Preparation of Modified Polycarboxylate by Pyrrolidone for Using as a Dispersant in Cobalt Blue Nano-Pigment Slurry
Abstract
1. Introduction
2. Results and Discussion
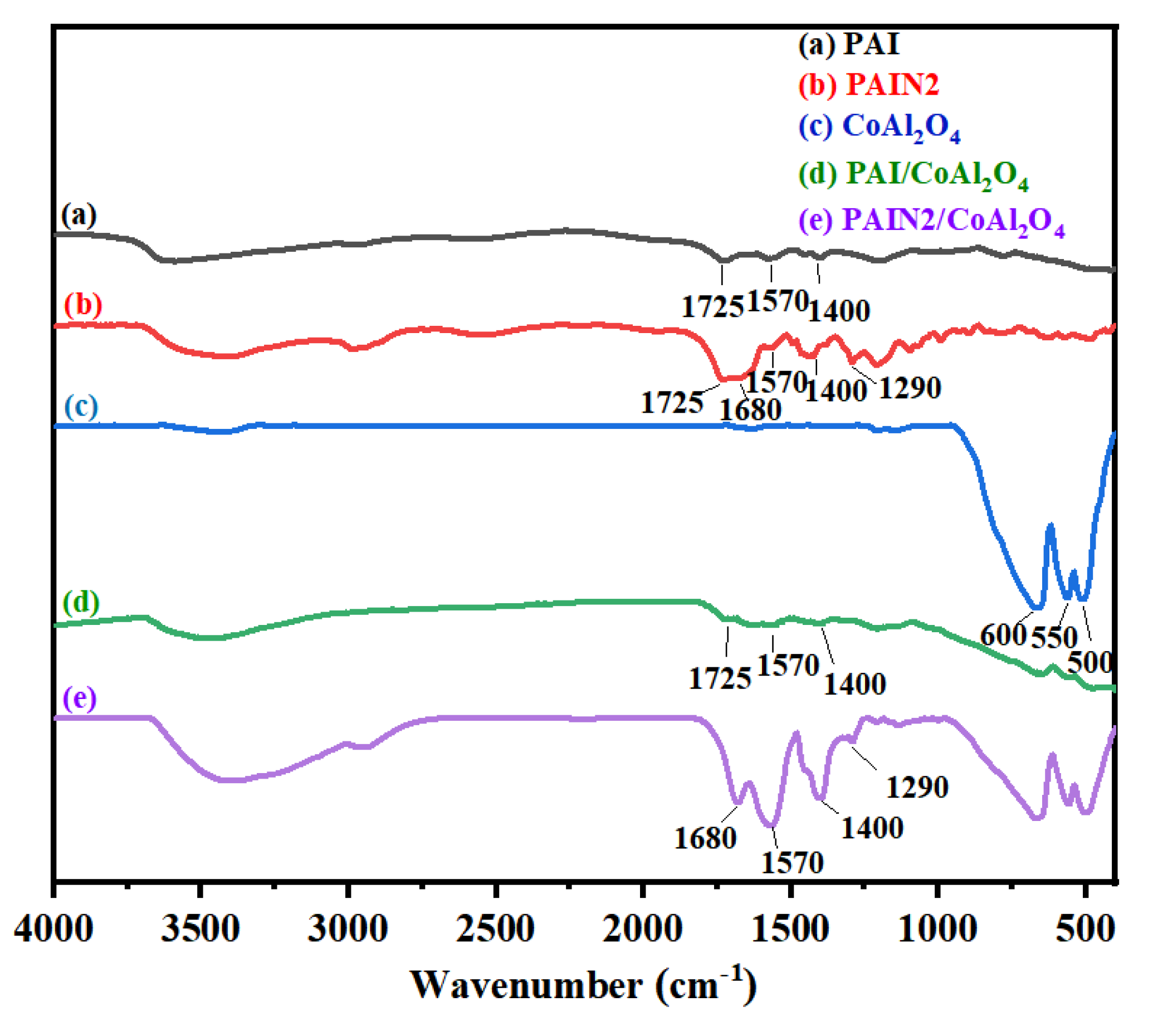
2.1. Characterizations of PAI and PAIN
2.2. Determination of the Optimum Wet Grinding Condition for Preparing CoAl2O4 Nano-Pigment Slurry
2.3. Characterization and Performance of CoAl2O4 Nano-Pigment Slurry
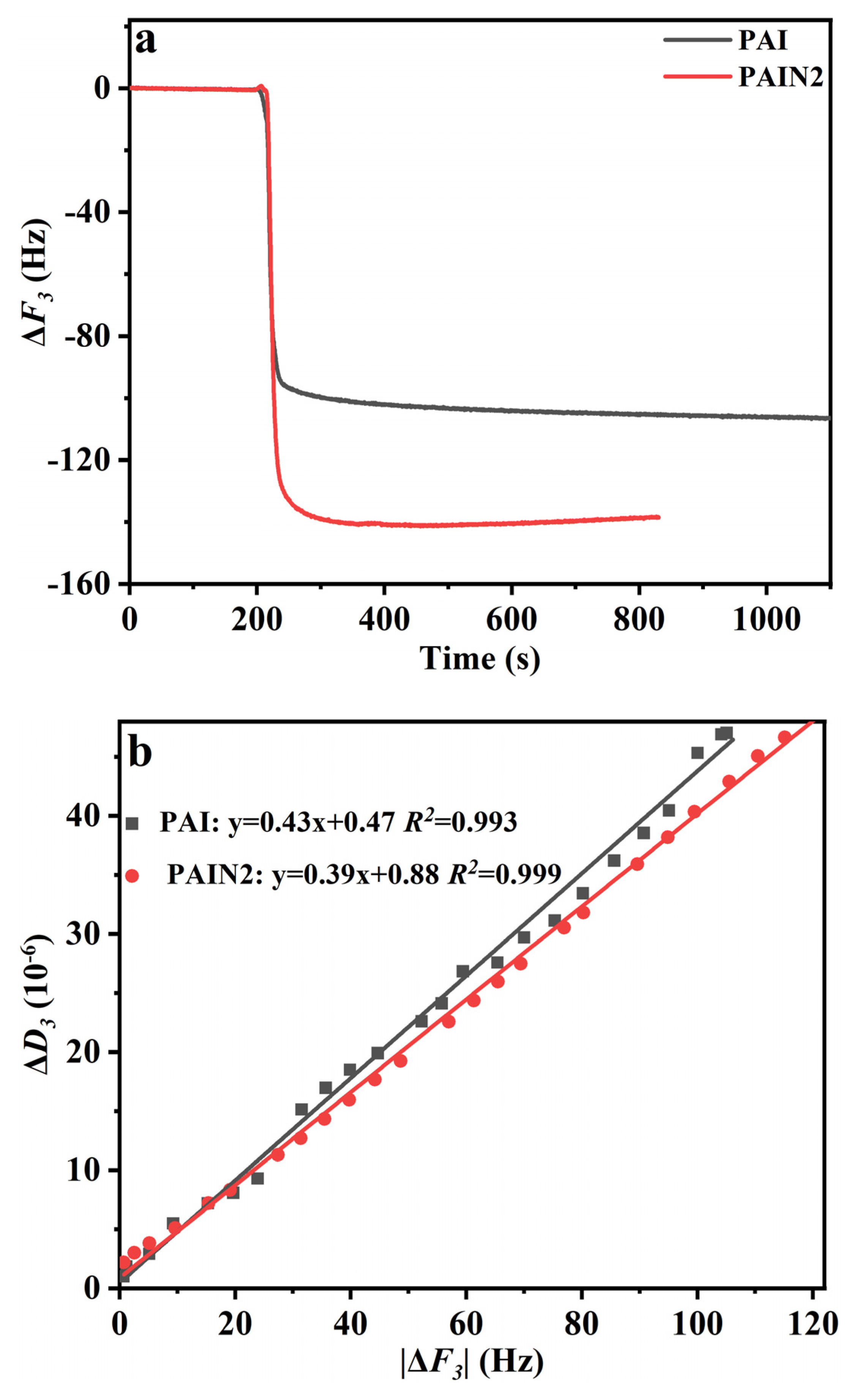
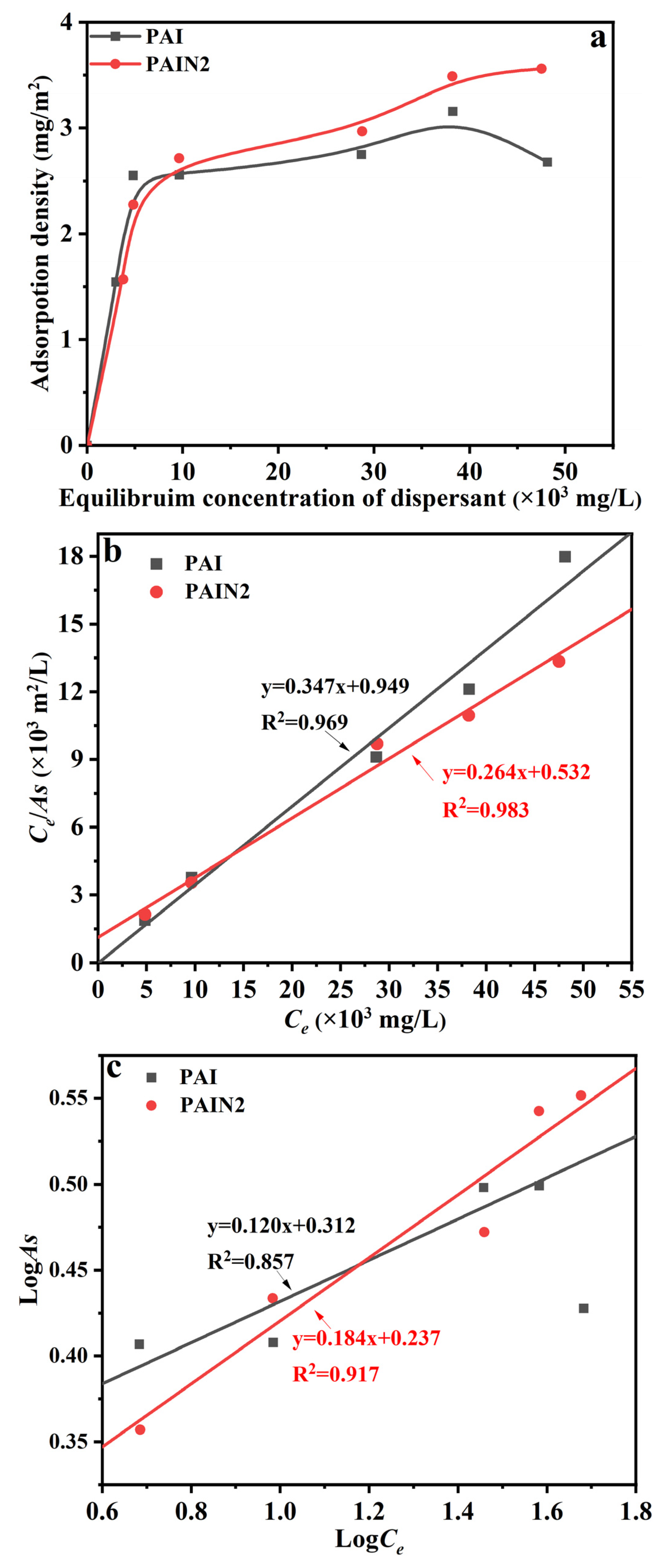
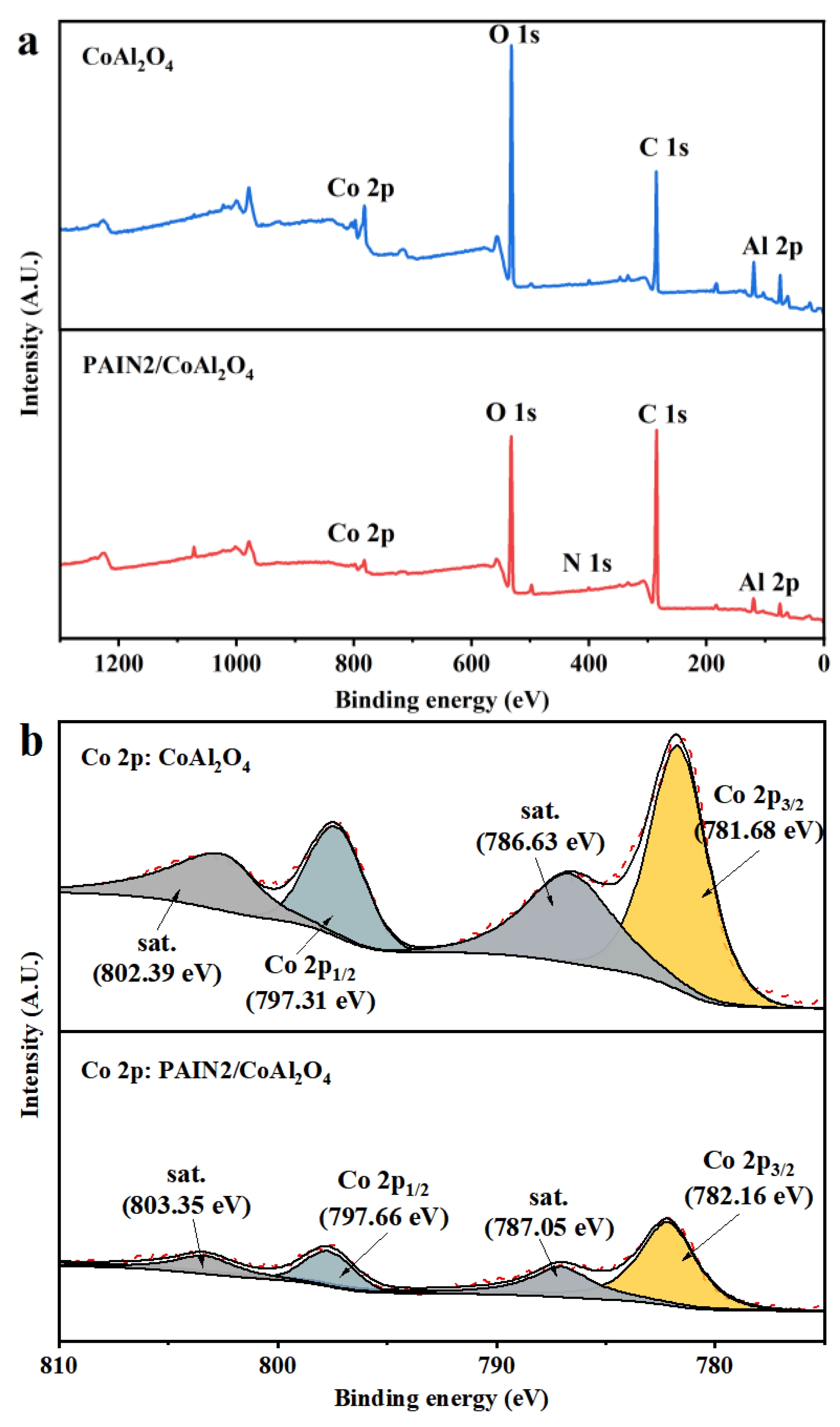
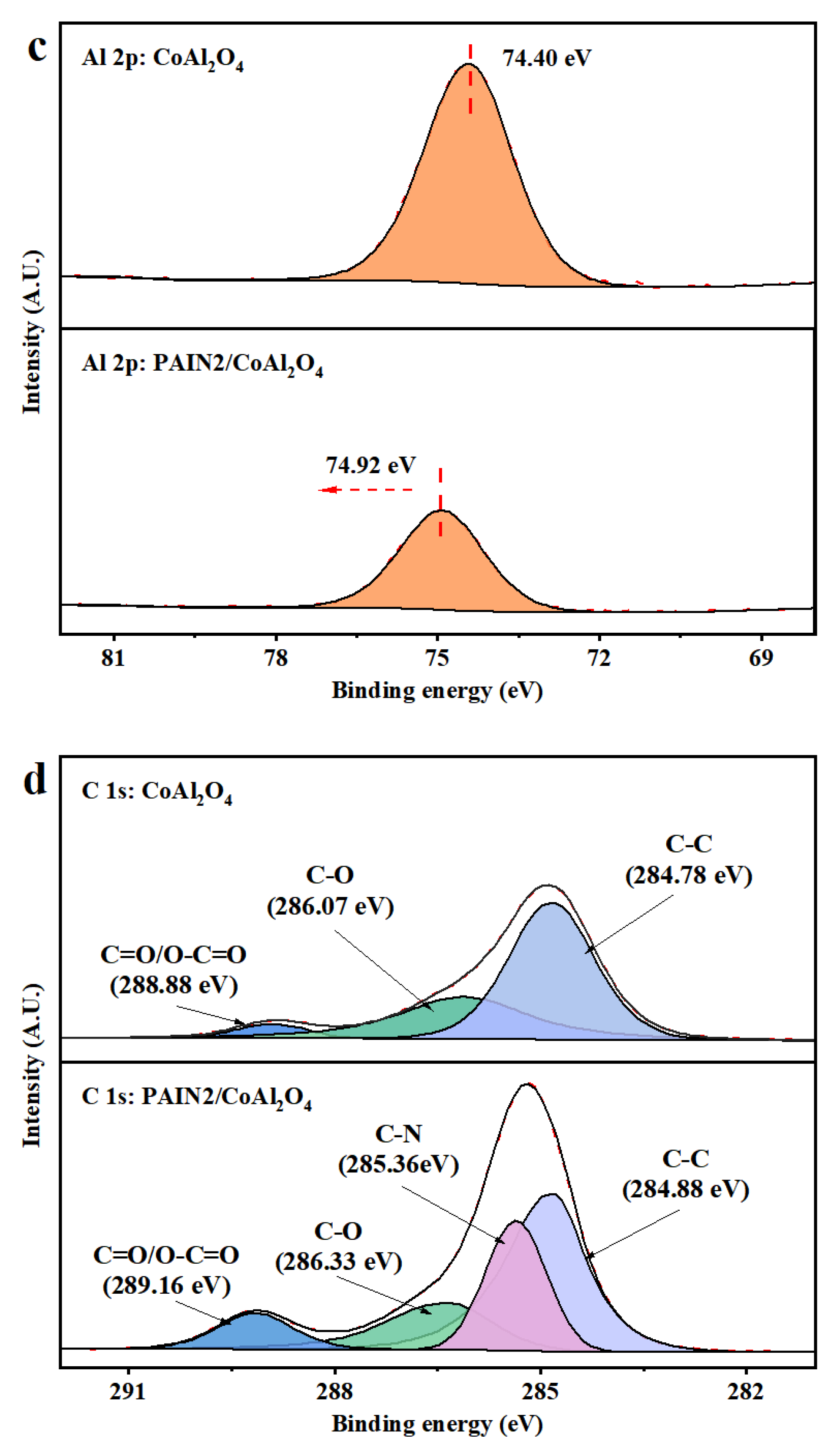
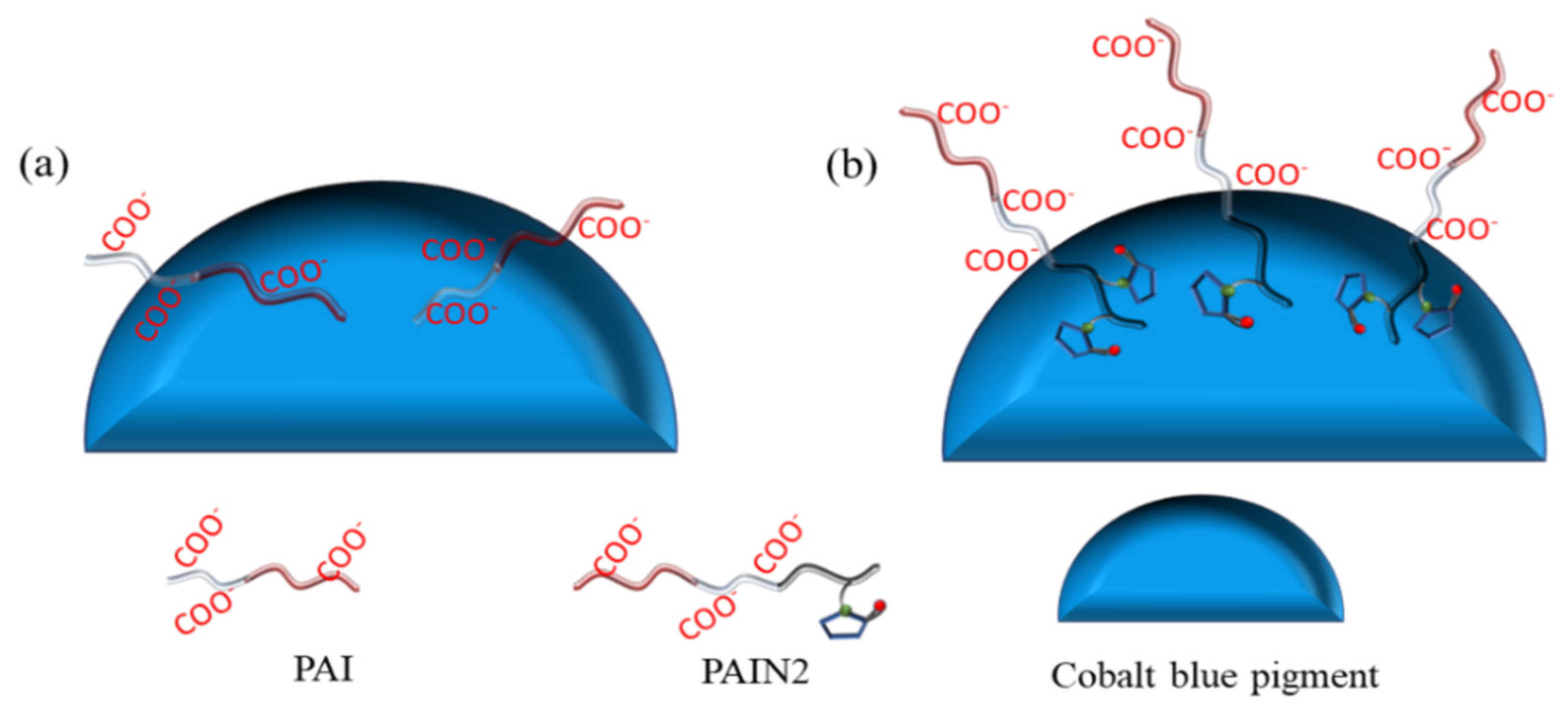
2.4. Research on the Dispersion Stability Mechanism of PAIN on CoAl2O4 Nano-Pigment Slurry
3. Materials and Methods
3.1. Materials
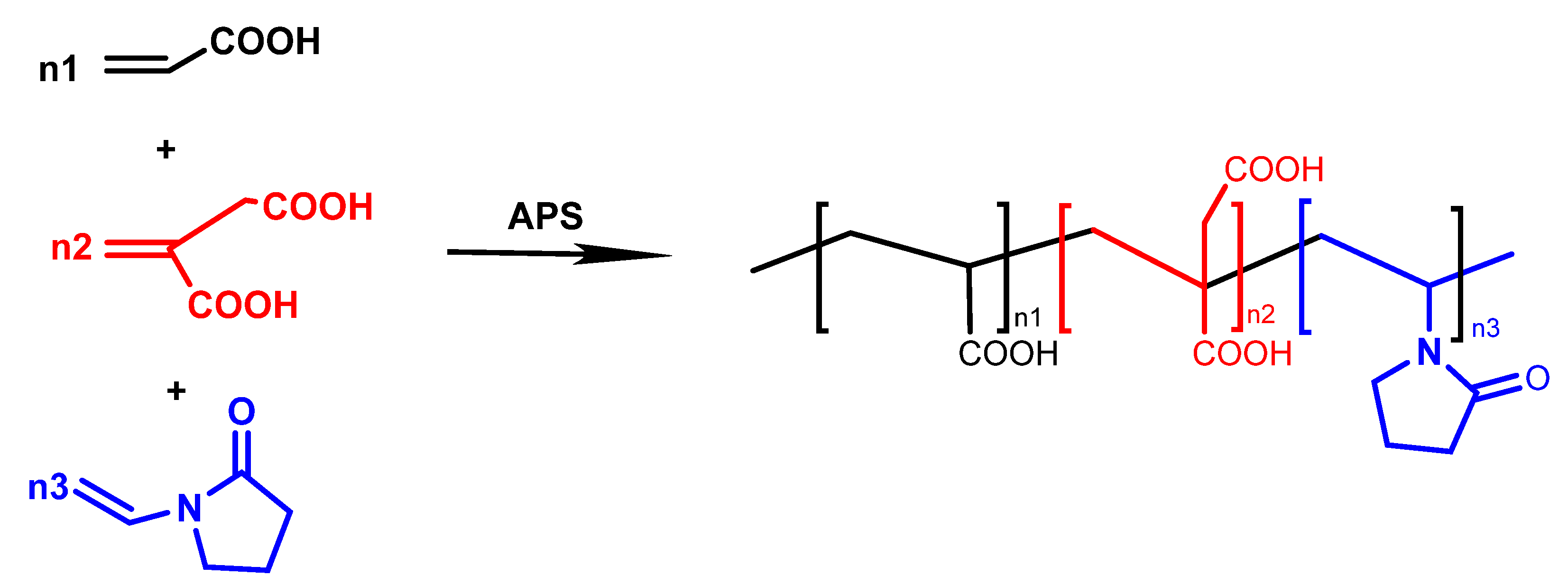
3.2. Synthesis of PAIN
3.3. Structural Characterizations of PAIN
3.4. Preparations of CoAl2O4 Nano-Pigment Slurry [4]
3.5. Performance Characterizations of CoAl2O4 Nano-Pigment Slurry
3.6. Dispersion Stability Mechanism Study of PAIN on CoAl2O4 Nano-Pigment Slurry
3.6.1. QCM Measurements
3.6.2. Adsorption Capacity Measurements of PAIN on CoAl2O4
3.6.3. Zeta Potential Measurements
3.6.4. XPS Measurements
3.6.5. FT–IR Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, Z.D.; Wang, Y.M.; Huang, H.N.; Ling, Z.Y.; Dai, Y.G.; Ke, S.J. Recent development on preparation of ceramic inks in ink-jet printing. Ceram. Int. 2015, 41, 12515–12528. [Google Scholar]
- Kim, J.H.; Noh, H.G.; Kim, U.S.; Cho, W.S.; Choi, J.H.; Lee, Y.O. Recent advances in the ink-jet printing ceramic tile using colorant ceramic-ink. J. Korean Ceram. Soc. 2013, 50, 498–503. [Google Scholar]
- Ferrari, G.; Zannini, P. Thermal behavior of vehicles and digital inks for inkjet decoration of ceramic tiles. Thermochim. Acta 2016, 639, 41–46. [Google Scholar]
- Yang, R.; Tang, Q.Q.; Qian, Y.; Pang, Y.X.; Yang, D.J.; Zheng, D.F.; Zhou, M.S. Preparation of sulfomethylated lignin grafted by pyrrolidone for utilization as a dispersant in nano pigment paste. Ceram. Int. 2023, 49, 16578–16586. [Google Scholar]
- Güngör, G.L.; Kara, A.; Gardini, D.; Blosi, M.; Dondi, M.; Zanelli, C. Ink-jet printability of aqueous ceramic inks for digital decoration of ceramic tiles. Dye. Pigment. 2016, 127, 148–154. [Google Scholar]
- Obata, S.; Yokoyama, H.; Oishi, T.; Usui, M.; Sakurada, O.; Hashiba, M. Preparation of aqueous pigment slurry for decorating whiteware by ink jet printing. J. Mater. Sci. 2004, 39, 2581–2584. [Google Scholar]
- Kwon, J.W.; Sim, H.S.; Lee, J.H.; Hwang, K.T.; Han, K.S.; Kim, J.H.; Kim, U.S. Optimization of aqueous nano ceramic ink and printing characterization for digital ink-jet printing. J. Korean Ceram. Soc. 2017, 54, 478–483. [Google Scholar]
- Salavati-Niasari, M.; Farhadi-Khouzani, M.; Davar, F. Bright blue pigment CoAl2O4 nanocrystals prepared by modified sol-gel method. J. Sol-Gel Sci. Technol. 2009, 52, 321–327. [Google Scholar]
- Costa, A.F.; Pimentel, P.M.; Aquino, F.M.; Melo, D.M.A.; Melo, M.A.F.; Santos, I.M.G. Gelatin synthesis of CuFe2O4 and CuFeCrO4 ceramic pigments. Mater. Lett. 2013, 112, 58–61. [Google Scholar]
- Shin, D.Y.; Kim, K.N.; Han, S.M. Synthesis and characterization of nano-size CoAl2O4 spinel powder using sol-gel process. Mater. Sci. Forum 2007, 544–545, 869–872. [Google Scholar]
- Melo, D.M.A.; Cunha, J.D.; Fernandes, J.D.G.; Bernardi, M.I.; Melo, M.A.F.; Martinelli, A.E. Evaluation of CoAl2O4 as ceramic pigments. Mater. Res. Bull. 2003, 38, 1559–1564. [Google Scholar]
- He, X.M.; Wang, F.; Liu, H.; Niu, L.J.; Wang, X.Z. Synthesis and color properties of the TiO2@CoAl2O4 blue pigments with low cobalt content applied in ceramic glaze. J. Am. Ceram. Soc. 2018, 101, 2578–2588. [Google Scholar]
- Dong, X.B.; Zhang, X.W.; Yu, X.C.; Jiang, Z.G.; Liu, X.R.; Li, C.Q.; Sun, Z.M.; Zheng, S.L.; Dionysiou, D.D. A novel rutile TiO2/AlPO4 core-shell pigment with substantially suppressed photoactivity and enhanced dispersion stability. Powder Technol. 2020, 366, 537–545. [Google Scholar]
- Kim, J.H.; Son, B.R.; Yoon, D.H.; Hwang, K.T.; Noh, H.G.; Cho, W.S.; Kim, U.S. Characterization of blue CoAl2O4 nano-pigment synthesized by ultrasonic hydrothermal method. Ceram. Int. 2012, 38, 5707–5712. [Google Scholar]
- Katsuki, H.; Shiraishi, A.; Komarneni, S.; Moon, W.J.; Toh, S.; Kaneko, K. Rapid synthesis of monodispersed α-Fe2O3 nanoparticles from Fe(NO3)3 solution by microwave irradiation. J. Ceram. Soc. Jpn. 2004, 112, 384–387. [Google Scholar]
- Chen, Z.Z.; Shi, E.W.; Li, W.J.; Zheng, Y.Q.; Zhuang, J.Y.; Bing, X.; Tang, L.A. Preparation of nanosized cobalt aluminate powders by a hydrothermal method. Mat. Sci. Eng. B-Adv. 2004, 107, 217–223. [Google Scholar]
- De Oliveira, A.L.M.; Ferreira, J.M.; Silva, M.R.S.; Braga, G.S.; Soledade, L.E.B.; Aldeiza, M.A.M.M.; Paskocimas, C.A.; Lima, S.J.G.; Longo, E.; de Souza, A.G.; et al. Yellow ZnxNi1−xWO4 pigments obtained using a polymeric precursor method. Dye. Pigment. 2008, 77, 210–216. [Google Scholar]
- Gama, L.; Ribeiro, M.A.; Barros, B.S.; Kiminami, R.H.A.; Weber, I.T.; Costa, A.C.F.M. Synthesis and characterization of the NiAl2O4, CoAl2O4 and ZnAl2O4 spinels by the polymeric precursors method. J. Alloys Comp. 2009, 483, 453–455. [Google Scholar]
- Manova, E.; Kunev, B.; Paneva, D.; Mitov, I.; Petrov, L.; Estournès, C.; D’Orléan, C.; Rehspringer, J.; Kurmoo, M. Mechano-synthesis, characterization, and magnetic properties of nanoparticles of cobalt ferrite, CoFe2O4. Chem. Mater. 2004, 16, 5689–5696. [Google Scholar]
- Kuscer, D.; Stavber, G.; Trefalt, G.; Kosec, M. Formulation of an aqueous titania suspension and its patterning with ink-jet printing technology. J. Am. Ceram. Soc. 2012, 95, 487–493. [Google Scholar]
- Kuo, M.S.; Chang, S.J.; Hsieh, P.H.; Huang, Y.C.; Li, C.C. Efficient dispersants for TiO2 nanopowder in organic suspensions. J. Am. Ceram. Soc. 2016, 99, 445–451. [Google Scholar]
- Luo, F.Z.; Fan, X.H.; Liao, H.M.; Wu, Z.J.; Zhou, Z.S. Study on the preparation of ceramic surface decorative inks. Foshan Ceram. 2016, 26, 22–24. [Google Scholar]
- Zhen, B.W.; Xie, X.L.; Tang, Q.; Li, C.J.; Zhu, W.F.; Wang, M.H.; Guo, H.; Wang, L.J. Effect of kaolinite particle size on stability of Pickering emulsion. J. Synthetic Cryst. 2019, 48, 705–711. [Google Scholar]
- Shih, C.J.; Hon, M.H. Stabilization of aqueous Si3N4 suspensions with ammonium salt of poly(acrylic acid) at various pH. Mater. Chem. Phys. 1998, 57, 125–133. [Google Scholar]
- Li, C.C.; Liu, W.I.; Chen, Y.S. Efficient dispersants for the dispersion of gallium zinc oxide nanopowder in aqueous suspensions. J. Am. Ceram. Soc. 2017, 100, 920–928. [Google Scholar]
- De Laat, A.W.M.; Derks, W.P.T. Colloidal stabilization of BaTiO3 with poly(vinyl alcohol) in water. Colloid. Surfaces A 1993, 71, 147–153. [Google Scholar]
- Li, C.C.; Chang, S.J.; Wu, C.W.; Chang, C.W.; Yu, R.H. Newly designed diblock dispersant for powder stabilization in water-based suspensions. J. Colloid Interf. Sci. 2017, 506, 180–187. [Google Scholar]
- Qiao, M.; Ran, Q.P.; Wu, S.S. Novel star-like surfactant as dispersant for multi-walled carbon nanotubes in aqueous suspensions at high concentration. Appl. Surf. Sci. 2018, 433, 975–982. [Google Scholar]
- Monteiro, S.; Dias, A.; Mendes, A.; Mendes, J.; Serra, A.; Rocha, N.; Coelho, J.; Magalhães, F. Stabilization of nano-TiO2 aqueous dispersions with poly(ethylene glycol)-b-poly(4-vinyl pyridine) block copolymer and their incorporation in photocatalytic acrylic varnishes. Prog. Org. Coat. 2014, 77, 1741–1749. [Google Scholar]
- North, S.M.; Armes, S.P. Aqueous one-pot synthesis of well-defined zwitterionic diblock copolymers by RAFT polymerization: An efficient and environmentally-friendly route to a useful dispersant for aqueous pigments. Green Chem. 2021, 23, 1248–1258. [Google Scholar]
- Zhang, L.L.; Yang, L.H.; Bao, L.J.; Peng, X.Q.; Xie, G.F.; Bi, Y.M. Preparation and application of amphiphilic block copolymers based on poly(N-vinylpyrrolidone). Yunnan Chem. Technol. 2022, 49, 7–9. [Google Scholar]
- Wang, J.; Liu, Q.; Li, K.Y.; He, Y.W. Preparation and properties of N-vinyl pyrrolidone UV pressure sensitive adhesive. Adhesion 2022, 49, 13–16. [Google Scholar]
- Chin, R.M.; Chang, S.J.; Li, C.C.; Chang, C.W.; Yu, R.H. Preparation of highly dispersed and concentrated aqueous suspensions of nanodiamonds using novel diblock dispersants. J. Colloid Interf. Sci. 2018, 520, 119–126. [Google Scholar]
- Tang, Q.Q.; Wu, H.; Zhou, M.S.; Yang, D.J. Preparation of a novel high-performance lignin-based anionic adsorption resin for efficient removal of Cr(VI) in aqueous solutions. Ind. Crop. Prod. 2023, 199, 116720. [Google Scholar]
- Liu, Y.; Jia, L.T.; Hou, B.; Sun, D.K.; Li, D.B. Cobalt aluminate-modified alumina as a carrier for cobalt in Fischer-Tropsch synthesis. Appl. Catal. A-Gen. 2017, 530, 30–36. [Google Scholar]
- Fang, X.; Wu, S.B.; Wu, Y.H.; Yang, W.; Li, Y.L.; He, J.Y.; Hong, P.D.; Nie, M.X.; Xie, C.; Wu, Z.J.; et al. High-efficiency adsorption of norfloxacin using octahedral UIO-66-NH2 nanomaterials: Dynamics, thermodynamics, and mechanisms. Appl. Surf. Sci. 2020, 518, 146226. [Google Scholar]
- Zhou, X.; Luo, H.P.; Sheng, B.; Chen, X.Y.; Wang, Y.H.; Chen, Q.Y.; Zhou, J. Cu2+/Cu+ cycle promoted PMS decomposition with the assistance of Mo for the degradation of organic pollutant. J. Hazard. Mater. 2021, 411, 125050. [Google Scholar] [PubMed]
- Xu, L.H.; Zeng, H.B.; Zhang, X.J.; Cosnier, S.; Marks, R.S.; Shan, D. Highly active M2P2O7@NC (M=Co and Zn) for bifunctional electrocatalysts for ORR and HER. J. Catal. 2019, 377, 20–27. [Google Scholar]
- Huang, G.H.; Pan, Z.D.; Wang, Y.M. Preparation and characterization of waterborne ceramic ink with submicron-sized praseodymium-doped zirconium silicate pigment by water-based diblock polymer dispersants. Ceram. Int. 2020, 46, 21910–21919. [Google Scholar]
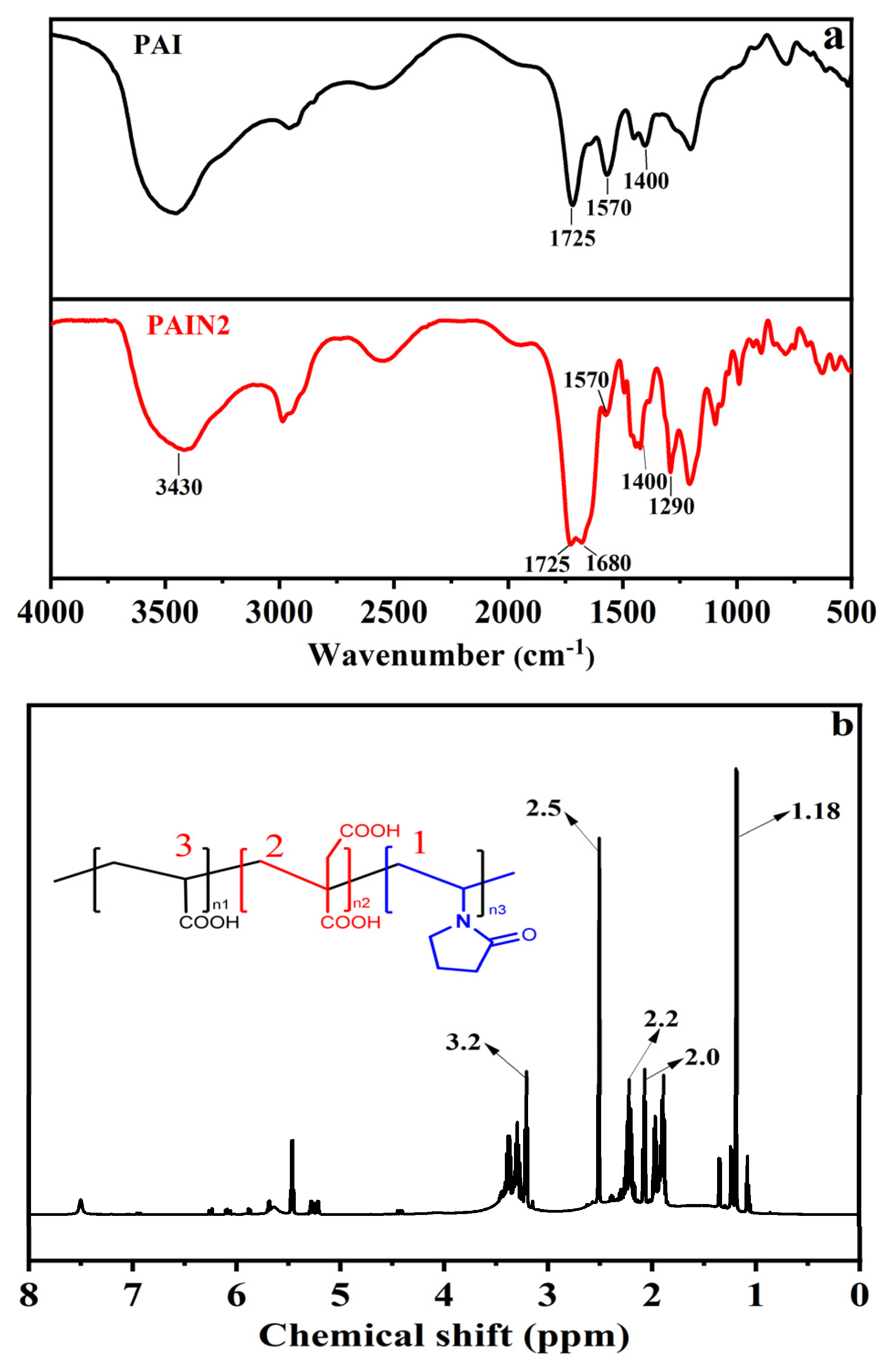

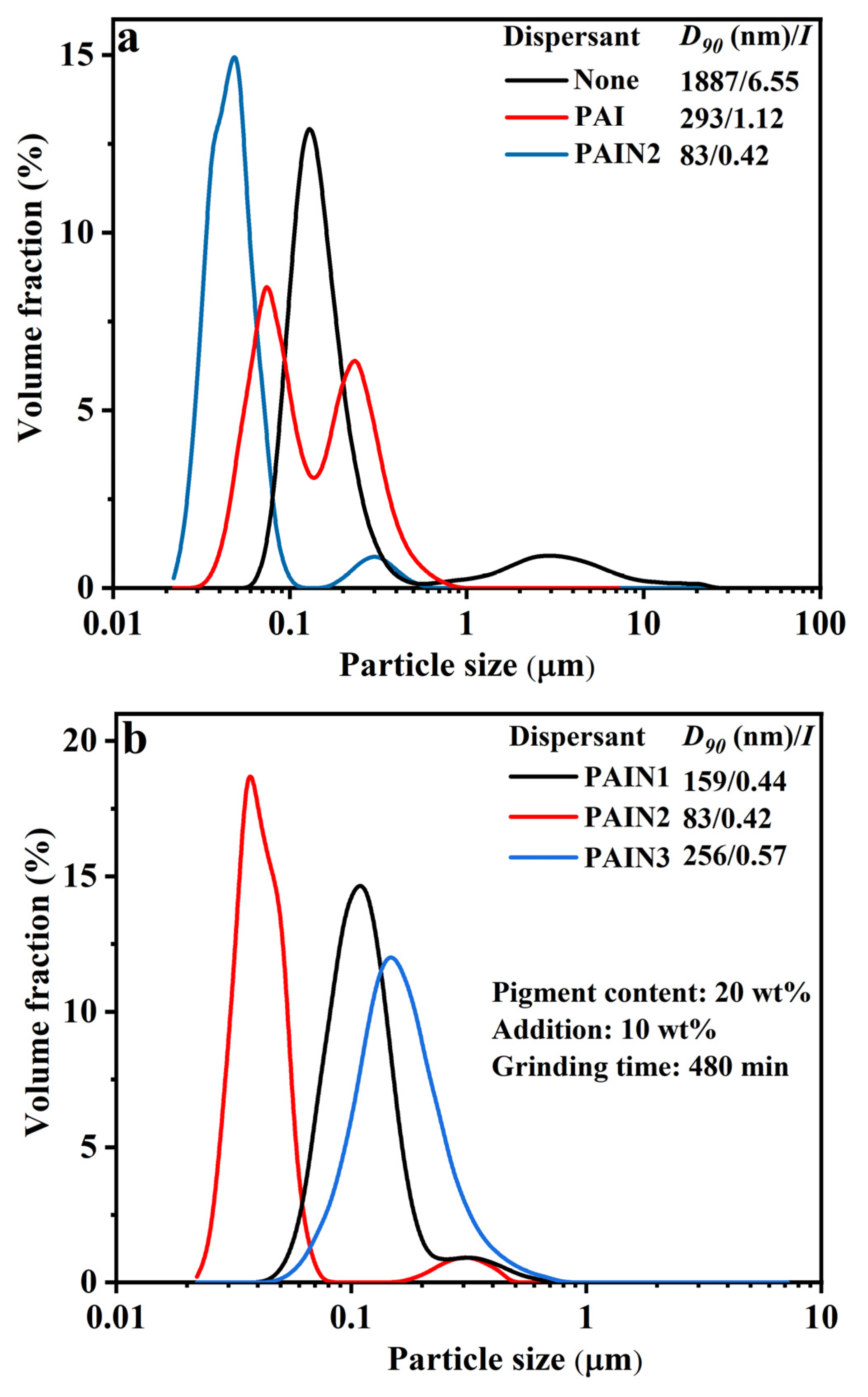
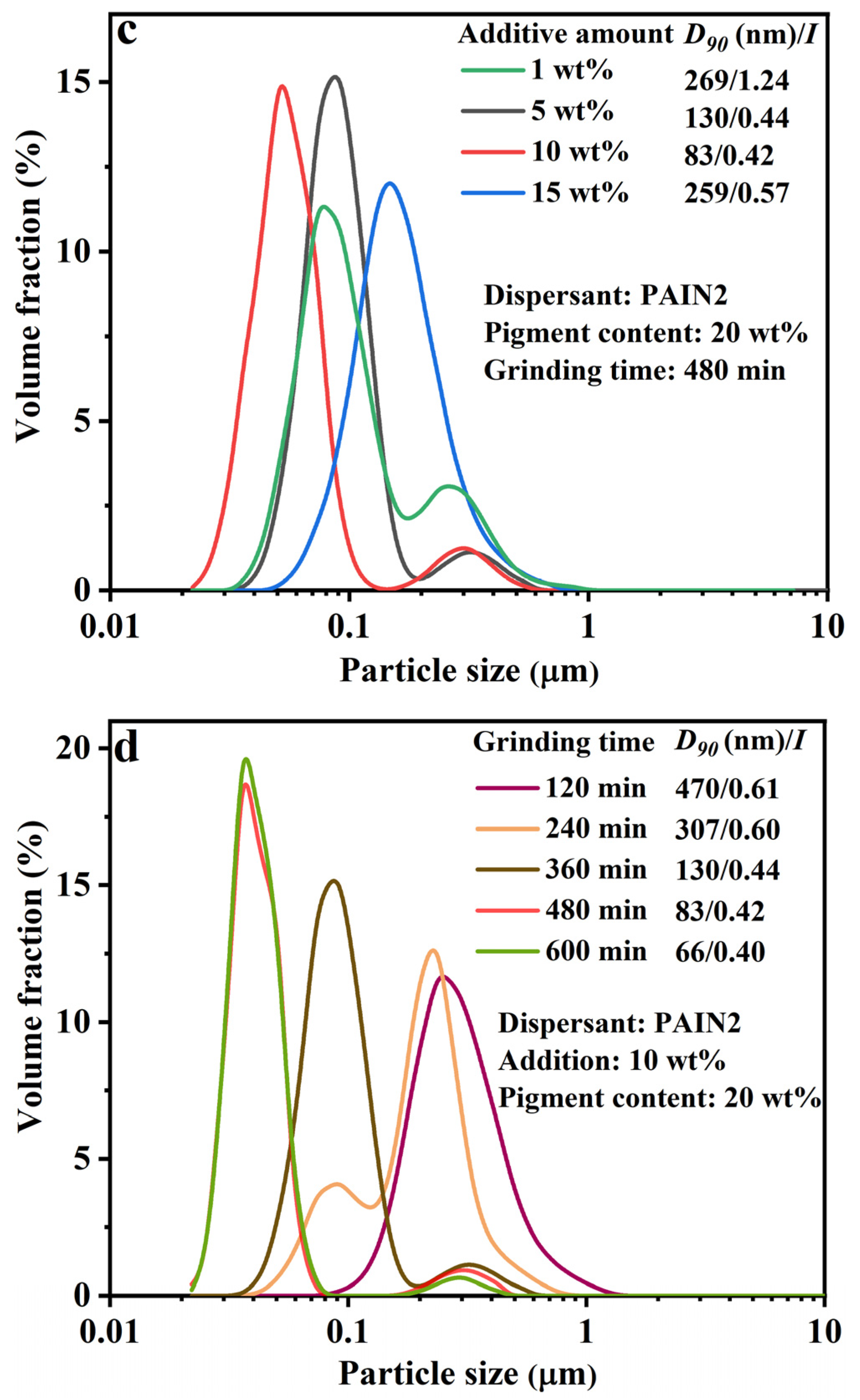

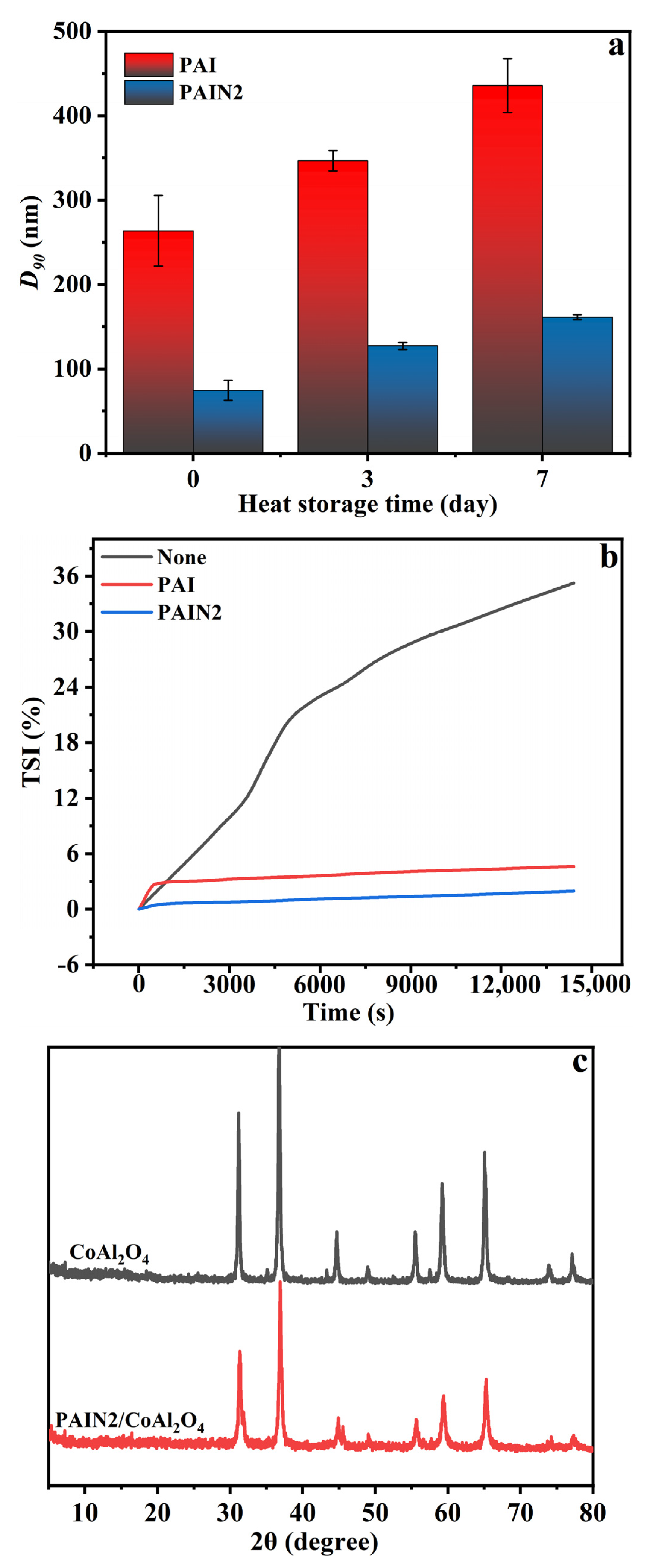

| Dispersant | C% | H% | N% | Pyrrolidone Ligand Content (mmol/g) | Mw | Mn | PDI |
|---|---|---|---|---|---|---|---|
| PAI | 44.51 | 4.23 | 0 | -- | 43,890 | 12,590 | 3.5 |
| PAIN1 | 43.21 | 5.53 | 2.64 | 1.89 | 42,830 | 15,130 | 2.8 |
| PAIN2 | 47.78 | 6.41 | 4.43 | 3.16 | 27,620 | 13,150 | 2.1 |
| PAIN3 | 43.10 | 7.51 | 4.73 | 3.38 | 14,240 | 6470 | 2.2 |
| Dispersant | a/D90 (nm) | V (nm/s) | D (nm) | X (nm) |
|---|---|---|---|---|
| None | 1887 | 5428 | 5428 | 481 |
| PAI | 293 | 131 | 131 | 1221 |
| PAIN2 | 83 | 11 | 11 | 2295 |
| Dispersant | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| As (mg/m2) | K1 (mg/L) | R2 | n | Kf | R2 | |
| PAI | 2.9 | 2.752 | 0.969 | 7.207 | 2.051 | 0.857 |
| PAIN2 | 3.8 | 2.021 | 0.983 | 5.712 | 1.726 | 0.917 |
| Dispersant | Molar Ratio of AA, IA and NVP (n1:n2:n3) | Additive Amount of APS (wt%) | Temperature (°C) | Reaction Time (h) |
|---|---|---|---|---|
| PAI | 1:1:0 | 3 | 85 | 8 |
| PAIN1 | 1:1:0.5 | 1 | 85 | 6 |
| PAIN2 | 1:1:1 | 1 | 80 | 6 |
| PAIN3 | 1:1:2 | 1 | 80 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Q.; Yang, R.; Li, J.; Zhou, M.; Yang, D. Preparation of Modified Polycarboxylate by Pyrrolidone for Using as a Dispersant in Cobalt Blue Nano-Pigment Slurry. Molecules 2024, 29, 3940. https://doi.org/10.3390/molecules29163940
Tang Q, Yang R, Li J, Zhou M, Yang D. Preparation of Modified Polycarboxylate by Pyrrolidone for Using as a Dispersant in Cobalt Blue Nano-Pigment Slurry. Molecules. 2024; 29(16):3940. https://doi.org/10.3390/molecules29163940
Chicago/Turabian StyleTang, Qianqian, Rong Yang, Jinnuo Li, Mingsong Zhou, and Dongjie Yang. 2024. "Preparation of Modified Polycarboxylate by Pyrrolidone for Using as a Dispersant in Cobalt Blue Nano-Pigment Slurry" Molecules 29, no. 16: 3940. https://doi.org/10.3390/molecules29163940
APA StyleTang, Q., Yang, R., Li, J., Zhou, M., & Yang, D. (2024). Preparation of Modified Polycarboxylate by Pyrrolidone for Using as a Dispersant in Cobalt Blue Nano-Pigment Slurry. Molecules, 29(16), 3940. https://doi.org/10.3390/molecules29163940




