The Impact of Fermentation on the Antioxidant Activity of Food Products
Abstract
1. Introduction
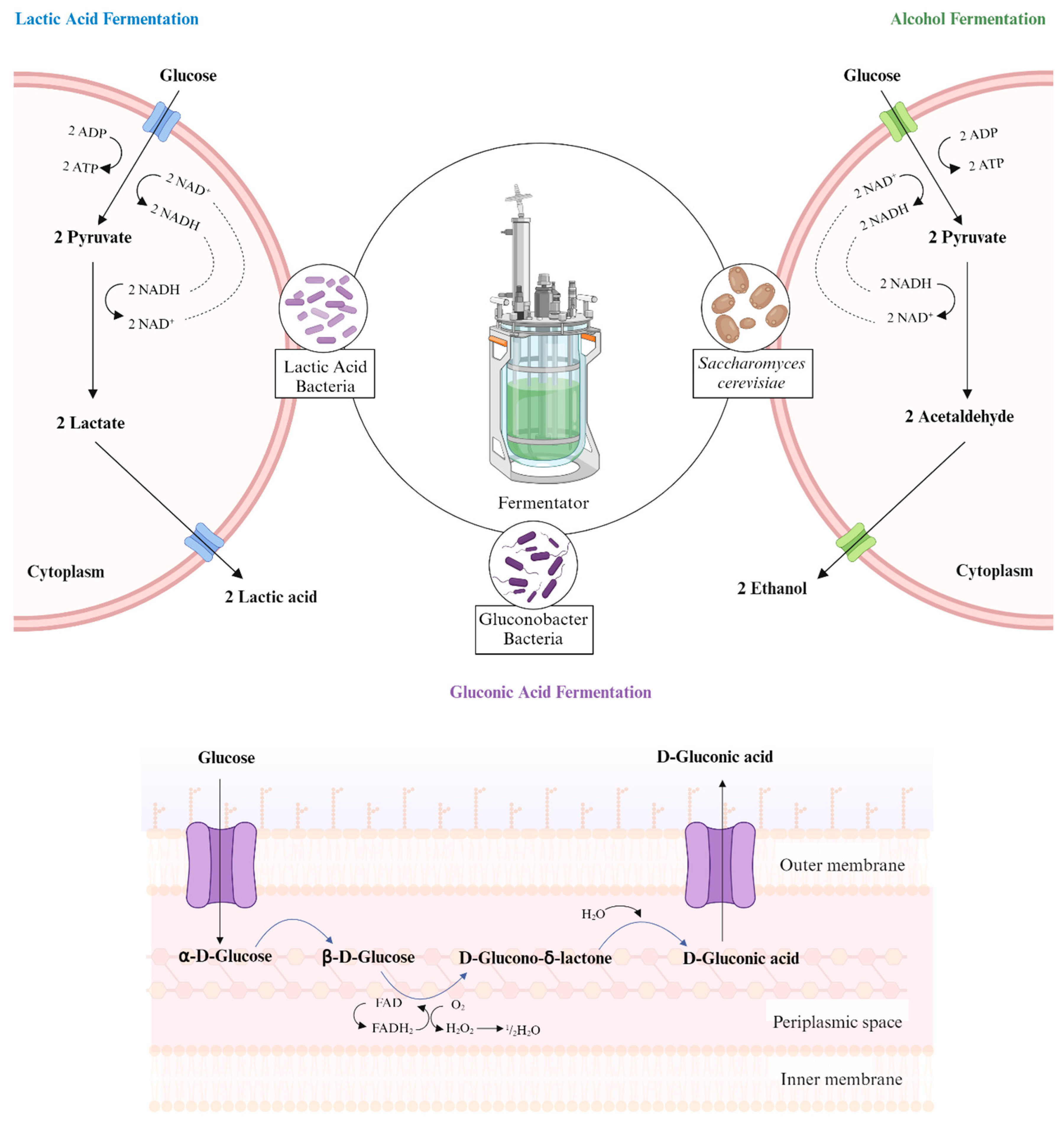
2. Understanding Fermentation
3. Impact of Fermentation on Antioxidant Activity
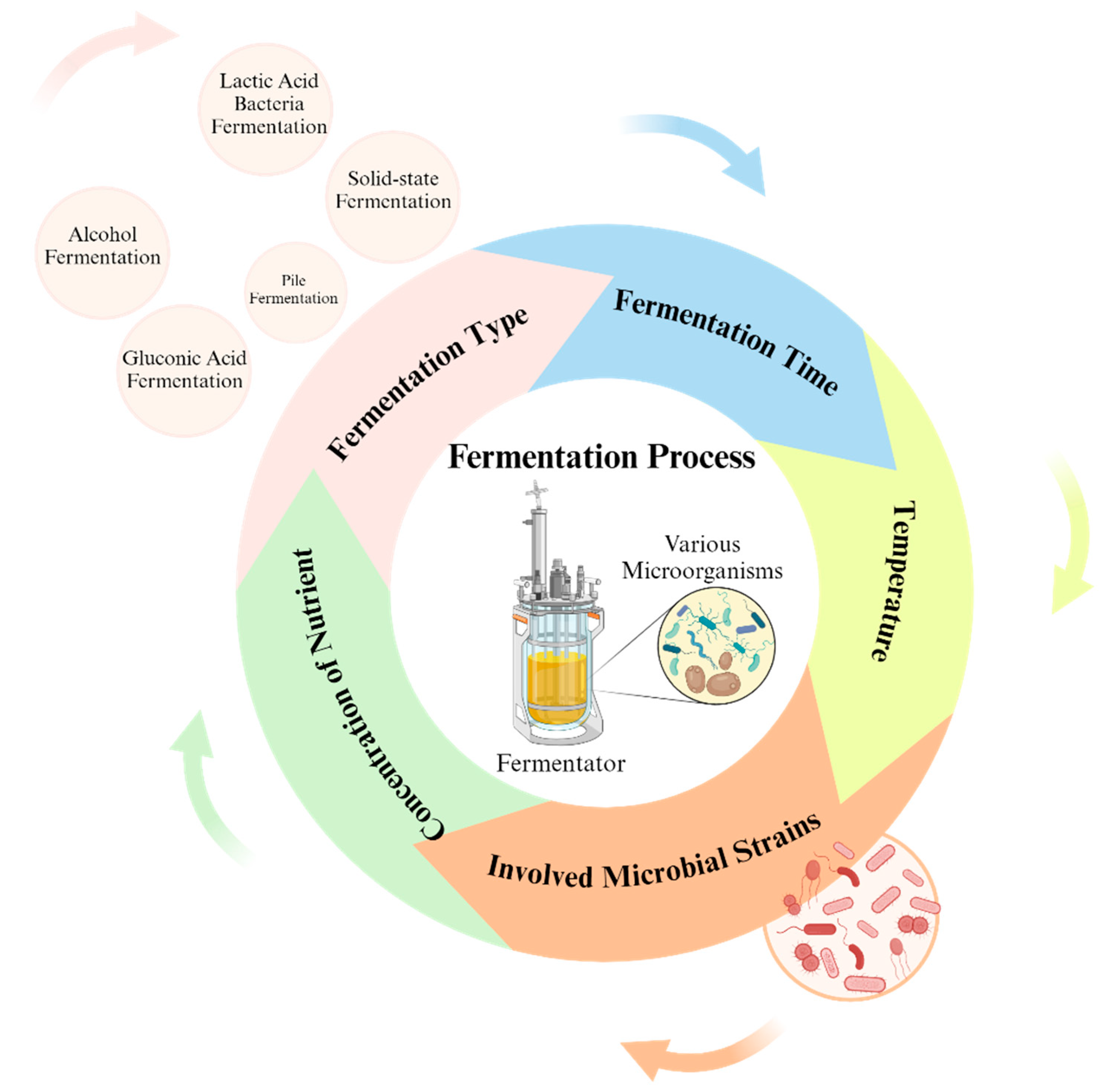
3.1. Factors Influencing the Impact of Fermentation on Antioxidant Activity
3.1.1. Microbial Strain
3.1.2. Fermentation Time
3.1.3. Other Factors
4. Antioxidant Profiles of Fermented Food
4.1. Dairy Products
4.2. Other Products
| Categories of Product | Fermented Product | Fermentation Type | Fermented by | Outcome | References |
|---|---|---|---|---|---|
| Plant-based milk and milk products | Oat and soy milk | Lactic acid bacteria fermentation | Lactiplantibacillus plantarum | -Exhibit radical scavenging activity | [124] |
| Cashew milk-based yogurt | Lactic acid bacteria fermentation | Lacticaseibacillus rhamnosus Lacticaseibacillus casei Lactiplantibacillus plantarum | -Increase phenolic content -Increase flavonoid content | [129] | |
| Chickpea yam milk | Lactic acid bacteria fermentation | Lacticaseibacillus rhamnosus | -Exhibit radical scavenging activity | [12] | |
| Soymilk | Lactic acid bacteria fermentation | Lactiplantibacillus plantarum Levilactobacillus brevis Limosilactobacillus reuteri | -Increase phenolic content | [130] | |
| Soymilk | Lactic acid bacteria fermentation | Lactiplantibacillus plantarum | -Exhibit an increasing β-galactosidase activity -Exhibit radical scavenging activity | [127] | |
| Rice milk | Lactic acid bacteria fermentation | Lactic acid bacteria | -Exhibit radical scavenging activity | [26] | |
| Hickory yogurt | * | Lactobacillus delbrueckii subsp. bulgaricus Streptococcus salivarius subsp. thermophilus | -Exhibit radical scavenging activity | [128] | |
| Sesame milk | Lactic acid bacteria fermentation | Lactiplantibacillus plantarum | -Exhibit an increasing β-galactosidase activity | [125] | |
| Plant samples, vegetables, and fruits | Lvjian okra | Lactic acid bacteria fermentation | Lactiplantibacillus plantarum | -Exhibit radical scavenging activity -Exhibit ferric reducing power | [133] |
| Avocado leaf extracts | Lactic acid bacteria fermentation | Pediococcus acidilactici Pediococcus pentosaceus Leuconostoc mesenteroides subsp. mesenteroides Levilactobacillus brevis Lactiplantibacillus plantarum subsp. plantarum Lactiplantibacillus plantarum | -Increase phenolic content | [76] | |
| Coix seed | * | Saccharomyces cerevisiae | -Exhibit radical scavenging activity | [137] | |
| Dark tea | Solid-state fermentation | Bacillus subtilis | -Altering catechin amount | [53] | |
| Coprinus comatus | Liquid fermentation | * | -Exhibit scavenging activity | [80] | |
| Corn bran | Solid-state fermentation | Lactiplantibacillus plantarum Limosilactobacillus reuteri | -Increase phenolic content | [54] | |
| Coffee beans | Solid-state fermentation | Hanseniaspora osmophila Hanseniaspora Vineae Schizosaccharomyces osmophilus Lactiplantibacillus plantarum | -Increase phenolic content | [8] | |
| Garlic | * | * | -Increase phenolic content -Increase flavonoid content | [132] | |
| Green tea | Lactic acid bacteria fermentation followed by acetic acid fermentation | Acetobacter pasteurianus Lacticaseibacillus paracasei Saccharomyces cerevisiae | -Exhibit radical scavenging activity | [90] | |
| Wheat bran | Solid-state fermentation | Lactiplantibacillus plantarum Saccharomyces cerevisiae | -Increase phenolic content | [50] | |
| Pollen | Solid-state fermentation | Lactobacillus rhamnosus | -Increase phenolic content -Increase flavonoids content -Exhibit scavenging activity | [51] | |
| Angelica pubescens | Submerged fermentation | * | -Exhibit scavenging activity | [30] | |
| Pogostemon cablin | |||||
| Paeonia lactiflora | |||||
| Alpinia oxyphylla | |||||
| Melaleuca leucadendron | |||||
| Osmanthus fragrans | |||||
| Glycyrrhiza uralensis | |||||
| Phellodendron chinense | |||||
| Rice | |||||
| Edible grass | Lactic acid bacteria fermentation | Lactiplantibacillus plantarum Lactobacillus rhamnosus | -Exhibit scavenging activity | [49] | |
| Chestnut inner shell | Alcohol fermentation | Aspergillus sojae | -Improve bioactive components | [32] | |
| Barley grain | * | Lactiplantibacillus plantarum | -Improve bioactive components -Exhibit scavenging activity | [122] | |
| Wheat sourdough | Lactic acid bacteria fermentation | Lactiplantibacillus plantarum Lactobacillus casei | -Increase polyphenol content | [79] | |
| African nightshade leaves | Lactic acid bacteria fermentation | Lactiplantibacillus plantarum Weissella cibaria Leuconostoc pseudomesenteroides | -Increase phenolic content -Exhibit radical scavenging activity | [42] | |
| Dandelion (Taraxacum officinale) | Solid-state fermentation | Lactiplantibacillus plantarum Saccharomyces cerevisiae | -Increase flavonoid content | [1] | |
| Soybean flour | Solid-state fermentation | Lactobacillus casei | -Exhibit an improvement of nutritive value -Increase flavonoids content -Exhibit an increase flavonoids metabolite -Exhibit scavenging activity | [55] | |
| Rice bran | Lactic acid bacteria fermentation | Lactiplantibacillus plantarum | -Exhibit scavenging activity | [71] | |
| Wheat bran | |||||
| Cyperus rotundus L. | Lactic acid bacteria fermentation | Lactiplantibacillus plantarum | -Exhibit radical scavenging activity | [131] | |
| Lavandula angustifolia extract | * | Pediococcus pentosaceus | -Inhibit ROS generation | [10] | |
| Soybean | * | Lactobacillus delbrueckii subsp. bulgaricus Streptococcus salivarius subsp. thermophilus | -Exhibit radical scavenging activity | [6] | |
| Green coffee beans | * | Saccharomyces cerevisiae Saccharomycopsis fibuligera | -Increase flavonoid content | [4] | |
| Kombucha tea | * | * | -Improve bioactive content -Exhibit radical scavenging activity | [123] | |
| Black rice bran | Solid-state fermentation | Aspergillus awamori Aspergillus oryzae | -Increase phenolic content | [7] | |
| Rice bran | Lactic acid bacteria fermentation | Lactococcus lactis Lactiplantibacillus plantarum | -Increase phenolic content -Exhibit radical scavenging activity | [77] | |
| Diospyros lotus fruit | * | Microbacterium flavum Lactiplantibacillus plantarum | -Exhibit radical scavenging activity -Exhibit an inhibitory effect on α-glucosidase activities | [126] | |
| Rice flour and black gram flour | Solid-state fermentation | Yeast | -Increase phenolic content -Improve increase bioactive content | [5] | |
| Lablab purpureus | Solid-state fermentation | Aspergillus oryzae Aspergillus awamori | -Increase phenolic content -Exhibit α-amylase activity | [21] | |
| Rice bran | Solid-state fermentation | Aspergillus oryzae Rhizopus oryzae | -Exhibit radical scavenging activity | [33] | |
| Quinoa seeds | * | Saccharomyces cerevisiae | -Increase phenolic content | [68] | |
| Myrtle (Myrtus communis) berries | Lactic acid bacteria fermentation | Lactiplantibacillus plantarum | -Exhibit radical scavenging activity -Increase phenolic content | [138] | |
| Red cabbage | Lactic acid bacteria fermentation | Lactiplantibacillus plantarum Lactobacillus delbrueckii subsp. acidophilus | -Increase phenolic content | [29] | |
| Pu-erh tea | Pile-fermentation | * | -Exhibit scavenging activity | [57] | |
| Beverage | Mead | Alcohol fermentation | Saccharomyces bayanus Saccharomyces cerevisiae | -Exhibit scavenging activity | [39] |
| Pomegranate juice | Lactic acid bacteria fermentation | Lactiplantibacillus plantarum | -Increase phenolic content | [73] | |
| Goji juice | * | Bacillus velezensis Bacillus licheniformis Limosilactobacillus reuteri Lacticaseibacillus rhamnosus Lactiplantibacillus plantarum | -Increase phenolic content -Exhibit scavenging activity | [139] | |
| Pomegranate juice | Lactic acid bacteria fermentation | Lactiplantibacillus plantarum Lactobacillus delbrueckii subsp. acidophilus | -Exhibit scavenging activity -Increase phenolic content | [9] | |
| Apple juice | Lactic acid bacteria fermentation | Lactiplantibacillus plantarum | -Exhibit scavenging activity | [28] | |
| Pear juice | Lactic acid bacteria fermentation | Lactiplantibacillus plantarum Lactobacillus helveticus Lacticaseibacillus casei | -Increase phenolic content -Improve the formation of alcohols, esters, acids, and terpenoids -Reduce the content of aldehydes and ketones -Exhibit scavenging activity | [44] | |
| Strawberry juice | Lactic acid bacteria fermentation | Lactiplantibacillus plantarum Lactobacillus delbrueckii subsp. acidophilus | -Increase phenolic content -Exhibit scavenging activity | [70] | |
| Wolfberry and longan juice | Lactic acid bacteria fermentation | Lacticaseibacillus paracasei Lactococcus lactis subsp. lactis | -Alter metabolite profile | [46] | |
| Cupuassu | Lactic acid bacteria fermentation | Lactobacillus casei | -Increase phenolic content | [134] | |
| Chamerion angustifolium | Solid-state fermentation | * | -Increase flavonoid content | [81] | |
| Mulberry juice | Lactic acid bacteria fermentation | Lactiplantibacillus plantarum | -Increase phenolic content -Increase flavonoid content -Increase anthocyanin content | [87] | |
| Lactic acid bacteria fermentation | Lactiplantibacillus plantarum Lactobacillus delbrueckii subsp. acidophilus Lacticaseibacillus paracasei | -Increase phenolic content -Increase flavonoid content -Increase anthocyanin content -Exhibit scavenging activity | [72] | ||
| Murta (Ugni molinae) juice | Lactic acid bacteria fermentation | Leuconostoc mesenteroides | -Increase phenolic content -Improve bioactive content -Exhibit scavenging activity | [20] | |
| Apple juice | Lactic acid bacteria fermentation | Lactiplantibacillus plantarum Lactobacillus helveticus Lacticaseibacillus casei Lactobacillus delbrueckii subsp. acidophilus Lacticaseibacillus paracasei Bifidobacterium lactis | -Exhibit scavenging activity | [45] | |
| Goji juice | Lactic acid bacteria fermentation | Lacticaseibacillus paracasei Lacticaseibacillus rhamnosus Lactiplantibacillus plantarum | -Exhibit scavenging activity | [31] | |
| Kombucha beverage | * | Symbiotic cultures of bacteria and yeasts | -Increase polyphenol content | [82] | |
| Strawberry beverage | Gluconic fermentation | Gephyroberyx japonicus | -Increase cell viability -Reduce oxidative stress | [3] | |
| Alcohol fermentation | Saccharomyces cerevisiae | ||||
| Cabernet sauvignon wine | Mixed fermentation | Pichia kudriavzevii Saccharomyces cerevisiae | -Increase phenolic content | [60] | |
| Sauce | Porphyra yezoensis | Lactic acid bacteria fermentation | Lactiplantibacillus plantarum Lacticaseibacillus casei | -Increase free amino acids | [140] |
| Lactic acid bacteria fermentation | Lactobacillus fermentum Lactobacillus casei Streptococcus salivarius subsp. thermophilus | -Exhibit scavenging activity | [74] | ||
| Himanthalia elongata | Solid-state fermentation | Lactobacillus casei Lacticaseibacillus paracasei Lactobacillus rhamnosus Bacillus subtilis | -Decrease phenolic content | [136] |
4.3. Waste and Byproducts
| Wastes and Byproducts | Fermentation Type | Fermented by | Outcome | References |
|---|---|---|---|---|
| Cauliflower byproducts | Lactic acid bacteria fermentation | Levilactobacillus brevis Lactiplantibacillus plantarum | -Exhibit scavenging activity | [141] |
| Siraitia grosvenorii pomace | Solid-state fermentation | Eurotium cristatum | -Increase phenolic content | [142] |
| Apple pomace and pomegranate peel powders | * | Lactobacillus bulgaricus Streptococcus salivarius subsp. thermophilus | -Increase phenolic content | [144] |
| Kiwifruit pulp | Lactic acid bacteria fermentation | Lactiplantibacillus plantarum | -Increase phenolic content -Increase flavonoid content -Exhibit scavenging activity -Reduce oxidative stress | [2] |
| Sea bass samples | Lactic acid bacteria fermentation | Lactiplantibacillus plantarum | -Increase phenolic content | [48] |
| Rice bran byproduct | Solid-state fermentation | Aspergillus oryzae | -Improve bioactive components -Exhibit scavenging activity | [143] |
| Apple pomace | Solid-state fermentation | Phanerochaete chrysosporium | -Increase phenolic content | [11] |
| Red bayberry pomace | Microbial fermentation | Yeast powder | -Increase flavonoid content | [59] |
| Lactic acid bacteria fermentation | Yeast powder and lactic acid bacteria powder | |||
| Alcohol fermentation | Yeast powder and acetic bacteria | |||
| Microbial fermentation | Yeast powder, lactic acid bacteria powder, and acetic bacteria | |||
| Fruit and vegetable wastes | Solid-state fermentation | Blakeslea trispora | -Increase β-carotene production -Exhibit scavenging activity | [40] |
| Orange pomace | Solid-state fermentation | Paecilomyces variotii | -Increase phenolic content | [27] |
| Prunus armeniaca L. pomace | Solid-state fermentation | Aspergillus niger Rhizopus oligosporus | -Increase phenolic content -Exhibit scavenging activity | [61] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, N.; Song, M.; Wang, N.; Wang, Y.; Wang, R.; An, X.; Qi, J. The Effects of Solid-State Fermentation on the Content, Composition and in Vitro Antioxidant Activity of Flavonoids from Dandelion. PLoS ONE 2020, 15, e0239076. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, R.; Zhang, Y.; Yang, Y.; Sun, X.; Zhang, Q.; Yang, N. Biotransformation of Phenolics and Metabolites and the Change in Antioxidant Activity in Kiwifruit Induced by Lactobacillus Plantarum Fermentation. J. Sci. Food Agric. 2020, 100, 3283–3290. [Google Scholar] [CrossRef] [PubMed]
- Hornedo-Ortega, R.; Krisa, S.; Carmen García-Parrilla, M.; Richard, T. Effects of Gluconic and Alcoholic Fermentation on Anthocyanin Composition and Antioxidant Activity of Beverages Made from Strawberry. LWT—Food Sci. Technol. 2016, 69, 382–389. [Google Scholar] [CrossRef]
- Haile, M.; Kang, W. Antioxidant Activity, Total Polyphenol, Flavonoid and Tannin Contents of Fermented Green Coffee Beans with Selected Yeasts. Fermentation 2019, 5, 29. [Google Scholar] [CrossRef]
- Rani, P.; Kumar, A.; Purohit, S.R.; Rao, P.S. Impact of Fermentation and Extrusion Processing on Physicochemical, Sensory and Bioactive Properties of Rice-Black Gram Mixed Flour. LWT 2018, 89, 155–163. [Google Scholar] [CrossRef]
- Tonolo, F.; Moretto, L.; Folda, A.; Scalcon, V.; Bindoli, A.; Bellamio, M.; Feller, E.; Rigobello, M.P. Antioxidant Properties of Fermented Soy during Shelf Life. Plant Foods Hum. Nutr. 2019, 74, 287–292. [Google Scholar] [CrossRef]
- Shin, H.-Y.; Kim, S.-M.; Lee, J.H.; Lim, S.-T. Solid-State Fermentation of Black Rice Bran with Aspergillus Awamori and Aspergillus Oryzae: Effects on Phenolic Acid Composition and Antioxidant Activity of Bran Extracts. Food Chem. 2019, 272, 235–241. [Google Scholar] [CrossRef]
- Therdtatha, P.; Jareontanahun, N.; Chaisuwan, W.; Yakul, K.; Paemanee, A.; Manassa, A.; Moukamnerd, C.; Phimolsiripol, Y.; Sommano, S.R.; Seesuriyachan, P. Production of Functional Arabica and Robusta Green Coffee Beans: Optimization of Fermentation with Microbial Cocktails to Improve Antioxidant Activity and Metabolomic Profiles. Biocatal. Agric. Biotechnol. 2023, 53, 102869. [Google Scholar] [CrossRef]
- Mousavi, Z.E.; Mousavi, S.M.; Razavi, S.H.; Hadinejad, M.; Emam-Djomeh, Z.; Mirzapour, M. Effect of Fermentation of Pomegranate Juice by Lactobacillus Plantarum and Lactobacillus Acidophilus on the Antioxidant Activity and Metabolism of Sugars, Organic Acids and Phenolic Compounds. Food Biotechnol. 2013, 27, 1–13. [Google Scholar] [CrossRef]
- Ha, J.H.; Kim, A.R.; Lee, K.-S.; Xuan, S.H.; Kang, H.C.; Lee, D.H.; Cha, M.Y.; Kim, H.J.; An, M.; Park, S.N. Anti-Aging Activity of Lavandula Angustifolia Extract Fermented with Pediococcus Pentosaceus DK1 Isolated from Diospyros Kaki Fruit in UVB-Irradiated Human Skin Fibroblasts and Analysis of Principal Components. J. Microbiol. Biotechnol. 2019, 29, 21–29. [Google Scholar] [CrossRef]
- Ajila, C.M.; Gassara, F.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Valéro, J.R. Polyphenolic Antioxidant Mobilization in Apple Pomace by Different Methods of Solid-State Fermentation and Evaluation of Its Antioxidant Activity. Food Bioprocess Technol. 2012, 5, 2697–2707. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Xie, B.; Sun, Z. Influence of Lactic Acid Bacteria Fermentation on Physicochemical Properties and Antioxidant Activity of Chickpea Yam Milk. J. Food Qual. 2021, 2021, 5523356. [Google Scholar] [CrossRef]
- Elfahri, K.R.; Vasiljevic, T.; Yeager, T.; Donkor, O.N. Anti-Colon Cancer and Antioxidant Activities of Bovine Skim Milk Fermented by Selected Lactobacillus Helveticus Strains. J. Dairy Sci. 2016, 99, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.; Şahutoğlu, A.S.; Sarıtaş, S.; Duman, H.; Arslan, A.; Pekdemir, B.; Karav, S. Role of Milk Glycome in Prevention, Treatment, and Recovery of COVID-19. Front. Nutr. 2022, 9, 779. [Google Scholar] [CrossRef]
- Sarıtaş, S.; Duman, H.; Karav, S. Nutritional and Functional Aspects of Fermented Algae. Int. J. Food Sci. Technol. 2024, 59, 5270–5284. [Google Scholar] [CrossRef]
- Gholamhosseinpour, A.; Hashemi, S.M.B. Ultrasound Pretreatment of Fermented Milk Containing Probiotic Lactobacillus Plantarum AF1: Carbohydrate Metabolism and Antioxidant Activity. J. Food Process Eng. 2019, 42, e12930. [Google Scholar] [CrossRef]
- Arslan, A.; Duman, H.; Kaplan, M.; Uzkuç, H.; Bayraktar, A.; Ertürk, M.; Alkan, M.; Frese, S.A.; Duar, R.M.; Henrick, B.M.; et al. Determining Total Protein and Bioactive Protein Concentrations in Bovine Colostrum. J. Vis. Exp. 2021, 178, e63001. [Google Scholar] [CrossRef]
- Sarıtaş, S.; Duman, H.; Pekdemir, B.; Rocha, J.M.; Oz, F.; Karav, S. Functional Chocolate: Exploring Advances in Production and Health Benefits. Int. J. Food Sci. Technol. 2024, 59, 5303–5325. [Google Scholar] [CrossRef]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ayaz, M.; Ajmal, M.; Ellahi, M.Y.; Khalique, A. Antioxidant Capacity and Fatty Acids Characterization of Heat Treated Cow and Buffalo Milk. Lipids Health Dis. 2017, 16, 163. [Google Scholar] [CrossRef]
- Escobar-Beiza, N.; Pérez-Correa, J.R.; Franco, W. Fermentation of Murta (Ugni molinae) Juice: Effect on Antioxidant Activity and Control of Enzymes Associated with Glucose Assimilation. Int. J. Mol. Sci. 2023, 24, 15197. [Google Scholar] [CrossRef]
- Sadh, P.K.; Saharan, P.; Duhan, J.S. Bio-Augmentation of Antioxidants and Phenolic Content of Lablab Purpureus by Solid State Fermentation with GRAS Filamentous Fungi. Resour. Technol. 2017, 3, 285–292. [Google Scholar] [CrossRef]
- Chan, C.-L.; Gan, R.; Shah, N.P.; Corke, H. Enhancing Antioxidant Capacity of Lactobacillus Acidophilus-Fermented Milk Fortified with Pomegranate Peel Extracts. Food Biosci. 2018, 26, 185–192. [Google Scholar] [CrossRef]
- Muniandy, P.; Shori, A.B.; Baba, A.S. Influence of Green, White and Black Tea Addition on the Antioxidant Activity of Probiotic Yogurt during Refrigerated Storage. Food Packag. Shelf Life 2016, 8, 1–8. [Google Scholar] [CrossRef]
- Musa, M.A.; Mada, S.B.; Umar, U.A.; Abdulazeez, M. Fermented Horse Milk Exhibits Antioxidant Activity in Vitro. FUW Trends Sci. Technol. J. 2021, 6, 397–403. [Google Scholar]
- Shu, G.; Shi, X.; Chen, L.; Kou, J.; Meng, J.; Chen, H. Antioxidant Peptides from Goat Milk Fermented by Lactobacillus Casei L61: Preparation, Optimization, and Stability Evaluation in Simulated Gastrointestinal Fluid. Nutrients 2018, 10, 797. [Google Scholar] [CrossRef]
- Deeseenthum, S.; Luang-In, V.; Chunchom, S. Characteristics of Thai Pigmented Rice Milk Kefirs with Potential as Antioxidant and Anti-Inflammatory Foods. Pharmacogn. J. 2017, 10, 154–161. [Google Scholar] [CrossRef]
- Espinosa-Pardo, F.A.; Nakajima, V.M.; Macedo, G.A.; Macedo, J.A.; Martínez, J. Extraction of Phenolic Compounds from Dry and Fermented Orange Pomace Using Supercritical CO2 and Cosolvents. Food Bioprod. Process. 2017, 101, 1–10. [Google Scholar] [CrossRef]
- Li, Z.; Teng, J.; Lyu, Y.; Hu, X.; Zhao, Y.; Wang, M. Enhanced Antioxidant Activity for Apple Juice Fermented with Lactobacillus Plantarum ATCC14917. Molecules 2018, 24, 51. [Google Scholar] [CrossRef]
- Hunaefi, D.; Akumo, D.N.; Smetanska, I. Effect of Fermentation on Antioxidant Properties of Red Cabbages. Food Biotechnol. 2013, 27, 66–85. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Wu, H.-T.; Hwang, I.-E.; Chen, F.-F.; Yao, J.-Y.; Yin, Y.; Chen, M.-Y.; Liaw, L.-L.; Kuo, Y.-C. Identification of the High-Yield Monacolin K Strain from Monascus Spp. and Its Submerged Fermentation Using Different Medicinal Plants. Bot. Stud. 2022, 63, 20. [Google Scholar] [CrossRef]
- Duan, W.; Guan, Q.; Zhang, H.-L.; Wang, F.-Z.; Lu, R.; Li, D.-M.; Geng, Y.; Xu, Z.-H. Improving Flavor, Bioactivity, and Changing Metabolic Profiles of Goji Juice by Selected Lactic Acid Bacteria Fermentation. Food Chem. 2023, 408, 135155. [Google Scholar] [CrossRef]
- Ritthibut, N.; Lim, S.-T.; Oh, S.-J. In Vitro Cosmeceutical Activity of Alcoholic Extract from Chestnut Inner Shell Fermented with Aspergillus Sojae. Food Sci. Biotechnol. 2022, 31, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Abd Razak, D.L.; Abd Rashid, N.Y.; Jamaluddin, A.; Sharifudin, S.A.; Abd Kahar, A.; Long, K. Cosmeceutical Potentials and Bioactive Compounds of Rice Bran Fermented with Single and Mix Culture of Aspergillus Oryzae and Rhizopus Oryzae. J. Saudi Soc. Agric. Sci. 2017, 16, 127–134. [Google Scholar] [CrossRef]
- Babich, O.; Ivanova, S.; Michaud, P.; Budenkova, E.; Kashirskikh, E.; Anokhova, V.; Sukhikh, S. Fermentation of Micro- and Macroalgae as a Way to Produce Value-Added Products. Biotechnol. Rep. 2024, 41, e00827. [Google Scholar] [CrossRef]
- Ma, Y.; Li, B.; Zhang, X.; Wang, C.; Chen, W. Production of Gluconic Acid and Its Derivatives by Microbial Fermentation: Process Improvement Based on Integrated Routes. Front. Bioeng. Biotechnol. 2022, 10, 864787. [Google Scholar] [CrossRef]
- Chung, M.R.W.Y.; Tan, I.S.; Foo, H.C.Y.; Lam, M.K.; Lim, S. Potential of Macroalgae-Based Biorefinery for Lactic Acid Production from Exergy Aspect. Biomass Convers. Biorefin. 2023, 13, 2623–2653. [Google Scholar] [CrossRef]
- Harun, R.; Yip, J.W.S.; Thiruvenkadam, S.; Ghani, W.A.W.A.K.; Cherrington, T.; Danquah, M.K. Algal Biomass Conversion to Bioethanol—A Step-by-step Assessment. Biotechnol. J. 2014, 9, 73–86. [Google Scholar] [CrossRef]
- Garofalo, C.; Norici, A.; Mollo, L.; Osimani, A.; Aquilanti, L. Fermentation of Microalgal Biomass for Innovative Food Production. Microorganisms 2022, 10, 2069. [Google Scholar] [CrossRef]
- Czabaj, S.; Kawa-Rygielska, J.; Kucharska, A.; Kliks, J. Effects of Mead Wort Heat Treatment on the Mead Fermentation Process and Antioxidant Activity. Molecules 2017, 22, 803. [Google Scholar] [CrossRef]
- Kaur, P.; Ghoshal, G.; Jain, A. Bio-Utilization of Fruits and Vegetables Waste to Produce β-Carotene in Solid-State Fermentation: Characterization and Antioxidant Activity. Process Biochem. 2019, 76, 155–164. [Google Scholar] [CrossRef]
- Chen, Z.; Shi, J.; Yang, X.; Nan, B.; Liu, Y.; Wang, Z. Chemical and Physical Characteristics and Antioxidant Activities of the Exopolysaccharide Produced by Tibetan Kefir Grains during Milk Fermentation. Int. Dairy J. 2015, 43, 15–21. [Google Scholar] [CrossRef]
- Degrain, A.; Manhivi, V.; Remize, F.; Garcia, C.; Sivakumar, D. Effect of Lactic Acid Fermentation on Color, Phenolic Compounds and Antioxidant Activity in African Nightshade. Microorganisms 2020, 8, 1324. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Romeih, E.; Gamba, R.R.; Nagai, E.; Suzuki, T.; Koyanagi, T.; Enomoto, T. The Biological Activity of Fermented Milk Produced by Lactobacillus Casei ATCC 393 during Cold Storage. Int. Dairy J. 2019, 91, 1–8. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Lei, H. Phenolics Profile, Antioxidant Activity and Flavor Volatiles of Pear Juice: Influence of Lactic Acid Fermentation Using Three Lactobacillus Strains in Monoculture and Binary Mixture. Foods 2021, 11, 11. [Google Scholar] [CrossRef]
- Wu, C.; Li, T.; Qi, J.; Jiang, T.; Xu, H.; Lei, H. Effects of Lactic Acid Fermentation-Based Biotransformation on Phenolic Profiles, Antioxidant Capacity and Flavor Volatiles of Apple Juice. LWT 2020, 122, 109064. [Google Scholar] [CrossRef]
- Zheng, Z.; Wei, L.; Zhu, M.; Qian, Z.; Liu, J.; Zhang, L.; Xu, Y. Effect of Lactic Acid Bacteria Co-Fermentation on Antioxidant Activity and Metabolomic Profiles of a Juice Made from Wolfberry and Longan. Food Res. Int. 2023, 174, 113547. [Google Scholar] [CrossRef] [PubMed]
- Taha, S.; El Abd, M.; De Gobba, C.; Abdel-Hamid, M.; Khalil, E.; Hassan, D. Antioxidant and Antibacterial Activities of Bioactive Peptides in Buffalo’s Yoghurt Fermented with Different Starter Cultures. Food Sci. Biotechnol. 2017, 26, 1325–1332. [Google Scholar] [CrossRef]
- Martí-Quijal, F.J.; Tornos, A.; Príncep, A.; Luz, C.; Meca, G.; Tedeschi, P.; Ruiz, M.-J.; Barba, F.J. Impact of Fermentation on the Recovery of Antioxidant Bioactive Compounds from Sea Bass Byproducts. Antioxidants 2020, 9, 239. [Google Scholar] [CrossRef]
- Li, X.; He, T.; Mao, J.; Sha, R. Effects of Lactic Acid Bacteria Fermentation on Physicochemical Properties, Functional Compounds and Antioxidant Activity of Edible Grass. Fermentation 2022, 8, 647. [Google Scholar] [CrossRef]
- Mahmoud, E.; Sorour, M.; Hussein, M.; Hassan, M. Impact of Solid State Fermentation on Chemical Composition, Functional Properties, and Antioxidant Activity of Wheat Bran. J. Sohag Agrisci. 2022, 7, 38–47. [Google Scholar] [CrossRef]
- Adaškevičiūtė, V.; Kaškonienė, V.; Barčauskaitė, K.; Kaškonas, P.; Maruška, A. The Impact of Fermentation on Bee Pollen Polyphenolic Compounds Composition. Antioxidants 2022, 11, 645. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, D.; Su, X.; Duan, S.; Wan, J.; Yuan, W.; Liu, B.; Ma, Y.; Pan, Y. An Integrated Metagenomics/Metaproteomics Investigation of the Microbial Communities and Enzymes in Solid-State Fermentation of Pu-Erh Tea. Sci. Rep. 2015, 5, 10117. [Google Scholar] [CrossRef]
- Xiao, L.; Yang, C.; Zhang, X.; Wang, Y.; Li, Z.; Chen, Y.; Liu, Z.; Zhu, M.; Xiao, Y. Effects of Solid-State Fermentation with Bacillus Subtilis LK-1 on the Volatile Profile, Catechins Composition and Antioxidant Activity of Dark Teas. Food Chem. X 2023, 19, 100811. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.; Razavi, S.H.; Khodaiyan, F.; Blesa, J.; Esteve, M.J. Fermented Corn Bran: A by-Product with Improved Total Phenolic Content and Antioxidant Activity. LWT 2023, 184, 115090. [Google Scholar] [CrossRef]
- Li, S.; Jin, Z.; Hu, D.; Yang, W.; Yan, Y.; Nie, X.; Lin, J.; Zhang, Q.; Gai, D.; Ji, Y.; et al. Effect of Solid-State Fermentation with Lactobacillus Casei on the Nutritional Value, Isoflavones, Phenolic Acids and Antioxidant Activity of Whole Soybean Flour. LWT 2020, 125, 109264. [Google Scholar] [CrossRef]
- Li, Q.; Chai, S.; Li, Y.; Huang, J.; Luo, Y.; Xiao, L.; Liu, Z. Biochemical Components Associated with Microbial Community Shift During the Pile-Fermentation of Primary Dark Tea. Front. Microbiol. 2018, 9, 1509. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.; Chen, W.; Tan, X.; Wang, P. Impact of Fermentation Degree on the Antioxidant Activity of Pu-Erh- Tea In Vitro. J. Food Biochem. 2012, 36, 262–267. [Google Scholar] [CrossRef]
- de Lima, M.D.S.F.; da Silva, R.A.; da Silva, M.F.; da Silva, P.A.B.; Costa, R.M.P.B.; Teixeira, J.A.C.; Porto, A.L.F.; Cavalcanti, M.T.H. Brazilian Kefir-Fermented Sheep’s Milk, a Source of Antimicrobial and Antioxidant Peptides. Probiot. Antimicrob. Proteins 2018, 10, 446–455. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, J.; Yue, Y.; Feng, Z.; Chen, J.; Ye, X. Influence of Mixed Probiotics on the the Bioactive Composition, Antioxidant Activity and Appearance of Fermented Red Bayberry Pomace. LWT 2020, 133, 110076. [Google Scholar] [CrossRef]
- Liu, W.; Ji, R.; Aimaier, A.; Sun, J.; Pu, X.; Shi, X.; Cheng, W.; Wang, B. Adjustment of Impact Phenolic Compounds, Antioxidant Activity and Aroma Profile in Cabernet Sauvignon Wine by Mixed Fermentation of Pichia Kudriavzevii and Saccharomyces Cerevisiae. Food Chem. X 2023, 18, 100685. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Dulf, E.-H.; Pintea, A. Phenolic Compounds, Flavonoids, Lipids and Antioxidant Potential of Apricot (Prunus armeniaca L.) Pomace Fermented by Two Filamentous Fungal Strains in Solid State System. Chem. Cent. J. 2017, 11, 92. [Google Scholar] [CrossRef]
- Rahmawati, I.S.; Suntornsuk, W. Effects of Fermentation and Storage on Bioactive Activities in Milks and Yoghurts. Procedia Chem. 2016, 18, 53–62. [Google Scholar] [CrossRef]
- Ding, W.; Wang, L.; Zhang, J.; Ke, W.; Zhou, J.; Zhu, J.; Guo, X.; Long, R. Characterization of Antioxidant Properties of Lactic Acid Bacteria Isolated from Spontaneously Fermented Yak Milk in the Tibetan Plateau. J. Funct. Foods 2017, 35, 481–488. [Google Scholar] [CrossRef]
- El-Sayed, M.I.; Awad, S.; Abou-Soliman, N.H.I. Improving the Antioxidant Properties of Fermented Camel Milk Using Some Strains of Lactobacillus. Food Nutr. Sci. 2021, 12, 352–371. [Google Scholar] [CrossRef]
- Tonolo, F.; Fiorese, F.; Moretto, L.; Folda, A.; Scalcon, V.; Grinzato, A.; Ferro, S.; Arrigoni, G.; Bindoli, A.; Feller, E.; et al. Identification of New Peptides from Fermented Milk Showing Antioxidant Properties: Mechanism of Action. Antioxidants 2020, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Begunova, A.V.; Savinova, O.S.; Glazunova, O.A.; Moiseenko, K.V.; Rozhkova, I.V.; Fedorova, T.V. Development of Antioxidant and Antihypertensive Properties during Growth of Lactobacillus helveticus, Lactobacillus rhamnosus and Lactobacillus reuteri on Cow’s Milk: Fermentation and Peptidomics Study. Foods 2020, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Solieri, L.; Rutella, G.S.; Tagliazucchi, D. Impact of Non-Starter Lactobacilli on Release of Peptides with Angiotensin-Converting Enzyme Inhibitory and Antioxidant Activities during Bovine Milk Fermentation. Food Microbiol. 2015, 51, 108–116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carciochi, R.A.; Galván-D’Alessandro, L.; Vandendriessche, P.; Chollet, S. Effect of Germination and Fermentation Process on the Antioxidant Compounds of Quinoa Seeds. Plant Foods Hum. Nutr. 2016, 71, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Bolat, E.; Sarıtaş, S.; Duman, H.; Eker, F.; Akdaşçi, E.; Karav, S.; Witkowska, A.M. Polyphenols: Secondary Metabolites with a Biological Impression. Nutrients 2024, 16, 2550. [Google Scholar] [CrossRef]
- Chen, W.; Xie, C.; He, Q.; Sun, J.; Bai, W. Improvement in Color Expression and Antioxidant Activity of Strawberry Juice Fermented with Lactic Acid Bacteria: A Phenolic-Based Research. Food Chem. X 2023, 17, 100535. [Google Scholar] [CrossRef]
- Wang, M.; Lei, M.; Samina, N.; Chen, L.; Liu, C.; Yin, T.; Yan, X.; Wu, C.; He, H.; Yi, C. Impact of Lactobacillus Plantarum 423 Fermentation on the Antioxidant Activity and Flavor Properties of Rice Bran and Wheat Bran. Food Chem. 2020, 330, 127156. [Google Scholar] [CrossRef] [PubMed]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Wu, M.; Sackey, A.S.; Xiao, L.; Tahir, H.E. Effect of Lactobacillus Strains on Phenolic Profile, Color Attributes and Antioxidant Activities of Lactic-Acid-Fermented Mulberry Juice. Food Chem. 2018, 250, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Pontonio, E.; Montemurro, M.; Pinto, D.; Marzani, B.; Trani, A.; Ferrara, G.; Mazzeo, A.; Gobbetti, M.; Rizzello, C.G. Lactic Acid Fermentation of Pomegranate Juice as a Tool to Improve Antioxidant Activity. Front. Microbiol. 2019, 10, 1550. [Google Scholar] [CrossRef]
- Yang, J.; Gao, T.; Ge, F.; Sun, H.; Cui, Z.; Wei, Z.; Wang, S.; Show, P.L.; Tao, Y.; Wang, W. Porphyra Yezoensis Sauces Fermented with Lactic Acid Bacteria: Fermentation Properties, Flavor Profile, and Evaluation of Antioxidant Capacity In Vitro. Front. Nutr. 2022, 8, 810460. [Google Scholar] [CrossRef]
- Li, S.N.; Tang, S.H.; He, Q.; Hu, J.X.; Zheng, J. In Vitro Antioxidant and Angiotensin-Converting Enzyme Inhibitory Activity of Fermented Milk with Different Culture Combinations. J. Dairy Sci. 2020, 103, 1120–1130. [Google Scholar] [CrossRef] [PubMed]
- De Montijo-Prieto, S.; Razola-Díaz, M.D.C.; Barbieri, F.; Tabanelli, G.; Gardini, F.; Jiménez-Valera, M.; Ruiz-Bravo, A.; Verardo, V.; Gómez-Caravaca, A.M. Impact of Lactic Acid Bacteria Fermentation on Phenolic Compounds and Antioxidant Activity of Avocado Leaf Extracts. Antioxidants 2023, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Nisa, K.; Rosyida, V.T.; Nurhayati, S.; Indrianingsih, A.W.; Darsih, C.; Apriyana, W. Total Phenolic Contents and Antioxidant Activity of Rice Bran Fermented with Lactic Acid Bacteria. IOP Conf. Ser. Earth Environ. Sci. 2019, 251, 012020. [Google Scholar] [CrossRef]
- Kozłowska, M.; Ziarno, M.; Zaręba, D.; Ścibisz, I. Exploring the Possibility of Enriching Fermented Milks with Young Barley Leaves Powder Preparation. Fermentation 2023, 9, 731. [Google Scholar] [CrossRef]
- Pejcz, E.; Lachowicz-Wiśniewska, S.; Nowicka, P.; Wojciechowicz-Budzisz, A.; Spychaj, R.; Gil, Z. Effect of Inoculated Lactic Acid Fermentation on the Fermentable Saccharides and Polyols, Polyphenols and Antioxidant Activity Changes in Wheat Sourdough. Molecules 2021, 26, 4193. [Google Scholar] [CrossRef]
- Gu, D.; Tang, S.; Liu, C.; He, D.; Tian, J.; Yang, Y. Optimization of Liquid Fermentation Conditions for Coprinus Comatus to Enhance Antioxidant Activity. Prep. Biochem. Biotechnol. 2023, 54, 830–837. [Google Scholar] [CrossRef]
- Lasinskas, M.; Jariene, E.; Vaitkeviciene, N.; Kulaitiene, J.; Adamaviciene, A.; Hallmann, E. The Impact of Solid-Phase Fermentation on Flavonoids, Phenolic Acids, Tannins and Antioxidant Activity in Chamerion angustifolium (L.) Holub (Fireweed) Leaves. Plants 2023, 12, 277. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Kałduńska, J.; Kochman, J.; Janda, K. Chemical Profile and Antioxidant Activity of the Kombucha Beverage Derived from White, Green, Black and Red Tea. Antioxidants 2020, 9, 447. [Google Scholar] [CrossRef] [PubMed]
- Panchal, G.; Hati, S.; Sakure, A. Characterization and Production of Novel Antioxidative Peptides Derived from Fermented Goat Milk by L. fermentum. LWT 2020, 119, 108887. [Google Scholar] [CrossRef]
- Vicenssuto, G.M.; de Castro, R.J.S. Development of a Novel Probiotic Milk Product with Enhanced Antioxidant Properties Using Mango Peel as a Fermentation Substrate. Biocatal. Agric. Biotechnol. 2020, 24, 101564. [Google Scholar] [CrossRef]
- Gholamhosseinpour, A.; Hashemi, S.M.B.; Raoufi Jahromi, L.; Sourki, A.H. Conventional Heating, Ultrasound and Microwave Treatments of Milk: Fermentation Efficiency and Biological Activities. Int. Dairy J. 2020, 110, 104809. [Google Scholar] [CrossRef]
- Terzioğlu, M.E.; Bakırcı, İ. Comparison of Buffalo’s, Sheep’s and Goat’s Yoghurts in Terms of Their Antioxidant Activity, Angiotensin-Converting Enzyme (ACE) Inhibitory Activity, Volatile Compound Content and 5-Hydroxymethylfurfural (HMF) Content. Med. Weter. 2022, 79, 148–152. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Sackey, A.S.; Wu, M.; Xiao, L. Impact of Ultrasonication and Pulsed Light Treatments on Phenolics Concentration and Antioxidant Activities of Lactic-Acid-Fermented Mulberry Juice. LWT 2018, 92, 61–66. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Gholamhosseinpour, A. Effect of Ultrasonication Treatment and Fermentation by Probiotic Lactobacillus Plantarum Strains on Goat Milk Bioactivities. Int. J. Food Sci. Technol. 2020, 55, 2642–2649. [Google Scholar] [CrossRef]
- Gaspar-Pintiliescu, A.; Oancea, A.; Cotarlet, M.; Vasile, A.M.; Bahrim, G.E.; Shaposhnikov, S.; Craciunescu, O.; Oprita, E.I. Angiotensin-converting Enzyme Inhibition, Antioxidant Activity and Cytotoxicity of Bioactive Peptides from Fermented Bovine Colostrum. Int. J. Dairy Technol. 2020, 73, 108–116. [Google Scholar] [CrossRef]
- Xu, H.; Hong, J.H.; Kim, D.; Jin, Y.H.; Pawluk, A.M.; Mah, J.-H. Evaluation of Bioactive Compounds and Antioxidative Activity of Fermented Green Tea Produced via One- and Two-Step Fermentation. Antioxidants 2022, 11, 1425. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Mousavi Khaneghah, A.; Kontominas, M.G.; Eş, I.; Sant’Ana, A.S.; Martinez, R.R.; Drider, D. Fermentation of Sarshir (Kaymak) by Lactic Acid Bacteria: Antibacterial Activity, Antioxidant Properties, Lipid and Protein Oxidation and Fatty Acid Profile. J. Sci. Food Agric. 2017, 97, 4595–4603. [Google Scholar] [CrossRef]
- Kaplan, M.; Baydemir, B.; Günar, B.B.; Arslan, A.; Duman, H.; Karav, S. Benefits of A2 Milk for Sports Nutrition, Health and Performance. Front. Nutr. 2022, 9, 935344. [Google Scholar] [CrossRef]
- Arslan, A.; Kaplan, M.; Duman, H.; Bayraktar, A.; Ertürk, M.; Henrick, B.M.; Frese, S.A.; Karav, S. Bovine Colostrum and Its Potential for Human Health and Nutrition. Front. Nutr. 2021, 8, 651721. [Google Scholar] [CrossRef]
- Bolat, E.; Eker, F.; Yılmaz, S.; Karav, S.; Oz, E.; Brennan, C.; Proestos, C.; Zeng, M.; Oz, F. BCM-7: Opioid-like Peptide with Potential Role in Disease Mechanisms. Molecules 2024, 29, 2161. [Google Scholar] [CrossRef] [PubMed]
- Eker, F.; Akdaşçi, E.; Duman, H.; Yalçıntaş, Y.M.; Canbolat, A.A.; Kalkan, A.E.; Karav, S.; Šamec, D. Antimicrobial Properties of Colostrum and Milk. Antibiotics 2024, 13, 251. [Google Scholar] [CrossRef]
- Duman, H.; Karav, S. Bovine Colostrum and Its Potential Contributions for Treatment and Prevention of COVID-19. Front. Immunol. 2023, 14, 1214514. [Google Scholar] [CrossRef]
- Eker, F.; Bolat, E.; Pekdemir, B.; Duman, H.; Karav, S. Lactoferrin: Neuroprotection against Parkinson’s Disease and Secondary Molecule for Potential Treatment. Front. Aging Neurosci. 2023, 15, 1204149. [Google Scholar] [CrossRef]
- Bunyatratchata, A.; Parc, A.L.; de Moura Bell, J.M.L.N.; Cohen, J.L.; Duman, H.; Arslan, A.; Kaplan, M.; Barile, D.; Karav, S. Release of Bifidogenic N-Glycans from Native Bovine Colostrum Proteins by an Endo-β-N-Acetylglucosaminidase. Enzyme Microb. Technol. 2023, 162, 110138. [Google Scholar] [CrossRef]
- Kaplan, M.; Arslan, A.; Duman, H.; Karyelioğlu, M.; Baydemir, B.; Günar, B.B.; Alkan, M.; Bayraktar, A.; Tosun, H.İ.; Ertürk, M.; et al. Production of Bovine Colostrum for Human Consumption to Improve Health. Front. Pharmacol. 2022, 12, 796824. [Google Scholar] [CrossRef]
- Yalçıntaş, Y.M.; Duman, H.; Rocha, J.M.; Bartkiene, E.; Karav, S.; Ozogul, F. Role of Bovine Colostrum against Various Diseases. Food Biosci. 2024, 61, 104818. [Google Scholar] [CrossRef]
- Bolat, E.; Eker, F.; Kaplan, M.; Duman, H.; Arslan, A.; Saritaş, S.; Şahutoğlu, A.S.; Karav, S. Lactoferrin for COVID-19 Prevention, Treatment, and Recovery. Front. Nutr. 2022, 9, 992733. [Google Scholar] [CrossRef]
- Karav, S.; German, J.; Rouquié, C.; Le Parc, A.; Barile, D. Studying Lactoferrin N-Glycosylation. Int. J. Mol. Sci. 2017, 18, 870. [Google Scholar] [CrossRef]
- Yalçıntaş, Y.M.; Baydemir, B.; Duman, H.; Eker, F.; Bayraktar Biçen, A.; Ertürk, M.; Karav, S. Exploring the Impact of Colostrum Supplementation on Athletes: A Comprehensive Analysis of Clinical Trials and Diverse Properties. Front. Immunol. 2024, 15, 1395437. [Google Scholar] [CrossRef]
- Huang, Z.; Habib, A.; Ding, X.; Lv, H. Physiochemical and Microbial Analysis of Tibetan Yak Milk Yogurt in Comparison to Locally Available Yogurt. Molecules 2023, 28, 5242. [Google Scholar] [CrossRef]
- Moreno-Montoro, M.; Olalla-Herrera, M.; Rufián-Henares, J.Á.; Martínez, R.G.; Miralles, B.; Bergillos, T.; Navarro-Alarcón, M.; Jauregi, P. Antioxidant, ACE-Inhibitory and Antimicrobial Activity of Fermented Goat Milk: Activity and Physicochemical Property Relationship of the Peptide Components. Food Funct. 2017, 8, 2783–2791. [Google Scholar] [CrossRef]
- Satir, G.; Guzel-Seydim, Z.B. Influence of Kefir Fermentation on the Bioactive Substances of Different Breed Goat Milks. LWT—Food Sci. Technol. 2015, 63, 852–858. [Google Scholar] [CrossRef]
- Perna, A.; Intaglietta, I.; Simonetti, A.; Gambacorta, E. Donkey Milk for Manufacture of Novel Functional Fermented Beverages. J. Food Sci. 2015, 80, 12862. [Google Scholar] [CrossRef]
- Ayyash, M.; Al-Nuaimi, A.K.; Al-Mahadin, S.; Liu, S.-Q. In Vitro Investigation of Anticancer and ACE-Inhibiting Activity, α-Amylase and α-Glucosidase Inhibition, and Antioxidant Activity of Camel Milk Fermented with Camel Milk Probiotic: A Comparative Study with Fermented Bovine Milk. Food Chem. 2018, 239, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.H.; Ryu, H.; Zhang, T.; Lee, C.H.; Seo, H.G.; Han, S.G. Green Tea Powder Supplementation Enhances Fermentation and Antioxidant Activity of Set-Type Yogurt. Food Sci. Biotechnol. 2018, 27, 1419–1427. [Google Scholar] [CrossRef]
- Felfoul, I.; Borchani, M.; Samet-bali, O.; Attia, H.; Ayadi, M.A. Effect of Ginger (Zingiber officinalis) Addition on Fermented Bovine Milk: Rheological Properties, Sensory Attributes and Antioxidant Potential. J. New Sci. 2017, 44, 2400–2409. [Google Scholar]
- Zhang, T.; Jeong, C.H.; Cheng, W.N.; Bae, H.; Seo, H.G.; Petriello, M.C.; Han, S.G. Moringa Extract Enhances the Fermentative, Textural, and Bioactive Properties of Yogurt. LWT 2019, 101, 276–284. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Casale, M.; Paini, M.; Casazza, A.A.; Lanteri, S.; Perego, P. Production of a Novel Fermented Milk Fortified with Natural Antioxidants and Its Analysis by NIR Spectroscopy. LWT—Food Sci. Technol. 2015, 62, 376–383. [Google Scholar] [CrossRef]
- Ramos, L.R.; Santos, J.S.; Daguer, H.; Valese, A.C.; Cruz, A.G.; Granato, D. Analytical Optimization of a Phenolic-Rich Herbal Extract and Supplementation in Fermented Milk Containing Sweet Potato Pulp. Food Chem. 2017, 221, 950–958. [Google Scholar] [CrossRef]
- Ge, X.; Tang, N.; Huang, Y.; Chen, X.; Dong, M.; Rui, X.; Zhang, Q.; Li, W. Fermentative and Physicochemical Properties of Fermented Milk Supplemented with Sea Buckthorn (Hippophae eleagnaceae L.). LWT 2022, 153, 112484. [Google Scholar] [CrossRef]
- Ozcan, T.; Yilmaz-Ersan, L.; Akpinar-Bayizit, A.; Delikanli, B. Antioxidant Properties of Probiotic Fermented Milk Supplemented with Chestnut Flour (Castanea sativa Mill). J. Food Process. Preserv. 2017, 41, e13156. [Google Scholar] [CrossRef]
- Liu, D. Effect of Fuzhuan Brick-tea Addition on the Quality and Antioxidant Activity of Skimmed Set-type Yoghurt. Int. J. Dairy Technol. 2018, 71, 22–33. [Google Scholar] [CrossRef]
- El-Fattah, A.A.; Sakr, S.; El-Dieb, S.; Elkashef, H. Angiotensin-Converting Enzyme Inhibition and Antioxidant Activity of Commercial Dairy Starter Cultures. Food Sci. Biotechnol. 2016, 25, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Abdollahzadeh, S.M.; Zahedani, M.R.; Rahmdel, S.; Hemmati, F.; Mazloomi, S.M. Development of Lactobacillus Acidophilus-Fermented Milk Fortified with Date Extract. LWT 2018, 98, 577–582. [Google Scholar] [CrossRef]
- Sung, J.-M.; Kim, Y.-B.; Kum, J.-S.; Choi, Y.-S.; Seo, D.-H.; Choi, H.-W.; Park, J.-D. Effects of Freeze-Dried Mulberry on Antioxidant Activities and Fermented Characteristics of Yogurt during Refrigerated Storage. Korean J. Food Sci. Anim. Resour. 2015, 35, 807–814. [Google Scholar] [CrossRef]
- Prestes, A.A.; Verruck, S.; Vargas, M.O.; Canella, M.H.M.; Silva, C.C.; da Silva Barros, E.L.; Dantas, A.; de Oliveira, L.V.A.; Maran, B.M.; Matos, M.; et al. Influence of Guabiroba Pulp (Campomanesia xanthocarpa o. Berg) Added to Fermented Milk on Probiotic Survival under in Vitro Simulated Gastrointestinal Conditions. Food Res. Int. 2021, 141, 110135. [Google Scholar] [CrossRef]
- Ayyash, M.; Al-Dhaheri, A.S.; Al Mahadin, S.; Kizhakkayil, J.; Abushelaibi, A. In Vitro Investigation of Anticancer, Antihypertensive, Antidiabetic, and Antioxidant Activities of Camel Milk Fermented with Camel Milk Probiotic: A Comparative Study with Fermented Bovine Milk. J. Dairy Sci. 2018, 101, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, X.; Wu, C.; Meng, R.; Gu, Y.; Xiao, X. Phytochemical Profiles and Antioxidant Activity of Fermented Barley with Lactiplantibacillus Plantarum Dy-1. Food Biotechnol. 2022, 36, 266–282. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.-P.; Renard, T.; Rollan, S.; Taillandier, P. Impact of Fermentation Conditions on the Production of Bioactive Compounds with Anticancer, Anti-Inflammatory and Antioxidant Properties in Kombucha Tea Extracts. Process Biochem. 2019, 83, 44–54. [Google Scholar] [CrossRef]
- Aziz, T.; Xingyu, H.; Sarwar, A.; Naveed, M.; Shabbir, M.A.; Khan, A.A.; Ulhaq, T.; Shahzad, M.; Zhennai, Y.; Shami, A.; et al. Assessing the Probiotic Potential, Antioxidant, and Antibacterial Activities of Oat and Soy Milk Fermented with Lactiplantibacillus Plantarum Strains Isolated from Tibetan Kefir. Front. Microbiol. 2023, 14, 1265188. [Google Scholar] [CrossRef]
- Fitrotin, U.; Utami, T.; Hastuti, P.; Journal, U.S.-I.R. Antioxidant Properties of Fermented Sesame Milk Using Lactobacillus Plantarum Dad 13. Int. Res. J. Biol. Sci. 2015, 4, 56–61. [Google Scholar]
- Zhang, Z.-P.; Ma, J.; He, Y.-Y.; Lu, J.; Ren, D.-F. Antioxidant and Hypoglycemic Effects of Diospyros Lotus Fruit Fermented with Microbacterium Flavum and Lactobacillus Plantarum. J. Biosci. Bioeng. 2018, 125, 682–687. [Google Scholar] [CrossRef]
- Hwang, C.E.; Cho, K.M.; Kim, S.C.; Joo, O.S. Change in Physicochemical Properties, Phytoestrogen Content, and Antioxidant Activity during Lactic Acid Fermentation of Soy Powder Milk Obtained from Colored Small Soybean. Korean J. Food Preserv. 2018, 25, 696–705. [Google Scholar] [CrossRef]
- Su, N.; Ren, L.; Ye, H.; Sui, Y.; Li, J.; Ye, M. Antioxidant Activity and Flavor Compounds of Hickory Yogurt. Int. J. Food Prop. 2017, 20, 1894–1903. [Google Scholar] [CrossRef]
- Shori, A.B.; Aljohani, G.S.; Al-zahrani, A.J.; Al-sulbi, O.S.; Baba, A.S. Viability of Probiotics and Antioxidant Activity of Cashew Milk-Based Yogurt Fermented with Selected Strains of Probiotic lactobacillus spp. LWT 2022, 153, 112482. [Google Scholar] [CrossRef]
- Niyibituronsa, M.; Onyango, A.N.; Gaidashova, S.; Imathiu, S.; Boevre, M.D.; Leenknecht, D.; Neirnck, E.; Saeger, S.D.; Vermeir, P.; Raes, K. The Growth of Different Probiotic Microorganisms in Soymilk from Different Soybean Varieties and Their Effects on Anti-Oxidant Activity and Oligosaccharide Content. J. Food Res. 2019, 8, 41. [Google Scholar] [CrossRef]
- Kadum, H. Metabolomic Profiling Elucidated by 1H- NMR and the Correlation with Antibacterial and Antioxidant Activity of (Cyperus Rotundus L) Fermented by Lactic Acid Bacteria. J. Pure Appl. Microbiol. 2019, 13, 1475–1482. [Google Scholar] [CrossRef]
- Tahir, Z.; Saeed, F.; Nosheen, F.; Ahmed, A.; Anjum, F.M. Comparative Study of Nutritional Properties and Antioxidant Activity of Raw and Fermented (Black) Garlic. Int. J. Food Prop. 2022, 25, 116–127. [Google Scholar] [CrossRef]
- Wang, X.; Hu, K.; Chen, Y.; Lai, J.; Zhang, M.; Li, J.; Li, Q.; Zhao, N.; Liu, S. Effect of Lactiplantibacillus Plantarum Fermentation on the Physicochemical, Antioxidant Activity and Immunomodulatory Ability of Polysaccharides from Lvjian Okra. Int. J. Biol. Macromol. 2024, 257, 128649. [Google Scholar] [CrossRef]
- Pereira, A.L.F.; Feitosa, W.S.C.; Abreu, V.K.G.; Lemos, T.D.O.; Gomes, W.F.; Narain, N.; Rodrigues, S. Impact of Fermentation Conditions on the Quality and Sensory Properties of a Probiotic Cupuassu (Theobroma grandiflorum) Beverage. Food Res. Int. 2017, 100, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Suazo, Y.; Davidov-Pardo, G.; Arozarena, I. Effect of Fermentation and Roasting on the Phenolic Concentration and Antioxidant Activity of Cocoa from Nicaragua. J. Food Qual. 2014, 37, 50–56. [Google Scholar] [CrossRef]
- Martelli, F.; Favari, C.; Mena, P.; Guazzetti, S.; Ricci, A.; Del Rio, D.; Lazzi, C.; Neviani, E.; Bernini, V. Antimicrobial and Fermentation Potential of Himanthalia Elongata in Food Applications. Microorganisms 2020, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yan, M.; Xu, H.; Liang, H.; Zhang, J.; Li, M.; Wang, C. Antioxidant and Antiaging Activity of Fermented Coix Seed Polysaccharides on Caenorhabditis Elegans. Nutrients 2023, 15, 2474. [Google Scholar] [CrossRef] [PubMed]
- Curiel, J.A.; Pinto, D.; Marzani, B.; Filannino, P.; Farris, G.A.; Gobbetti, M.; Rizzello, C.G. Lactic Acid Fermentation as a Tool to Enhance the Antioxidant Properties of Myrtus Communis Berries. Microb. Cell Fact. 2015, 14, 67. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, H.; Liu, H.; Ma, R.; Ma, J.; Fang, H. Fermentation by Multiple Bacterial Strains Improves the Production of Bioactive Compounds and Antioxidant Activity of Goji Juice. Molecules 2019, 24, 3519. [Google Scholar] [CrossRef]
- Gao, T.; Chen, J.; Xu, J.; Gu, H.; Zhao, P.; Wang, W.; Pan, S.; Tao, Y.; Wang, H.; Yang, J. Screening of a Novel Lactiplantibacillus Plantarum MMB-05 and Lacticaseibacillus Casei Fermented Sandwich Seaweed Scraps: Chemical Composition, In Vitro Antioxidant, and Volatile Compounds Analysis by GC-IMS. Foods 2022, 11, 2875. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, D.; Zhang, C.; Niu, H.; Xin, X.; Chen, J.; Yi, H.; Liu, D. The Impact of Levilactobacillus Brevis YSJ3 and Lactiplantibacillus Plantarum JLSC2-6 Co-Culture on Gamma-Aminobutyric Acid Yield, Volatile and Non-Volatile Metabolites, Antioxidant Activity, and Bacterial Community in Fermented Cauliflower Byproducts. Food Chem. 2024, 432, 137169. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Liu, X.; Peng, F.; Wang, Q.; Xiao, Y.; Liu, S. Metabolite Profiling, Antioxidant and Anti-Aging Activities of Siraitia Grosvenorii Pomace Processed by Solid-State Fermentation with Eurotium cristatum. Process Biochem. 2023, 133, 109–120. [Google Scholar] [CrossRef]
- Punia, S.; Sandhu, K.S.; Grasso, S.; Purewal, S.S.; Kaur, M.; Siroha, A.K.; Kumar, K.; Kumar, V.; Kumar, M. Aspergillus Oryzae Fermented Rice Bran: A Byproduct with Enhanced Bioactive Compounds and Antioxidant Potential. Foods 2020, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Ali, A.; Sarfraz, A.; Hong, Q.; Boran, H. Effect of Freeze-Drying on Apple Pomace and Pomegranate Peel Powders Used as a Source of Bioactive Ingredients for the Development of Functional Yogurt. J. Food Qual. 2022, 2022, 3327401. [Google Scholar] [CrossRef]
| Fermented Product | Fermentation Type | Fermented by | Outcomes | Concentration of Activities | References | |
|---|---|---|---|---|---|---|
| Buffalo yogurt | Lactic acid bacteria fermentation | Streptococcus salivarius subsp. thermophilus Lactobacillus delbrueckii subsp. bulgaricus | -Exhibit radical scavenging activity -Exhibit angiotensin I-converting enzyme inhibitory activity | Radical scavenging activity (mg TE 100 g−1) | Angiotensin I-converting enzyme inhibitory activity (%) | [86] |
| 7.06 ± 0.04 | 28.82 ± 0.04 | |||||
| Goat yogurt | 8.18 ± 0.05 | 34.69 ± 0.04 | ||||
| Sheep yogurt | 9.34 ± 0.02 | 38.51 ± 0.08 | ||||
| Milk | Lactic acid bacteria fermentation | Lactobacillus pentosus Limosilactobacillus fermentum Lacticaseibacillus paracasei subsp. tolerans | -Exhibit radical scavenging activity | 12.7–16.3% | [114] | |
| -Increase total phenolic content | 36–97 μg mL−1 | |||||
| -Increase total flavonoid content | 0.54–0.55 mg RU mL−1 | |||||
| Horse milk | * | Lactobacillus bulgaricus Streptococcus salivarius subsp. thermophilus | -Exhibit radical scavenging activity | 63.04 ± 6.85% | [24] | |
| Camel milk | Lactic acid bacteria fermentation | Lactobacillus helveticus Lacticaseibacillus casei subsp. casei Lacticaseibacillus paracasei Lacticaseibacillus rhamnosus | -Exhibit radical scavenging activity | 0.322 mg GAE mL−1 | [64] | |
| Milk | * | Lactobacillus delbrueckii subsp. bulgaricus Streptococcus salivarius subsp. thermophilus | -Increase phenolic content | 89.25–532.24 mg GAE L−1 | [120] | |
| Milk | * | Lactiplantibacillus plantarum Bifidobacterium animalis ssp. lactis Streptococcus salivarius subsp. thermophilus | -Exhibit radical scavenging activity | 90.35–95.62% | [75] | |
| Kefir | Submerged fermentation | Lactic acid bacteria | -Exhibit radical scavenging activity | [84] | ||
| -Exhibit total reducing capacity | * | |||||
| Milk | Lactic acid bacteria fermentation | Lactobacillus delbrueckii subsp. acidophilus | -Exhibit radical scavenging activity | 64.7% | [85] | |
| -Exhibit an inhibitory effect on α-amylase | * | |||||
| -Increase exopolysaccharide content | * | |||||
| Goat milk | Lactic acid bacteria fermentation | Lactiplantibacillus plantarum | -Exhibit an inhibitory effect on α-amylase and α-glucosidase activities -Increase exopolysaccharide content | * | [88] | |
| Milk | Lactic acid bacteria fermentation | Lactobacillus helveticus Limosilactobacillus reuteri Lacticaseibacillus rhamnosus | -Exhibit radical scavenging activity | 415–1045 µM TE | [66] | |
| -Exhibit angiotensin I-converting enzyme inhibitory activity | 0.18–2.63 mg mL−1 | |||||
| Milk | * | Streptococcus salivarius subsp. thermophilus Lactobacillus delbrueckii subsp. bulgaricus L. delbrueckii subsp. Acidophilus | -Exhibit radical scavenging activity | 2.16–24.44% | [65] | |
| Bovine colostrum | * | Candida lipolytica | -Exhibit radical scavenging activity | 63–92% | [89] | |
| -Exhibit angiotensin I-converting enzyme inhibitory activity | 72.85–78.52% | |||||
| Goat milk | Lactic acid bacteria fermentation | Lactobacillus fermentum | -Exhibit radical scavenging activity | 0.32–55.73% | [83] | |
| -Exhibit higher proteolytic activity | 1.57–8.44 mg mL−1 | |||||
| Yogurt | Lactic acid bacteria fermentation | Streptococcus salivarius subsp. thermophilus Lactobacillus delbrueckii subsp. acidophilus Bifidobacterium longum subsp. longum | -Increase phenolic content | * | [111] | |
| -Exhibit radical scavenging activity | 73.32% in DPPH and 86.29% in ABTS assays | |||||
| Milk | Lactic acid bacteria fermentation | Lactiplantibacillus plantarum | -Exhibit radical scavenging activity | * | [16] | |
| Milk | Lactic acid bacteria fermentation | Lacticaseibacillus casei | -Exhibit angiotensin I-converting enzyme inhibitory activity | 42.78–52.28% | [43] | |
| Skim camel milk | Lactic acid bacteria fermentation | Limosilactobacillus reuteri Lactiplantibacillus plantarum | -Exhibit radical scavenging activity | 30–70% | [108] | |
| -Exhibit angiotensin I-converting enzyme inhibitory activity | * | |||||
| Milk | Lactic acid bacteria fermentation | Lactobacillus delbrueckii subsp. acidophilus | -Increase phenolic content | 2.6–6.8 GAE mL−1 | [22] | |
| -Exhibit radical scavenging activity | 23–42 μmol TE mL−1 | |||||
| Camel milk | Lactic acid bacteria fermentation | Lacticaseibacillus lactis Lactobacillus delbrueckii subsp. acidophilus | -Exhibit an inhibitory effect on α-amylase and α-glucosidase activities | * | [121] | |
| -Exhibit angiotensin I-converting enzyme inhibitory activity | * | |||||
| -Exhibit radical scavenging activity | 30–50% | |||||
| Goat milk | Lactic acid bacteria fermentation | Lacticaseibacillus casei | -Exhibit radical scavenging activity | 56.50–88.01% | [25] | |
| Yogurt | Lactic acid bacteria fermentation | Lactobacillus delbrueckii subsp. acidophilus Bifidobacterium longum subsp. longum Streptococcus salivarius subsp. thermophilus | -Exhibit radical scavenging activity | * | [109] | |
| Kefir | Microbial fermentation | Lactic acid bacteria Yeast | -Exhibit radical scavenging activity | * | [58] | |
| Milk | * | Lactobacillus delbrueckii subsp. acidophilus | -Exhibit ferric reducing power | 22.24 mg AE 100 g−1 | [118] | |
| Buffalo yogurt | Lactic acid bacteria fermentation | Lactobacillus delbrueckii subsp. acidophilus Lactobacillus helveticus Lactobacillus delbrueckii subsp. bulgaricus Streptococcus salivarius subsp. thermophilus | -Exhibit radical scavenging activity | 70.14–81.62% | [47] | |
| Milk | Lactic acid bacteria fermentation | Streptococcus salivarius subsp. thermophilus Lactobacillus delbrueckii subsp. acidophilus Bifidobacterium animalis spp. lactis | -Increase phenolic content | 333–2409 mg GAE L−1 | [113] | |
| Sarshir | Lactic acid bacteria fermentation | Lactiplantibacillus plantarum | -Exhibit radical scavenging activity | 53.1 ± 1.8% | [91] | |
| Goat milk | Lactic acid bacteria fermentation | Lactiplantibacillus plantarum | -Exhibit angiotensin I-converting enzyme inhibitory activity -Exhibit radical scavenging activity | * | [105] | |
| Yogurt | Lactic acid bacteria fermentation | Streptococcus salivarius subsp. thermophilus Lactobacillus delbrueckii subsp. bulgaricus | -Increase phenolic content | 0.165–0.223 mg GAE mL−1 | [110] | |
| -Exhibit radical scavenging activity | 80.23–95.56% | |||||
| -Exhibit metal chelating activity | 95.88–96.75% | |||||
| Milk | * | Lactobacillus delbrueckii subsp. acidophilus Lacticaseibacillus rhamnosus Bifidobacterium animalis ssp. lactis | -Increase phenolic content | 51.403–62.367 mg GAE 100 g−1 | [115] | |
| -Exhibit radical scavenging activity | 12.759–13.312 mg TE 100 mL−1 | |||||
| Yak yogurt | Spontaneous fermentation | * | -Exhibit radical scavenging activity | * | [63] | |
| Yogurt | Lactic acid bacteria fermentation | Lactobacillus delbrueckii subsp. bulgaricus Lactobacillus delbrueckii subsp. acidophilus Streptococcus salivarius subsp. thermophilus | -Exhibit an increasing β-galactosidase activity | 0.13–0.19 U mL−1 | [116] | |
| -Increase phenolic content | 1.23–3.26 mg mL−1 | |||||
| -Exhibit radical scavenging activity | 24–63% | |||||
| -Exhibit metal chelating activity | * | |||||
| Milk | Lactic acid bacteria fermentation | Lactic acid bacteria | -Exhibit angiotensin I-converting enzyme inhibitory activity | 26.31–75.87% | [117] | |
| -Exhibit radical scavenging activity | 42.78–83.57% | |||||
| -Exhibit metal chelating activity | * | |||||
| Milk and yogurt | Lactic acid bacteria fermentation | Streptococcus salivarius subsp. thermophilus Lactobacillus delbrueckii subsp. bulgaricus Bifidobacterium animalis ssp. lactis | -Exhibit radical scavenging activity | * | [62] | |
| Bovine skim milk | * | Lactobacillus helveticus | -Exhibit radical scavenging activity | 41.34–48.01% | [13] | |
| -Exhibit proteolytic activity | * | |||||
| Yogurt | Lactic acid bacteria fermentation | Lactobacillus delbrueckii subsp. acidophilus Bifidobacterium animalis ssp. lactis Lacticaseibacillus casei Streptococcus salivarius subsp. thermophilus Lactobacillus delbrueckii subsp. bulgaricus | -Increase phenolic content | 2.811–3.220 mg GAE mL−1 | [23] | |
| -Exhibit radical scavenging activity | 97.71–98.53% | |||||
| Bovine milk | Lactic acid bacteria fermentation | Lacticaseibacillus casei Lacticaseibacillus rhamnosus | -Exhibit angiotensin I-converting enzyme inhibitory activity | 48–100% | [67] | |
| -Exhibit radical scavenging activity | * | |||||
| Donkey milk | Lactic acid bacteria fermentation | Streptococcus salivarius subsp. thermophilus Lactobacillus delbrueckii subsp. bulgaricus | -Exhibit radical scavenging activity | 63.38–66.21% | [107] | |
| Kefir | * | Saccharomyces cerevisiae Kazachstania exigua Acetobacter okinawensis Leuconostoc pseudomesenteroides Lactococcus lactis subsp. Lactis | -Exhibit radical scavenging activity | 1.22 mg mL−1 | [41] | |
| Kefir | Lactic acid bacteria fermentation | * | -Increase phenolic content | 266.62–1232.33 mg GAE mL−1 | [106] | |
| -Exhibit radical scavenging activity | 15.68 μmol TE mL−1 | |||||
| Yogurt | Lactic acid bacteria fermentation | Lactobacillus delbrueckii subsp. bulgaricus Lacticaseibacillus casei Streptococcus salivarius subsp. thermophilus Bifidobacterium longum subsp. longum | -Increase phenolic content | 51.66–145.86 mg GAE g−1 | [119] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarıtaş, S.; Portocarrero, A.C.M.; Miranda López, J.M.; Lombardo, M.; Koch, W.; Raposo, A.; El-Seedi, H.R.; de Brito Alves, J.L.; Esatbeyoglu, T.; Karav, S.; et al. The Impact of Fermentation on the Antioxidant Activity of Food Products. Molecules 2024, 29, 3941. https://doi.org/10.3390/molecules29163941
Sarıtaş S, Portocarrero ACM, Miranda López JM, Lombardo M, Koch W, Raposo A, El-Seedi HR, de Brito Alves JL, Esatbeyoglu T, Karav S, et al. The Impact of Fermentation on the Antioxidant Activity of Food Products. Molecules. 2024; 29(16):3941. https://doi.org/10.3390/molecules29163941
Chicago/Turabian StyleSarıtaş, Sümeyye, Alicia C. Mondragon Portocarrero, Jose M. Miranda López, Mauro Lombardo, Wojciech Koch, António Raposo, Hesham R. El-Seedi, José Luiz de Brito Alves, Tuba Esatbeyoglu, Sercan Karav, and et al. 2024. "The Impact of Fermentation on the Antioxidant Activity of Food Products" Molecules 29, no. 16: 3941. https://doi.org/10.3390/molecules29163941
APA StyleSarıtaş, S., Portocarrero, A. C. M., Miranda López, J. M., Lombardo, M., Koch, W., Raposo, A., El-Seedi, H. R., de Brito Alves, J. L., Esatbeyoglu, T., Karav, S., & Witkowska, A. M. (2024). The Impact of Fermentation on the Antioxidant Activity of Food Products. Molecules, 29(16), 3941. https://doi.org/10.3390/molecules29163941