fly ashes as the alternative fillers for natural rubber composites: A waste to wealth approach. Ind. Crops Prod. 2022, 188, 115589. [Google Scholar] [CrossRef]


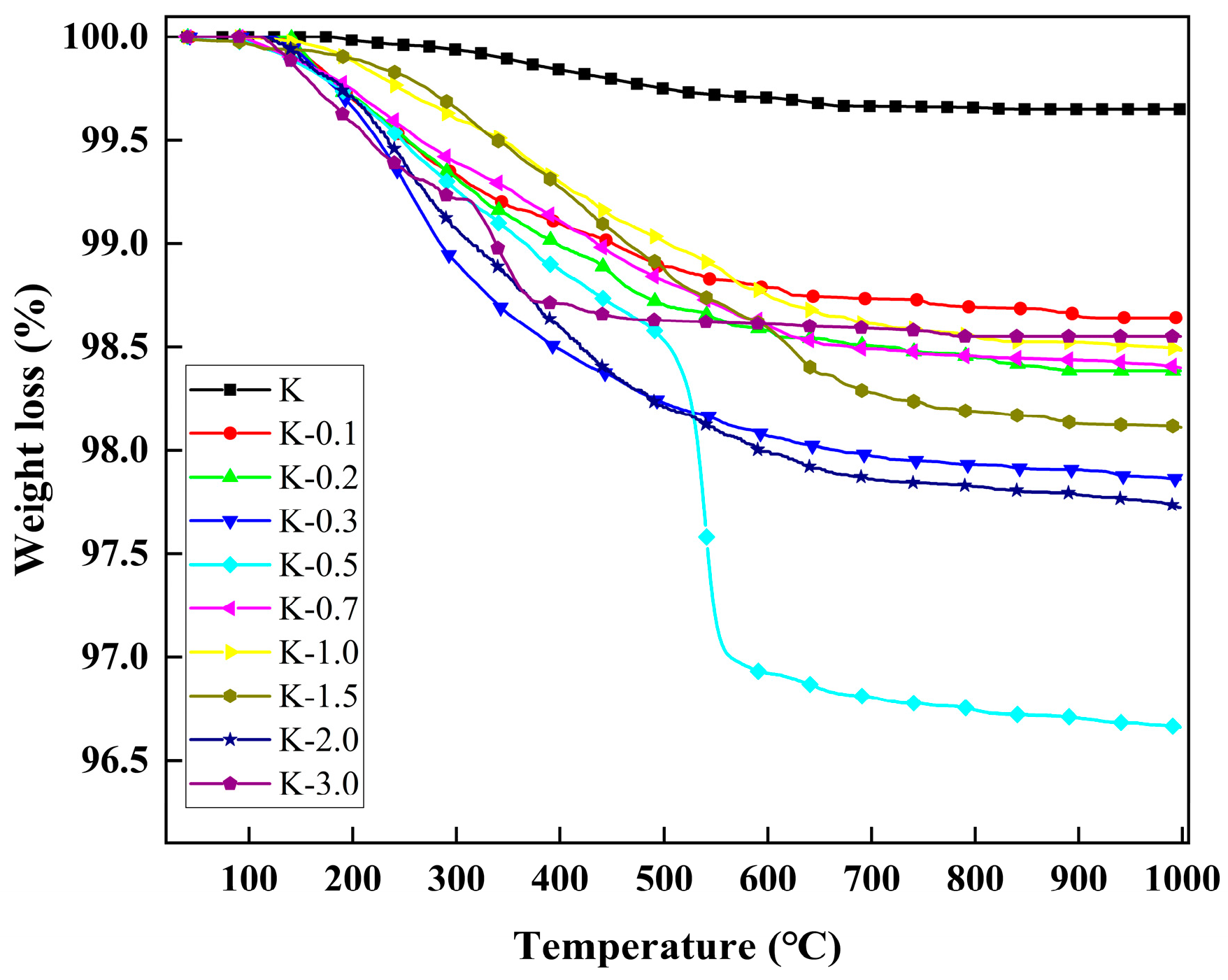
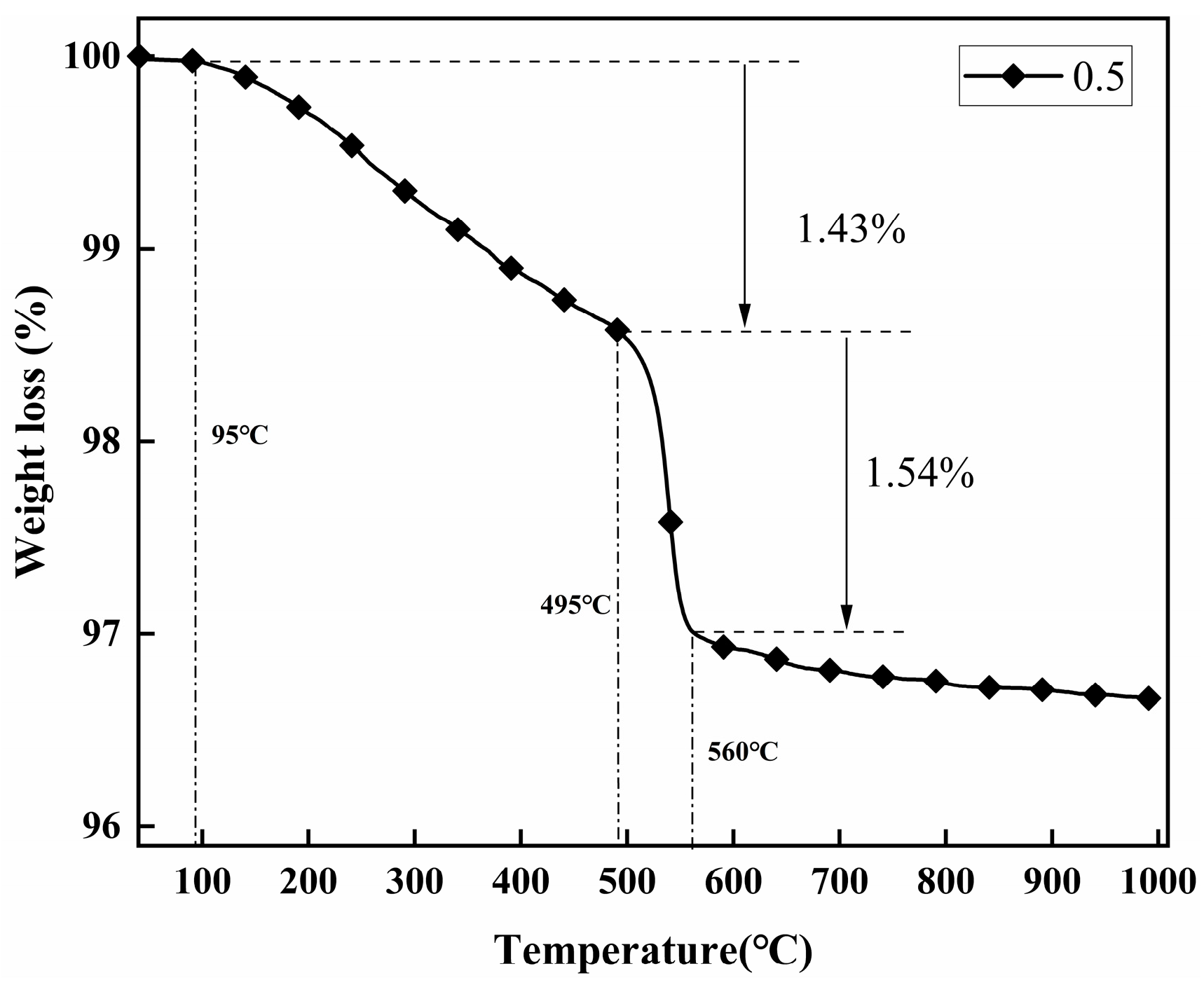
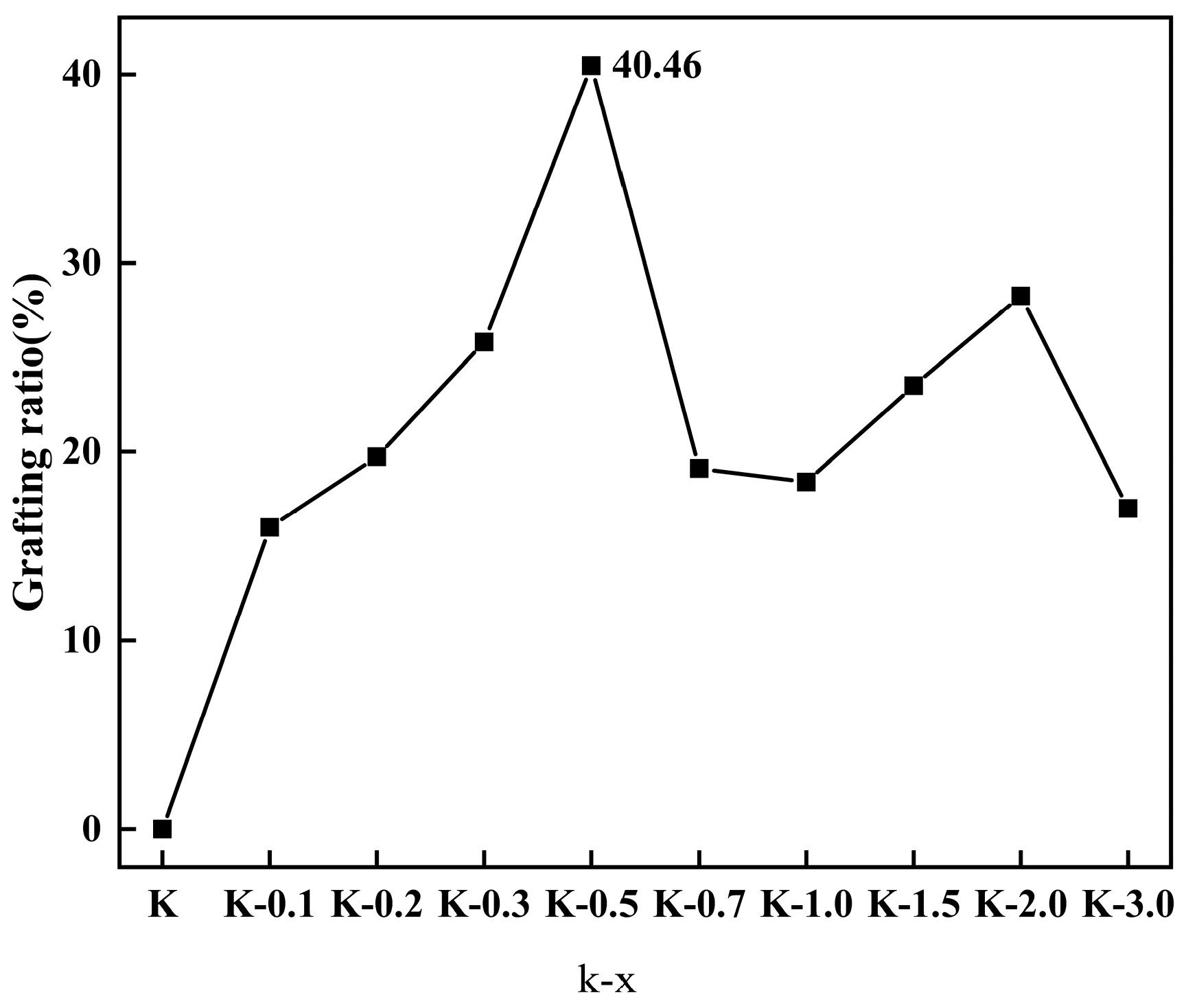
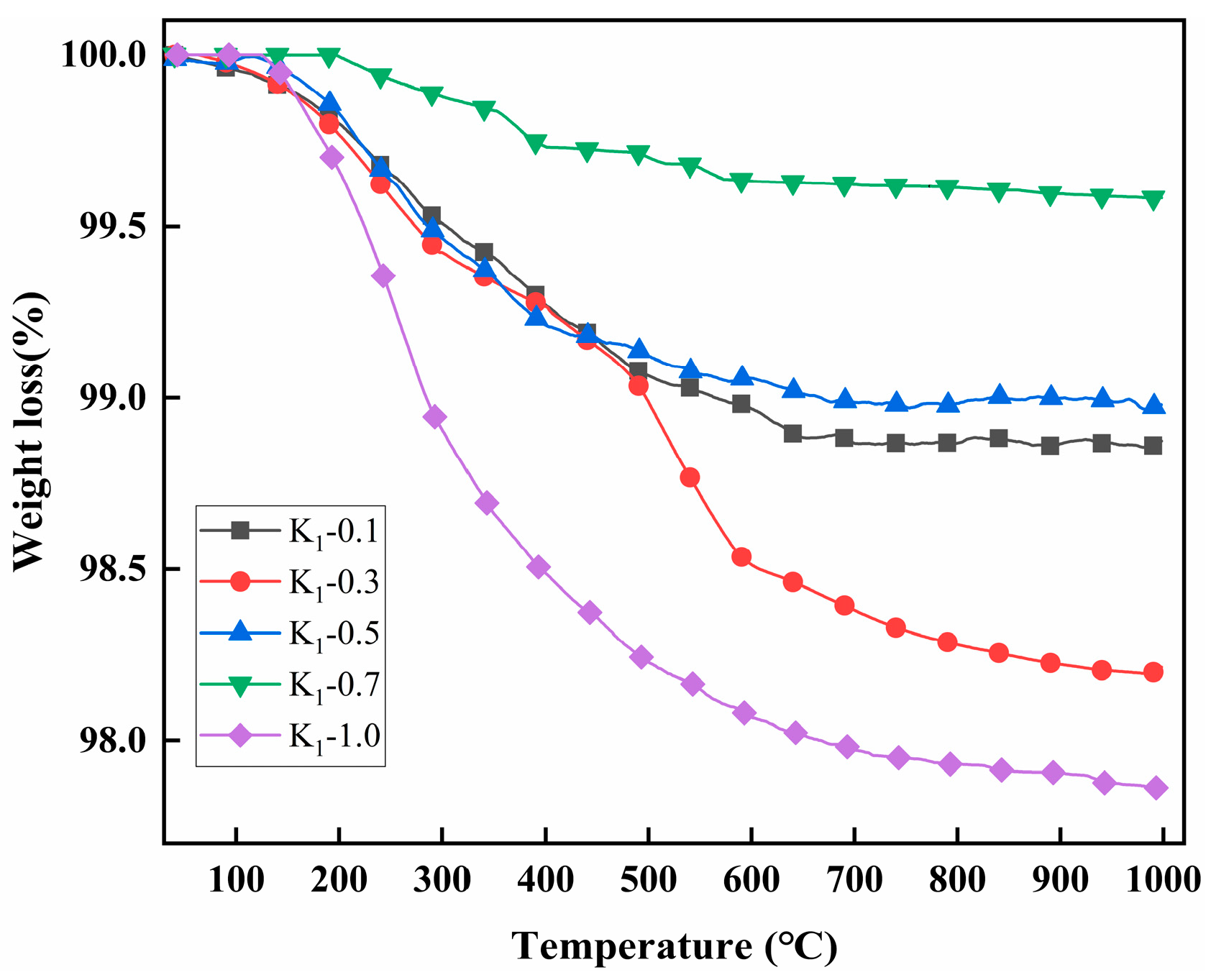



| Samples | M1 (mg) | M2 (mg) | Weight Loss (mg) | Weight (%) | Grafting Ratio (%) |
|---|---|---|---|---|---|
| K | 20.34 | 20.26 | 0.07 | 0.35 | 0.00 |
| K-0.1 | 20.15 | 19.88 | 0.27 | 1.34 | 16.00 |
| K-0.2 | 20.81 | 20.48 | 0.33 | 1.60 | 19.73 |
| K-0.3 | 20.36 | 19.92 | 0.44 | 2.14 | 25.81 |
| K-0.5 | 20.44 | 19.76 | 0.68 | 3.34 | 40.46 |
| K-0.7 | 20.29 | 19.97 | 0.32 | 1.59 | 19.11 |
| K-1.0 | 20.56 | 20.25 | 0.31 | 1.51 | 18.39 |
| K-1.5 | 20.55 | 20.16 | 0.40 | 1.93 | 23.50 |
| K-2.0 | 20.91 | 20.43 | 0.48 | 2.28 | 28.25 |
| K-3.0 | 20.21 | 19.93 | 0.29 | 1.42 | 17.00 |
| Element | Fe2O3 | TiO2 | CaO | K2O | P2O5 | MgO | Na2O | Al2O3 | SiO2 |
|---|---|---|---|---|---|---|---|---|---|
| Con.(%) | 0.419 | 0.678 | 0.177 | 0.263 | 0.382 | 0.206 | 0.424 | 49.48 | 47.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Tang, X.; Shi, J.; Zhou, C.; Li, L.; Sun, H.; Northwood, D.O.; Waters, K.E.; Ma, H. Controlling and Tuning the Dispersion Properties of Calcined Kaolinite Particles in Various Organic Solvents via the Modification Method Using Triethoxyvinylsilane and 3-Mercaptopropionic Acid. Molecules 2024, 29, 4129. https://doi.org/10.3390/molecules29174129
Yuan Y, Tang X, Shi J, Zhou C, Li L, Sun H, Northwood DO, Waters KE, Ma H. Controlling and Tuning the Dispersion Properties of Calcined Kaolinite Particles in Various Organic Solvents via the Modification Method Using Triethoxyvinylsilane and 3-Mercaptopropionic Acid. Molecules. 2024; 29(17):4129. https://doi.org/10.3390/molecules29174129
Chicago/Turabian StyleYuan, Yongbing, Xinyu Tang, Junkang Shi, Congshan Zhou, Lijun Li, Honghong Sun, Derek O. Northwood, Kristian E. Waters, and Hao Ma. 2024. "Controlling and Tuning the Dispersion Properties of Calcined Kaolinite Particles in Various Organic Solvents via the Modification Method Using Triethoxyvinylsilane and 3-Mercaptopropionic Acid" Molecules 29, no. 17: 4129. https://doi.org/10.3390/molecules29174129
APA StyleYuan, Y., Tang, X., Shi, J., Zhou, C., Li, L., Sun, H., Northwood, D. O., Waters, K. E., & Ma, H. (2024). Controlling and Tuning the Dispersion Properties of Calcined Kaolinite Particles in Various Organic Solvents via the Modification Method Using Triethoxyvinylsilane and 3-Mercaptopropionic Acid. Molecules, 29(17), 4129. https://doi.org/10.3390/molecules29174129







