Surface Modification of Bi2O3 Nanoparticles with Biotinylated β-Cyclodextrin as a Biocompatible Therapeutic Agent for Anticancer and Antimicrobial Applications
Abstract
1. Introduction
2. Results and Discussion
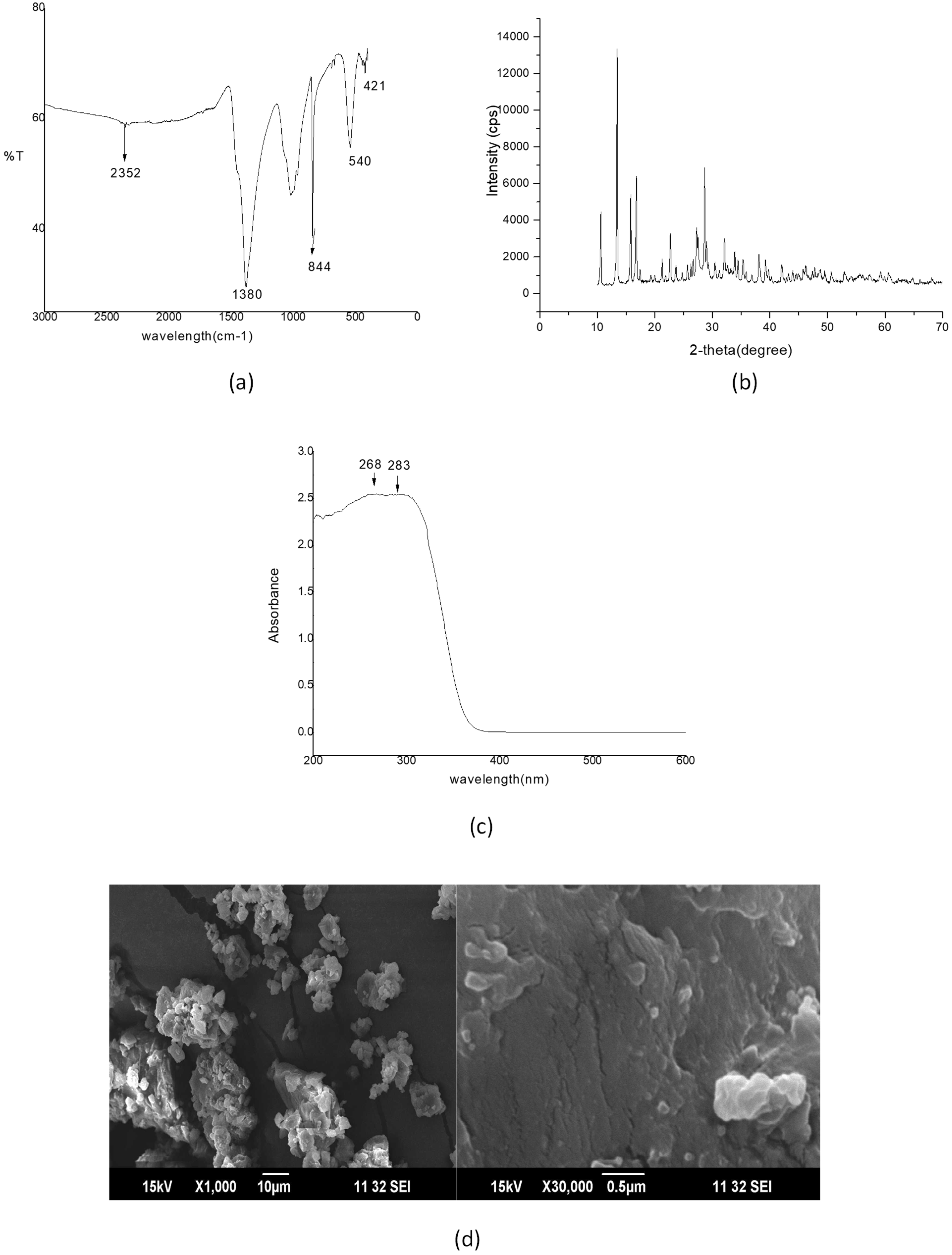
2.1. Characterization of Bi2O3 NPs
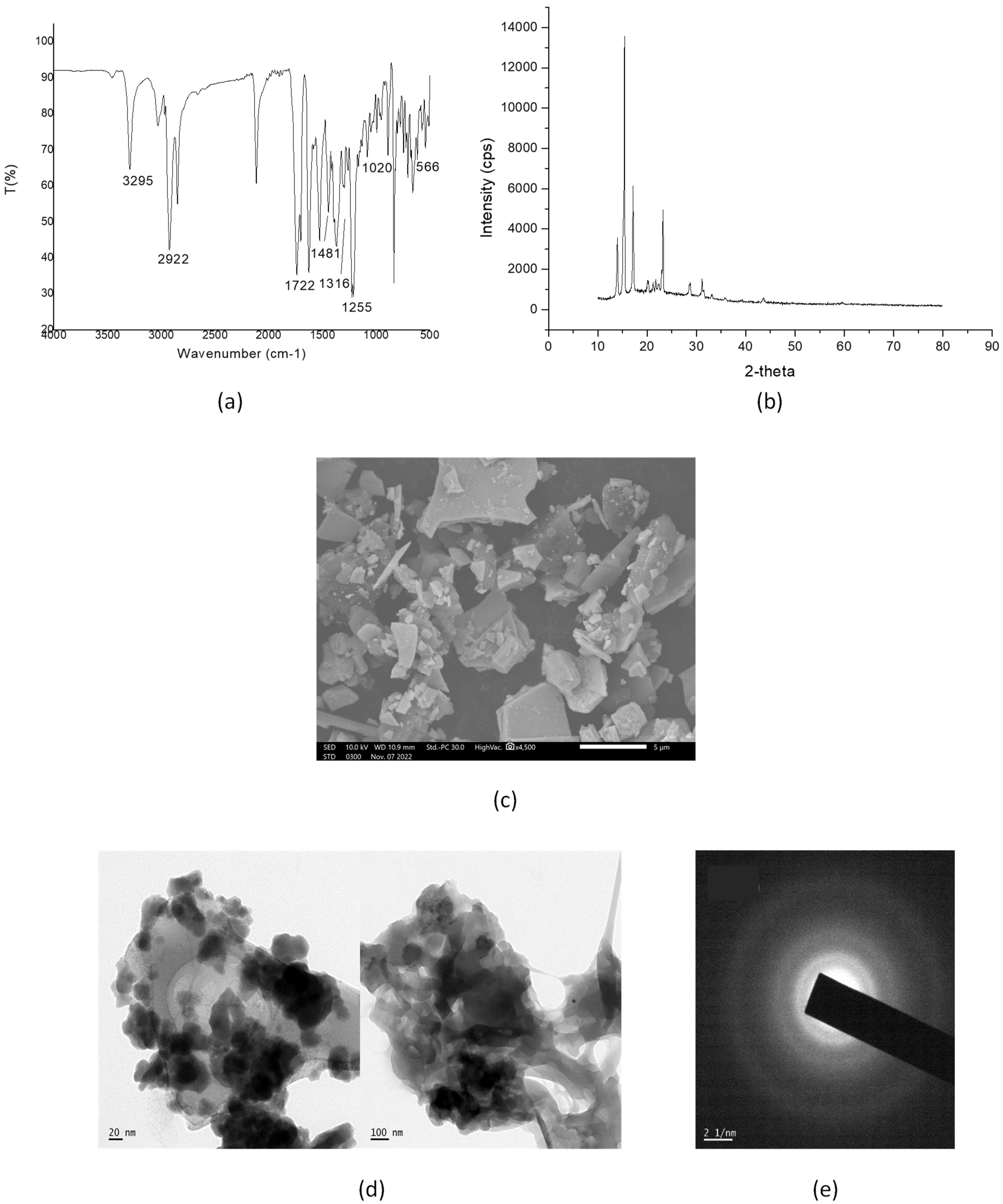
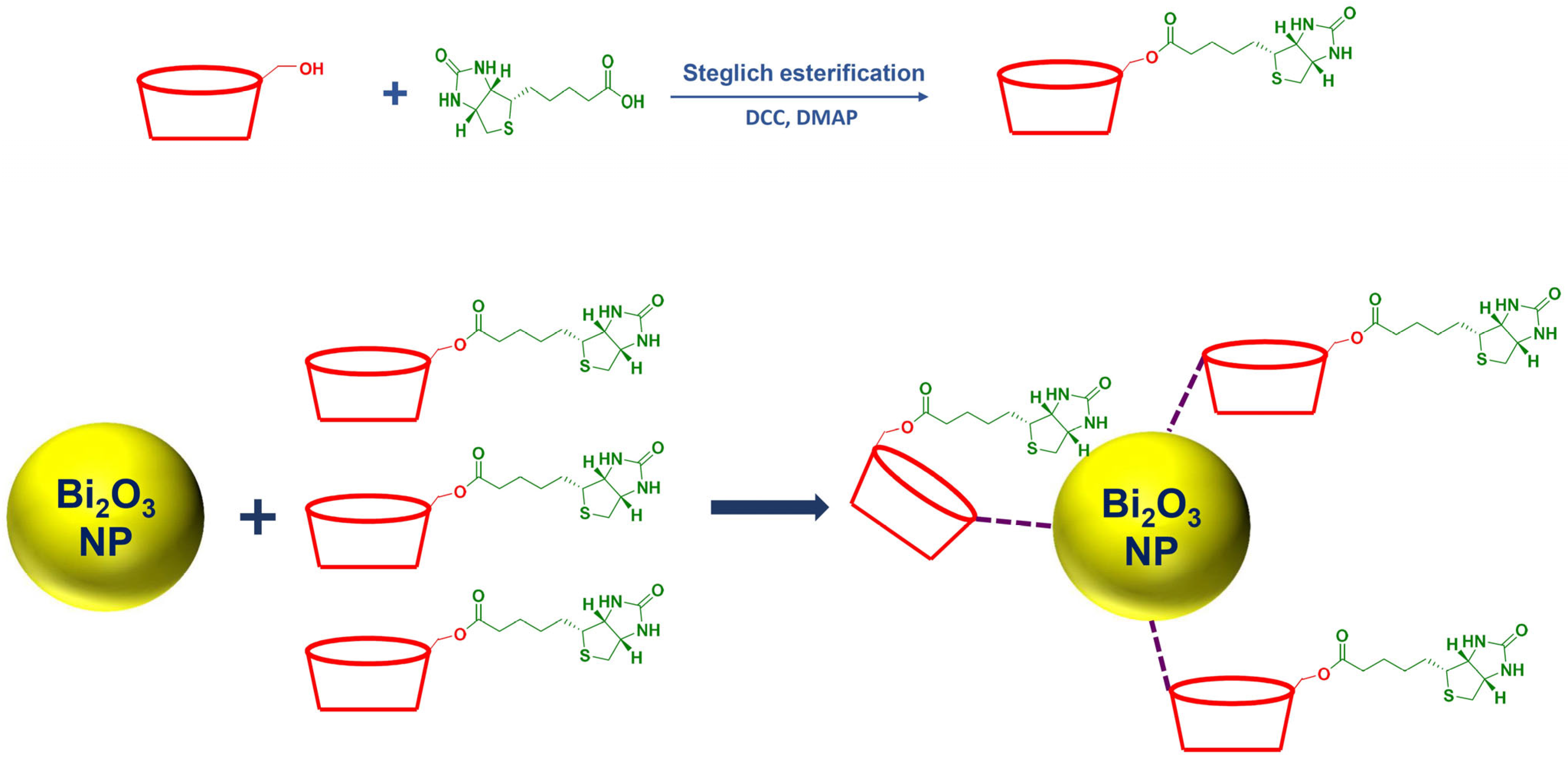
2.2. Characterization of the Surface-Modified Bi2O3 Nanoparticles Using Biotinylated β-Cyclodextrin
2.3. Antimicrobial Studies
2.4. In Vitro Cytotoxicity Studies
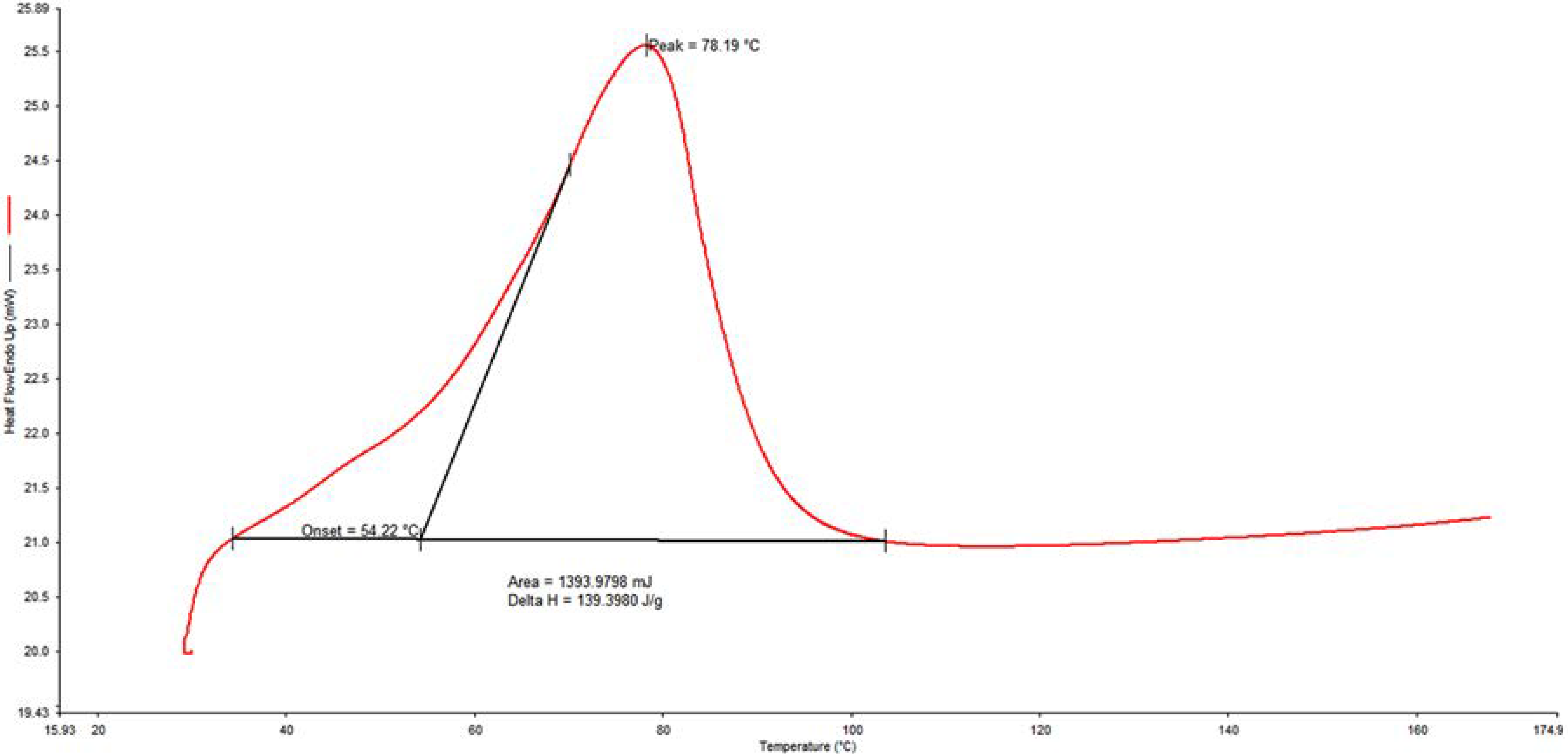
2.5. Thermal Studies
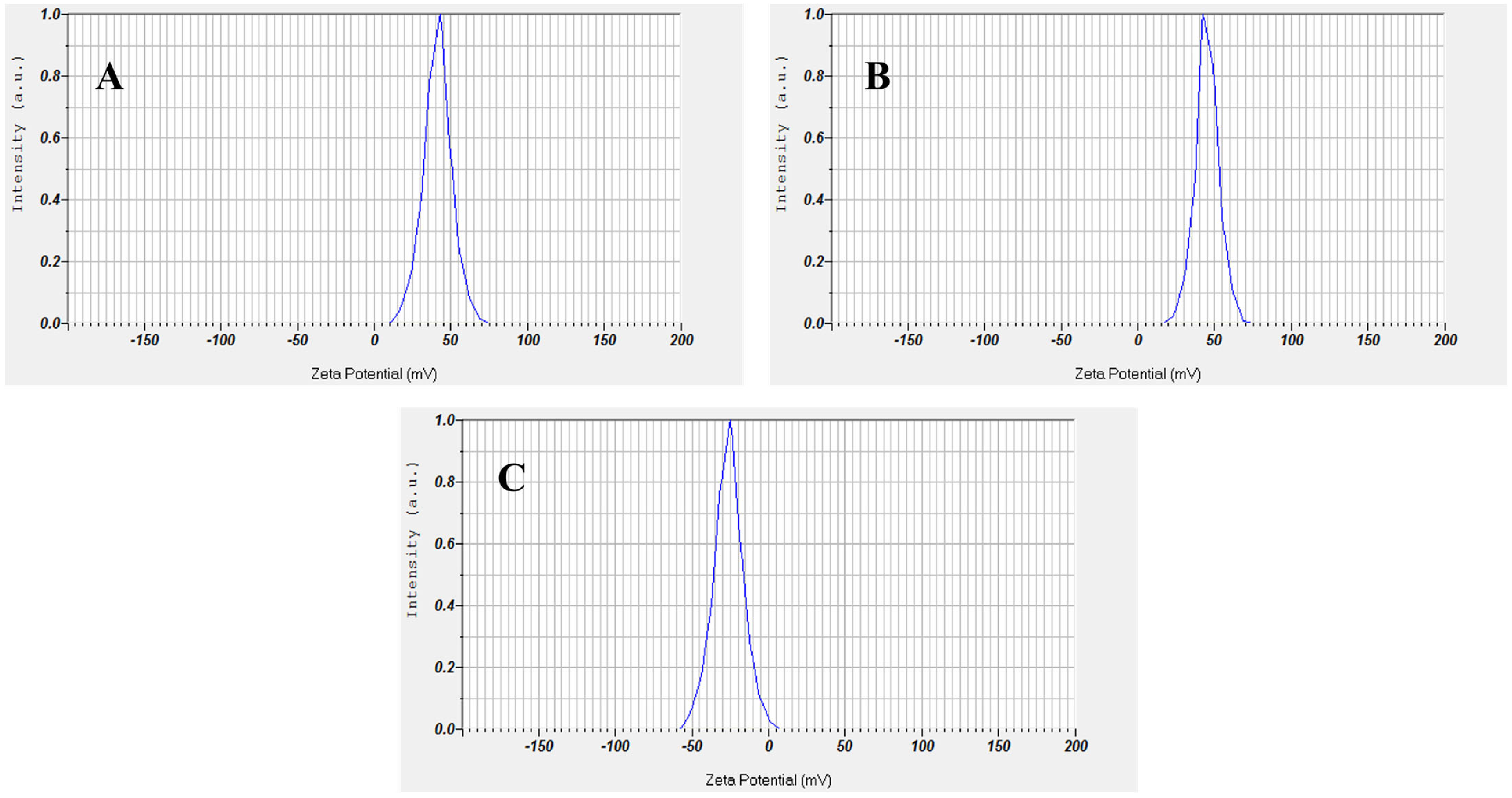
2.6. Zeta Potential Analysis
3. Experimental
3.1. Materials and Methods
3.2. Synthesis of Bi2O3 NPs
3.3. Synthesis of Surface-Modified Bi2O3 Nanoparticles Using Biotinylated β-Cyclodextrin
3.4. Antimicrobial Activity
3.5. In Vitro Cytotoxicity Assays—MTT Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zarschler, K.; Rocks, L.; Licciardello, N.; Boselli, L.; Polo, E.; Garcia, K.P.; De Cola, L.; Stephan, H.; Dawson, K.A. Ultrasmall Inorganic Nanoparticles: State-of-the-Art and Perspectives for Biomedical Applications. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1663–1701. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K. Construction of Functional Biomaterials by Biomolecular Self-Assembly. Bull. Chem. Soc. Jpn. 2017, 90, 873–884. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.; Park, Y.I.; Lee, N.; Hyeon, T. Recent Development of Inorganic Nanoparticles for Biomedical Imaging. ACS Cent. Sci. 2018, 4, 324–336. [Google Scholar] [CrossRef]
- Diana, E.J.; Mathew, T. V Synthesis and Characterization of Surface-Modified Ultrafine Titanium Dioxide Nanoparticles with an Antioxidant Functionalized Biopolymer as a Therapeutic Agent: Anticancer and Antimicrobial Evaluation. Colloids Surf. B Biointerfaces 2022, 220, 112949. [Google Scholar] [CrossRef]
- Jiao, M.; Zhang, P.; Meng, J.; Li, Y.; Liu, C.; Luo, X.; Gao, M. Recent Advancements in Biocompatible Inorganic Nanoparticles towards Biomedical Applications. Biomater. Sci. 2018, 6, 726–745. [Google Scholar] [CrossRef] [PubMed]
- Kanchana, U.S.; Mathew, T.V. Surface Functionalization of ZnO Nanoparticles with Functionalized Bovine Serum Albumin as a Biocompatible Photochemical and Antimicrobial Agent. Surf. Interfaces 2021, 24, 101056. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Bahrampour, S.; Mozafari, M.; Gasik, M. Surface Functionalization of Anodized Tantalum with Mn3O4 Nanoparticles for Effective Corrosion Protection in Simulated Inflammatory Condition. Ceram. Int. 2022, 48, 3148–3156. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Yarmand, B.; Mozafari, M. Enhanced Corrosion Resistance and In-Vitro Biodegradation of Plasma Electrolytic Oxidation Coatings Prepared on AZ91 Mg Alloy Using ZnO Nanoparticles-Incorporated Electrolyte. Surf. Coat. Technol. 2019, 360, 153–171. [Google Scholar] [CrossRef]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered Nanoparticles Interacting with Cells: Size Matters. J. Nanobiotechnol. 2014, 12, 5. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective Use of Nanocarriers as Drug Delivery Systems for the Treatment of Selected Tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [PubMed]
- Mathew, T.V.; Kuriakose, S. Synthesis and Characterization of Sodium-Lithium Niobate Ceramic Structures and Their Composites with Biopolymers. J. Adv. Ceram. 2013, 2, 11–20. [Google Scholar] [CrossRef]
- Mathew, T.V.; Kuriakose, S. 4-(1-Pyrenyl)Butyric Acid-Functionalised Chitosan as a Matrix for AgNP: Photoresponsive and Thermal Properties. J. Polym. Res. 2013, 20, 291. [Google Scholar] [CrossRef]
- Yang, C.; Liu, G.; Chen, J.; Zeng, B.; Shen, T.; Qiu, D.; Huang, C.; Li, L.; Chen, D.; Chen, J.; et al. Chitosan and Polyhexamethylene Guanidine Dual-Functionalized Cotton Gauze as a Versatile Bandage for the Management of Chronic Wounds. Carbohydr. Polym. 2022, 282, 119130. [Google Scholar] [CrossRef]
- Leontie, L.; Caraman, M.; Alexe, M.; Harnagea, C. Structural and Optical Characteristics of Bismuth Oxide Thin Films. Surf. Sci. 2002, 507–510, 480–485. [Google Scholar] [CrossRef]
- Azad, A.M.; Larose, S.; Akbar, S.A. Bismuth Oxide-Based Solid Electrolytes for Fuel Cells. J. Mater. Sci. 1994, 29, 4135–4151. [Google Scholar] [CrossRef]
- Mahmoud, W.E.; Al-Ghamdi, A.A. Synthesis and Properties of Bismuth Oxide Nanoshell Coated Polyaniline Nanoparticles for Promising Photovoltaic Properties. Polym. Adv. Technol. 2011, 22, 877–881. [Google Scholar] [CrossRef]
- Sergeev, A.; Golev, I. High-Temperature Superconducting Materials Based on Bismuth with a Low Critical Current. Mater. Today Proc. 2019, 11, 489–493. [Google Scholar] [CrossRef]
- Reverberi, A.P.; Varbanov, P.S.; Vocciante, M.; Fabiano, B. Bismuth Oxide-Related Photocatalysts in Green Nanotechnology: A Critical Analysis. Front. Chem. Sci. Eng. 2018, 12, 878–892. [Google Scholar] [CrossRef]
- Bhande, S.S.; Mane, R.S.; Ghule, A.V.; Han, S.H. A Bismuth Oxide Nanoplate-Based Carbon Dioxide Gas Sensor. Scr. Mater. 2011, 12, 1081–1084. [Google Scholar] [CrossRef]
- Fruth, V.; Popa, M.; Berger, D.; Ramer, R.; Gartner, M.; Ciulei, A.; Zaharescu, M. Deposition and Characterisation of Bismuth Oxide Thin Films. J. Eur. Ceram. Soc. 2005, 25, 2171–2174. [Google Scholar] [CrossRef]
- Bartoli, M.; Jagdale, P.; Tagliaferro, A. A Short Review on Biomedical Applications of Nanostructured Bismuth Oxide and Related Nanomaterials. Materials 2020, 13, 5234. [Google Scholar] [CrossRef] [PubMed]
- Kumar Trivedi, M.; Mohan Tallapragada, R.; Branton, A.; Trivedi, D.; Nayak, G.; Latiyal, O.; Jana, S. Evaluation of Atomic, Physical, and Thermal Properties of Bismuth Oxide Powder: An Impact of Biofield Energy Treatment. Am. J. Nano Res. Appl. 2015, 2015, 94–98. [Google Scholar]
- Kim, H.W.; Myung, J.H.; Shim, S.H. One-Dimensional Structures of Bi2O3 Synthesized via Metalorganic Chemical Vapor Deposition Process. Solid State Commun. 2006, 137, 196–198. [Google Scholar] [CrossRef]
- Irmawati, R.; Nasriah, M.N.N.; Taufiq-Yap, Y.H.; Hamid, S.B.A. Characterization of Bismuth Oxide Catalysts Prepared from Bismuth Trinitrate Pentahydrate: Influence of Bismuth Concentration. Catal. Today 2004, 93–95, 701–709. [Google Scholar] [CrossRef]
- Zaid, M.; Marwah, A.; Farah, K. Low Temperature Photosynthesis of Bismuth Oxide Nano Powder. Earth. J. Chem. Sci. 2019, 2, 303–307. [Google Scholar] [CrossRef]
- Shinde, P.V.; Ghule, B.G.; Shaikh, S.F.; Shinde, N.M.; Sangale, S.S.; Jadhav, V.V.; Yoon, S.-Y.; Kim, K.H.; Mane, R.S. Microwave-Assisted Hierarchical Bismuth Oxide Worm-like Nanostructured Films as Room-Temperature Hydrogen Gas Sensors. J. Alloy. Compd. 2019, 802, 244–251. [Google Scholar] [CrossRef]
- Dong, W.; Zhu, C. Optical Properties of Surface-Modified Bi2O3 Nanoparticles. J. Phys. Chem. Solids 2003, 64, 265–271. [Google Scholar] [CrossRef]
- Shokuhfar, A.; Nasir, K.; Esmaeilirad, A.; Mazinani, V.; Mallahi, M.; Shokuhfar, A.; Vaezi, M.R.; Esmaeilirad, A.; Mazinani, V. Synthesis and Characterization of Bismuth Oxide Nanoparticles via Sol-Gel Method. Am. J. Eng. Res. 2014, 03, 162–165. [Google Scholar]
- Yang, Q.; Li, Y.; Yin, Q.; Wang, P.; Cheng, Y.B. Hydrothermal Synthesis of Bismuth Oxide Needles. Mater. Lett. 2002, 55, 46–49. [Google Scholar] [CrossRef]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle Surface Functionalization: How to Improve Biocompatibility and Cellular Internalization. Front. Mol. Biosci. 2020, 7, 381. [Google Scholar] [CrossRef]
- Szejtli Introduction and General Overview of Cyclodextrin Chemistry. ChemInform 2010, 29, 1750. [CrossRef]
- Liu, L.; Guo, Q.X. The Driving Forces in the Inclusion Complexation of Cyclodextrins. J. Incl. Phenom. 2002, 42, 1–14. [Google Scholar] [CrossRef]
- Jia, F.; Yang, X.; Li, Z. Synthesis and Application of Colloidal Beta-Cyclodextrin-Decorated Silver Nanoparticles for Rapid Determination of Malachite Green in Environmental Water Using Surface-Enhanced Raman Spectroscopy. RSC Adv. 2016, 6, 92723–92728. [Google Scholar] [CrossRef]
- Sawant, V.J.; Bamane, S.R. PEG-Beta-Cyclodextrin Functionalized Zinc Oxide Nanoparticles Show Cell Imaging with High Drug Payload and Sustained PH Responsive Delivery of Curcumin in to MCF-7 Cells. J. Drug Deliv. Sci. Technol. 2018, 43, 397–408. [Google Scholar] [CrossRef]
- Uekama, K.; Hirayama, F.; Irie, T. Cyclodextrin Drug Carrier Systems. Chem. Rev. 1998, 98, 2045–2076. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K. Organic Reactions Mediated by Cyclodextrins. Chem. Rev. 1998, 98, 2013–2033. [Google Scholar] [CrossRef]
- Easton, C.J.; Lincoln, S.F. Chiral Discrimination by Modified Cyclodextrins. Chem. Soc. Rev. 1996, 163–170. [Google Scholar] [CrossRef]
- Hedges, A.R. Industrial Applications of Cyclodextrins. Chem. Rev. 1998, 98, 2035–2044. [Google Scholar] [CrossRef]
- Zempleni, J.; Wijeratne, S.S.K.; Hassan, Y.I. Biotin. BioFactors 2009, 35, 36–46. [Google Scholar] [CrossRef]
- González, M.; Bagatolli, L.A.; Echabe, I.; Arrondo, J.L.R.; Argaraña, C.E.; Cantor, C.R.; Fidelio, G.D. Interaction of Biotin with Streptavidin. Thermostability and Conformational Changes upon Binding. J. Biol. Chem. 1997, 272, 11288–11294. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, J.; Deshmane, S.V.; Purohit, R.N.; Biyani, K.R. Behavioural Study of Cyclodextrin Inclusion Complex on Enhancement of Solubility of Aceclofenac. Indian Drugs 2015, 52, 19–23. [Google Scholar] [CrossRef]
- Nosrati, H.; Barzegari, P.; Danafar, H.; Kheiri Manjili, H. Biotin-Functionalized Copolymeric PEG-PCL Micelles for in Vivo Tumour-Targeted Delivery of Artemisinin. Artif. Cells Nanomed. Biotechnol. 2019, 47, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Sahari, M.A.; Moghimi, H.R.; Hadian, Z.; Barzegar, M.; Mohammadi, A. Improved Physical Stability of Docosahexaenoic Acid and Eicosapentaenoic Acid Encapsulated Using Nanoliposome Containing α-Tocopherol. Int. J. Food Sci. Technol. 2016, 51, 1075–1086. [Google Scholar] [CrossRef]
- Das, S.; Ng, W.K.; Tan, R.B.H. Are Nanostructured Lipid Carriers (NLCs) Better than Solid Lipid Nanoparticles (SLNs): Development, Characterizations and Comparative Evaluations of Clotrimazole-Loaded SLNs and NLCs. Eur. J. Pharm. Sci. 2012, 47, 139–151. [Google Scholar] [CrossRef]
- Aslantürk, Ö.S.; Larramendy, M.; Soloneski, S. Genotoxicity—A predictable risk to our actual world. In In Vitro Cytotoxicity and Cell Viability Assays: Principles, Advantages, and Disadvantages; 2018; pp. 1–19. [Google Scholar] [CrossRef]
- Ishiyama, M.; Tominaga, H.; Shiga, M.; Sasamoto, K.; Ohkura, Y.; Ueno, K. A Combined Assay of Cell Viability and in Vitro Cytotoxicity with a Highly Water-Soluble Tetrazolium Salt, Neutral Red and Crystal Violet. Bio. Pharm. Bull 1996, 19, 1518–1520. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Stone, V.; Johnston, H.; Schins, R.P.F. Development of in Vitro Systems for Nanotoxicology: Methodological Considerations in Vitro Methods for Nanotoxicology Vicki Stone et Al. Crit. Rev. Toxicol. 2009, 39, 613–626. [Google Scholar] [CrossRef]

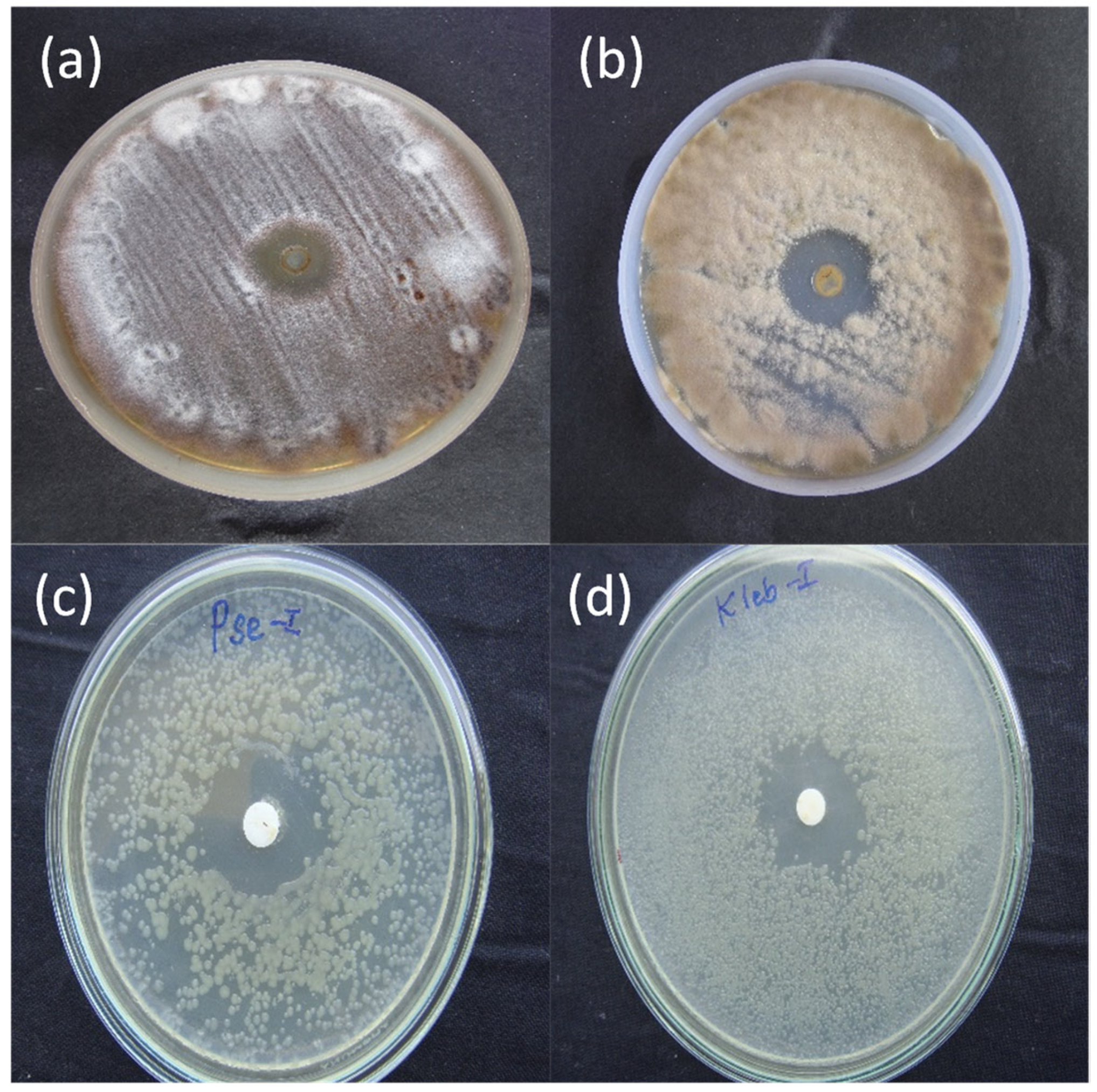

| Bacterial Strain Tested | Inhibition Zone (mm) ± Standard Deviation Observed for BiONP-β-CD-Biotin System |
|---|---|
| S. pneumoniae | 14.26 ± 0.53 |
| S. aureus | 17.54 ± 0.21 |
| P. aeruginosa | 15.31 ± 0.36 |
| K. pneumoniae | 12.23 ± 0.24 |
| Hep G2 Cancer Cell Lines | |||||
|---|---|---|---|---|---|
| Drug Concentration | 6.25 | 12.5 | 25 | 50 | 100 |
| Percentage of cell viability | 91.69 ± 0.55 | 79.58 ± 0.32 | 73.78 ± 0.72 | 61.00 ± 0.43 | 40.11 ± 0.60 |
| MCF-7 cancer cell lines | |||||
| Drug Concentration | 6.25 | 12.5 | 25 | 50 | 100 |
| Percentage of cell viability | 68.78 ± 0.51 | 63.99 ± 0.36 | 60.42 ± 0.52 | 48.76 ± 0.30 | 41.57 ± 0.12 |
| A549 cancer cell lines | |||||
| Drug Concentration | 6.25 | 12.5 | 25 | 50 | 100 |
| Percentage of cell viability | 67.45 ± 0.97 | 63.14 ± 0.72 | 57.62 ± 0.76 | 50.46 ± 0.81 | 44.58 ± 0.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alex, J.; Mathew, T.V. Surface Modification of Bi2O3 Nanoparticles with Biotinylated β-Cyclodextrin as a Biocompatible Therapeutic Agent for Anticancer and Antimicrobial Applications. Molecules 2023, 28, 3604. https://doi.org/10.3390/molecules28083604
Alex J, Mathew TV. Surface Modification of Bi2O3 Nanoparticles with Biotinylated β-Cyclodextrin as a Biocompatible Therapeutic Agent for Anticancer and Antimicrobial Applications. Molecules. 2023; 28(8):3604. https://doi.org/10.3390/molecules28083604
Chicago/Turabian StyleAlex, Jogy, and Thomas V. Mathew. 2023. "Surface Modification of Bi2O3 Nanoparticles with Biotinylated β-Cyclodextrin as a Biocompatible Therapeutic Agent for Anticancer and Antimicrobial Applications" Molecules 28, no. 8: 3604. https://doi.org/10.3390/molecules28083604
APA StyleAlex, J., & Mathew, T. V. (2023). Surface Modification of Bi2O3 Nanoparticles with Biotinylated β-Cyclodextrin as a Biocompatible Therapeutic Agent for Anticancer and Antimicrobial Applications. Molecules, 28(8), 3604. https://doi.org/10.3390/molecules28083604









