Abstract
Mobilization of heavy metals in the environment has been a matter of concern for several decades due to their toxicity for humans, environments, and other living organisms. In recent years, use of inexpensive and abundantly available biosorbents generated from fibrous plant-based food-waste materials to remove heavy metals has garnered considerable research attention. The aim of this review is to investigate the applicability of using fibrous plant-based food waste, which comprises different components such as pectin, hemicellulose, cellulose, and lignin, to remove heavy metals from wastewater. This contribution confirms that plant-fiber-based food waste has the potential to bind heavy metals from wastewater and aqueous solutions. The binding capacities of these biosorbents vary depending on the source, chemical structure, type of metal, modification technology applied, and process conditions used to improve functionalities. This review concludes with a discussion of arguments and prospects, as well as future research directions, to support valorization of fibrous plant-based food waste as an efficient and promising strategy for water purification.
1. Introduction
“Heavy metals” are associated with environmental pollution, food contamination, and toxicity and have adverse effects on terrestrial and aquatic ecosystems and animal and human health [1,2]. Hazardous heavy metals and metalloids, such as arsenic (As), cadmium (Cd), chromium (Cr), lead (Pb), and mercury (Hg), and several essential heavy metals, such as copper (Cu), iron (Fe), manganese (Mn), nickel (Ni), and zinc (Zn), above threshold levels, have been identified as priority contaminants and one of the key environmental issues of global concern over the last several decades due to their mobility in terrestrial and natural aquatic ecosystems and their carcinogenic nature [3,4]. Municipal and industrial wastewaters frequently include a variety of heavy-metal ions, posing a serious threat to the aquatic ecosystem and environment [5]. This is because heavy metals are stable and persistent environmental pollutants due to their nonbiodegradability and high toxicity [6,7]. Furthermore, they possess a tendency to bioaccumulate and biomagnify through the food chain, causing serious threats to humans and other living organisms directly and indirectly [8]. Consequently, metal-polluted wastewater must be treated prior to discharge into the environment. Table 1 lists several common heavy metals, their sources, and associated health problems.

Table 1.
Sources of toxic heavy metals, permissible limits (WHO), and their health effects.
Several technologies have been employed for eliminating heavy metals from wastewater, contaminated aquatic media, and industrial effluents over the last three decades, including chemical precipitation [26], solvent extraction [27], coagulation–flocculation [28], advanced oxidation [29], membrane filtration [30], reverse osmosis [31], ion exchange [26], ozonation [32], photocatalysis [33], adsorption [34,35], biosorption/bioaccumulation [36], bioleaching [37], phytoextraction using hydroponic systems coupled with bioremediation [38], phytofiltration [39], electroremediation [34], etc. However, there is no single best method to provide adequate treatment, as each treatment has its own distinct benefits and shortcomings, not only in terms of cost but also in terms of consistency, efficacy, practicability, viability, and operational difficulties (Table 2) as well as environmental impact [40].

Table 2.
Advantages and limitations of common technologies used for the removal of heavy metal from wastewater.
Among them, sorption of heavy metals from aqueous media is hailed as a promising and frequently employed technique due to its high removal efficiency for metal ions, even at trace concentrations, and ease of operation compared to conventional techniques [45]. However, use of sorption is limited due to the high cost and insufficient regeneration of frequently used adsorbents such as commercial activated carbon [34,35]. Both non-plant- and plant-based materials are employed as low-cost adsorbents. Zeolites, clay, chitosan, red mud, dairy sludge, and metal oxides are all used as adsorbents in non-plant-based materials [46]. Prospects for using plant-based waste as adsorbents, including industrial byproducts and agricultural waste, are deemed highly promising [47]. On the basis of the theories of a “circular bioeconomy” and “green chemistry”, transformation of agricultural waste and residues into products with added value is viewed as a cheap, renewable, abundantly accessible, and ecologically beneficial process [47].
Various fibrous plant-based food biomasses have been employed as precursors for production of adsorbents, such as plant leaves [48], lentil husks [49], agricultural peels [50], coconut biomass [51], etc. However, they need to be treated or modified before being used as adsorbents for metal ions. This is due to the fact that application of untreated plant waste may result in a number of problems, such as decreased sorption capacity, increased biological and chemical oxygen demand, and an increase in total organic carbon due to the discharge of soluble organic carbon remaining in the plant materials [52]. Thus, while application of biosorption for removal of hazardous metals using inexpensive raw materials has attracted substantial interest, various obstacles must be solved before these materials can be employed commercially. On the other hand, various food and agricultural waste are created globally and could be used as soil supplements to improve soil health and crop yield [53]. However, direct application of such waste may endanger soil health, particularly soil chemical and microbiological characteristics [54]. Bioconversion of agricultural and food waste into nonhazardous and stable soil additives is therefore a potential option. This would not only decrease the dangers connected with environmental burdens but also assure safe disposal and use of the end product as sustainable soil additives [55].
Food waste results in roughly 20 million tons (Mt) of CO2-equivalent GHG emissions annually [56]. According to a recent estimate by the Food and Agricultural Organization (FAO), the worldwide food-waste market is valued at over 750 billion USD annually [57]. Forty percent of domestic food output is wasted annually in the United States and Canada, amounting to 165 billion and 27 billion USD, respectively [58]. In this context, recycling or reusing fibrous plant-based food waste for developing affordable purification technology in the water, soil, and food industries could be an attractive component of circular bioeconomies as well as provide greater environmental benefits. Recently, plant fibers produced from agricultural waste have been characterized as excellent adsorbents for environmental remediation of effluents [59,60,61].
Plant fibers are found as structural elements in all higher plants [62]. Examples of plant fibers mainly include lignocellulose-based materials made of lignin, hemicellulose, and cellulose; when mixed with polyphenols, pectin, and proteins, they are utilized for sorption of trace metal ions [63] and dyes [64] as well as oil removal [65] from water. The components of fibers vary not only in physiological activity and chemical structure from one source to another but also in their capacity to bind essential elements such as Ca, Cu, Fe, and Zn [66] and heavy metals such as As, Cd, Hg, and Pb [67]. The performance of fibers depends on several factors: physicochemical parameters, functionality, and modification technology to improve functionality [68,69]. Fibers are found in plant-based foods such as nuts and seeds (beans, split peas, soybeans, corn, sunflowers, barley, oats, wheat, almonds, pumpkins, lentils, etc.), legumes or vegetables (cauliflower, carrots, broccoli, celery, cabbage, turnip greens, brussels sprouts, potatoes, artichokes, eggplants, beets, cauliflower, endives, turnips, fennel, onions, leeks, rutabagas, etc.), and fruits (guavas, mangoes, strawberries, pomegranates, bananas, prunes, apples, raspberries, pears, avocados, blackberries, oranges, pineapples, etc.). Thereby, plant-based food wastes contain plant fibers. Fiber is a blanket term that applies to any type of carbohydrate that humans cannot digest. In a characterization study of dietary fiber lignins from 11 fruits and vegetables using the DFRC method, Bunzel and Seiler [70] found that apples, kiwis, pears, asparagus, carrots, curly kale, kohlrabi, radishes, small radishes, rhubarb, and spinach contained 9.8, 11.9, 12.9, 18.0, 10.3, 33,4, 6.2, 12.6, 18.3, 26.7, and 28.5% insoluble fiber, respectively. Natural fibers of plant origin (plant fibers) can come from different parts of a plant.
The purpose of this review is to highlight potential applications and research in the field of biosorption, utilizing a variety of low-cost materials, most notably fibrous plant-based food waste, including their biomass parts and fiber components, for heavy-metal remediation from wastewater. Nevertheless, the influences of fiber structures and properties on the sorption process, the mechanisms of their actions, and the regeneration capabilities of fibrous plant-based food waste are reviewed. Finally, the main challenges and prospects for heavy-metal sorption using fibrous plant-based food waste in water or soil are highlighted for future research directions. This review specifies that it encompasses research published between 1997 and 2022 and that the search criteria included “fibrous plant-based food waste”, “biosorbent”, “plant fibers”, “heavy metal”, and “wastewater”.
2. Fibrous Plant-Based Food Waste for Sorbing Heavy Metals
2.1. Plant-Fiber Components
Plant fibers are found as structural elements in agricultural crops and in their botanical parts, such as nuts, grains, or seeds (beans, split peas, soybeans, corn, sunflowers, barley, oats, wheat, almonds, pumpkins, etc.); lentils, legumes, or vegetables (cauliflower, carrots, broccoli, celery, cabbage, turnip greens, brussels sprouts, potatoes, artichokes, eggplants, beets, cauliflower, endives, turnips, fennel, onions, leeks, rutabagas, etc.); and fruits (guavas, mangoes, strawberries, pomegranates, bananas, prunes, apples, raspberries, pears, avocados, blackberries, oranges, pineapples, etc.). Plant fibers have a complex structure that is really made up of a cell wall and a central lumen channel. The middle lamella, the primary wall, and the secondary wall are the three components that make up a cell wall [71]. The primary wall is made up of disorganized cellulose in a pectin, hemicellulose, and lignin matrix. The secondary wall is composed of crystalline cellulose and is separated into three sections: the exterior, middle, and interior secondary walls [72]. The chemical components of plant fibers, including cellulose, lignin, hemicellulose, pectin, and wax, can be different depending on their sources and origins [71]. In food science, cellulose, lignin, pectin, and hemicellulose derived or extracted from fibrous plant foods or contained in plant foods or plant-fiber matrices are designated as cellulose fiber, lignin fiber, pectin fiber, and hemicellulose fiber, respectively.
The main component of plant cells is generally cellulose, which is arranged in microfibrils and surrounded by hemicellulose, which includes xylans, mannans, glucomannans, galactans, and arabinogalactans as well as lignin, pectin, and trace amounts of protein [59,73]. Fibers include functional groups such as carboxyl, phenolic, lactonic, and hydroxyl groups that bind to metals and remove them from aqueous environments. These functional groups interact with metal ions and act as hydrogen-ion replacements. Over a wide pH range, the process includes electrostatic and dispersive interactions between cations and the acidic surface area [66]. Feng and Guo [74] showed how Pb2+, Cd2+, and Ni2+ ions were attached to modified orange peel by inclusion of carboxyl and hydroxyl groups. The constancy of metal-fiber complexes varies with the type of metal, the experimental settings, the fiber sources, and other factors, according to published studies [59,66]. Al-Ghouti and Li [75] revealed that raw date pits may be utilized to remove Cu2+ and Cd2+ through the processes of complexation, coordination, chelation, ion exchange, and adsorption.
2.2. Fibrous Plant-Based Biomass Parts
The different parts of fibrous plant-based biomasses, considered low-cost potential metal biosorbents, are leaves, stems, stalks, roots, bagasse, seeds, shells, peels, husks, bark, and fibers [46,76,77]. Various plant fiber-based biomasses have been widely used as natural materials, pretreated or chemically modified, for heavy-metal removal from aqueous media, including wastewater and aqueous solutions. These are carrot residue [78]; potato peel [79]; sunflower stalks and leaves [80]; coconut shells [81]; seed shells [82]; coffee husks [83]; sugar-beet pulp [84,85]; crude olive stones [86]; olive-oil waste and hydrolyzed olive cake [87,88]; apple peel beads [89]; citrus peels [90], the shells of hazelnuts and almonds [91]; physic seed hulls [92]; rice husks [93]; neem bark [94]; tea waste [95]; sunflower, potato, canola and walnut shells [80]; sugarcane bagasse [96]; bamboo charcoal [97]; pistachio-hull waste [98]; cashew-nut shells [99]; agave bagasse [100]; Rosa damascena leaf powder [101]; ajwa date pits [102]; chemically modified orange peel [74]; banana peel and chemically modified banana peel [103]; orange and potato immobilized on sodium alginate beads [104]; olive-oil waste and hydrolyzed olive cake [87,88]; banana thrunk fibers [105]; and cellulose fibers extracted from pineapple leaves [106] (Table 3).

Table 3.
Commonly used fibrous plant-based food waste for heavy-metal removal from wastewater and aqueous solutions.
2.3. Factors Influencing the Sorption Efficiencies of Fibrous Plants
The capacities of biowaste-derived sorbents for metal-ion sorption are, however, dependent on the physicochemical properties of the prepared sorbents. The most important properties of these sorbents are cation exchange capacity (CEC), pore distribution, porosity, specific surface area, surface functional groups, etc. [113,114]. Lyu and Wang [115] stated that larger specific surface areas led to higher metal (Cd2+, Cu2+, Pb2+, and Zn2+) sorption from their aqueous solutions using insoluble fiber from soybean dregs (okara). They described that the smaller particle size of insoluble okara fiber demonstrated a higher oil-holding capacity (OHC), CEC, and sorption capacity of heavy metals. In another study, ultramicro-grinding of insoluble fiber from carrot pomace decreased the particle size of the total fiber and increased its Brunauer–Emmett–Teller surface area from 0.374 to 1.835 m2/g, leading to an increase in the water-holding capacity (WHC), swelling capacity (SC), and OHC, as well as the nitrite- and Pb2+-ion-adsorbing capacities [116]. Furthermore, Al-Ghouti and Li [75] discovered that the volume of solute (Cu2+ and Cd2+) adsorbed increases as the particle size of the adsorbents decreases. The crystallinity of the cellulosic structure also affects sorption kinetics [117]. Amorphous regions have a positive correlation, while crystalline structures have a negative correlation with heavy-metal sorption [118]. With a rise in pH, the negative charge density of a fiber surface improves, which leads to an increase in sorption of heavy metals [119]. Higher Cd2+, Cu2+, and Pb2+ bind to biosorbents by having more acidic functional groups and negative zeta potential [120]. Similarly, Wang and Yang [121] observed that there is a positive correlation between efficiency of removing heavy metals and pH, and that removal efficiency improved when the pH was increased to 7.
Nevertheless, the biological origins of plants and types of processing have a considerable influence on sorption properties. For instance, beet pectin demonstrated a higher affinity for Pb2+ and Cu2+, while citrus pectin did so for Ni2+ and apple pectin did so for Co2+ [122]. Requena and González [123] showed that CEC significantly varies depending on the source of fiber; for example, CEC values of 3.5, 4.1, 2.6, 2.7, 2.6, and 1.3 meq/g were reported for ashen agave bagasse, green agave bagasse, cabuche, prickly pear peel, palm flowers, and the leaves of smooth amaranth, respectively. Nevertheless, sorption capacity largely depends on solution ion strength [124]. The higher charge density of Cu2+ (116 C mm−3) results in increased ion sorption compared to Pb2+ (32 C mm−3) and Cd2+ (59 C mm−3) [120]. Several critical factors affecting sorption efficiency of heavy metals are presented in Figure 1.
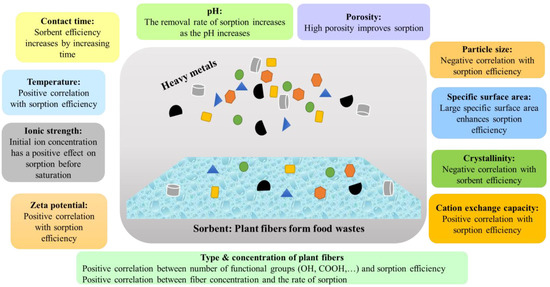
Figure 1.
Critical factors affecting sorption efficiency of heavy metals from aqueous media.
Fiber concentration also possesses substantial effects on sorption efficiency; efficiency of ion sorption increases linearly with increasing concentrations of fiber due to concurrent production of more active sites (OH and COOH) on macromolecules. Sorption efficacy may decrease beyond the optimal sorbent dose due to increased aggregation of adsorbent molecules [120]. Pb2+ sorption increased for 21.78, 23.41, and 26.98 mg/L at various polymer concentrations, of 1, 2, and 3%, respectively, and at a pH of 5.0, according to Basiri and Shekarforoush [125]. Additionally, it was discovered that sorption effectiveness changes with temperature; it increases with rising temperatures before dropping after a period of time. Guiza [126] studied Cu-ion sorption from aqueous solutions by cellulose from waste orange peel. That team observed that sorption was dependent on solution pH, adsorbent dosage, contact time, metal-ion concentration, and agitation speed. According to Pal and Giri [127], the sorption efficiency of guar gum increased at up to a temperature of 40 °C and then decreased at 40 °C. The efficiency increased due to the possibility that a temperature increase would enhance the ions’ mobility and mobilize more Pb2+ towards the giant adsorbent molecules, which would then increase their contact with the surfaces of the adsorbents. However, the efficiency dropped beyond 40 °C due to dominant desorption of Pb2+ because of the increased Brownian movement [128].
2.4. Different Modification Technologies for Enhancing Sorption Efficiency
The ability of fibrous plants and their components, such as cellulose, hemicellulose, and lignin, to remove heavy metals from effluents has been intensively studied. Cellulose, as a plant-fiber component, works as a skeleton of natural plant-cell walls, whereas lignin and hemicellulose are distributed in the fibrous plant matrix, resulting in poor functionalities of fibers [129]. Subsequently, it is imperative to develop a suitable method for increasing the functionalities of fibers to enhance usage of plant byproducts, such as biosorbents [130]. The effectiveness of these materials can be enhanced through various types of modification techniques. Several technical approaches, including mechanical, chemical, enzymatic, and/or biological technologies, have been developed to disrupt plant cellular integrity and isolate fibers with altered structural, physicochemical, and functional characteristics to enhance sorption efficiency [68,69].
Physical modification involves altering the physical structures of fibrous plant materials, such as pore size and surface area, to increase their sorption capacities. Methods such as grinding, milling, and sieving can be used to affect physical changes. Huang and Liao [131] demonstrated that homogenization via mechanical shearing resulted in damage to the cellulose and crystallization regions of citrus peels, as well as an increase in the specific surface area and the total number of charged ions. Similarly, the molecules of cellulose and lignin were destroyed and transformed into tiny molecules during a steam explosion, increasing the sorption capacities for heavy metals [118]. Xu and Wang [132] showed that high hydrostatic pressure may significantly enhance the ability of insoluble fibers for water retention and swelling, oil holding and cation exchange, and glucose adsorption. Meanwhile, the twin-screw extrusion treatment has been shown to reduce the OHC of orange-peel fiber and increase the lead-binding ability of garlic-skin fiber [133].
Chemical modification involves treating fibrous plants with chemical reagents to introduce functional groups to their surfaces, thereby enhancing their sorption capacities. Using oxidizing compounds such as sodium chlorite and sodium periodate, for example, it is possible to introduce carboxylic and hydroxyl groups. Following a chemical treatment, Wang and Li [134] found that kiwifruit fiber treated with NaOH has greater thermal stability, but kiwifruit fiber treated with citric acid delivers higher sorption capacities for water, oil, bile acid, nitrite ions, and glucose. In this regard, Adegoke and Akinnawo [135] employed numerous surface-modification treatments, such as acid, alkaline, magnetic, and grafting modifications, for improving sorption of heavy metals, including As, Cd, Cr, Cu, Co, Fe, Hg, Mn, Ni, Pb, and Zn. They revealed that acidic treatment mostly favors the sorption process. It has been proven that some pretreatments, such as hydrochloric acid, tartaric acid, sodium carbonate, and sodium hydroxide, can effectively increase the rate of heavy-metal sorption by rice husks [136,137].
Biological modification entails treating fibrous plant materials with microorganisms or enzymes to alter their surface properties, such as charge and hydrophobicity, thereby increasing their sorption capacities. As a biological surface functionalization, cationic surfactant can be applied to remediate heavy metals in wastewater, in which case, a cationic surfactant could change the negative surface charge of a biosorbent to a positive surface charge and would have the profound ability to uptake metal anions rather than cations. Rastogi and Tiwari [138] used agroindustrial waste to synthesize a biosurfactant via submerged fermentation using Bacillus haynesii, and the biosurfactant could significantly remediate Pb2+. In addition, Dong and Du [139] implied that modified wheat straw with polyethylenimine (a highly branched molecule containing amine groups) has a paramount impact on Cu2+ purification from aqueous solutions. Furthermore, Chu and Zhao [140] used Bacillus natto to ferment millet bran. As a result of degradation of cellulose and hemicellulose by fermentation, the modified millet bran fiber developed more porous and loose structures, which increased its sorption capacity.
In conclusion, the relationship between various types of modification techniques and the respective components of the plant-fiber sorbent materials used for heavy-metal sorption is complex and dependent on the specific modification technique employed, the type of plant fiber used, and the degree of modification. The selection of an effective modification technique for fibrous plant-based sorbent materials used for heavy-metal sorption requires careful consideration of these factors.
3. Industrial Applications of Fibrous Plant-Based Materials and Plant-Fiber Components for Environmental Remediation of Aqueous Solutions and Wastewater
The ability of plant-fiber components, such as pectin, to bind metals is advantageous for removing heavy metals from aqueous systems, as reported in many studies [141,142]. Kumar and Kumar [143] produced ferrous ion-loaded pectin hydrogels for removal of arsenic (As) from aqueous solutions. They proposed that their compound can be used as a vehicle for water purification because of its high yield, biodegradability, and low cost [143]. Jakóbik-Kolon and Bok-Badura [144] developed calcium-crosslinked pectin (30.2% DE, degree of esterification) beads in combination with various biopolymers, karaya gum, arabic gum, and xanthan for Zn removal from water; it demonstrated the best swelling and Zn sorption at a pH of 4. In addition, Zn removal was also facilitated by physical sorption of Zn2+ into the complex [144]. Hastuti and Hadi [145] successfully removed ~ 44% of Pb from water using pectin derived from carrot peel at a pH of 6. Metal removal from water was accomplished using a high concentration of methoxylated nopal pectin (65% DE). After pectin treatment, more than 90% of Ca, Cu, Zn, Cr, and Ni; 67% of Pb; and 44% of Cd were eliminated by ionic contact and polar covalent bond formation [142]. Tarmizi and Ismail [146] performed another investigation on use of apple pectin (DE: 70–75%, 5 mg/L) and magnesium chloride (15 mg/L) at an alkaline pH (10). This mixture reduced the turbidity of a water supply by up to 97.71% and the iron content by 92.23% but did not significantly reduce the concentrations of other cations, such as Cd, As, Cr, and Cu. This result is most likely due to the high esterification degree of pectin, which has a limited number of accessible sites for cations. This is also attributed to the high content of Fe in the untreated sample, which favored Fe removal more than would other electrolytes [146].
In another study, Shukla and Pai [147] evaluated the potential of coir, a low-cost lignocellulosic fiber, for removal of heavy-metal ions such as Ni2+, Zn2+, and Fe2+ from aqueous solutions. Additionally, the fiber was chemically changed by oxidization with hydrogen peroxide before use as an adsorbent. Coir fibers were used to perform Langmuir-type adsorption. Modified coir fibers adsorbed 4.33, 7.88, and 7.49 mg/g of Ni2+, Zn2+, and Fe2+, respectively, compared to the 2.51, 1.83, and 2.84 mg/g by unmodified coir fibers [147]. The adsorption ability was retained only when an intermediary stage of regeneration with a diluted NaOH solution was performed following desorption. The higher metal-ion uptake in modified coir has been attributed to an ion-exchange process [147]. Notably, fibers could be regenerated with alkali and reused three times with maximum efficiency, boosting their reusability and function as a reversible ion exchanger [147]. Feng and Guo [74] demonstrated that the adsorption capacity of modified orange peel increased 4.2, 4.6, and 16.5-fold for Pb2+, Cd2+, and Ni2+ from wastewater, respectively, compared to that of unmodified orange peel. Furthermore, the adsorbed Pb2+, Cd2+, and Ni2+ ions could be recovered using a 0.05 mol/L HCl solution, and the wasted sorbent could be regenerated and reused due to immobilized behavior, which makes the biosorption process more cost-effective. Tangtubtim and Saikrasun [148] reported that alkali-treated pineapple fiber immobilized with polyethyleneimine could be used as a potential adsorbent to remove Cu2+ and Pb2+ from aqueous solutions. Hu and Huang [149] investigated Pb2+, Cd2+, Zn2+, and Cu2+ adsorption by cellulose, lignin, and hemicellulose. The results demonstrated that the highest percentage of heavy-metal removal was achieved by hemicellulose, followed by cellulose and lignin.
Pejic and Vukcevic [150] investigated the sorption capacity of short-hemp-fiber waste for Pb2+, Cd2+, and Zn2+ ions in aqueous media. They demonstrated that the sorption characteristics of hemp fibers improved by gradual reduction of the amount of lignin or hemicelluloses in the hemp fibers via chemical treatment. Short hemp fibers can bind metal ions (Pb2+, Cd2+, and Zn2+) from both single and ternary metal-ion solutions. The maximal total sorption capabilities of Pb2+, Cd2+, and Zn2+ ions were the same in single solutions, i.e., 0.078 mmol/g, while in ternary mixtures, they were 0.074, 0.035, and 0.035 mmol/g, respectively [150]. Mongioví and Morin-Crini [151] used plant fibers of hemp and flax in the form of felt as biosorbents to remove Al, Cd, Co, Cu, Mn, Ni, and Zn from aqueous solutions. The flax-based felt had higher biosorption capacities with respect to the studied metals than did the hemp-based felt. The highest removal efficiency was always obtained for Cu ions, and the following order of Cu > Cd > Zn > Ni > Co > Al > Mn was found for both examined biosorbents. In another study, Demirbas [152] studied adsorption of heavy-metal ions (Co2+ and Hg2+) by modified lignin from Ailanthus altissima wood using alkali glycerol delignification. Imran-Shaukat and Wahi [153] used various agricultural waste biomasses to adsorb metal ions for their cellulosic constituents, such as lignin, hemicellulose, lipids, extractives, sugars, proteins, and starch, which contain functional groups to participate in heavy-metal complexation. A study by Agarwal and Upadhyay [154] demonstrated that the olive stone is capable of removing Cu2+ from effluents.
Reshmy and Philip [155] reviewed the most practical and recent information on applying nanocellulose in heavy-metal remediation from wastewater. Faster kinetics, efficiency across a wide pH and temperature range, and low cost are the most important features of nanocellulose. Cheng and Chen [156] stated recent developments for sugarcane bagasse fiber and sugarcane-bagasse-fiber cellulose nanocrystals (SBFCNCs) as green materials in manufacturing of composites and heavy-metal sorbents. They mentioned that SBFCNCs have a high specific surface area, chemical accessibility, hydrophilic properties, and functionalization flexibility to enhance their sorption capacity towards heavy metals. Nevertheless, cellulose, pectin, starch, guar, and xanthan gums have been used for sustainable water treatment [157].
4. Challenges and Future Perspectives of Using Plant Fiber-Based Materials as Heavy-Metal Biosorbents
4.1. Effects of Process Conditions on Fibrous Plant-Based Food Waste
Recent years have witnessed a boom in biosorption-related research. It is unclear, however, whether such a substantial increase in published output has appreciably increased our understanding of the process or facilitated economic exploitation, which is so frequently the primary motivation for such studies. Most of that research focused on characterization of selected biomass types in adsorbing particular substances from solutions and the influences of physicochemical factors on biosorption. Most studies focused on metals, although a rising number also examined organic contaminants [158]. Despite the tremendous rise in creation of various biosorbents, there are still several issues related to these materials, including pH stability, sorption capacity, and durability, that must be addressed for future applications [159]. Further studies could be performed to focus on the sorption mechanism at the biosorbent–water interface.
4.2. Modification of Fibrous Plant-Based Food Waste and Process Intensification
It has been observed that modified fibers extracted from plant-based food yield better outcomes than unmodified fibers. Different modifications, such as physical, enzymatic, bacterial, and chemical treatments, can be used to increase fiber porosity and surface area, thereby increasing the number of sorption sites and binding functional groups on biosorbent surfaces [69]. A combination of multiple methods in the context of process intensification should be explored for increasing heavy-metal removal efficiency and possibly reducing costs.
4.3. Regeneration and Reusability of Fibrous Plant-Based Food Waste
Biosorption studies have been conducted for many years, but commercial use has yet to be achieved. The lack of studies on regeneration of adsorbents and their sustainable disposal is one of the major challenges to scaling up. Biosorption, on the other hand, may entail many functional groups on the surface of biomasses and is frequently nonselective, suggesting that its application to metal combinations (a frequent occurrence in waste streams) would be troublesome. As ion-exchange resins may be produced to include a single metal-binding functional group with a high affinity, they are more ideal for selective recovery of target chemicals and are more predictable for particular metal ions. The lack of selectivity and reduced resilience of plant biomass-based systems compared to ion exchange resins are frequently highlighted as key obstacles to commercialization of biosorption [160]. Suspended biomass is ineffective and unreliable in repeated, long-term applications, and its subsequent separation from treated effluents is problematic. Immobilized and/or granular biomass preparation may address the robustness and separation issues, but not the specificity issue. In addition, it should be highlighted that (bio)sorption technology moves sorbate from one medium to another, which poses concerns regarding safe disposal of loaded biosorbents, sorbate recovery, and regeneration or replacement of biosorbents [158]. The creation of particular metal-binding molecules and/or tailored highly specific fibrous plant-based biosorbents is hailed as a promising research direction; however, practical application appears to have made little progress.
4.4. Effects of Possible Competition between Heavy Metals on Their Sorption
Most studies were conducted using one model heavy metal at a time; however, the presence of a single heavy metal in nature, such as wastewater or polluted water, is a rare situation. For an effective ion-exchange process, it is essential to comprehend the mechanism of competitive sorption of coexisting metals on biosorbents [161]. Additionally, use of deionized water as an experimental solution for sorption of heavy-metal ions rather than the more-complex river water or wastewater is another limitation in sorption studies [162]. The effect of multiple metals in real wastewater and polluted soil on the kinetic rate of sorption can be further investigated using plant fibers.
4.5. Possible Practical Applications of Fibrous Plant-Based Food Waste in Water Purification
Considering the sorption capacity of fibrous plant-based food waste, use of those fibers for water remediation, such as removal of chemical residues, oil spills, and organic wastewater, could be a substantial and cost-effective technique to minimize pollution of aquifers with metal ions, marine ecosystems with oil spills, and water bodies with organic dyes. Moreover, plant-fiber components, such as the pectin derived from apples, could be used to lower turbidity of water supplies or to reduce iron and arsenic ions [146]. Therefore, further studies could be performed on sorption of Fe or As using fiber-based sorbents in the potable water of Asia, such as in Cambodia, Afghanistan, China, Japan [163], Nepal or Bangladesh [164], and India [165]; in the surface water and groundwater of Australia, Brazil, and Mexico for gold mining [163,165]; to minimize deposition of As in the sediments of natural reservoirs, such as the Haiwee Reservoir (Olancha, CA) [166]; etc.
4.6. Possible Practical Applications of Fibrous Plant-Based Food Waste in Remediation of Heavy-Metal-Polluted Soil
Sorption of metal ions by fibrous plant-based food waste could be an interesting option for treatment of metal-polluted soil, soil leachates, or groundwater. In a study, cocoa shells, a byproduct of the chocolate industry and rich in fibers, proteins, polyphenols, methylxanthines, etc., were used as an efficient natural adsorbent to remove Pb and other metals (Cu and Zn) from acid soil leachates [167]. The fibers of cocoa shells are mainly composed of pectin and cellulose [168]. Those results showed that around 1060–2730 mg Pb/kg could be removed from contaminated soil leachates [167]. This demonstrated that the uptake of ions in cocoa shells is dominated by ion-exchange reactions with Ca, Mg, and K ions and protons. The carboxyl and amine functional groups played a key role in the Pb uptake process. Derakhshan-Nejad and Jung [169] used raw rice husks and maple leaves for agricultural soil purification from Pb, Cu, Cd, and Zn using immobilization techniques.
Yang and Li [170] used extracts from food wastes (pineapple peel, lemon peel, grapefruit peel, and gardening crab-apple fruit) to develop a two-stage sequential washing method (extracts and/or citric acid coupled with extracts) for facile remediation of metal-contaminated agricultural soil. The removal mechanisms of Cd and Cu in soil and eluents by pineapple-peel washing agents and residues are attributed to acid activation, cation exchange, and complexation between metal ions and carboxyl groups.
5. Conclusions
Heavy-metal sorption is a promising approach due to its ease of use and excellent removal efficacy over a wide pH range. However, preparing suitable sorbent materials can be expensive, and some, such as commercially activated carbons, cannot be regenerated after use, making large-scale applications unsustainable. Conversion of fibrous plant-based food waste into low-cost sorbents is a renewable and ecologically benign strategy based on a “circular bioeconomy” and “green chemistry”. However, untreated plant waste can reduce sorption capacity, increase biological and chemical oxygen demand, and increase total organic carbon due to release of soluble organic carbon from plant materials. Fibrous plant-based food wastes and fibers extracted from nuts, cereals, fruits, and vegetable waste materials could be excellent sorbents for eliminating several detrimental and poisonous compounds, such as heavy metals, from wastewater and aqueous solutions. Thus, the use of fibrous plant-based food waste as biosorbents for heavy-metal remediation shows tremendous promise as a cost-effective and environmentally friendly water purification solution. To investigate biosorption technology on an industrial scale, however, several challenges, including pH stability, sorption capacity, durability, and regeneration of adsorbents, must be overcome. Further study should focus on optimizing binding capacity and process conditions to maximize efficacy. Certainly, this review article contributes to the field by providing an insight of the potential of using fibrous plant-based food waste as biosorbents for removal of heavy metals from effluents. It confirms that this waste can bind heavy metals and provides valuable insights into the factors that influence its binding capacity, such as the waste’s source, its chemical structure, and the type of metal.
Author Contributions
A.K. (Ahasanul Karim): conceptualization, writing—original draft, and writing—review and editing; Z.R.: writing—original draft and writing—review and editing; A.K. (Antoine Karam): supervision and writing—review and editing; S.K.: conceptualization, supervision, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The project was funded by the Natural Sciences and Engineering Research Council of Canada (CRSNG RDC 538873-19).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.; Sadeghi, M. Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Ayati, A.; Ghanbari, S.; Orooji, Y.; Tanhaei, B.; Karimi, F.; Alizadeh, M.; Rouhi, J.; Fu, L.; Sillanpää, M. Recent advances in removal techniques of Cr (VI) toxic ion from aqueous solution: A comprehensive review. J. Mol. Liq. 2021, 329, 115062. [Google Scholar] [CrossRef]
- Lin, H.; Tang, Y.; Dong, Y.; Wei, Z.; Liu, C. Characterization of heavy metal migration, the microbial community, and potential bioremediating genera in a waste-rock pile field of the largest copper mine in Asia. J. Clean. Prod. 2022, 351, 131569. [Google Scholar] [CrossRef]
- Malik, L.A.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and removal of heavy metal ions: A review. Environ. Chem. Lett. 2019, 17, 1495–1521. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E. Bioaccumulation of non-essential hazardous heavy metals and metalloids in freshwater fish. Risk to human health. Environ. Chem. Lett. 2018, 16, 903–917. [Google Scholar] [CrossRef]
- Kahlon, S.K.; Sharma, G.; Julka, J.; Kumar, A.; Sharma, S.; Stadler, F.J. Impact of heavy metals and nanoparticles on aquatic biota. Environ. Chem. Lett. 2018, 16, 919–946. [Google Scholar] [CrossRef]
- Bilal, M.; Ihsanullah, I.; Younas, M.; Shah, M.U.H. Recent advances in applications of low-cost adsorbents for the removal of heavy metals from water: A critical review. Sep. Purif. Technol. 2021, 278, 119510. [Google Scholar] [CrossRef]
- Iqbal, M.O.; Yahya, E.B. In vivo assessment of reversing aminoglycoside antibiotics nephrotoxicity using Jatropha mollissima crude extract. Tissue Cell 2021, 72, 101525. [Google Scholar] [CrossRef]
- Asere, T.G.; Stevens, C.V.; Du Laing, G. Use of (modified) natural adsorbents for arsenic remediation: A review. Sci. Total Environ. 2019, 676, 706–720. [Google Scholar] [CrossRef]
- Pyrzynska, K. Removal of cadmium from wastewaters with low-cost adsorbents. J. Environ. Chem. Eng. 2019, 7, 102795. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, J.; Chen, R.; Yu, P.; Guo, S.; Wang, X. Adsorption and reduction of chromium (VI) from aqueous solution using polypyrrole/calcium rectorite composite adsorbent. Water Res. 2019, 160, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Demirbaş, E. Adsorption of cobalt (II) ions from aqueous solution onto activated carbon prepared from hazelnut shells. Adsorpt. Sci. Technol. 2003, 21, 951–963. [Google Scholar] [CrossRef]
- Fowler Jr, J.F. Cobalt. Dermatitis 2016, 27, 3–8. [Google Scholar] [CrossRef]
- Pavan Kumar, G.; Malla, K.A.; Yerra, B.; Srinivasa Rao, K. Removal of Cu (II) using three low-cost adsorbents and prediction of adsorption using artificial neural networks. Appl. Water Sci. 2019, 9, 44. [Google Scholar] [CrossRef]
- Ahamad, K.U.; Jawed, M. Kinetics, equilibrium and breakthrough studies for Fe (II) removal by wooden charcoal: A low-cost adsorbent. Desalination 2010, 251, 137–145. [Google Scholar] [CrossRef]
- Fiuza, T.E.R.; Borges, J.F.M.; da Cunha, J.B.M.; Antunes, S.R.M.; de Andrade, A.V.C.; Antunes, A.C.; de Souza, É.C.F. Iron-based inorganic pigments from residue: Preparation and application in ceramic, polymer, and paint. Dye. Pigment. 2018, 148, 319–328. [Google Scholar] [CrossRef]
- Cha, N.-R.; Lee, J.-K.; Lee, Y.-R.; Jeong, H.-J.; Kim, H.-K.; Lee, S.-Y. Determination of iron, copper, zinc, lead, nickel and cadmium in cosmetic matrices by flame atomic absorption spectroscopy. Anal. Lett. 2010, 43, 259–268. [Google Scholar] [CrossRef]
- Makimura, Y.; Rougier, A.; Tarascon, J.-M. Pulsed laser deposited iron fluoride thin films for lithium-ion batteries. Appl. Surf. Sci. 2006, 252, 4587–4592. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Kontoghiorghe, C.N. Iron and chelation in biochemistry and medicine: New approaches to controlling iron metabolism and treating related diseases. Cells 2020, 9, 1456. [Google Scholar] [CrossRef]
- Mve, M.Z.; Makani, T.; Eba, F. Removal of Mn (II) from aqueous solutions by activated carbons prepared from Coula edulis nut shell. J. Environ. Sci. Technol. 2016, 9, 226. [Google Scholar] [CrossRef]
- Duan, C.; Ma, T.; Wang, J.; Zhou, Y. Removal of heavy metals from aqueous solution using carbon-based adsorbents: A review. J. Water Process Eng. 2020, 37, 101339. [Google Scholar] [CrossRef]
- Xia, M.; Chen, Z.; Li, Y.; Li, C.; Ahmad, N.M.; Cheema, W.A.; Zhu, S. Removal of Hg (II) in aqueous solutions through physical and chemical adsorption principles. RSC Adv. 2019, 9, 20941–20953. [Google Scholar] [CrossRef]
- Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, A. Removal of nickel (II) from aqueous solution by biosorption on A. barbadensis Miller waste leaves powder. Appl. Water Sci. 2019, 9, 96. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J. Removal of chromium from wastewater by membrane filtration, chemical precipitation, ion exchange, adsorption electrocoagulation, electrochemical reduction, electrodialysis, electrodeionization, photocatalysis and nanotechnology: A review. Environ. Chem. Lett. 2020, 18, 2055–2068. [Google Scholar] [CrossRef]
- Gasser, M.; Rahman, R.A. Sustainability of solvent extraction techniques in pollution prevention and control. In Handbook of Advanced Approaches Towards Pollution Prevention and Control; Rahman, R.O.A., Hussain, C.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 2, pp. 33–66. [Google Scholar]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent advancement of coagulation–flocculation and its application in wastewater treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Obotey Ezugbe, E.; Rathilal, S. Membrane technologies in wastewater treatment: A review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Arola, K.; Van der Bruggen, B.; Mänttäri, M.; Kallioinen, M. Treatment options for nanofiltration and reverse osmosis concentrates from municipal wastewater treatment: A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 2049–2116. [Google Scholar] [CrossRef]
- Malik, S.N.; Ghosh, P.C.; Vaidya, A.N.; Mudliar, S.N. Hybrid ozonation process for industrial wastewater treatment: Principles and applications: A review. J. Water Process Eng. 2020, 35, 101193. [Google Scholar] [CrossRef]
- Vikrant, K.; Kim, K.-H.; Dong, F.; Giannakoudakis, D.A. Photocatalytic platforms for removal of ammonia from gaseous and aqueous matrixes: Status and challenges. ACS Catal. 2020, 10, 8683–8716. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Kumar, R.; Rauwel, P.; Rauwel, E. Nanoadsorbants for the removal of heavy metals from contaminated water: Current scenario and future directions. Processes 2021, 9, 1379. [Google Scholar] [CrossRef]
- Filote, C.; Roșca, M.; Hlihor, R.M.; Cozma, P.; Simion, I.M.; Apostol, M.; Gavrilescu, M. Sustainable application of biosorption and bioaccumulation of persistent pollutants in wastewater treatment: Current practice. Processes 2021, 9, 1696. [Google Scholar] [CrossRef]
- Kamizela, T.; Worwag, M. Processing of water treatment sludge by bioleaching. Energies 2020, 13, 6539. [Google Scholar] [CrossRef]
- Delgado-González, C.R.; Madariaga-Navarrete, A.; Fernández-Cortés, J.M.; Islas-Pelcastre, M.; Oza, G.; Iqbal, H.; Sharma, A. Advances and applications of water phytoremediation: A potential biotechnological approach for the treatment of heavy metals from contaminated water. Int. J. Environ. Res. Public Health 2021, 18, 5215. [Google Scholar] [CrossRef]
- Meitei, M.D.; Prasad, M.N.V. Potential of Typha latifolia L. for phytofiltration of iron-contaminated waters in laboratory-scale constructed microcosm conditions. Appl. Water Sci. 2021, 11, 47. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Zhu, Y.; Fan, W.; Zhou, T.; Li, X. Removal of chelated heavy metals from aqueous solution: A review of current methods and mechanisms. Sci. Total Environ. 2019, 678, 253–266. [Google Scholar] [CrossRef]
- Nguyen, T.; Ngo, H.; Guo, W.; Zhang, J.; Liang, S.; Yue, Q.; Li, Q.; Nguyen, T. Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. Bioresour. Technol. 2013, 148, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B.H. Adsorption of heavy metal ions by various low-cost adsorbents: A review. Int. J. Environ. Anal. Chem. 2022, 102, 342–379. [Google Scholar] [CrossRef]
- Agarwal, A.; Upadhyay, U.; Sreedhar, I.; Singh, S.A.; Patel, C.M. A review on valorization of biomass in heavy metal removal from wastewater. J. Water Process Eng. 2020, 38, 101602. [Google Scholar] [CrossRef]
- Mathew, B.B.; Jaishankar, M.; Biju, V.G.; Beeregowda, K.N. Role of bioadsorbents in reducing toxic metals. J. Toxicol. 2016, 2016, 4369604. [Google Scholar] [CrossRef]
- Malik, D.; Jain, C.; Yadav, A.K. Removal of heavy metals from emerging cellulosic low-cost adsorbents: A review. Appl. Water Sci. 2017, 7, 2113–2136. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Pashalidis, I. Environmental applications of Luffa cylindrica-based adsorbents. J. Mol. Liq. 2020, 319, 114127. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Ighalo, J.O. Biosorption of pollutants by plant leaves: An empirical review. J. Environ. Chem. Eng. 2019, 7, 103100. [Google Scholar] [CrossRef]
- Basu, M.; Guha, A.K.; Ray, L. Adsorption of lead on lentil husk in fixed bed column bioreactor. Bioresour. Technol. 2019, 283, 86–95. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Pashalidis, I.; Hosseini-Bandegharaei, A.; Giannakoudakis, D.A.; Robalds, A.; Usman, M.; Escudero, L.B.; Zhou, Y.; Colmenares, J.C.; Núñez-Delgado, A. Agricultural biomass/waste as adsorbents for toxic metal decontamination of aqueous solutions. J. Mol. Liq. 2019, 295, 111684. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Vilar, V.J.; Botelho, C.M.; Boaventura, R.A. Coconut-based biosorbents for water treatment—A review of the recent literature. Adv. Colloid Interface Sci. 2010, 160, 1–15. [Google Scholar] [CrossRef]
- Ngah, W.W.; Hanafiah, M.M. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: A review. Bioresour. Technol. 2008, 99, 3935–3948. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Tanvir, B.; Xiukang, W.; Brtnicky, M.; Ditta, A.; Kucerik, J.; Subhani, Z.; Nazir, M.Z.; Radziemska, M.; Saeed, Q. Co-composted biochar enhances growth, physiological, and phytostabilization efficiency of brassica napus and reduces associated health risks under chromium stress. Front. Plant Sci. 2021, 12, 775785. [Google Scholar] [CrossRef] [PubMed]
- Urra, J.; Alkorta, I.; Garbisu, C. Potential benefits and risks for soil health derived from the use of organic amendments in agriculture. Agronomy 2019, 9, 542. [Google Scholar] [CrossRef]
- Brtnicky, M.; Kintl, A.; Holatko, J.; Hammerschmiedt, T.; Mustafa, A.; Kucerik, J.; Vitez, T.; Prichystalova, J.; Baltazar, T.; Elbl, J. EFFECT of digestates derived from the fermentation of maize-legume intercropped culture and maize monoculture application on soil properties and plant biomass production. Chem. Biol. Technol. Agric. 2022, 9, 43. [Google Scholar] [CrossRef]
- Graham-Rowe, E.; Jessop, D.C.; Sparks, P. Identifying motivations and barriers to minimising household food waste. Resour. Conserv. Recycl. 2014, 84, 15–23. [Google Scholar] [CrossRef]
- Footprint, F.W. Food Wastage Footprint: Impacts on Natural Resources: Summary Report; Food & Agriculture Org: Québec City, QC, Canada, 2013. [Google Scholar]
- Nanda, S.; Isen, J.; Dalai, A.K.; Kozinski, J.A. Gasification of fruit wastes and agro-food residues in supercritical water. Energy Convers. Manag. 2016, 110, 296–306. [Google Scholar] [CrossRef]
- Demirbas, A. Heavy metal adsorption onto agro-based waste materials: A review. J. Hazard. Mater. 2008, 157, 220–229. [Google Scholar] [CrossRef]
- Daneshfozoun, S.; Abdullah, M.; Abdullah, B. Preparation and characterization of magnetic biosorbent based on oil palm empty fruit bunch fibers, cellulose and Ceiba pentandra for heavy metal ions removal. Ind. Crop. Prod. 2017, 105, 93–103. [Google Scholar] [CrossRef]
- Abdić, Š.; Memić, M.; Šabanović, E.; Sulejmanović, J.; Begić, S. Adsorptive removal of eight heavy metals from aqueous solution by unmodified and modified agricultural waste: Tangerine peel. Int. J. Environ. Sci. Technol. 2018, 15, 2511–2518. [Google Scholar] [CrossRef]
- Van Dam, J.E.G.; Gorshkova, T.A. Cell Walls and Fibers: Fiber Formation; Elsevier: Amsterdam, The Netherlands, 2003; pp. 87–96. [Google Scholar]
- Neris, J.B.; Luzardo, F.H.M.; da Silva, E.G.P.; Velasco, F.G. Evaluation of adsorption processes of metal ions in multi-element aqueous systems by lignocellulosic adsorbents applying different isotherms: A critical review. Chem. Eng. J. 2019, 357, 404–420. [Google Scholar] [CrossRef]
- Değermenci, G.D.; Değermenci, N.; Ayvaoğlu, V.; Durmaz, E.; Çakır, D.; Akan, E. Adsorption of reactive dyes on lignocellulosic waste; characterization, equilibrium, kinetic and thermodynamic studies. J. Clean. Prod. 2019, 225, 1220–1229. [Google Scholar] [CrossRef]
- Dong, T.; Xu, G.; Wang, F. Oil spill cleanup by structured natural sorbents made from cattail fibers. Ind. Crop. Prod. 2015, 76, 25–33. [Google Scholar] [CrossRef]
- Nawirska, A. Binding of heavy metals to pomace fibers. Food Chem. 2005, 90, 395–400. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, W.; Wu, B.; Wu, P.; Duan, Y.; Yang, Q.; Ma, H. Modification of garlic skin dietary fiber with twin-screw extrusion process and in vivo evaluation of Pb binding. Food Chem. 2018, 268, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Berglund, L.; Breedveld, L.; Oksman, K. Toward eco-efficient production of natural nanofibers from industrial residue: Eco-design and quality assessment. J. Clean. Prod. 2020, 255, 120274. [Google Scholar] [CrossRef]
- Fayaz, G.; Soleimanian, Y.; Mhamadi, M.; Turgeon, S.L.; Khalloufi, S. The applications of conventional and innovative mechanical technologies to tailor structural and functional features of dietary fibers from plant wastes: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2149–2199. [Google Scholar] [CrossRef]
- Bunzel, M.A.; Seiler, A.; Steinhart, H. Characterization of dietary fiber lignins from fruits and vegetables using the DFRC method. J. Agric. Food Chem. 2005, 53, 9553–9559. [Google Scholar] [CrossRef]
- Latif, R.; Wakeel, S.; Zaman Khan, N.; Noor Siddiquee, A.; Lal Verma, S.; Akhtar Khan, Z. Surface treatments of plant fibers and their effects on mechanical properties of fiber-reinforced composites: A review. J. Reinf. Plast. Compos. 2019, 38, 15–30. [Google Scholar] [CrossRef]
- Fidelis, M.E.A.; Pereira, T.V.C.; Gomes, O.d.F.M.; de Andrade Silva, F.; Toledo Filho, R.D. The effect of fiber morphology on the tensile strength of natural fibers. J. Mater. Res. Technol. 2013, 2, 149–157. [Google Scholar] [CrossRef]
- Al-Asheh, S.; Duvnjak, Z. Sorption of cadmium and other heavy metals by pine bark. J. Hazard. Mater. 1997, 56, 35–51. [Google Scholar] [CrossRef]
- Feng, N.; Guo, X.; Liang, S.; Zhu, Y.; Liu, J. Biosorption of heavy metals from aqueous solutions by chemically modified orange peel. J. Hazard. Mater. 2011, 185, 49–54. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Li, J.; Salamh, Y.; Al-Laqtah, N.; Walker, G.; Ahmad, M.N. Adsorption mechanisms of removing heavy metals and dyes from aqueous solution using date pits solid adsorbent. J. Hazard. Mater. 2010, 176, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Varghese, A.G.; Paul, S.A.; Latha, M.S. Remediation of heavy metals and dyes from wastewater using cellulose-based adsorbents. Environ. Chem. Lett. 2019, 17, 867–877. [Google Scholar] [CrossRef]
- Paranjape, P.; Sadgir, P. Heavy Metal Removal Using Plant Origin Biomass and Agricultural Waste-Derived Biomass from Aqueous Media: A Review. Water Conserv. Sci. Eng. 2023, 8, 9. [Google Scholar] [CrossRef]
- Nasernejad, B.; Zadeh, T.E.; Pour, B.B.; Bygi, M.E.; Zamani, A. Camparison for biosorption modeling of heavy metals (Cr (III), Cu (II), Zn (II)) adsorption from wastewater by carrot residues. Process Biochem. 2005, 40, 1319–1322. [Google Scholar] [CrossRef]
- El-Azazy, M.; El-Shafie, A.S.; Issa, A.A.; Al-Sulaiti, M.; Al-Yafie, J.; Shomar, B.; Al-Saad, K. Potato peels as an adsorbent for heavy metals from aqueous solutions: Eco-structuring of a green adsorbent operating Plackett–Burman design. J. Chem. 2019, 2019, 4926240. [Google Scholar] [CrossRef]
- Feizi, M.; Jalali, M. Removal of heavy metals from aqueous solutions using sunflower, potato, canola and walnut shell residues. J. Taiwan Inst. Chem. Eng. 2015, 54, 125–136. [Google Scholar] [CrossRef]
- Alalwan, H.A.; Kadhom, M.A.; Alminshid, A.H. Removal of heavy metals from wastewater using agricultural byproducts. J. Water Supply Res. Technol.-Aqua 2020, 69, 99–112. [Google Scholar] [CrossRef]
- Maina, I.W.; Obuseng, V.; Nareetsile, F. Use of Moringa oleifera (Moringa) seed pods and Sclerocarya birrea (Morula) nut shells for removal of heavy metals from wastewater and borehole water. J. Chem. 2016, 2016, 9312952. [Google Scholar] [CrossRef]
- Thi Quyen, V.; Pham, T.-H.; Kim, J.; Thanh, D.M.; Thang, P.Q.; Van Le, Q.; Jung, S.H.; Kim, T. Biosorbent derived from coffee husk for efficient removal of toxic heavy metals from wastewater. Chemosphere 2021, 284, 131312. [Google Scholar] [CrossRef]
- Mata, Y.; Blázquez, M.; Ballester, A.; González, F.; Muñoz, J. Studies on sorption, desorption, regeneration and reuse of sugar-beet pectin gels for heavy metal removal. J. Hazard. Mater. 2010, 178, 243–248. [Google Scholar] [CrossRef]
- Castro, L.; Blázquez, M.L.; González, F.; Muñoz, J.A.; Ballester, A. Biosorption of Zn (II) from industrial effluents using sugar beet pulp and F. vesiculosus: From laboratory tests to a pilot approach. Sci. Total Environ. 2017, 598, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Nieto, L.M.; Alami, S.B.D.; Hodaifa, G.; Faur, C.; Rodríguez, S.; Giménez, J.A.; Ochando, J. Adsorption of iron on crude olive stones. Ind. Crop. Prod. 2010, 32, 467–471. [Google Scholar] [CrossRef]
- Petrella, A.; Spasiano, D.; Acquafredda, P.; De Vietro, N.; Ranieri, E.; Cosma, P.; Rizzi, V.; Petruzzelli, V.; Petruzzelli, D. Heavy metals retention (Pb (II), Cd (II), Ni (II)) from single and multimetal solutions by natural biosorbents from the olive oil milling operations. Process Saf. Environ. Prot. 2018, 114, 79–90. [Google Scholar] [CrossRef]
- Fernández-González, R.; Martín-Lara, M.Á.; Blázquez, G.; Pérez, A.; Calero, M. Recovering Metals from aqueous solutions by biosorption onto hydrolyzed olive cake. Water 2019, 11, 2519. [Google Scholar] [CrossRef]
- Singh, R.; Martin, C.; Barr, D.; Rosengren, R. Immobilised apple peel bead biosorbent for the simultaneous removal of heavy metals from cocktail solution. Cogent Environ. Sci. 2019, 5, 1673116. [Google Scholar] [CrossRef]
- Šabanović, E.; Memić, M.; Sulejmanović, J.; Selović, A. Simultaneous adsorption of heavy metals from water by novel lemon-peel based biomaterial. Pol. J. Chem. Technol. 2020, 22, 46–53. [Google Scholar] [CrossRef]
- Bulut, Y.; Tez, Z. Adsorption studies on ground shells of hazelnut and almond. J. Hazard. Mater. 2007, 149, 35–41. [Google Scholar] [CrossRef]
- Mohammad, M.; Maitra, S.; Ahmad, N.; Bustam, A.; Sen, T.; Dutta, B.K. Metal ion removal from aqueous solution using physic seed hull. J. Hazard. Mater. 2010, 179, 363–372. [Google Scholar] [CrossRef]
- Xu, X.; Cao, X.; Zhao, L. Comparison of rice husk-and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: Role of mineral components in biochars. Chemosphere 2013, 92, 955–961. [Google Scholar] [CrossRef]
- Maheshwari, U.; Gupta, S. Removal of Cr (VI) from wastewater using activated neem bark in a fixed-bed column: Interference of other ions and kinetic modelling studies. Desalination Water Treat. 2016, 57, 8514–8525. [Google Scholar] [CrossRef]
- Tripathi, A.; Ranjan, M.R. Heavy metal removal from wastewater using low cost adsorbents. Bioremediat. Biodegrad. 2015, 6, 1000315. [Google Scholar] [CrossRef]
- Chao, H.-P.; Chang, C.-C.; Nieva, A. Biosorption of heavy metals on Citrus maxima peel, passion fruit shell, and sugarcane bagasse in a fixed-bed column. J. Ind. Eng. Chem. 2014, 20, 3408–3414. [Google Scholar] [CrossRef]
- Wang, F.Y.; Wang, H.; Ma, J.W. Adsorption of cadmium (II) ions from aqueous solution by a new low-cost adsorbent—Bamboo charcoal. J. Hazard. Mater. 2010, 177, 300–306. [Google Scholar] [CrossRef]
- Moussavi, G.; Barikbin, B. Biosorption of chromium (VI) from industrial wastewater onto pistachio hull waste biomass. Chem. Eng. J. 2010, 162, 893–900. [Google Scholar] [CrossRef]
- SenthilKumar, P.; Ramalingam, S.; Sathyaselvabala, V.; Kirupha, S.D.; Sivanesan, S. Removal of copper (II) ions from aqueous solution by adsorption using cashew nut shell. Desalination 2011, 266, 63–71. [Google Scholar] [CrossRef]
- Cholico-González, D.; Ortiz Lara, N.; Fernández Macedo, A.M.; Chavez Salas, J. Adsorption Behavior of Pb(II), Cd(II), and Zn(II) onto Agave Bagasse, Characterization, and Mechanism. ACS Omega 2020, 12, 3302–3314. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Al-Yasi, H.M.; Galal, T.M.; Hamza, R.Z.; Abdelkader, T.G.; Ali, E.F.; Hassan, S.H. Statistical optimization, kinetic, equilibrium isotherm and thermodynamic studies of copper biosorption onto Rosa damascena leaves as a low-cost biosorbent. Sci. Rep. 2022, 12, 8583. [Google Scholar] [CrossRef]
- Azam, M.; Wabaidur, S.M.; Khan, M.R.; Al-Resayes, S.I.; Islam, M.S. Heavy metal ions removal from aqueous solutions by treated ajwa date pits: Kinetic, isotherm, and thermodynamic approach. Polymers 2022, 14, 914. [Google Scholar] [CrossRef] [PubMed]
- Massocatto, C.; Paschoal, E.; Buzinaro, N.; Oliveria, T.; Tarley, C.; Caetano, J.; Gonçalves Jr, A.; Dragunski, D.; Diniz, K. Preparation and evaluation of kinetics and thermodynamics studies of lead adsorption onto chemically modified banana peels. Desalination Water Treat. 2013, 51, 5682–5691. [Google Scholar] [CrossRef]
- Nathan, R.J.; Martin, C.E.; Barr, D.; Rosengren, R.J. Simultaneous removal of heavy metals from drinking water by banana, orange and potato peel beads: A study of biosorption kinetics. Appl. Water Sci. 2021, 11, 28. [Google Scholar] [CrossRef]
- Sathasivam, K.; Haris, M.R.H.M. Banana trunk fibers as an efficient biosorbent for the removal of Cd(II), Cu(II), Fe(II) and Zn(II) from aqueous solutions. J. Chil. Chem. 2010, 55, 278–282. [Google Scholar] [CrossRef]
- Daochalermwong, A.; Chanka, N.; Songsrirote, K.; Dittanet, P.; Niamnuy, C.; Seubsai, A. Removal of Heavy Metal Ions Using Modified Celluloses Prepared from Pineapple Leaf Fiber. ACS Omega 2020, 5, 5285–5296. [Google Scholar] [CrossRef] [PubMed]
- Priyantha, N.; Kotabewatta, P. Biosorption of heavy metal ions on peel of Artocarpus nobilis fruit: 1—Ni (II) sorption under static and dynamic conditions. Appl. Water Sci. 2019, 9, 37. [Google Scholar] [CrossRef]
- Tran, H.N.; Chao, H.-P. Adsorption and desorption of potentially toxic metals on modified biosorbents through new green grafting process. Environ. Sci. Pollut. Res. 2018, 25, 12808–12820. [Google Scholar] [CrossRef] [PubMed]
- Schiewer, S.; Patil, S.B. Pectin-rich fruit wastes as biosorbents for heavy metal removal: Equilibrium and kinetics. Bioresour. Technol. 2008, 99, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Aman, T.; Kazi, A.A.; Sabri, M.U.; Bano, Q. Potato peels as solid waste for the removal of heavy metal copper (II) from waste water/industrial effluent. Colloids Surf. B Biointerfaces 2008, 63, 116–121. [Google Scholar] [CrossRef]
- Zhu, B.; Fan, T.; Zhang, D. Adsorption of copper ions from aqueous solution by citric acid modified soybean straw. J. Hazard. Mater. 2008, 153, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Huang, C.; Ou, S. In vitro binding capacities of three dietary fibers and their mixture for four toxic elements, cholesterol, and bile acid. J. Hazard. Mater. 2011, 186, 236–239. [Google Scholar] [CrossRef]
- Almendros, A.; Martín-Lara, M.; Ronda, A.; Pérez, A.; Blázquez, G.; Calero, M. Physico-chemical characterization of pine cone shell and its use as biosorbent and fuel. Bioresour. Technol. 2015, 196, 406–412. [Google Scholar] [CrossRef]
- Pathirana, C.; Ziyath, A.M.; Jinadasa, K.; Egodawatta, P.; Goonetilleke, A. Mathematical modelling of the influence of physico-chemical properties on heavy metal adsorption by biosorbents. Chemosphere 2020, 255, 126965. [Google Scholar] [CrossRef] [PubMed]
- Lyu, B.; Wang, H.; Swallah, M.S.; Fu, H.; Shen, Y.; Guo, Z.; Tong, X.; Li, Y.; Yu, H.; Jiang, L. Structure, properties and potential bioactivities of high-purity insoluble fibre from soybean dregs (Okara). Food Chem. 2021, 364, 130402. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Ren, B.; Diao, Z.; Chen, Y.; Qiao, Q.; Liu, X. Physicochemical properties and intestinal protective effect of ultra-micro ground insoluble dietary fibre from carrot pomace. Food Funct. 2016, 7, 3902–3909. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Wang, X.; Li, M.; Lei, H.; Xu, H. Cellulose nanocrystals prepared from wheat bran: Characterization and cytotoxicity assessment. Int. J. Biol. Macromol. 2019, 140, 225–233. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Yuan, F.; Fan, R.; Gao, Y. Preparation and physicochemical properties of soluble dietary fiber from orange peel assisted by steam explosion and dilute acid soaking. Food Chem. 2015, 185, 90–98. [Google Scholar] [CrossRef]
- Song, Y.; Su, W.; Mu, Y.C. Modification of bamboo shoot dietary fiber by extrusion-cellulase technology and its properties. Int. J. Food Prop. 2018, 21, 1219–1232. [Google Scholar] [CrossRef]
- Pathirana, C.; Ziyath, A.M.; Jinadasa, K.; Egodawatta, P.; Sarina, S.; Goonetilleke, A. Quantifying the influence of surface physico-chemical properties of biosorbents on heavy metal adsorption. Chemosphere 2019, 234, 488–495. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L.; Wu, J.; Zhang, H.; Zhu, L.; Zhan, X. Potential application of a low-viscosity and high-transparency xanthan gum produced from Xanthomonas campestris CCTCC M2015714 in foods. Prep. Biochem. Biotechnol. 2018, 48, 402–407. [Google Scholar] [CrossRef]
- Kartel, M.T.; Kupchik, L.A.; Veisov, B.K. Evaluation of pectin binding of heavy metal ions in aqueous solutions. Chemosphere 1999, 38, 2591–2596. [Google Scholar] [CrossRef] [PubMed]
- Requena, M.C.; González, C.N.A.; Barragán, L.A.P.; Correia, T.; Esquivel, J.C.C.; Herrera, R.R. Functional and physico-chemical properties of six desert-sources of dietary fiber. Food Biosci. 2016, 16, 26–31. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, C.; Liu, F.; Yuan, Y.; Wu, H.; Li, A. Effects of ionic strength on removal of toxic pollutants from aqueous media with multifarious adsorbents: A review. Sci. Total Environ. 2019, 646, 265–279. [Google Scholar] [CrossRef]
- Basiri, S.; Shekarforoush, S.S.; Mazkour, S.; Modabber, P.; Kordshouli, F.Z. Evaluating the potential of mucilaginous seed of psyllium (Plantago ovata) as a new lead biosorbent. Bioact. Carbohydr. Diet. Fibre 2020, 24, 100242. [Google Scholar] [CrossRef]
- Guiza, S. Biosorption of heavy metal from aqueous solution using cellulosic waste orange peel. Ecol. Eng. 2017, 99, 134–140. [Google Scholar] [CrossRef]
- Pal, A.; Giri, A.; Bandyopadhyay, A. Influence of hydrodynamic size and zeta potential of a novel polyelectrolyte poly (acrylic acid) grafted guar gum for adsorption of Pb (II) from acidic waste water. J. Environ. Chem. Eng. 2016, 4, 1731–1742. [Google Scholar] [CrossRef]
- Jain, M.; Yadav, M.; Kohout, T.; Lahtinen, M.; Garg, V.K.; Sillanpää, M. Development of iron oxide/activated carbon nanoparticle composite for the removal of Cr (VI), Cu (II) and Cd (II) ions from aqueous solution. Water Resour. Ind. 2018, 20, 54–74. [Google Scholar] [CrossRef]
- Hussain, S.; Jõudu, I.; Bhat, R. Dietary fiber from underutilized plant resources—A positive approach for valorization of fruit and vegetable wastes. Sustainability 2020, 12, 5401. [Google Scholar] [CrossRef]
- Gan, J.; Xie, L.; Peng, G.; Xie, J.; Chen, Y.; Yu, Q. Systematic review on modification methods of dietary fiber. Food Hydrocoll. 2021, 119, 106872. [Google Scholar] [CrossRef]
- Huang, J.; Liao, J.; Qi, J.; Jiang, W.; Yang, X. Structural and physicochemical properties of pectin-rich dietary fiber prepared from citrus peel. Food Hydrocoll. 2021, 110, 106140. [Google Scholar] [CrossRef]
- Xu, G.; Wang, L.; Xie, Y.; Tao, M.; Zhang, W. Highly selective and efficient adsorption of Hg2+ by a recyclable aminophosphonic acid functionalized polyacrylonitrile fiber. J. Hazard. Mater. 2018, 344, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Amezquita, L.E.; Tejada-Ortigoza, V.; Pérez-Carrillo, E.; Serna-Saldívar, S.O.; Campanella, O.H.; Welti-Chanes, J. Functional and compositional changes of orange peel fiber thermally-treated in a twin extruder. LWT 2019, 111, 673–681. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Wang, Y.; Liu, Z.; Ni, Y. Effects of extraction methods on the structural characteristics and functional properties of dietary fiber extracted from kiwifruit (Actinidia deliciosa). Food Hydrocoll. 2021, 110, 106162. [Google Scholar] [CrossRef]
- Adegoke, K.A.; Akinnawo, S.O.; Ajala, O.A.; Adebusuyi, T.A.; Maxakato, N.W.; Bello, O.S. Progress and challenges in batch and optimization studies on the adsorptive removal of heavy metals using modified biomass-based adsorbents. Bioresour. Technol. Rep. 2022, 19, 101115. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Mandal, S.; Das, S. Adsorption of Zn (II) from aqueous solution by using different adsorbents. Chem. Eng. J. 2006, 123, 43–51. [Google Scholar] [CrossRef]
- Yakout, S.; Daifullah, A.; El-Reefy, S. Adsorption of naphthalene, phenanthrene and pyrene from aqueous solution using low-cost activated carbon derived from agricultural wastes. Adsorpt. Sci. Technol. 2013, 31, 293–302. [Google Scholar] [CrossRef]
- Rastogi, S.; Tiwari, S.; Ratna, S.; Kumar, R. Utilization of agro-industrial waste for biosurfactant production under submerged fermentation and its synergistic application in biosorption of Pb2+. Bioresour. Technol. Rep. 2021, 15, 100706. [Google Scholar] [CrossRef]
- Dong, J.; Du, Y.; Duyu, R.; Shang, Y.; Zhang, S.; Han, R. Adsorption of copper ion from solution by polyethylenimine modified wheat straw. Bioresour. Technol. Rep. 2019, 6, 96–102. [Google Scholar] [CrossRef]
- Chu, J.; Zhao, H.; Lu, Z.; Lu, F.; Bie, X.; Zhang, C. Improved physicochemical and functional properties of dietary fiber from millet bran fermented by Bacillus natto. Food Chem. 2019, 294, 79–86. [Google Scholar] [CrossRef]
- Moslemi, M. Reviewing the recent advances in application of pectin for technical and health promotion purposes: From laboratory to market. Carbohydr. Polym. 2021, 254, 117324. [Google Scholar] [CrossRef]
- Ibarra-Rodríguez, D.; Lizardi-Mendoza, J.; López-Maldonado, E.A.; Oropeza-Guzmán, M.T. Capacity of ‘nopal’pectin as a dual coagulant-flocculant agent for heavy metals removal. Chem. Eng. J. 2017, 323, 19–28. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.; Chauhan, K.; Gupta, R.; Ahn, J.-H.; Chauhan, G.S. Removal of As (V) from water by pectin based active hydrogels following geochemical approach. Bioresour. Technol. 2009, 100, 1474–1477. [Google Scholar] [CrossRef]
- Jakóbik-Kolon, A.; Bok-Badura, J.; Karoń, K.; Mitko, K.; Milewski, A. Hybrid pectin-based biosorbents for zinc ions removal. Carbohydr. Polym. 2017, 169, 213–219. [Google Scholar] [CrossRef]
- Hastuti, B.; Hadi, S.; Totiana, F. Isolation of pectin from carrot peel as biosorbent of Pb (II) ion. In IOP Conference Series: Earth and Environmental Science, Proceedings of the 2018 5th International Conference on Coastal and Ocean Engineering (ICCOE 2018), Shanghai, China, 27–29 April 2018; IOP Publishing: Bristol, UK, 2018; p. 12039. [Google Scholar]
- Tarmizi, A.N.M.; Ismail, N.; Ahmad, H. Preliminary study on heavy metal removal and turbidity reduction from groundwater by using apple pectin (bioflocculant). Int. J. Environ. Eng. 2018, 9, 271–281. [Google Scholar] [CrossRef]
- Shukla, S.; Pai, R.S.; Shendarkar, A.D. Adsorption of Ni (II), Zn (II) and Fe (II) on modified coir fibres. Sep. Purif. Technol. 2006, 47, 141–147. [Google Scholar] [CrossRef]
- Tangtubtim, S.; Saikrasun, S. Effective removals of copper (II) and lead (II) cations from aqueous solutions by polyethyleneimine-immobilized pineapple fiber. Bioresour. Technol. Rep. 2019, 7, 100188. [Google Scholar] [CrossRef]
- Hu, G.; Huang, S.; Chen, H.; Wang, F. Binding of four heavy metals to hemicelluloses from rice bran. Food Res. Int. 2010, 43, 203–206. [Google Scholar] [CrossRef]
- Pejic, B.; Vukcevic, M.; Kostic, M.; Skundric, P. Biosorption of heavy metal ions from aqueous solutions by short hemp fibers: Effect of chemical composition. J. Hazard. Mater. 2009, 164, 146–153. [Google Scholar] [CrossRef]
- Mongioví, C.; Morin-Crini, N.; Lacalamita, D.; Bradu, C.; Raschetti, M.; Placet, V.; Ribeiro, A.R.L.; Ivanovska, A.; Kostić, M.; Crini, G. Biosorbents from plant fibers of hemp and flax for metal removal: Comparison of their biosorption properties. Molecules 2021, 26, 4199. [Google Scholar] [CrossRef]
- Demirbas, A. Adsorption of Co (II) and Hg (II) from water and wastewater onto modified lignin. Energy Sources Part A 2007, 29, 117–123. [Google Scholar] [CrossRef]
- Imran-Shaukat, M.; Wahi, R.; Ngaini, Z. The application of agricultural wastes for heavy metals adsorption: A meta-analysis of recent studies. Bioresour. Technol. Rep. 2022, 17, 100902. [Google Scholar] [CrossRef]
- Agarwal, A.; Upadhyay, U.; Sreedhar, I.; Anitha, K. Simulation studies of Cu (II) removal from aqueous solution using olive stone. Clean. Mater. 2022, 5, 100128. [Google Scholar] [CrossRef]
- Reshmy, R.; Philip, E.; Madhavan, A.; Pugazhendhi, A.; Sindhu, R.; Sirohi, R.; Awasthi, M.K.; Pandey, A.; Binod, P. Nanocellulose as green material for remediation of hazardous heavy metal contaminants. J. Hazard. Mater. 2022, 424, 127516. [Google Scholar] [CrossRef]
- Cheng, S.; Chen, T.; Xu, W.; Huang, J.; Jiang, S.; Yan, B. Application research of biochar for the remediation of soil heavy metals contamination: A review. Molecules 2020, 25, 3167. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano) materials for sustainable water treatment: A review. Carbohydr. Polym. 2021, 251, 116986. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- Elgarahy, A.; Elwakeel, K.; Mohammad, S.; Elshoubaky, G. A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Clean. Eng. Technol. 2021, 4, 100209. [Google Scholar] [CrossRef]
- Eccles, H. Treatment of metal-contaminated wastes: Why select a biological process? Trends Biotechnol. 1999, 17, 462–465. [Google Scholar] [CrossRef] [PubMed]
- El-Bayaa, A.; Badawy, N.; Abd AlKhalik, E. Effect of ionic strength on the adsorption of copper and chromium ions by vermiculite pure clay mineral. J. Hazard. Mater. 2009, 170, 1204–1209. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, L.; Fu, F.; Bowman, S.; Wang, P.; Li, Y.; Zhang, Y. The heavy metal adsorption and plant cultivation performance of grafting modified plant medium made with recycled fibers. J. Clean. Prod. 2021, 329, 129788. [Google Scholar] [CrossRef]
- Haldar, D.; Duarah, P.; Purkait, M.K. MOFs for the treatment of arsenic, fluoride and iron contaminated drinking water: A review. Chemosphere 2020, 251, 126388. [Google Scholar] [CrossRef] [PubMed]
- Giles, D.E.; Mohapatra, M.; Issa, T.B.; Anand, S.; Singh, P. Iron and aluminium based adsorption strategies for removing arsenic from water. J. Environ. Manag. 2011, 92, 3011–3022. [Google Scholar] [CrossRef]
- Devi, R.R.; Umlong, I.M.; Das, B.; Borah, K.; Thakur, A.J.; Raul, P.K.; Banerjee, S.; Singh, L. Removal of iron and arsenic (III) from drinking water using iron oxide-coated sand and limestone. Appl. Water Sci. 2014, 4, 175–182. [Google Scholar] [CrossRef]
- Kneebone, P.; O’day, P.; Jones, N.; Hering, J. Deposition and fate of arsenic in iron-and arsenic-enriched reservoir sediments. Environ. Sci. Technol. 2002, 36, 381–386. [Google Scholar] [CrossRef]
- Meunier, N.; Blais, J.-F.; Tyagi, R.D. Removal of heavy metals from acid soil leachate using cocoa shells in a batch counter-current sorption process. Hydrometallurgy 2004, 73, 225–235. [Google Scholar] [CrossRef]
- Barišić, V.; Jozinović, A.; Flanjak, I.; Šubarić, D.; Babić, J.; Miličević, B.; Doko, K.; Ačkar, Đ. Difficulties with use of cocoa bean shell in food production and high voltage electrical discharge as a possible solution. Sustainability 2020, 12, 3981. [Google Scholar] [CrossRef]
- Derakhshan-Nejad, Z.; Jung, M.C. Remediation of multi-metal contaminated soil using biochars from rice husk and maple leaves. J. Mater. Cycles Waste Manag. 2019, 21, 457–468. [Google Scholar] [CrossRef]
- Yang, S.; Li, Y.; Liu, G.-M.; Si, S.-C.; Zhu, X.; Tu, C.; Li, L.-Z.; Luo, Y.-M. Sequential washing and eluent regeneration with agricultural waste extracts and residues for facile remediation of meta-contaminated agricultural soils. Sci. Total Environ. 2022, 835, 155548. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).