Influence of Bi3+ Doping on Electrochemical Properties of Ti/Sb-SnO2/PbO2 Electrode for Zinc Electrowinning
Abstract
1. Introduction
2. Results and Discussion
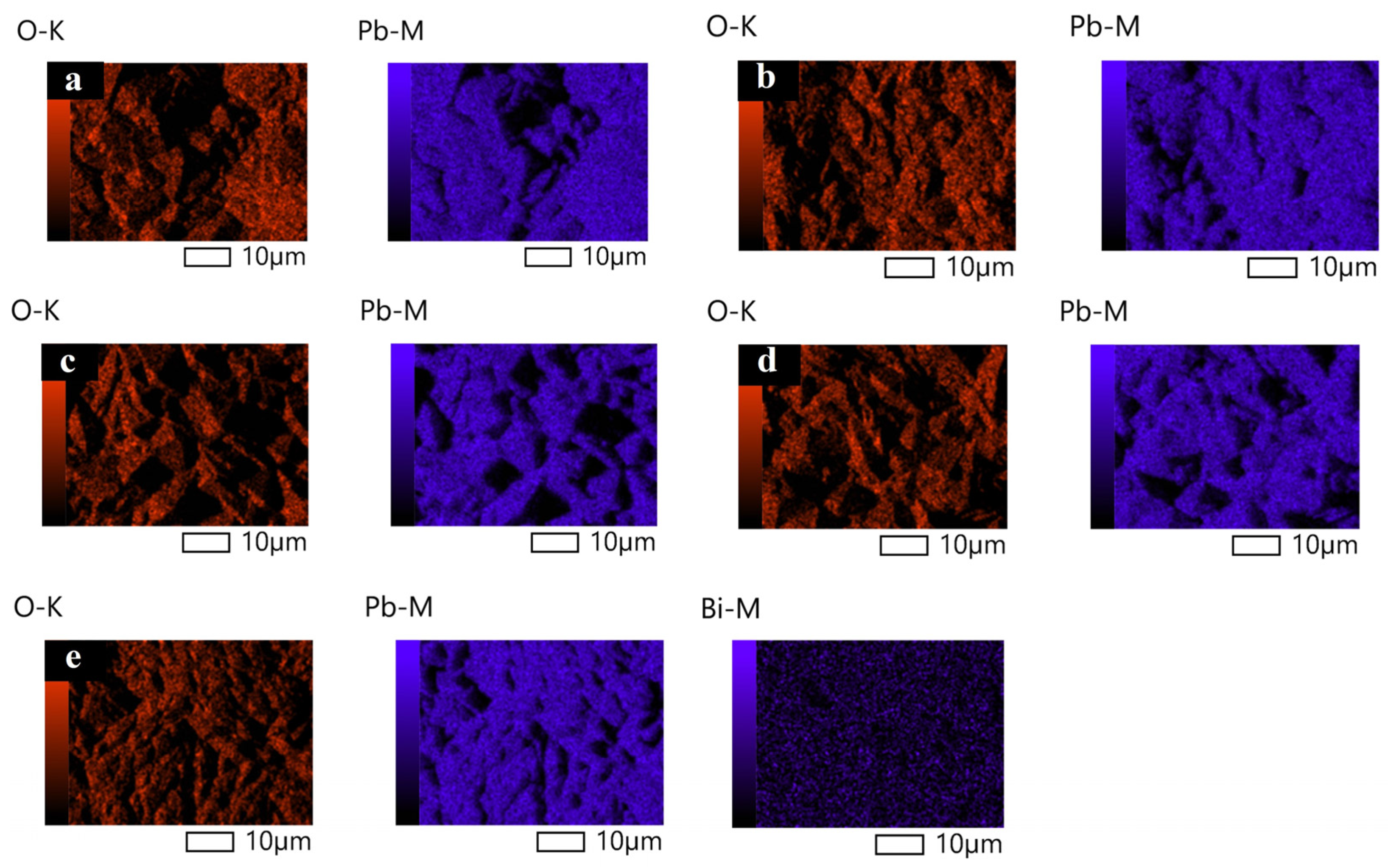
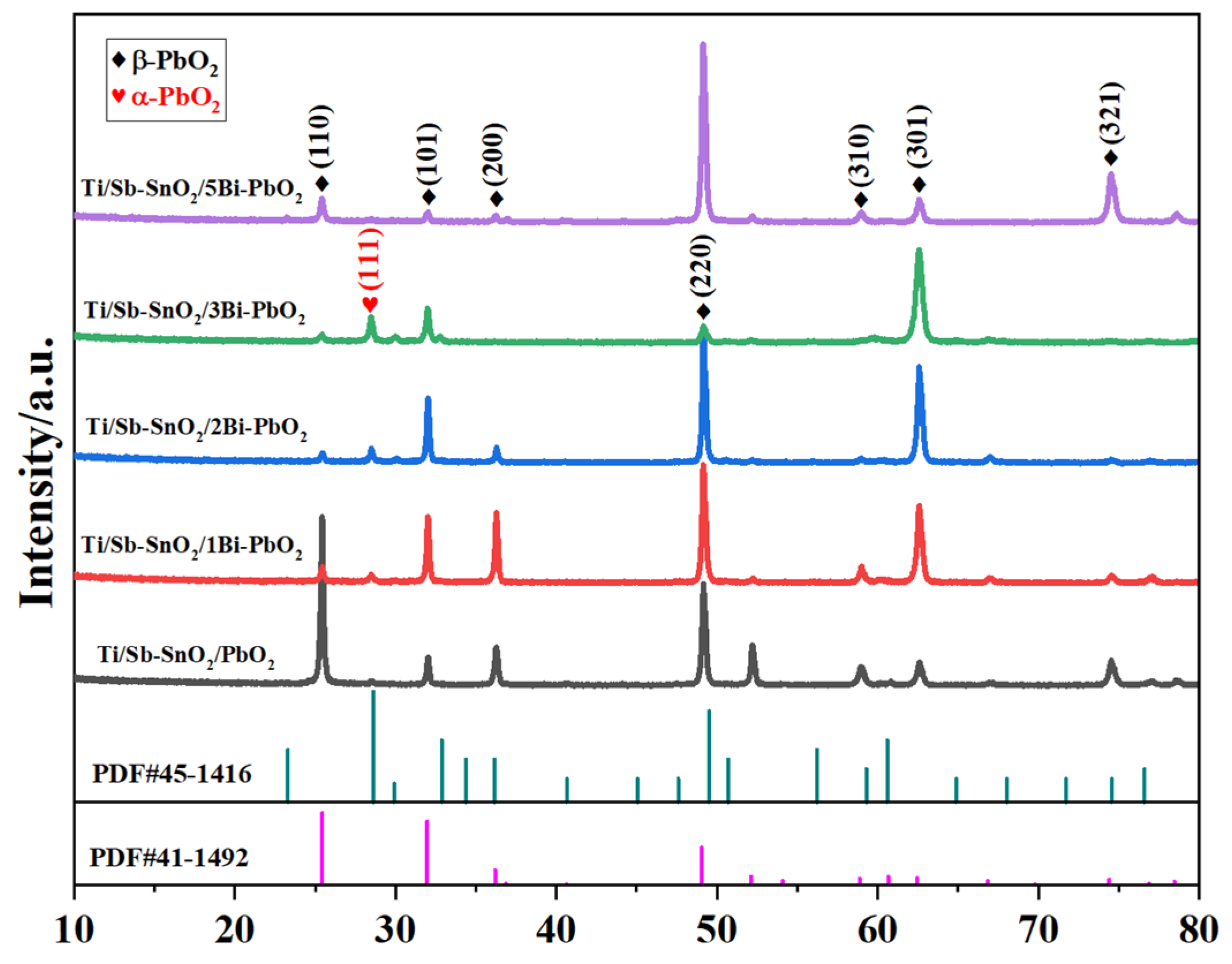
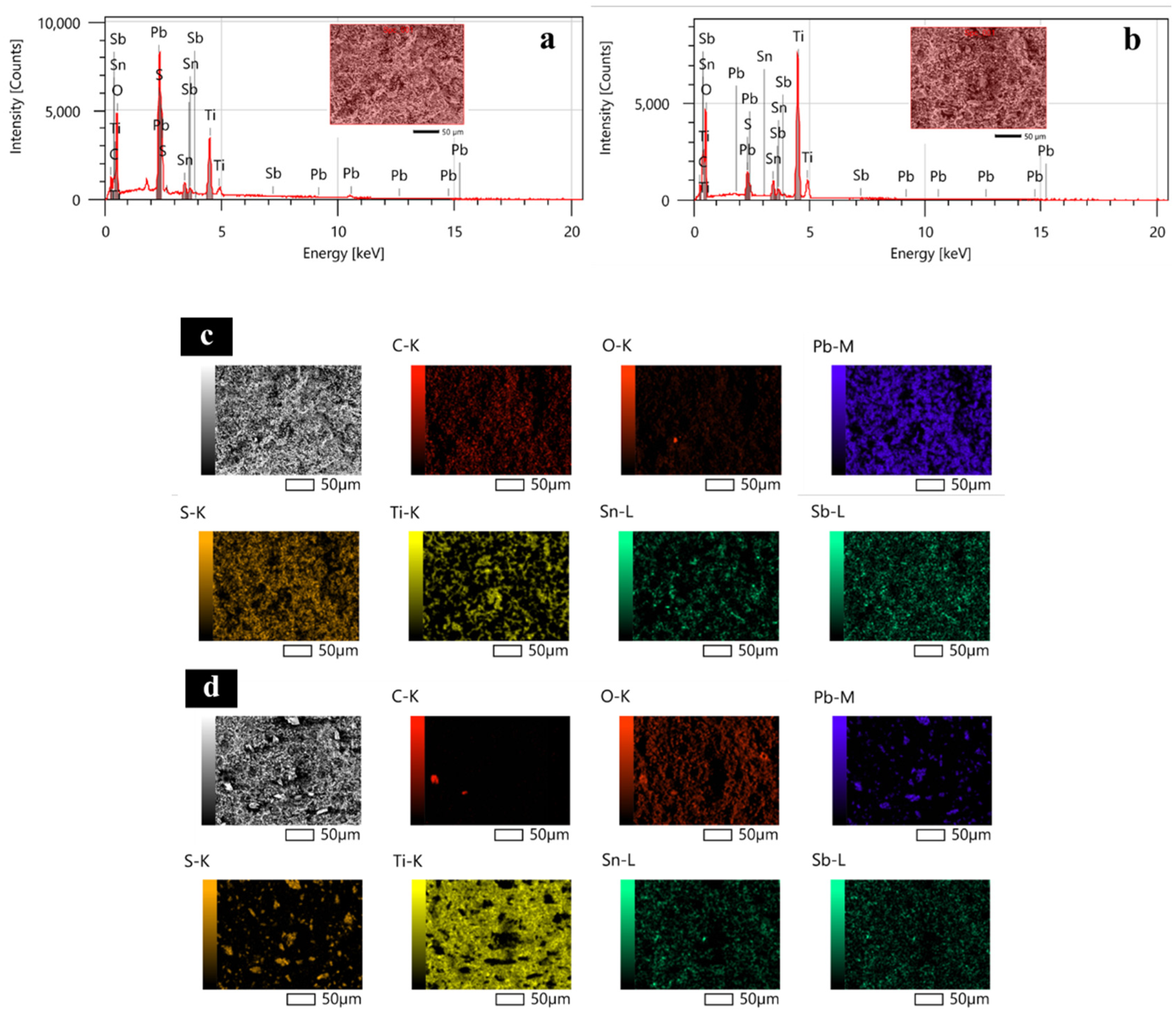
2.1. Surface Morphology and Crystal Structure
2.2. XPS Analysis
2.3. Hydrophilicity Analysis
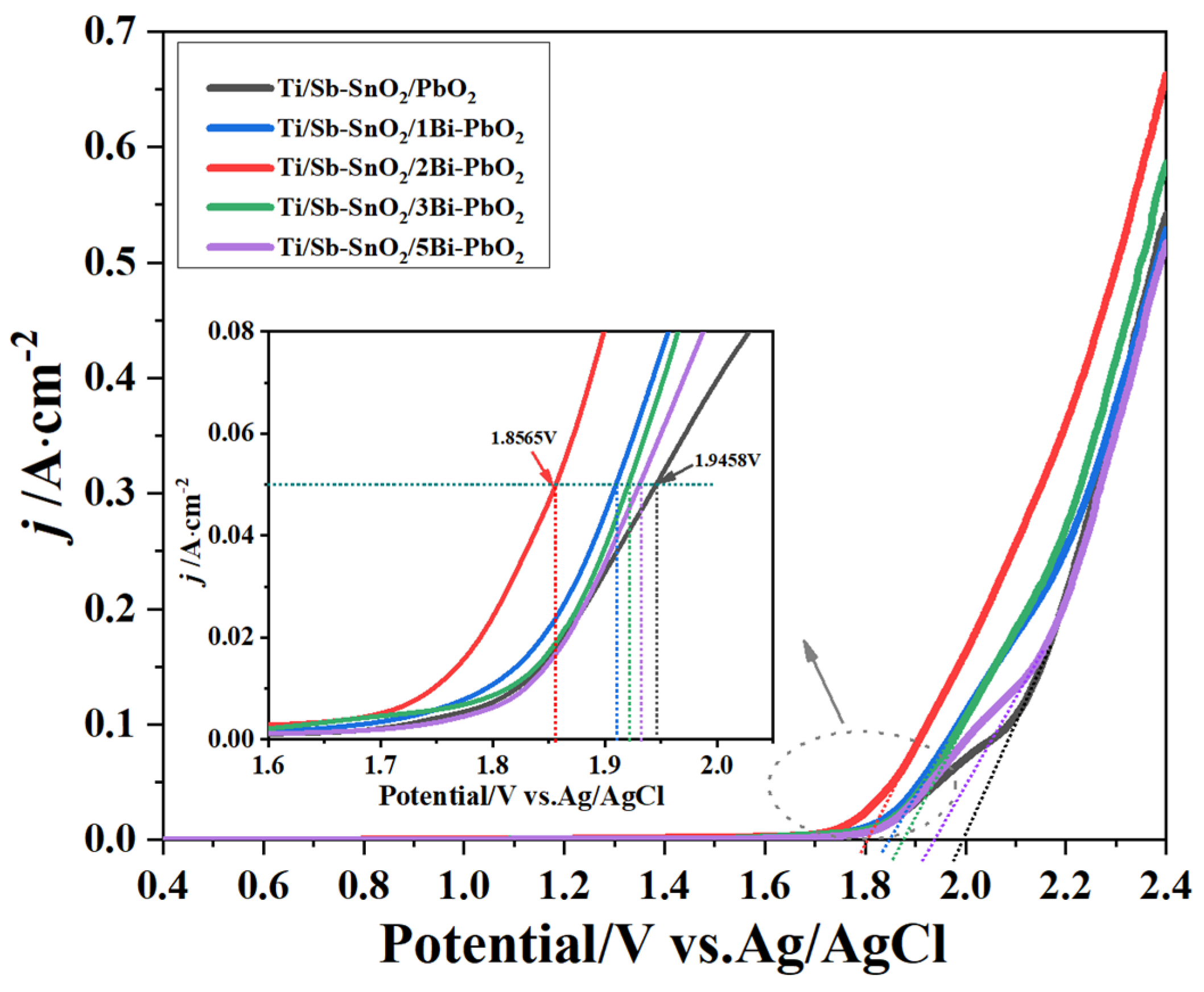
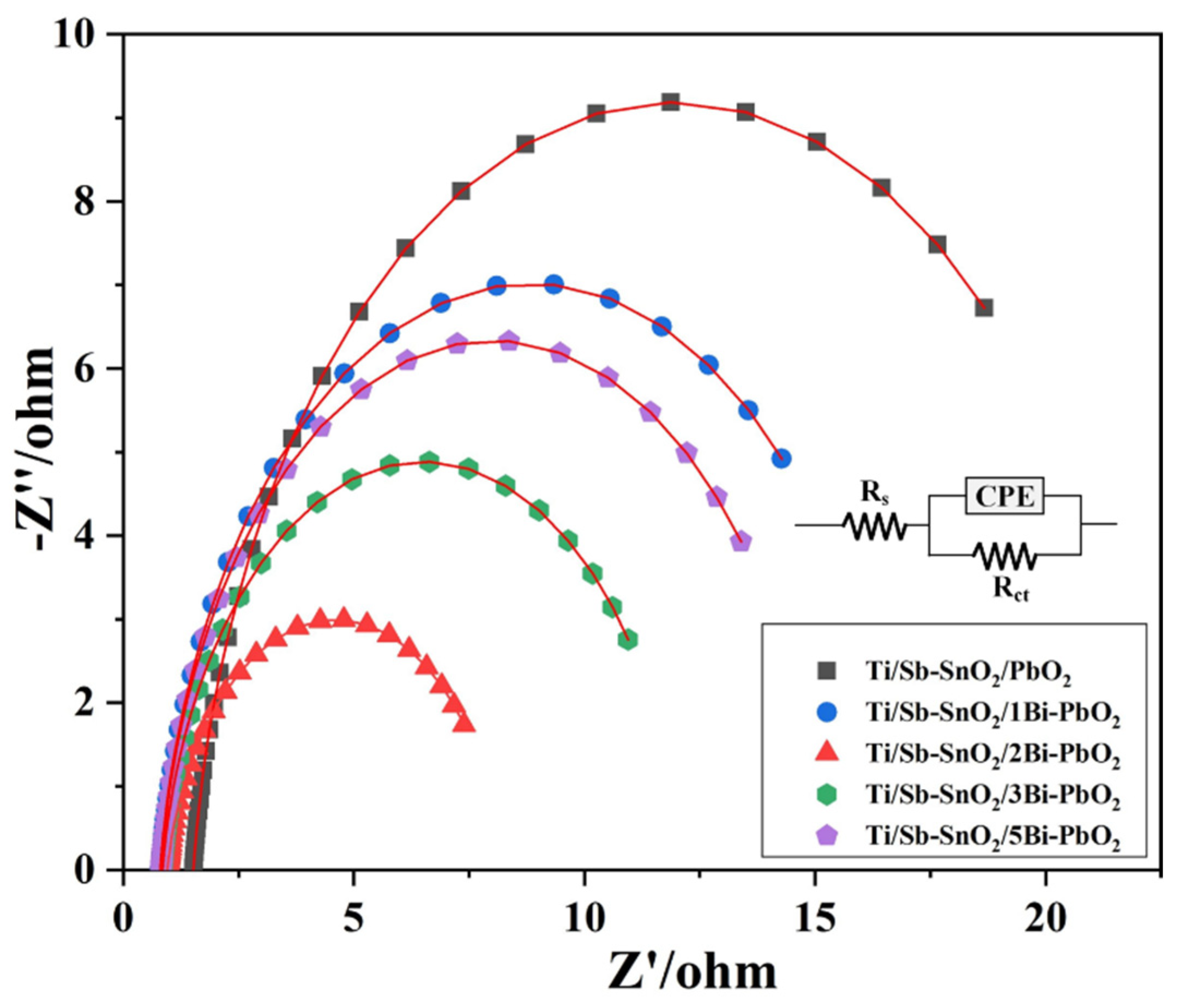
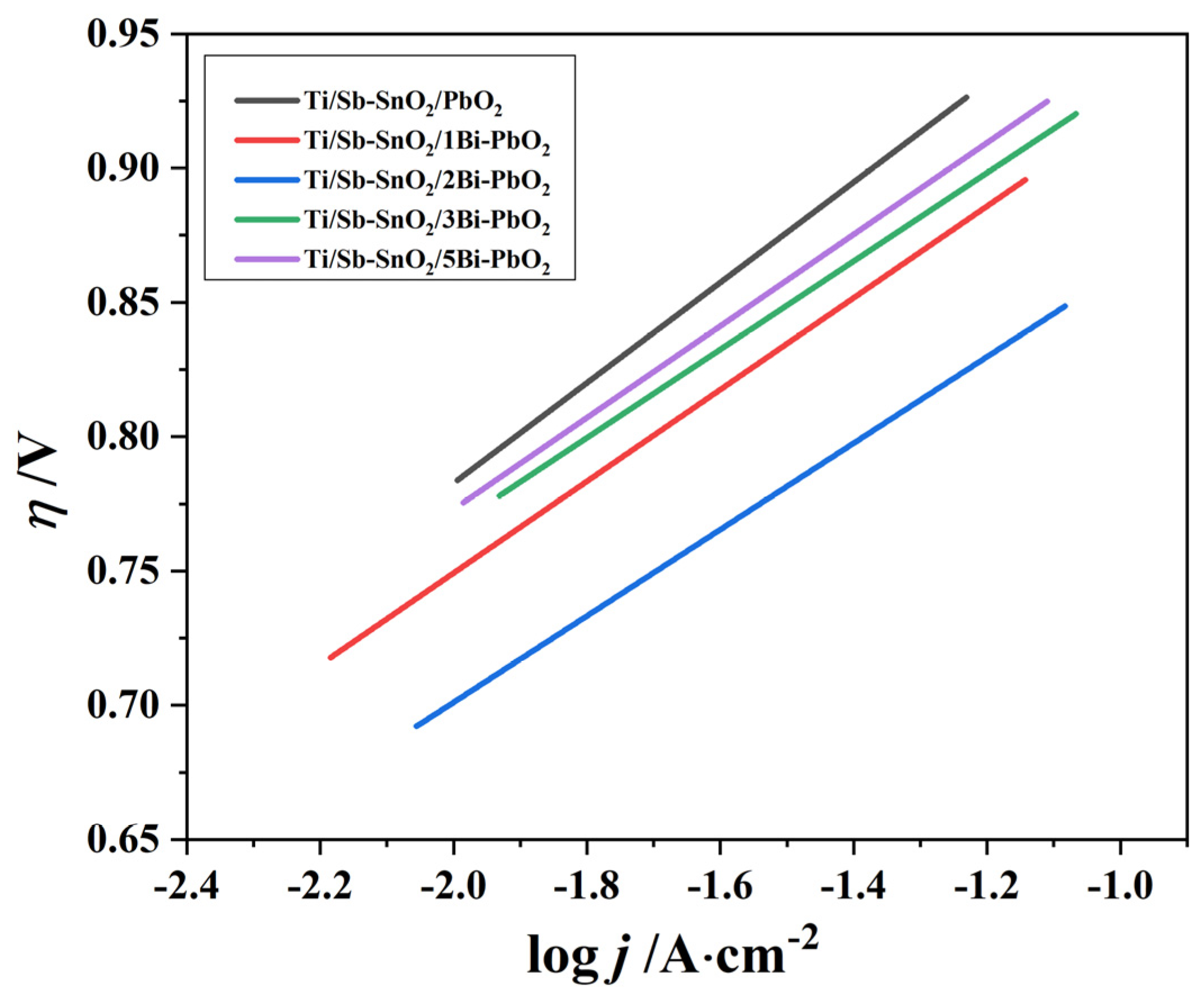
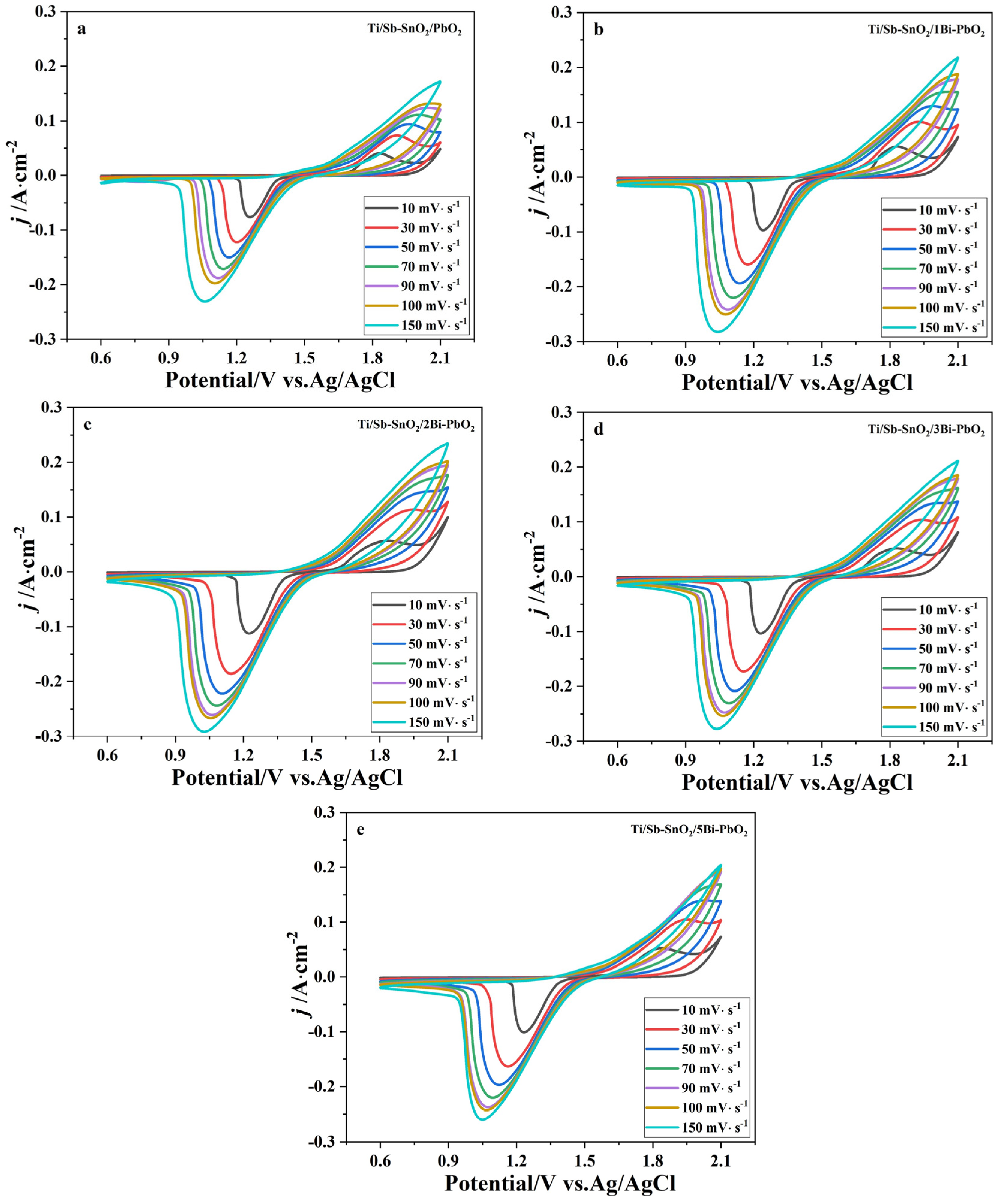
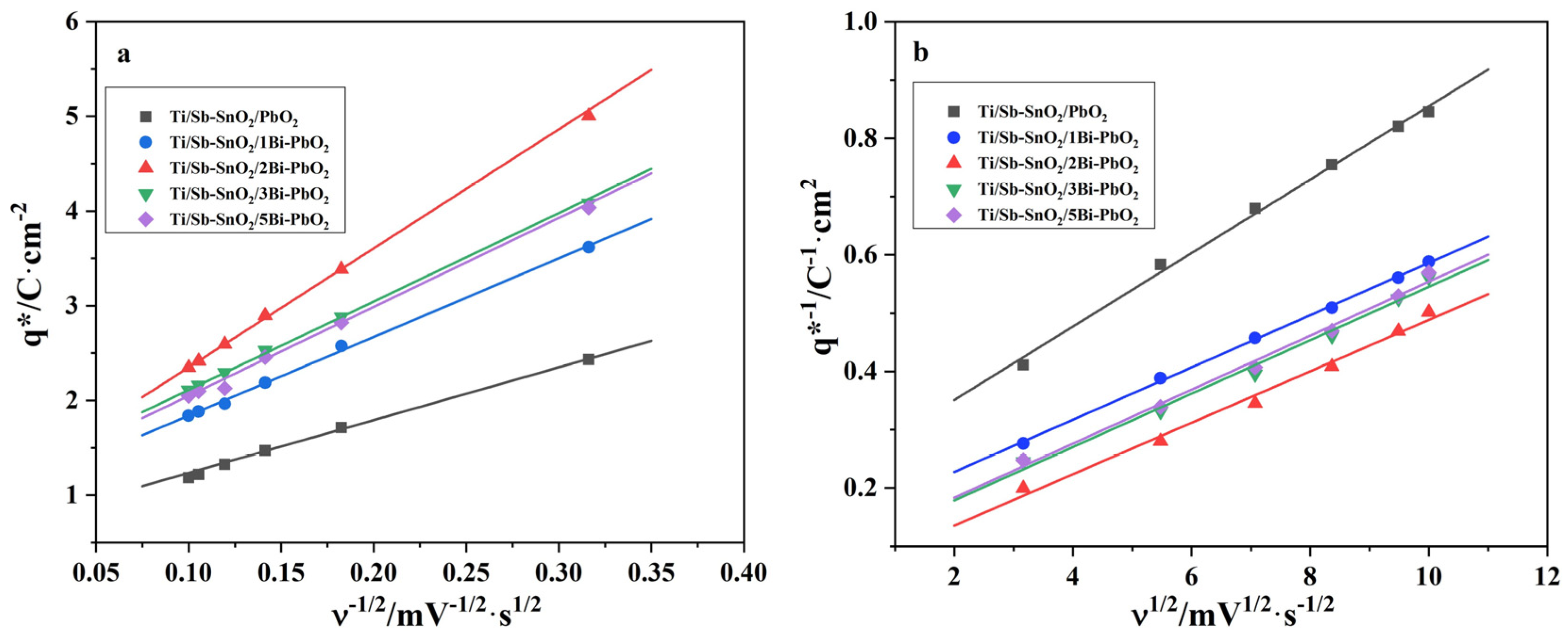
2.4. Electrochemical Property
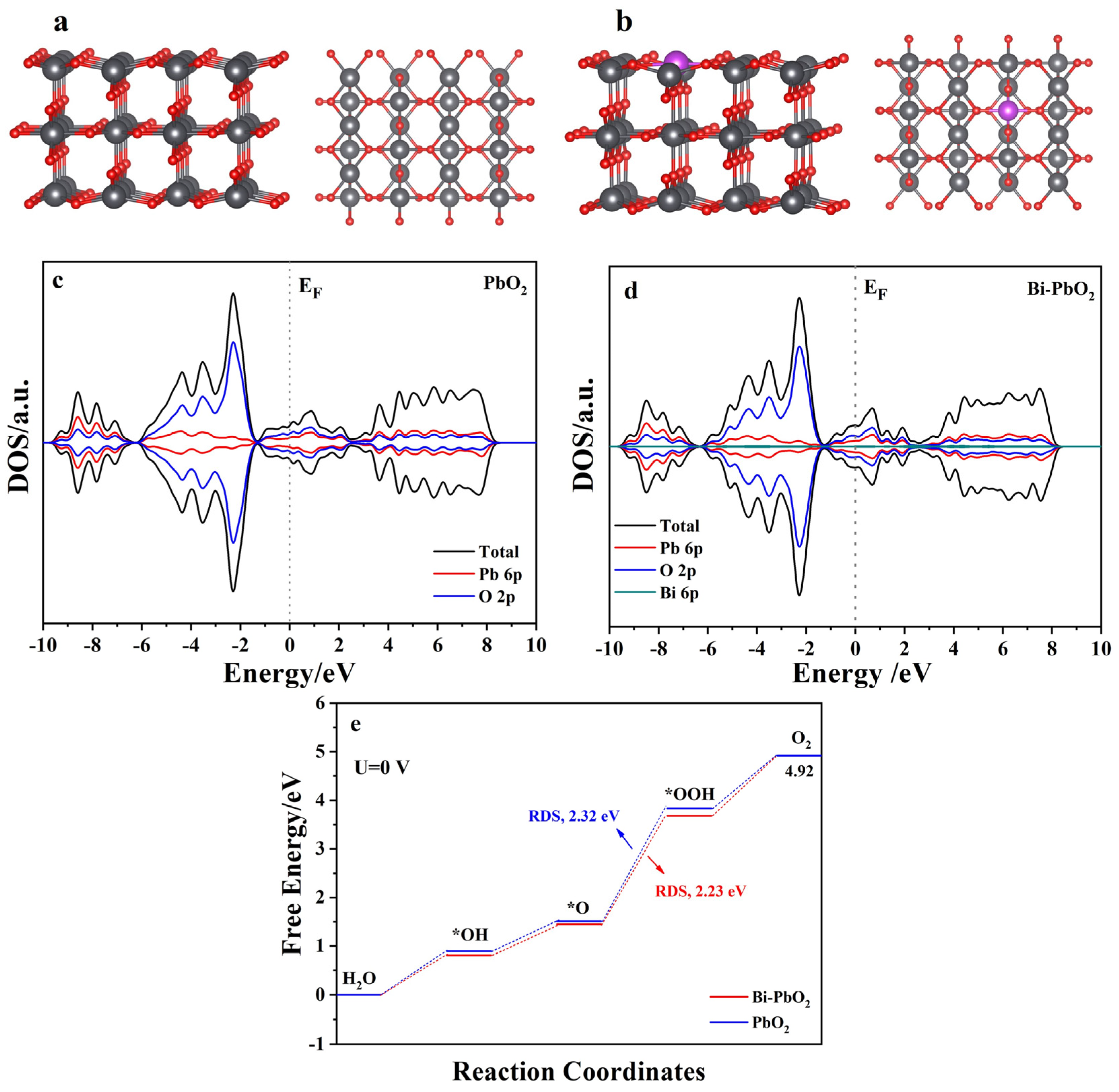
2.5. DFT Calculations
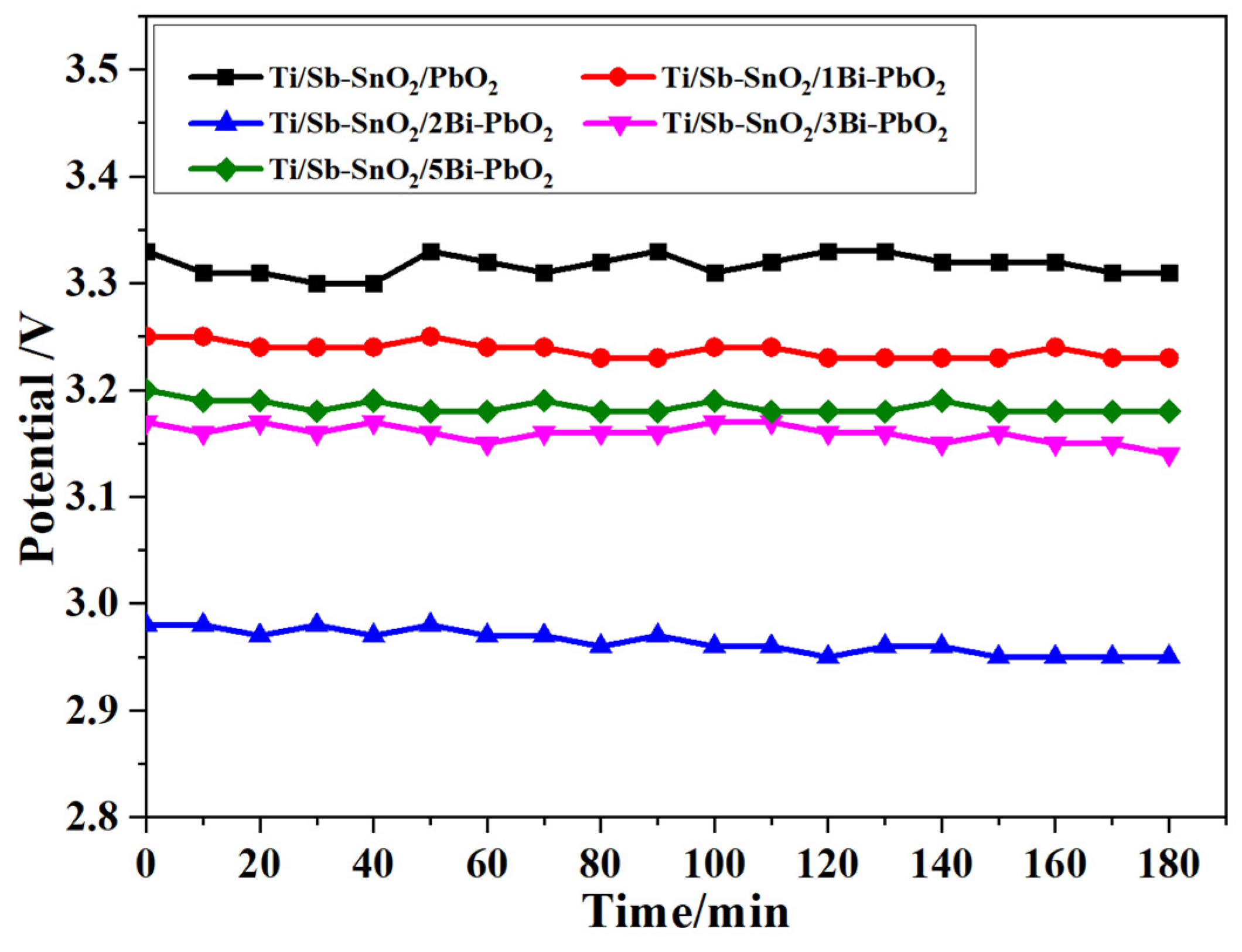
2.6. Electrochemical Stability Test
2.7. Simulated Zinc Electrowinning Experiment
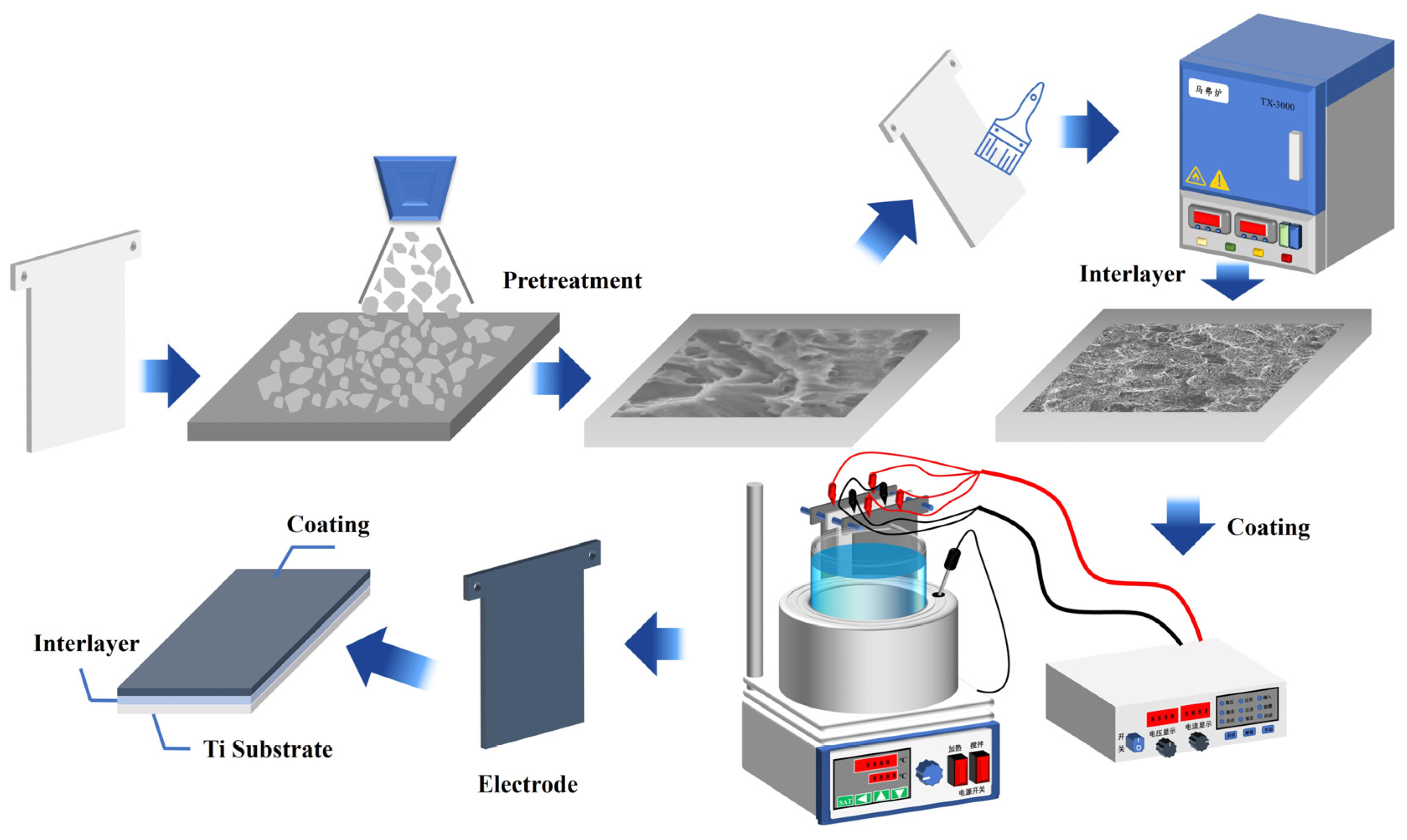
3. Experimental Details
3.1. Pretreatment of Ti Sheet
3.2. Preparation of Sb-SnO2 Interlayer
3.3. Synthesis of Bi3+ Doped Ti/Sb-SnO2/PbO2 Electrode
3.4. Characterization and Electrochemical Measurements
3.5. Theoretical Calculation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ortiz, I.Y.; Santos, A.R.; Costa, A.M.; Mavropoulos, E.; Tanaka, M.N.; Silva, M.H.P.; Camargo, S.S., Jr. In vitro assessment of zinc apatite coatings on titanium surfaces. Ceram. Int. 2016, 42, 15502–15510. [Google Scholar] [CrossRef]
- Li, S.; Chen, W.; Huang, X.; Ding, L.; Ren, Y.; Xu, M.; Zhu, J.; Miao, Z.; Liu, H. Enabling wasted A4 papers as a promising carbon source to construct partially graphitic hierarchical porous carbon for high-performance aqueous Zn-ion storage. ACS Appl. Mater. Interfaces 2024, 16, 10126–10137. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Wang, H.; Li, J.; Solomon, J.; Zhu, Y.; Carlson, B. Novel measures for spatter prediction in laser welding of thin-gage zinc-coated steel. Int. J. Heat Mass Tran. 2021, 167, 120830. [Google Scholar] [CrossRef]
- Rudnik, E. Recovery of zinc from zinc ash by leaching in sulphuric acid and electrowinning. Hydrometallurgy 2019, 188, 256–263. [Google Scholar] [CrossRef]
- Xu, X.; Li, D.; Chen, L.; Liu, M.; Liu, C.; Jia, J. Improve the energy efficiency: Effects of additives on longtime zinc electrowinning. Hydrometallurgy 2020, 193, 105326. [Google Scholar] [CrossRef]
- Karbasi, M.; Alamdari, E.K.; Dehkordi, E.A. Electrochemical performance of Pb-Co composite anode during Zinc electrowinning. Hydrometallurgy 2019, 183, 51–59. [Google Scholar] [CrossRef]
- Skaftun, S.M.; Sunde, S.; Haarberg, G.M.; Seland, F. Deposition and removal of manganese oxide in zinc electrowinning. Electrochim. Acta 2024, 484, 144040. [Google Scholar] [CrossRef]
- Xue, Y.; Hao, X.; Liu, X.; Zhang, N. Recovery of zinc and iron from steel mill dust-an overview of available technologies. Materials 2022, 15, 4127. [Google Scholar] [CrossRef] [PubMed]
- Valdrez, I.V.; Almeida, M.F.; Dias, J.M. Direct recovery of Zn from wasted alkaline batteries through selective anode’s separation. J. Environ. Manage. 2022, 321, 115979. [Google Scholar] [CrossRef]
- Kim, H.K.; Jang, H.; Jin, X.; Kim, M.G.; Hwang, S.J. A crucial role of enhanced Volmer-Tafel mechanism in improving the electrocatalytic activity via synergetic optimization of host, interlayer, and surface features of 2D nanosheets. Appl. Catal. B Environ. 2022, 312, 121391. [Google Scholar] [CrossRef]
- Abedini, A.; Valmoozi, A.A.E.; Afghahi, S.S.S. Anodized graphite as an advanced substrate for electrodeposition of PbO2. Mater. Today Commun. 2022, 31, 103464. [Google Scholar] [CrossRef]
- Sangeetha, V.; Sivakumar, V.; Sudha, A.; Kannan, K. Electrochemical degradation of sago wastewater using Ti/PbO2 electrode: Optimisation using response surface methodology. Int. J. Electrochem. Sci. 2014, 10, 1506–1516. [Google Scholar] [CrossRef]
- Tao, Y.; Luo, K.; Chang, L.; Chen, B.; Huang, H.; He, Y.; Guo, Z. The influence of rare earth La on properties of lead-based alloy anode for zinc electrowinning. J. Solid State Electr. 2022, 26, 2555–2564. [Google Scholar] [CrossRef]
- Li, H.; Yuan, T.; Li, R.; Wang, W.; Zheng, D.; Yuan, J. Electrochemical properties of powder-pressed Pb-Ag-PbO2 anodes. Trans. Nonferr. Met. Soc. 2019, 29, 2422–2429. [Google Scholar] [CrossRef]
- Ye, W.; Xu, F.; Jiang, L.; Duan, N.; Li, J.; Zhang, F.; Zhang, G.; Chen, L. A novel functional lead-based anode for efficient lead dissolution inhibition and slime generation reduction in zinc electrowinning. J. Clean. Prod. 2021, 284, 124767. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, J.; Jiang, L. Industrial tests of composite porous Pb-Ag anode in zinc electrowinning plant. Ionics 2023, 29, 377–385. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, T.; Li, R.; Zhu, X.; Li, H.; Lin, W.; Li, L.; Zheng, D. Electrochemical corrosion behaviors of Pb-Ag anodes by electric current pulse assisted casting. J. Electroanal. Chem. 2019, 847, 113250. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, X.; Yuan, M.; Long, B.; Wang, H.; Tang, J.; Yang, J. Electrochemical properties of Pb-0.6wt% Ag powder-pressed alloy in sulfuric acid electrolyte containing Cl−/Mn2+ ions. Hydrometallurgy 2018, 177, 218–226. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Jiang, W.; Chen, C.; Xu, R. Facile synthesis MnCo2O4 modifying PbO2 composite electrode with enhanced OER electrocatalytic activity for zinc electrowinning. Sep. Purif. Technol. 2021, 272, 118916. [Google Scholar] [CrossRef]
- Tang, C.; Lu, Y.; Wang, F.; Niu, H.; Xue, J. Influence of a MnO2-WC interlayer on the stability and electrocatalytic activity of titanium-based PbO2 anodes. Electrochim. Acta 2020, 331, 135381. [Google Scholar] [CrossRef]
- Zhang, F.; Zuo, J.; Jin, W.; Xu, F.; Jiang, L.; Xi, D.; Wen, Y.; Li, J.; Yu, Z.; Li, Z.; et al. Size effect of γ-MnO2 precoated anode on lead-containing pollutant reduction and its controllable fabrication in industrial-scale for zinc electrowinning. Chemosphere 2022, 287, 132457. [Google Scholar] [CrossRef]
- Chen, S.; Li, J.; Liu, L.; He, Q.; Zhou, L.; Yang, T.; Wang, X.; He, P.; Zhang, H.; Jia, B. Fabrication of Co/Pr co-doped Ti/PbO2 anode for efficiently electrocatalytic degradation of β-naphthoxyacetic acid: Efficiency and mechanism. Chemosphere 2020, 256, 127139. [Google Scholar]
- Duan, P.; Qian, C.; Wang, X.; Jia, X.; Jiao, L.; Chen, Y. Fabrication and characterization of Ti/polyaniline-Co/PbO2-Co for efficient electrochemical degradation of cephalexin in secondary effluents. Environ. Res. 2022, 214, 113842. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Li, W.; Wang, Z.; Yang, C.; Xu, H.; Yan, W.; Yang, L.; Wang, G.; Yang, J.; Feng, L.; et al. Variable activity and selectivity for electrochemical oxidation wastewater treatment using a magnetically assembled electrode based on Ti/PbO2 and carbon nanotubes. Sep. Purif. Technol. 2022, 301, 122008. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Chen, B. Effect of Ce(NO3)4 on the electrochemical properties of Ti/PbO2-TiO2-Ce(NO3)4 electrode for zinc electrowinning. Appl. Phys. A-Mater. 2019, 125, 150. [Google Scholar] [CrossRef]
- Chen, S.; Chen, B.; Wang, S.; Yan, W.; He, Y.; Guo, Z.; Xu, R. Ag doping to boost the electrochemical performance and corrosion resistance of Ti/Sn-Sb-RuOx/α-PbO2/β-PbO2 electrode in zinc electrowinning. J. Alloys Compd. 2020, 815, 152551. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Chen, B. Effect of CeO2 and graphite powder on the electrochemical performance of Ti/PbO2 anode for zinc electrowinning. Ceram. Int. 2018, 44, 19735–19742. [Google Scholar] [CrossRef]
- Dan, Y.; Lu, H.; Liu, X.; Lin, H.; Zhao, J. Ti/PbO2+nano-Co3O4 composite electrode material for electrocatalysis of O2 evolution in alkaline solution. Int. J. Hydrog. Energ. 2011, 36, 1949–1954. [Google Scholar] [CrossRef]
- He, S.; Xu, R.; Hu, G.; Chen, B. Electrosynthesis and performance of WC and Co3O4 co-doped α-PbO2 electrodes. RSC Adv. 2016, 6, 3362–3371. [Google Scholar] [CrossRef]
- Hakimi, F.; Rashchi, F.; Ghalekhani, M.; Dolati, A.; Astaraei, F.R. Effect of a synthesized pulsed electrodeposited Ti/PbO2-RuO2 nanocomposite on zinc electrowinning. Ind. Eng. Chem. Res. 2021, 60, 11737–11748. [Google Scholar] [CrossRef]
- Dolatabadi, M.; Ehrampoush, M.H.; Pournamdari, M.; Ebrahimi, A.A.; Fallahzadeh, H.; Ahmadzadeh, S. Simultaneous electrochemical degradation of pesticides from the aqueous environment using Ti/SnO2-Sb2O3/PbO2/Bi electrode; process modeling and mechanism insight. Chemosphere 2023, 311, 137001. [Google Scholar] [CrossRef]
- Figueredo-Sobrinho, F.A.A.; Souza Lucas, F.W.; Fill, T.P.; Rodrigues-Filho, E.; Mascaro, L.H.; Silva Casciano, P.N.; Lima-Neto, P.; Correia, A.N. Insights into electrodegradation mechanism of tebuconazole pesticide on Bi-doped PbO2 electrodes. Electrochim. Acta 2015, 154, 278–286. [Google Scholar] [CrossRef]
- Rahmani, A.; Ansari, A.; Seid-mohammadi, A.; Leili, M.; Nematollahi, D.; Shabanloo, A. Bismuth-doped 3D carbon felt/PbO2 electrocatalyst for degradation of diuron herbicide and improvement of pesticide wastewater biodegradability. J. Environ. Chem. Eng. 2023, 11, 109118. [Google Scholar] [CrossRef]
- Chang, L.; Zhou, Y.; Duan, X.; Liu, W.; Xu, D. Preparation and characterization of carbon nanotube and Bi co-doped PbO2 electrode. J. Taiwan Inst. Chem. Eng. 2014, 45, 1338–1346. [Google Scholar] [CrossRef]
- Gao, G.; Zhang, X.; Wang, P.; Ren, Y.; Meng, X.; Ding, Y.; Zhang, T.; Jiang, W. Electrochemical degradation of doxycycline hydrochloride on Bi/Ce co-doped Ti/PbO2 anodes: Efficiency and mechanism. J. Environ. Chem. Eng. 2022, 10, 108430. [Google Scholar] [CrossRef]
- Shmychkova, O.; Luk’yanenko, T.; Yakubenko, A.; Amadelli, R.; Velichenko, A. Electrooxidation of some phenolic compounds at Bi-doped PbO2. Appl. Catal. B-Environ. 2015, 162, 346–351. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Yu, J.; Chen, Y.; Liu, M.; Shao, Z. Enhancing electrocatalytic activity of perovskite oxides by tuning cation deficiency for oxygen reduction and evolution reactions. Chem. Mater. 2016, 28, 1691–1697. [Google Scholar] [CrossRef]
- Wan, C.; Zhao, L.; Wu, C.; Lin, L.; Liu, X. Bi5+ doping improves the electrochemical properties of Ti/SnO2-Sb/PbO2 electrode and its electrocatalytic performance for phenol. J. Clean. Prod. 2022, 380, 135005. [Google Scholar] [CrossRef]
- Yan, Z.; Zhao, Y.; Zhang, Z.; Li, G.; Li, H.; Wang, J.; Feng, Z.; Tang, M.; Yuan, X.; Zhang, R.; et al. A study on the performance of IrO2-Ta2O5 coated anodes with surface treated Ti substrates. Electrochim. Acta 2015, 157, 345–350. [Google Scholar] [CrossRef]
- Xu, R.; Huang, L.; Zhou, J.; Zhan, P.; Guan, Y.; Kong, Y. Effects of tungsten carbide on electrochemical properties and microstructural features of Al/Pb-PANI-WC composite inert anodes used in zinc electrowinning. Hydrometallurgy 2012, 12, 8–15. [Google Scholar] [CrossRef]
- Chen, Y.; Hong, L.; Xue, H.; Han, W.; Wang, L.; Sun, X.; Li, J. Preparation and characterization of TiO2-NTs/SnO2-Sb electrodes by electrodeposition. J. Electroanal. Chem. 2010, 648, 119–127. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, J.; Li, H.; Xu, Y.; Tu, W.; Zhou, J. Self-supported N-doped hierarchical Co3O4 electrocatalyst with abundant oxygen vacancies for acidic water oxidation. Chem. Eng. J. 2023, 465, 142745. [Google Scholar] [CrossRef]
- Yang, H.; Gao, S.; Rao, D.; Zhang, C.; Zhou, X.; Yan, X. The regulation mechanism of cationic substitution in morphology-controlled oxy-spinel for oxygen evolution reaction. J. Catal. 2022, 407, 221–231. [Google Scholar] [CrossRef]
- Peng, Y.; Hajiyani, H.; Pentcheva, R. Influence of Fe and Ni doping on the OER performance at the Co3O4(001) surface: Insights from DFT+U calculations. ACS Catal. 2021, 11, 5601–5613. [Google Scholar] [CrossRef]
- Dziri, F.; Djaballah, Y.; Belbacha, E.; Bouzida, A.B. Thermodynamic reassessment of the Bi-Rb system supported by Ab-Initio Calculations. J. Phase Equilibria Diffus. 2022, 43, 382–392. [Google Scholar] [CrossRef]
- Shi, B.; Nicholls, R.J.; Yates, J.R. Accurate and efficient structure factors in ultrasoft pseudopotential and projector augmented wave DFT. Phys. Rev. B 2023, 108, 115112. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]

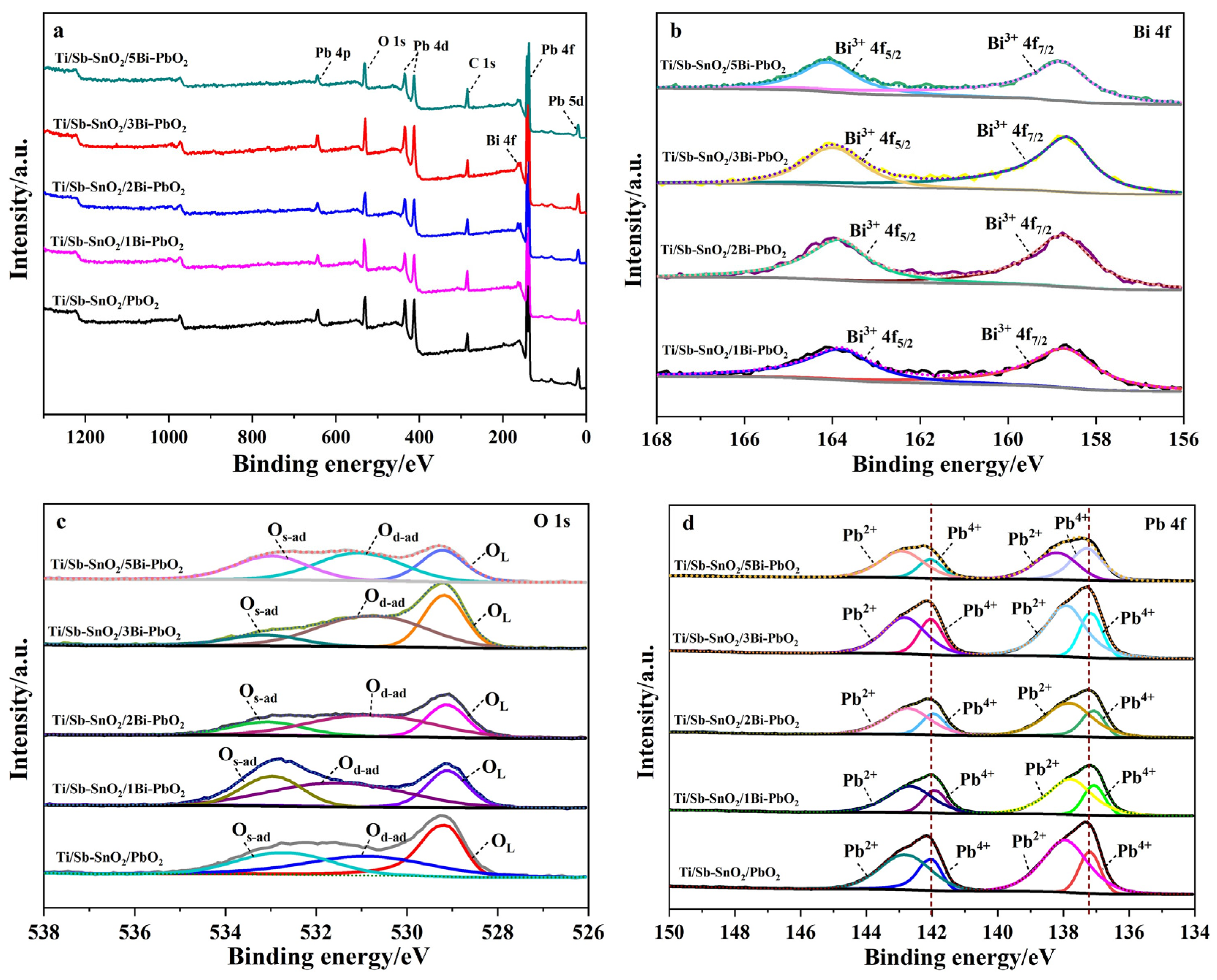



| Electrode | OL/eV | OL/% | Od-ad/eV | Od-ad/% | Os-ad/eV | Os-ad/% |
|---|---|---|---|---|---|---|
| Ti/Sb-SnO2/PbO2 | 529.16 | 36.4 | 530.85 | 35.8 | 532.65 | 27.8 |
| Ti/Sb-SnO2/1Bi-PbO2 | 529.11 | 26.6 | 531.48 | 47.3 | 532.95 | 26.1 |
| Ti/Sb-SnO2/2Bi-PbO2 | 529.12 | 28.5 | 530.74 | 51.5 | 533.07 | 20.0 |
| Ti/Sb-SnO2/3Bi-PbO2 | 529.18 | 32.8 | 530.68 | 50.3 | 533.12 | 16.8 |
| Ti/Sb-SnO2/5Bi-PbO2 | 529.22 | 25.4 | 531.04 | 41.6 | 532.97 | 33.0 |
| Electrode | Pb4+4f7/2/eV | Pb4+4f5/2/eV | Pb2+4f7/2/eV | Pb2+4f5/2/eV |
|---|---|---|---|---|
| Ti/Sb-SnO2/PbO2 | 137.20 | 142.01 | 137.96 | 142.82 |
| Ti/Sb-SnO2/1Bi-PbO2 | 137.07 | 141.92 | 137.80 | 142.67 |
| Ti/Sb-SnO2/2Bi-PbO2 | 137.06 | 141.96 | 137.79 | 142.74 |
| Ti/Sb-SnO2/3Bi-PbO2 | 137.15 | 142.04 | 137.88 | 142.83 |
| Ti/Sb-SnO2/5Bi-PbO2 | 137.29 | 142.06 | 138.17 | 142.92 |
| Materials | Rs/ohm | Rct/ohm | CPE/S·sec^n·cm−2 | n |
|---|---|---|---|---|
| Ti/Sb-SnO2/PbO2 | 1.521 | 20.80 | 0.0306 | 0.921 |
| Ti/Sb-SnO2/1Bi-PbO2 | 0.797 | 16.07 | 0.0369 | 0.914 |
| Ti/Sb-SnO2/2Bi-PbO2 | 0.952 | 7.30 | 0.0599 | 0.905 |
| Ti/Sb-SnO2/3Bi-PbO2 | 0.854 | 11.29 | 0.0388 | 0.908 |
| Ti/Sb-SnO2/5Bi-PbO2 | 0.823 | 14.37 | 0.0350 | 0.920 |
| Materials | a/V | b/V | η | |
|---|---|---|---|---|
| 500 A·m−2 | 1000 A·m−2 | |||
| Ti/Sb-SnO2/PbO2 | 1.141 | 0.187 | 0.898 | 0.954 |
| Ti/Sb-SnO2/1Bi-PbO2 | 1.091 | 0.171 | 0.869 | 0.920 |
| Ti/Sb-SnO2/2Bi-PbO2 | 1.023 | 0.161 | 0.814 | 0.862 |
| Ti/Sb-SnO2/3Bi-PbO2 | 1.096 | 0.164 | 0.882 | 0.931 |
| Ti/Sb-SnO2/5Bi-PbO2 | 1.114 | 0.171 | 0.892 | 0.944 |
| Materials | qT*/C·cm−2 | q*O/C·cm−2 | q*i/C·cm−2 | q*i/qT* |
|---|---|---|---|---|
| Ti/Sb-SnO2/PbO2 | 4.44 | 0.68 | 3.76 | 84.7% |
| Ti/Sb-SnO2/1Bi-PbO2 | 7.25 | 1.01 | 6.24 | 86.1% |
| Ti/Sb-SnO2/2Bi-PbO2 | 21.20 | 1.09 | 20.11 | 94.9% |
| Ti/Sb-SnO2/3Bi-PbO2 | 11.49 | 1.18 | 10.31 | 89.8% |
| Ti/Sb-SnO2/5Bi-PbO2 | 11.01 | 1.11 | 9.90 | 89.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Kang, X.; Xu, S.; Wei, Z.; Xu, S.; Liu, K.; Feng, Q.; Jia, B.; Wang, Y. Influence of Bi3+ Doping on Electrochemical Properties of Ti/Sb-SnO2/PbO2 Electrode for Zinc Electrowinning. Molecules 2024, 29, 4062. https://doi.org/10.3390/molecules29174062
Wu J, Kang X, Xu S, Wei Z, Xu S, Liu K, Feng Q, Jia B, Wang Y. Influence of Bi3+ Doping on Electrochemical Properties of Ti/Sb-SnO2/PbO2 Electrode for Zinc Electrowinning. Molecules. 2024; 29(17):4062. https://doi.org/10.3390/molecules29174062
Chicago/Turabian StyleWu, Jia, Xuanqi Kang, Shuangwen Xu, Zhen Wei, Shangyuan Xu, Kang Liu, Qing Feng, Bo Jia, and Yunhai Wang. 2024. "Influence of Bi3+ Doping on Electrochemical Properties of Ti/Sb-SnO2/PbO2 Electrode for Zinc Electrowinning" Molecules 29, no. 17: 4062. https://doi.org/10.3390/molecules29174062
APA StyleWu, J., Kang, X., Xu, S., Wei, Z., Xu, S., Liu, K., Feng, Q., Jia, B., & Wang, Y. (2024). Influence of Bi3+ Doping on Electrochemical Properties of Ti/Sb-SnO2/PbO2 Electrode for Zinc Electrowinning. Molecules, 29(17), 4062. https://doi.org/10.3390/molecules29174062






