The Classification, Molecular Structure and Biological Biosynthesis of Flavonoids, and Their Roles in Biotic and Abiotic Stresses
Abstract
1. Introduction
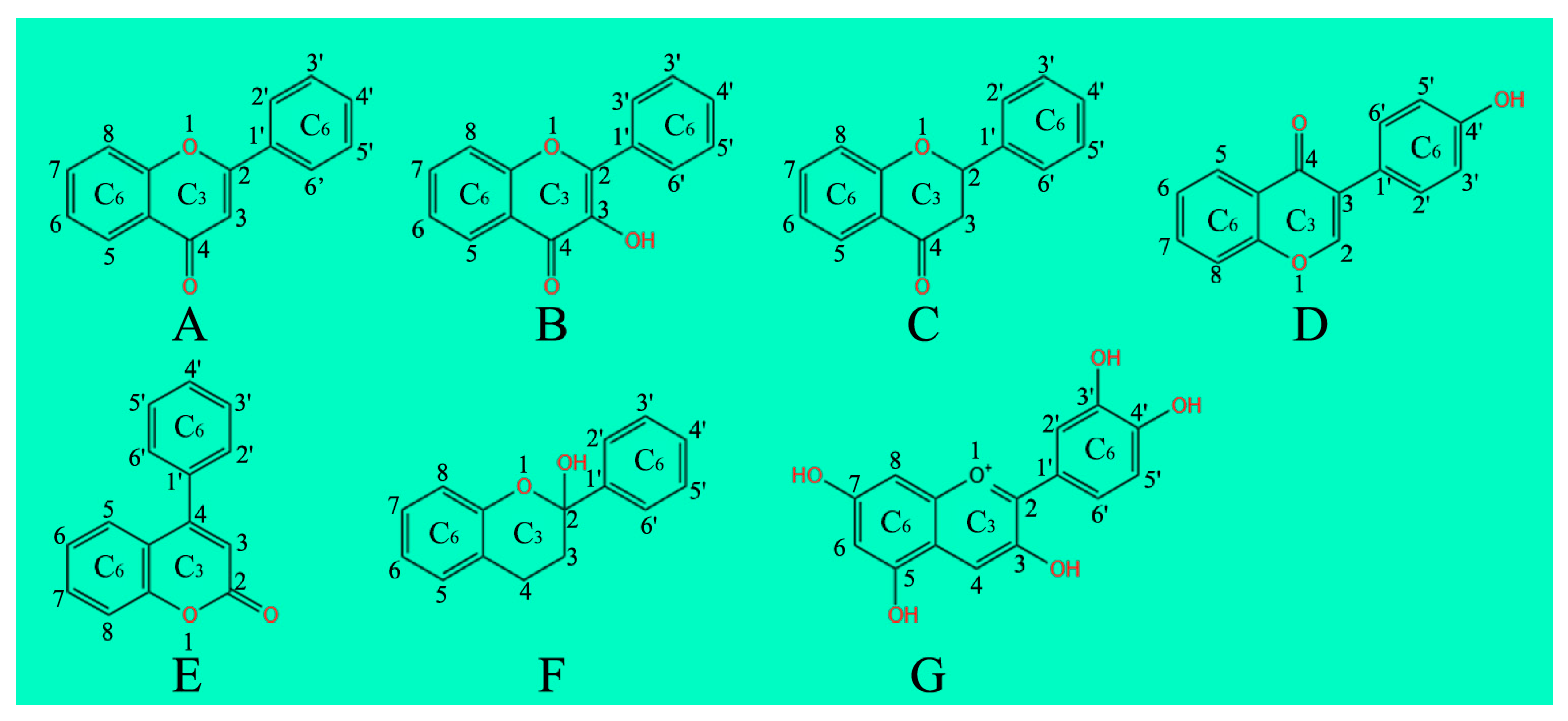
2. Flavonoids Classification
2.1. Flavones
2.2. Flavonols
2.3. Flavanones
2.4. Isoflavonoids
2.5. Neoflavonoids
2.6. Flavanols, Flavan-3-ols or Catechins
2.7. Anthocyanins
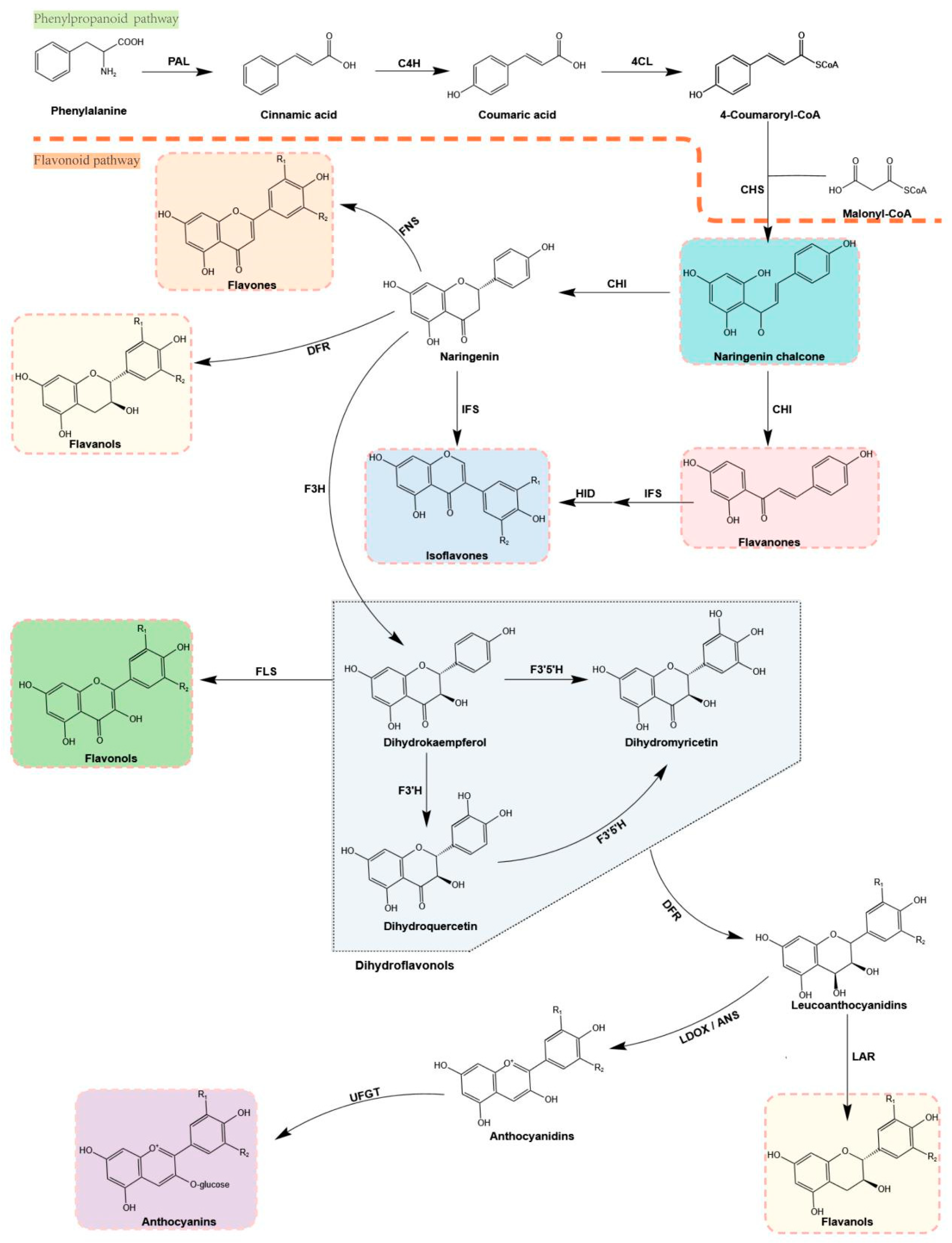
3. Flavonoid Biosynthesis in Plants
3.1. Regulation of Flavonoid Biosynthesis
3.2. Transcription Factors (TFs) Regulate Flavonoid Biosynthesis
3.3. Non-Coding RNA Regulates Flavonoid Biosynthesis
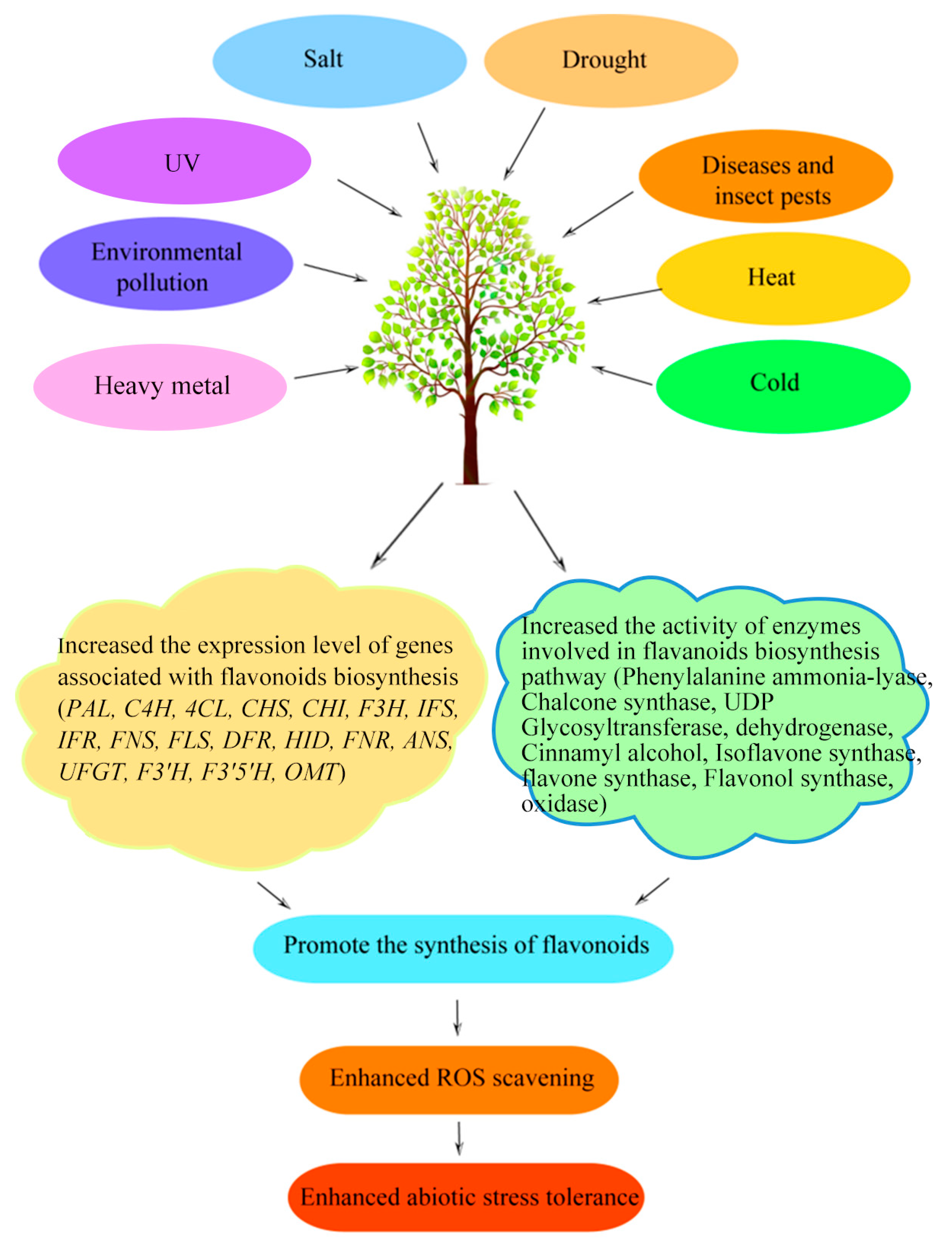
4. The Roles of Flavonoid Compounds in Various Stresses
4.1. Biotic Stress
4.1.1. The Roles of Flavonoids in the Invasion of Nematodes
4.1.2. The Roles of Flavonoids in the Invasion of Pathogenic Fungi
4.1.3. Antibacterial Effects of Flavonoids
4.2. Abiotic Stress
4.2.1. UV Stress
4.2.2. Cold Stress
4.2.3. Salt Stress
4.2.4. Drought Stress
4.2.5. Heavy Metal Stress
5. Transgenic Technologies Used in Enhancing Stress Resistance
5.1. Transgenic Technology Used in Enhancing UV and HL Resistance
5.2. Transgenic Technology Used in Enhancing Salt Resistance
5.3. Transgenic Technology Used in Enhancing Drought Resistance
5.4. Transgenic Technology Used in Enhancing Low Temperature Resistance
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Santos, E.L.; Maia, B.; Ferriani, A.P.; Teixeira, S. Flavonoids: Classification, biosynthesis and chemical ecology. Flavonoids-Biosynth. Hum. Health 2017, 13, 78–94. [Google Scholar]
- Jakimiuk, K.; Wink, M.; Tomczyk, M. Flavonoids of the Caryophyllaceae. Phytochem. Rev. 2022, 21, 179–218. [Google Scholar] [CrossRef]
- Martens, S.; Mithöfer, A. Flavones and flavone synthases. Phytochemistry 2005, 66, 2399–2407. [Google Scholar] [CrossRef]
- Yonekura-Sakakibara, K.; Higashi, Y.; Nakabayashi, R. The origin and evolution of plant flavonoid metabolism. Front. Plant Sci. 2019, 10, 943. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E. Effect of plant flavonoids on immune and inflammatory cell function. Flavonoids Living Syst. 1998, 39, 175–182. [Google Scholar]
- Karak, P. Biological activities of flavonoids: An overview. Int. J. Pharm. Sci. Res. 2019, 4, 1567–1574. [Google Scholar]
- Kozlowska, A.; Szostak-Wegierek, D. Flavonoids-food sources and health benefits. Rocz. Państwowego Zakładu Hig. 2014, 65, 53–78. [Google Scholar]
- García-Lafuente, A.; Guillamón, E.; Villares, A.; Rostagno, M.A.; Martínez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef] [PubMed]
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84. [Google Scholar]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Kisiriko, M.; Anastasiadi, M.; Terry, L.A.; Yasri, A.; Beale, M.H.; Ward, J.L. Phenolics from medicinal and aromatic plants: Characterisation and potential as biostimulants and bioprotectants. Molecules 2021, 26, 6343. [Google Scholar] [CrossRef]
- Samanta, A.; Das, G.; Das, S.K. Roles of flavonoids in plants. Carbon 2011, 100, 12–35. [Google Scholar]
- Kumar, V.; Suman, U.; Yadav, S.K. Flavonoid secondary metabolite: Biosynthesis and role in growth and development in plants. In Recent Trends and Techniques in Plant Metabolic Engineering; Springer: Singapore, 2018; pp. 19–45. [Google Scholar]
- Shirley, B.W. Flavonoid biosynthesis: ‘new’ functions for an ‘old’ pathway. Trends Plant Sci. 1996, 1, 377–382. [Google Scholar]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Bioch. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Baskar, V.; Venkatesh, R.; Ramalingam, S. Flavonoids (antioxidants systems) in higher plants and their response to stresses. In Antioxidants and Antioxidant Enzymes in Higher Plants; Springer: Cham, Switzerland, 2018; pp. 253–268. [Google Scholar]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Pollastri, S.; Tattini, M. Functional roles of flavonoids in photoprotection: New evidence, lessons from the past. Plant Physiol. Bioch. 2013, 72, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Sondi-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Hussain, S.; Rao, M.J.; Anjum, M.A.; Ejaz, S.; Zakir, I.; Ali, M.A.; Niaz, A.; Shakeel, A. Oxidative stress and antioxidant defense in plants under drought conditions. In Plant Abiotic Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2019; pp. 207–219. [Google Scholar]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- Bag, S.; Mondal, A.; Majumder, A.; Mondal, S.K.; Banik, A. Flavonoid mediated selective cross-talk between plants and beneficial soil microbiome. Phytochem. Rev. 2022, 21, 1739–1760. [Google Scholar] [CrossRef]
- Kaufman, P.B.; Chang, S.C.; Kirakosyan, A. Risks and benefits associated with genetically modified (GM) plants. In Recent Advances in Plant Biotechnology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 333–346. [Google Scholar]
- Van Esse, H.P.; Reuber, T.L.; Does, D. Genetic modification to improve disease resistance in crops. New Phytol. 2020, 225, 70–86. [Google Scholar] [CrossRef]
- Mubeen, H.; Naqvi, R.Z.; Masood, A.; Shoaib, M.W.; Raza, S. Gene transformation: Methods, uses and applications. J. Pharm. Biol. Sci. 2016, 4, 54. [Google Scholar]
- Niazian, M.; Noori, S.S.; Galuszka, P.; Mortazavian, S.M.M. Tissue culture-based Agrobacterium-mediated and in planta transformation methods. Soil Water Res. 2017, 53, 133–143. [Google Scholar] [CrossRef]
- Chownk, M.; Thakur, K.; Yadav, S.K. Retrospect and prospects of plant metabolic engineering. J. Plant Biochem. Biot. 2019, 28, 1–13. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Kim, K.M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Chowdhury, S.; Basu, A.; Kundu, S. Overexpression of a new osmotin-like protein gene (SindOLP) confers tolerance against biotic and abiotic stresses in sesame. Front. Plant Sci. 2017, 8, 410. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.K.; Singh, S.P. (Eds.) Plant Genome Engineering for Improved Flavonoids Production. In Plants as Bioreactors for Industrial Molecules; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2023; pp. 215–240. [Google Scholar]
- Shah, F.L.A.; Ramzi, A.B.; Baharum, S.N.; Noor, N.M.; Goh, H.H.; Leow, T.C.; Siti, N.O.; Suriana, S. Recent advancement of engineering microbial hosts for the biotechnological production of flavonoids. Mol. Biol. Rep. 2019, 46, 6647–6659. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Murtaza, G.; Liu, G.; Rahu, N.; Kalhoro, M.S.; Hussain, K.D. Flavonoids and type 2 diabetes: Evidence of efficacy in clinical and animal studies and delivery strategies to enhance their therapeutic efficacy. Pharmacol. Res. 2020, 152, 104629. [Google Scholar] [CrossRef]
- Dixon, R.A.; Achnine, L.; Kota, P.; Liu, C.J.; Reddy, M.S.; Wang, L. The phenylpropanoid pathway and plant defence—A genomics perspective. Mol. Plant Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef]
- Zakaryan, H.; Arabyan, E.; Oo, A.; Zandi, K. Flavonoids: Promising natural compounds against viral infections. Arch. Virol. 2017, 162, 2539–2551. [Google Scholar] [CrossRef]
- Yan, J.; Yu, L.; Xu, S.; Gu, W.; Zhu, W. Apigenin accumulation and expression analysis of apigenin biosynthesis relative genes in celery. Sci. Hortic. 2014, 165, 218–224. [Google Scholar] [CrossRef]
- Meyer, H.; Bolarinwa, A.; Wolfram, G.; Linseisen, J. Bioavailability of apigenin from apiin-rich parsley in humans. Ann. Nutr. Metab. 2006, 50, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food sources, bioavailability, metabolism, and bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Berim, A.; Gang, D.R. Methoxylated flavones: Occurrence, importance, biosynthesis. Phytochem. Rev. 2016, 15, 363–390. [Google Scholar] [CrossRef]
- Bose, S.; Sarkar, D.; Bose, A.; Mandal, S.C. Natural flavonoids and its pharmaceutical importance. Pharmacol. Rev. 2018, 94, 61–75. [Google Scholar]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary flavonoids: Cardioprotective potential with antioxidant effects and their pharmacokinetic, toxicological and therapeutic concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef]
- Khalid, M.; Bilal, M.; Huang, D.F. Role of flavonoids in plant interactions with the environment and against human pathogens—A review. J. Integr. Agric. 2019, 18, 211–230. [Google Scholar] [CrossRef]
- Khoo, B.Y.; Chua, S.L.; Balaram, P. Apoptotic effects of chrysin in human cancer cell lines. Int. J. Mol. Sci. 2010, 11, 2188–2199. [Google Scholar] [CrossRef]
- Šeruga, M.; Tomac, I. Influence of chemical structure of some flavonols on their electrochemical behaviour. Int. J. Electrochem. Sci. 2017, 12, 7616–7637. [Google Scholar] [CrossRef]
- de la Iglesia, R.; Milagro, F.I.; Campión, J.; Boqué, N.; Martínez, J.A. Healthy properties of proanthocyanidins. Biofactors 2010, 36, 159–168. [Google Scholar] [CrossRef]
- González-de-Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Carrera, C.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. Flavonol composition and antioxidant activity of onions (Allium cepa L.) based on the development of new analytical ultrasound-assisted extraction methods. Antioxidants 2021, 10, 273. [Google Scholar] [CrossRef]
- Proteggente, A.R.; Pannala, A.S.; Paganga, G.; Buren, L.V.; Wagner, E.; Wiseman, S.; Frans, P.; Dacombe, C.; Rice-Evans, C.A. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Radic. Tes. 2002, 36, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Fiol, M.; Adermann, S.; Neugart, S.; Rohn, S.; Mügge, C.; Schreiner, M.; Krumbein, A.; Kroh, L.W. Highly glycosylated and acylated flavonols isolated from kale (Brassica oleracea var. sabellica)—Structure–antioxidant activity relationship. Food Res. Int. 2012, 47, 80–89. [Google Scholar] [CrossRef]
- Stewart, A.J.; Bozonnet, S.; Mullen, W.; Jenkins, G.I.; Lean, M.E.; Crozier, A. Occurrence of flavonols in tomatoes and tomato-based products. J. Agric. Food Chem. 2000, 48, 2663–2669. [Google Scholar] [CrossRef] [PubMed]
- Sekhon-Loodu, S.; Catalli, A.; Kulka, M.; Wang, Y.; Shahidi, F.; Rupasinghe, H.V. Apple flavonols and n-3 polyunsaturated fatty acid–rich fish oil lowers blood C-reactive protein in rats with hypercholesterolemia and acute inflammation. Nutr. Res. 2014, 34, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Flamini, R.; Mattivi, F.; De, R.M.; Arapitsas, P.; Bavaresco, L. Advanced knowledge of three important classes of grape phenolics: Anthocyanins, stilbenes and flavonols. Int. J. Mol. Sci. 2013, 14, 19651–19669. [Google Scholar] [CrossRef]
- Larmo, P.S.; Yang, B.; Hurme, S.A.; Alin, J.A.; Kallio, H.P.; Salminen, E.K.; Tahvonen, R.L. Effect of a low dose of sea buckthorn berries on circulating concentrations of cholesterol, triacylglycerols, and flavonols in healthy adults. Eur. J. Nutr. 2009, 48, 277–282. [Google Scholar] [CrossRef]
- Xie, L.; Guo, Y.; Ren, C.; Cao, Y.; Li, J.; Lin, J.; Donald, G.; Zhao, X.; Zhang, B.; Sun, C.; et al. Unravelling the consecutive glycosylation and methylation of flavonols in peach in response to UV-B irradiation. Plant Cell Environ. 2022, 45, 2158–2175. [Google Scholar] [CrossRef]
- Rauter, A.P.; Ennis, M.; Hellwich, K.H.; Herold, B.J.; Horton, D.; Moss, G.P.; Schomburg, I. Nomenclature of flavonoids (IUPAC Recommendations 2017). Pure Appl. Chem. 2018, 90, 1429–1486. [Google Scholar] [CrossRef]
- Colombo, P.S.; Flamini, G.; Christodoulou, M.S.; Rodondi, G.; Vitalini, S.; Passarella, D.; Fico, G. Farinose alpine Primula species: Phytochemical and morphological investigations. Phytochemistry 2014, 98, 151–159. [Google Scholar] [CrossRef]
- Kozłowska, A.; Szostak-Węgierek, D. Flavonoids–food sources, health benefits, and mechanisms involved. In Bioactive Molecules in Food; Springer: Cham, Switzerland, 2019; pp. 53–78. [Google Scholar]
- Barreca, D.; Trombetta, D.; Smeriglio, A.; Mandalari, G.; Romeo, O.; Felice, M.R.; Gattuso, G.; Nabavi, S.M. Food flavonols: Nutraceuticals with complex health benefits and functionalities. Trends Food Sci. Technol. 2021, 117, 194–204. [Google Scholar] [CrossRef]
- Pereira, A.M.; Cidade, H.; Tiritan, M.E. Stereoselective Synthesis of Flavonoids: A Brief Overview. Molecules 2023, 28, 426. [Google Scholar] [CrossRef]
- Çetinkaya, S.; Akça, K.T.; Süntar, I. Flavonoids and anticancer activity: Structure–activity relationship. Stud. Nat. Prod. Chem. 2022, 74, 81–115. [Google Scholar]
- Peterson, J.J.; Dwyer, J.T.; Beecher, G.R.; Bhagwat, S.A.; Gebhardt, S.E.; Haytowitz, D.B.; Holden, J.M. Flavanones in oranges, tangerines (mandarins), tangors, and tangelos: A compilation and review of the data from the analytical literature. J. Food Compos. Anal. 2006, 19, S66–S73. [Google Scholar] [CrossRef]
- Vandercook, C.E.; Stephenson, R.G. Lemon juice composition. Identification of major phenolic compounds and estimation by paper chromatography. J. Agric. Food Chem. 1966, 14, 450–454. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.J.; Chen, J.B.; Cao, J.P.; Li, X.; Sun, C.D. Citrus flavonoids and their antioxidant evaluation. Crit. Rev. Food Sci. Nutr. 2022, 62, 3833–3854. [Google Scholar] [CrossRef] [PubMed]
- Najmanová, I.; Vopršalová, M.; Saso, L.; Mladěnka, P. The pharmacokinetics of flavanones. Crit. Rev. Food Sci. Nutr. 2020, 60, 3155–3171. [Google Scholar] [CrossRef] [PubMed]
- Abad-García, B.; Garmón-Lobato, S.; Berrueta, L.A.; Gallo, B.; Vicente, F. On line characterization of 58 phenolic compounds in Citrus fruit juices from Spanish cultivars by high-performance liquid chromatography with photodiode-array detection coupled to electrospray ionization triple quadrupole mass spectrometry. Talanta 2012, 99, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Nayak, R.; Patro, S.; Pradhan, B.; Sahu, B.; Behera, C.; Bhutia, S.K.; Jena, M. Chemical diversity of dietary phytochemicals and their mode of chemoprevention. Biotechnol. Rep. 2021, 30, e00633. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.J.; Beecher, G.R.; Bhagwat, S.A.; Dwyer, J.T.; Gebhardt, S.E.; Haytowitz, D.B.; Holden, J.M. Flavanones in grapefruit, lemons, and limes: A compilation and review of the data from the analytical literature. J. Food Compos. Anal. 2006, 19, S74–S80. [Google Scholar] [CrossRef]
- Seyedrezazadeh, E.; Kolahian, S.; Shahbazfar, A.A.; Ansarin, K.; Pour Moghaddam, M.; Sakhinia, M.; Sakhinia, E.; Vafa, M. Effects of the flavanone combination Hesperetin-Naringenin, and orange and grapefruit juices, on airway inflammation and remodeling in a murine asthma model. Phytother. Res. 2015, 29, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qian, J.; Li, J.; Xing, M.; Grierson, D.; Sun, C.; Xu, C.; Li, X.; Chen, K. Hydroxylation decoration patterns of flavonoids in horticultural crops: Chemistry, bioactivity, and biosynthesis. Hortic. Res. 2022, 9, uhab068. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Wang, J.; Zhu, Y.; Liu, S.; Zhou, X.; Zhang, H.; Wang, C.; Yang, W.; Tian, Z.; Cheng, H.; et al. An R2R3-type MYB transcription factor, GmMYB29, regulates isoflavone biosynthesis in soybean. PLoS Genet. 2017, 13, e1006770. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Smith, D.L. Flavonoids in agriculture: Chemistry and roles in, biotic and abiotic stress responses, and microbial associations. Agronomy 2020, 10, 1209. [Google Scholar] [CrossRef]
- Kopečná-Zapletalová, M.; Krasulová, K.; Anzenbacher, P.; Hodek, P.; Anzenbacherová, E. Interaction of isoflavonoids with human liver microsomal cytochromes P450: Inhibition of CYP enzyme activities. Xenobiotica 2017, 47, 324–331. [Google Scholar] [CrossRef]
- Hummelova, J.; Rondevaldova, J.; Balastikova, A.; Lapcik, O.; Kokoska, L. The relationship between structure and in vitro antibacterial activity of selected isoflavones and their metabolites with special focus on antistaphylococcal effect of demethyltexasin. Lett. Appl. Microbiol. 2015, 60, 242–247. [Google Scholar] [CrossRef]
- Lim, Y.J.; Jeong, H.Y.; Gil, C.S.; Kwon, S.J.; Na, J.K.; Lee, C.; Eom, S.H. Isoflavone accumulation and the metabolic gene expression in response to persistent UV-B irradiation in soybean sprouts. Food Chem. 2020, 303, 125376. [Google Scholar] [CrossRef]
- Hamayun, M.; Hussain, A.; Khan, S.A.; Irshad, M.; Khan, A.L.; Waqas, M.; Shahzad, R.; Iqbal, A.; Ullah, N.; Rehman, G.; et al. Kinetin modulates physio-hormonal attributes and isoflavone contents of soybean grown under salinity stress. Front. Plant Sci. 2015, 6, 377. [Google Scholar] [CrossRef]
- Meng, N.; Yu, B.J.; Guo, J.S. Ameliorative effects of inoculation with Bradyrhizobium japonicum on Glycine max and Glycine soja seedlings under salt stress. Plant Growth Regul. 2016, 80, 137–147. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant polyphenols as antioxidant and antibacterial agents for shelf-life extension of meat and meat products: Classification, structures, sources, and action mechanisms. Compr. Rev. Food Sci. F. 2017, 16, 1243–1268. [Google Scholar] [CrossRef]
- Mori-Yasumoto, K.; Hashimoto, Y.; Agatsuma, Y.; Fuchino, H.; Yasumoto, K.; Shirota, O.; Satake, M.; Sekita, S. Leishmanicidal phenolic compounds derived from Dalbergia cultrata. Nat. Prod. Res. 2021, 35, 4907–4915. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Huang, A.; Zhang, S.; Liu, R.; Ma, F. Identification of three Dalbergia species based on differences in extractive components. Molecules 2018, 23, 2163. [Google Scholar] [CrossRef]
- Lou, H.; Hu, L.; Lu, H.; Wei, T.; Chen, Q. Metabolic engineering of microbial cell factories for biosynthesis of flavonoids: A review. Molecules 2021, 26, 4522. [Google Scholar] [CrossRef]
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomas-Barberan, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef]
- Dutta, M.S.; Mahapatra, P.; Ghosh, A.; Basu, S. Estimation of the reducing power and electrochemical behavior of few flavonoids and polyhydroxybenzophenones substantiated by bond dissociation energy: A comparative analysis. Mol. Divers. 2022, 26, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Krysa, M.; Szymańska-Chargot, M.; Zdunek, A. FT-IR and FT-Raman fingerprints of flavonoids—A review. Food Chem. 2022, 393, 133430. [Google Scholar] [CrossRef]
- Pico, J.; Xu, K.; Guo, M.; Mohamedshah, Z.; Ferruzzi, M.G.; Martinez, M.M. Manufacturing the ultimate green banana flour: Impact of drying and extrusion on phenolic profile and starch bioaccessibility. Food Chem. 2019, 297, 124990. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, X.; Miao, Z.; Hassan, H.; Song, Y.; Fan, M. Screening for antioxidant and antibacterial activities of phenolics from Golden Delicious apple pomace. Chem. Cent. J. 2016, 10, 47. [Google Scholar] [CrossRef]
- Pal, D.; Verma, P. Flavonoids: A powerful and abundant source of antioxidants. Int. J. Pharm. Pharm. Sci. 2013, 5, 95–98. [Google Scholar]
- Ding, T.; Cao, K.; Fang, W.; Zhu, G.; Chen, C.; Wang, X.; Wang, L. Evaluation of phenolic components (anthocyanins, flavanols, phenolic acids, and flavonols) and their antioxidant properties of peach fruits. Sci. Hortic. 2020, 268, 109365. [Google Scholar] [CrossRef]
- Awad, M.A.; Hadi, F.H. Effect of aqueous extract of green tea on gene expression of CYP17, CYP11A, LH beta subunit and LHr genes in males wistar rats exposed to oxidative stress by streptozotocin. J. Madenat Alelem Univ. Coll. 2019, 11, 6–15. [Google Scholar]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Bartwal, A.; Mall, R.; Lohani, P.; Guru, S.K.; Arora, S. Role of secondary metabolites and brassinosteroids in plant defense against environmental stresses. J. Plant Growth Regul. 2013, 32, 216–232. [Google Scholar] [CrossRef]
- Yang, S.; Mi, L.; Wu, J.; Liao, X.; Xu, Z. Strategy for anthocyanins production: From efficient green extraction to novel microbial biosynthesis. Crit. Rev. Food Sci. Nutr. 2022, 1–16. [Google Scholar] [CrossRef]
- Chen, B.H.; Stephen Inbaraj, B. Nanoemulsion and nanoliposome based strategies for improving anthocyanin stability and bioavailability. Nutrients 2019, 11, 1052. [Google Scholar] [CrossRef]
- Qaisar, U.; Afzal, M.; Tayyeb, A. Commercial Application of Plant pigments. Int. J. Biotech Trend. Technol. 2019, 9, 18–22. [Google Scholar] [CrossRef]
- Fang, J. Classification of fruits based on anthocyanin types and relevance to their health effects. Nutrition 2015, 31, 1301–1306. [Google Scholar] [CrossRef]
- Veberic, R.; Slatnar, A.; Bizjak, J.; Stampar, F.; Mikulic-Petkovsek, M. Anthocyanin composition of different wild and cultivated berry species. LWT-Food Sci. Technol. 2015, 60, 509–517. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Slatnar, A.; Schmitzer, V.; Stampar, F.; Veberic, R.; Koron, D. Chemical profile of black currant fruit modified by different degree of infection with black currant leaf spot. Sci. Hortic. 2013, 150, 399–409. [Google Scholar] [CrossRef]
- Chaves-Silva, S.; Dos Santos, A.L.; Chalfun-Júnior, A.; Zhao, J.; Peres, L.E.; Benedito, V.A. Understanding the genetic regulation of anthocyanin biosynthesis in plants–tools for breeding purple varieties of fruits and vegetables. Phytochemistry 2018, 153, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Badhani, A.; Rawat, S.; Bhatt, I.D.; Rawal, R.S. Variation in Chemical Constituents and Antioxidant Activity in Y ellow H imalayan (R ubus ellipticus S mith) and Hill Raspberry (R ubus niveus T hunb.). J. Food Biochem. 2015, 39, 663–672. [Google Scholar] [CrossRef]
- Kim, A.N.; Lee, K.Y.; Jeong, E.J.; Cha, S.W.; Kim, B.G.; Kerr, W.L.; Choi, S.G. Effect of vacuum–grinding on the stability of anthocyanins, ascorbic acid, and oxidative enzyme activity of strawberry. Lwt 2021, 136, 110304. [Google Scholar] [CrossRef]
- Tohge, T.; de Souza, L.P.; Fernie, A.R. Current understanding of the pathways of flavonoid biosynthesis in model and crop plants. J. Exp. Bot. 2017, 68, 4013–4028. [Google Scholar] [CrossRef]
- Gho, Y.S.; Kim, S.; Jung, K.H. Phenylalanine ammonia-lyase family is closely associated with response to phosphate deficiency in rice. Genes Genom. 2020, 42, 67–76. [Google Scholar] [CrossRef]
- Kim, J.I.; Hidalgo-Shrestha, C.; Bonawitz, N.D.; Franke, R.B.; Chapple, C. Spatio-temporal control of phenylpropanoid biosynthesis by inducible complementation of a cinnamate 4-hydroxylase mutant. J. Exp. Bot. 2021, 72, 3061–3073. [Google Scholar] [CrossRef]
- Li, M.; Guo, L.; Wang, Y.; Li, Y.; Jiang, X.; Liu, Y.; Xie, D.; Gao, L.; Xia, T. Molecular and biochemical characterization of two 4-coumarate: CoA ligase genes in tea plant (Camellia sinensis). Plant Mol. Biol. 2022, 109, 579–593. [Google Scholar] [CrossRef]
- Sun, T.; Li, S.; Song, X.; Pei, G.; Diao, J.; Cui, J.; Shi, M.; Chen, L.; Zhang, W. Re-direction of carbon flux to key precursor malonyl-CoA via artificial small RNAs in photosynthetic Synechocystis sp. PCC 6803. Biotechnol. Biofuels 2018, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, H.; Yu, O. A plant malonyl-CoA synthetase enhances lipid content and polyketide yield in yeast cells. Appl. Microbiol. Biot. 2014, 98, 5435–5447. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, X.; Gong, Q.; Cao, J.; Shen, W.; Yin, X.; Grierson, D.; Zhang, B.; Xu, C.; Li, X. Three AP2/ERF family members modulate flavonoid synthesis by regulating type IV chalcone isomerase in citrus. Plant Biotechnol. J. 2021, 19, 671–688. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.X.; Han, X.J.; Wu, Y.F.; Lou, H.X. The function and catalysis of 2-oxoglutarate-dependent oxygenases involved in plant flavonoid biosynthesis. Int. J. Mol. Sci. 2014, 15, 1080–1095. [Google Scholar] [CrossRef]
- Dastmalchi, M.; Dhaubhadel, S. Soybean chalcone isomerase: Evolution of the fold, and the differential expression and localization of the gene family. Planta 2015, 241, 507–523. [Google Scholar] [CrossRef]
- Hacquard, S.; Wang, E.; Slater, H.; Martin, F. Impact of global change on the plant microbiome. Spec. Issue 2022, 234, 1907–1909. [Google Scholar] [CrossRef]
- Baba, S.A.; Ashraf, N. Functional characterization of flavonoid 3′-hydroxylase, CsF3′ H, from Crocus sativus L: Insights into substrate specificity and role in abiotic stress. Arch. Biochem. Biophys. 2019, 667, 70–78. [Google Scholar] [CrossRef]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, M. Flavonoids a bioactive compound from medicinal plants and its therapeutic applications. BioMed Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef]
- Sun, L.; Fan, X.; Zhang, Y.; Jiang, J.; Sun, H.; Liu, C. Transcriptome analysis of genes involved in anthocyanins biosynthesis and transport in berries of black and white spine grapes (Vitis davidii). Hereditas 2016, 153, 17. [Google Scholar] [CrossRef]
- Li, H.; Tian, J.; Yao, Y.Y.; Zhang, J.; Song, T.T.; Li, K.T.; Yao, Y.C. Identification of leucoanthocyanidin reductase and anthocyanidin reductase genes involved in proanthocyanidin biosynthesis in Malus crabapple plants. Plant Physiol. Biochem. 2019, 139, 141–151. [Google Scholar] [CrossRef]
- Ma, S.; Hu, R.; Ma, J.; Fan, J.; Wu, F.; Wang, Y.; Huang, L.; Feng, G.; Li, D.; Nei, G.; et al. Integrative analysis of the metabolome and transcriptome provides insights into the mechanisms of anthocyanins and proanthocyanidins biosynthesis in Trifolium repens. Ind. Crops Prod. 2022, 187, 115529. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham, U.H.; Patel, S.; Pan, X.; Naz, S.; Silva, A.S.; Saeed, F.; Suleria, H.A.R. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Dongbao, C.A.I.; Xusheng, L.I.; Jianxia, S.U.N.; Weibin, B.A.I. Advances in the Techniques of Stabilizing Anthocyanin. Future Food Sci. 2021, 1, 1. [Google Scholar]
- Guo, N.; Cheng, F.; Wu, J.; Liu, B.; Zheng, S.; Liang, J.; Wang, X. Anthocyanin biosynthetic genes in Brassica rapa. BMC Genom. 2014, 15, 426. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Zhao, Y.; Tao, R.; Yin, L.; Gao, L.; Strid, A.; Qian, M.; Li, J.; Li, Y.; Shen, J.; et al. Ethylene mediates the branching of the jasmonate-induced flavonoid biosynthesis pathway by suppressing anthocyanin biosynthesis in red Chinese pear fruits. Plant Biotechnol. J. 2020, 18, 1223–1240. [Google Scholar] [CrossRef]
- Xu, W.; Dubos, C.; Lepiniec, L. Transcriptional control of flavonoid biosynthesis by MYB–bHLH–WDR complexes. Trends Plant Sci. 2015, 20, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Fenn, M.A.; Giovannoni, J.J. Phytohormones in fruit development and maturation. Plant J. 2021, 105, 446–458. [Google Scholar] [CrossRef]
- Corso, M.; Perreau, F.; Mouille, G.; Lepiniec, L. Specialized phenolic compounds in seeds: Structures, functions, and regulations. Plant Sci. 2020, 296, 110471. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Y.; Zhai, Z.; Huang, T.; Zhao, D.; Peng, X.; Feng, C.; Xiao, Y.; Li, T. Transcriptomic analysis of light-dependent anthocyanin accumulation in bicolored cherry fruits. Plant Physiol. Biochem. 2018, 130, 663–677. [Google Scholar] [CrossRef]
- Sukumari Nath, V.; Kumar Mishra, A.; Kumar, A.; Matoušek, J.; Jakše, J. Revisiting the role of transcription factors in coordinating the defense response against citrus bark cracking viroid infection in commercial hop (Humulus Lupulus L.). Viruses 2019, 11, 419. [Google Scholar] [CrossRef]
- Li, K.; Xing, C.; Yao, Z.; Huang, X. Pbr MYB 21, a novel MYB protein of Pyrus betulaefolia, functions in drought tolerance and modulates polyamine levels by regulating arginine decarboxylase gene. Plant Biotechnol. J. 2017, 15, 1186–1203. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Xie, L.; Li, Y.; Liu, H.; Fu, X.; Chen, T.; Hassani, D.; Li, L.; Sun, X.; Tang, K. An R2R3-MYB transcription factor positively regulates the glandular secretory trichome initiation in Artemisia annua L. Front. Plant Sci. 2021, 12, 657156. [Google Scholar] [CrossRef] [PubMed]
- Czemmel, S.; Heppel, S.C.; Bogs, J. R2R3 MYB transcription factors: Key regulators of the flavonoid biosynthetic pathway in grapevine. Protoplasma 2012, 249, 109–118. [Google Scholar] [CrossRef]
- Anwar, M.; Yu, W.; Yao, H.; Zhou, P.; Allan, A.C.; Zeng, L. NtMYB3, an R2R3-MYB from narcissus, regulates flavonoid biosynthesis. Int. J. Mol. Sci. 2019, 20, 5456. [Google Scholar] [CrossRef]
- Xu, F.; Ning, Y.; Zhang, W.; Liao, Y.; Li, L.; Cheng, H.; Cheng, S. An R2R3-MYB transcription factor as a negative regulator of the flavonoid biosynthesis pathway in Ginkgo biloba. Funct. Integr. Genom. 2014, 14, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, B.; Zhong, Y.; Yao, L.; Cheng, L.; Wu, T. The soybean R2R3 MYB transcription factor GmMYB100 negatively regulates plant flavonoid biosynthesis. Plant Mol. Biol. 2015, 89, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Premathilake, A.T.; Ni, J.; Bai, S.; Tao, R.; Ahmad, M.; Teng, Y. R2R3-MYB transcription factor PpMYB17 positively regulates flavonoid biosynthesis in pear fruit. Planta 2020, 252, 59. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Linghu, B.; Hou, S.; Liu, R.; Wang, L.; Hao, Y.; Han, Y.; Zhou, M.; Liu, L.; Li, H. Tartary buckwheat FtMYB31 gene encoding an R2R3-MYB transcription factor enhances flavonoid accumulation in Tobacco. J. Plant Growth Regul. 2020, 39, 564–574. [Google Scholar] [CrossRef]
- Yuan, Y.; Qi, L.; Yang, J.; Wu, C.; Liu, Y.; Huang, L. A Scutellaria baicalensis R2R3-MYB gene, SbMYB8, regulates flavonoid biosynthesis and improves drought stress tolerance in transgenic tobacco. Plant Cell Tissue Organ Cult. 2015, 120, 961–972. [Google Scholar] [CrossRef]
- Cultrone, A.; Cotroneo, P.S.; Reforgiato Recupero, G. Cloning and molecular characterization of R2R3-MYB and bHLH-MYC transcription factors from Citrus sinensis. Tree Genet. Genomes 2010, 6, 101–112. [Google Scholar] [CrossRef]
- Qi, Y.; Zhou, L.; Han, L.; Zou, H.; Miao, K.; Wang, Y. PsbHLH1, a novel transcription factor involved in regulating anthocyanin biosynthesis in tree peony (Paeonia suffruticosa). Plant Physiol. Biochem. 2020, 154, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Transcriptional control of flavonoid biosynthesis: Fine-tuning of the MYB-bHLH-WD40 (MBW) complex. Plant Signal. Behav. 2014, 9, e27522. [Google Scholar] [CrossRef] [PubMed]
- Mano, H.; Ogasawara, F.; Sato, K.; Higo, H.; Minobe, Y. Isolation of a regulatory gene of anthocyanin biosynthesis in tuberous roots of purple-fleshed sweet potato. Plant Physiol. 2007, 143, 1252–1268. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Gao, L.; Wang, H.; Chen, X.; Wang, Y.; Yang, H.; Wei, C.; Wan, X.; Xia, T. The R2R3-MYB, bHLH, WD40, and related transcription factors in flavonoid biosynthesis. Funct. Integr. Genom. 2013, 13, 75–98. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Patterson, E.L.; Holalu, S.V.; Li, J.; Johnson, G.A.; Stanley, L.E.; Greenlee, A.B.; Peng, F.; Bradshaw, H.D., Jr.; Blinov, M.L.; et al. Two MYB proteins in a self-organizing activator-inhibitor system produce spotted pigmentation patterns. Curr. Biol. 2020, 30, 802–814. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, T.; Yu, Y.; Gou, L.; Yang, J.; Xu, J.; Pi, E. Regulatory mechanisms of bHLH transcription factors in plant adaptive responses to various abiotic stresses. Front. Plant Sci. 2021, 12, 677611. [Google Scholar] [CrossRef]
- Li, C.F.; Zhu, Y.; Yu, Y.; Zhao, Q.Y.; Wang, S.J.; Wang, X.C.; Yao, M.Z.; Luo, D.; Li, X.; Chen, L. Global transcriptome and gene regulation network for secondary metabolite biosynthesis of tea plant (Camellia sinensis). BMC Genom. 2015, 16, 560. [Google Scholar] [CrossRef]
- Qiu, Z.; Wang, H.; Li, D.; Yu, B.; Hui, Q.; Yan, S.; Huang, Z.; Cui, X.; Cao, B. Identification of candidate HY5-dependent and-independent regulators of anthocyanin biosynthesis in tomato. Plant Cell Physiol. 2019, 60, 643–656. [Google Scholar] [CrossRef]
- Outchkourov, N.S.; Karlova, R.; Hölscher, M.; Schrama, X.; Blilou, I.; Jongedijk, E.; Simon, C.D.; van Dijk, A.D.J.; Bosch, D.; Hall, R.D. Transcription factor-mediated control of anthocyanin biosynthesis in vegetative tissues. Plant Physiol. 2018, 176, 1862–1878. [Google Scholar] [CrossRef]
- Malacarne, G.; Coller, E.; Czemmel, S.; Vrhovsek, U.; Engelen, K.; Goremykin, V.; Bogs, J.; Moser, C. The grapevine VvibZIPC22 transcription factor is involved in the regulation of flavonoid biosynthesis. J. Exp. Bot. 2016, 67, 3509–3522. [Google Scholar] [CrossRef]
- Singh, D.; Singh, H.; Singh, N.; Dwivedi, S.; Trivedi, T.P. Tobacco HY5, NtHY5, positively regulates flavonoid biosynthesis and enhances salt stress tolerance. bioRxiv 2022, 1. [Google Scholar] [CrossRef]
- Morishita, T.; Kojima, Y.; Maruta, T.; Nishizawa-Yokoi, A.; Yabuta, Y.; Shigeoka, S. Arabidopsis NAC transcription factor, ANAC078, regulates flavonoid biosynthesis under high-light. Plant Cell Physiol. 2009, 50, 2210–2222. [Google Scholar] [CrossRef] [PubMed]
- An, X.H.; Tian, Y.; Chen, K.Q.; Liu, X.J.; Liu, D.D.; Xie, X.B.; Cheng, C.G.; Cong, P.H.; Hao, Y.J. MdMYB9 and MdMYB11 are involved in the regulation of the JA-induced biosynthesis of anthocyanin and proanthocyanidin in apples. Plant Cell Physiol. 2015, 56, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Skirycz, A.; Jozefczuk, S.; Stobiecki, M.; Muth, D.; Zanor, M.I.; Witt, I.; Mueller-Roeber, B. Transcription factor AtDOF4; 2 affects phenylpropanoid metabolism in Arabidopsis thaliana. New Phytol. 2007, 175, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liu, W.; Zhang, T.; Jiang, S.; Xu, H.; Wang, Y.; Zhang, Z.; Wang, C.; Chen, X. Transcriptomic analysis of red-fleshed apples reveals the novel role of MdWRKY11 in flavonoid and anthocyanin biosynthesis. J Agric. Food Chem. 2018, 66, 7076–7086. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Cao, M.; Pang, S.; Li, W.; Kato-Noguchi, H.; Jin, B. Identification and characterization of long non-coding RNAs regulating flavonoid biosynthesis in Ginkgo biloba leaves. Ind. Crop. Prod. 2020, 158, 112980. [Google Scholar] [CrossRef]
- Liu, X.; Tang, N.; Xu, F.; Chen, Z.; Zhang, X.; Ye, J.; Liao, Y.; Zhang, W.; Kim, S.; Wu, P.; et al. SMRT and Illumina RNA sequencing reveal the complexity of terpenoid biosynthesis in Zanthoxylum armatum. Tree Physiol. 2022, 42, 664–683. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, X.; Traore, S.M.; Xin, Z.; Yin, D. Genome-wide identification and analysis of long noncoding RNAs (lncRNAs) during seed development in peanut (Arachis hypogaea L.). BMC Plant Biol. 2020, 20, 192. [Google Scholar] [CrossRef]
- Sharma, A.; Badola, P.K.; Bhatia, C.; Sharma, D.; Trivedi, P.K. Primary transcript of miR858 encodes regulatory peptide and controls flavonoid biosynthesis and development in Arabidopsis. Nat. Plants 2020, 6, 1262–1274. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- ul Haq, S.; Khan, A.; Ali, M.; Khattak, A.M.; Gai, W.X.; Zhang, H.X.; Wei, M.; Gong, Z.H. Heat shock proteins: Dynamic biomolecules to counter plant biotic and abiotic stresses. Int. J. Mol. Sci. 2019, 20, 5321. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, R.; Huang, Y.; Gebrechristos, S.; Mikolajczyk, B.; Brown, H.; Prasad, R.; Varma, A.; Kathryn, E.; Bushley. Transcriptional responses of soybean roots to colonization with the root endophytic fungus Piriformospora indica reveals altered phenylpropanoid and secondary metabolism. Sci. Rep. 2018, 8, 10227. [Google Scholar] [CrossRef] [PubMed]
- Rodziewicz, P.; Swarcewicz, B.; Chmielewska, K.; Wojakowska, A.; Stobiecki, M. Influence of abiotic stresses on plant proteome and metabolome changes. Acta Physiol. Plant. 2014, 36, 1–19. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Role of plant metabolites in abiotic stress tolerance under changing climatic conditions with special reference to secondary compounds. Clim. Change Plant Abiotic Stress Toler. 2013, 26, 705–726. [Google Scholar]
- Mareri, L.; Parrotta, L.; Cai, G. Environmental Stress and Plants. Int. J. Mol. Sci. 2022, 23, 5416. [Google Scholar] [CrossRef]
- Ahmed, E.; Arshad, M.; Khan, M.Z.; Amjad, M.S.; Ahmad, S. Secondary metabolites and their multidimensional prospective in plant life. J. Pharm. Phytochem. 2017, 6, 205–214. [Google Scholar]
- Gupta, A.; Bano, A.; Rai, S.; Dubey, P.; Khan, F.; Pathak, N.; Sharma, S. Plant Growth Promoting Rhizobacteria (PGPR): A sustainable agriculture to rescue the vegetation from the effect of biotic stress: A Review. Lett. Appl. NanoBiosci. 2021, 10, 2459–2465. [Google Scholar]
- Singh, V.K.; Upadhyay, R.S. The hypersensitive response: A case of cell death induction in plants. Int. J. Eng. Res. Technol. 2013, 2, 1828–1832. [Google Scholar]
- Thakker, J.N.; Patel, S.; Dhandhukia, P.C. Induction of defense-related enzymes in banana plants: Effect of live and dead pathogenic strain of Fusarium oxysporum f. sp. cubense. Int. Schol. Res. Not. 2013, 2013, 601303. [Google Scholar] [CrossRef]
- Sindhu, S.S.; Sehrawat, A.; Sharma, R.; Dahiya, A. Biopesticides: Use of rhizosphere bacteria for biological control of plant pathogens. Def. Sci. J. 2016, 1, 135–148. [Google Scholar] [CrossRef]
- Molinari, S. Natural genetic and induced plant resistance, as a control strategy to plant-parasitic nematodes alternative to pesticides. Plant Cell Rep. 2011, 30, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Tuncsoy, B. Nematicidal activity of silver nanomaterials against plant-parasitic nematodes. In Silver Nanomaterials for Agri-Food Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 527–548. [Google Scholar]
- Oka, Y.; Koltai, H.; Bar-Eyal, M.; Mor, M.; Sharon, E.; Chet, I.; Spiegel, Y. New strategies for the control of plant-parasitic nematodes. Pest Manag. Sci. 2000, 56, 983–988. [Google Scholar] [CrossRef]
- Lindell, K.J.; Riley, I.T.; Davies, K.A.; Oldach, K.H. Characterization of resistance to Pratylenus thorni (Nematoda) in wheat (Triticum aestivum): Attraction, tension, utility, and reproduction. Phytopathology 2014, 104, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Desmedt, W.; Mangelinckx, S.; Kyndt, T.; Vanholme, B. A phytochemical perspective on plant defense against nematodes. Front Plant Sci. 2020, 11, 602079. [Google Scholar] [CrossRef]
- Makhwedzhana, M.M. Nematode Resistance and Resistance Mechanism in Sweet Potato Cultivars ‘Bophelo’, ‘Bosbok’ and Mvuvhelo’ to Meloidogyne incognita. Master’s Thesis, University of Limpopo, Mankweng, South Africa, 2018. [Google Scholar]
- Chin, S.; Behm, C.A.; Mathesius, U. Functions of flavonoids in plant–nematode interactions. Plants 2018, 7, 85. [Google Scholar] [CrossRef]
- Lozano, J.; Smant, G. Survival of plant-parasitic nematodes inside the host. Mol. Physiol. Basis Nematode Surviv. 2011, 13, 697–701. [Google Scholar]
- Al-Muwayhi, M.A.R. Biotic stress as a defense mechanism in soybean (Glycine max L.) toward microbial pathogen: Biochemical and physiological pathways study. Life Sci. J. 2020, 17, 24–36. [Google Scholar]
- Hammerschmidt, R. How glyphosate affects plant disease development: It is more than enhanced susceptibility. Pest Manag. Sci. 2018, 74, 1054–1063. [Google Scholar] [CrossRef]
- Walters, D. Plant defense: Warding off attack by pathogens, herbivores and parasitic plants. Q. Rev. Biol. 2011, 86, 356–357. [Google Scholar]
- Smant, G.; Jones, J. Suppression of plant defences by nematodes. Genomics and Molecular Genetics of Plant-Nematode Interactions; Springer: Dordrecht, The Netherlands, 2011; pp. 273–286. [Google Scholar]
- González-Fernández, R.; Prats, E.; Jorrín-Novo, J.V. Proteomics of plant pathogenic fungi. J. Biomed. Biotechnol. 2010, 2010, 932527. [Google Scholar] [CrossRef]
- Suprapta, D.N. Potential of microbial antagonists as biocontrol agents against plant fungal pathogens. J. ISSAAS 2012, 18, 1–8. [Google Scholar]
- Parmar, N.; Singh, K.H.; Sharma, D.; Singh, L.; Kumar, P.; Nanjundan, J.; Khan, Y.J.; Chauhan, D.K.; Thakur, A.K. Genetic engineering strategies for biotic and abiotic stress tolerance and quality enhancement in horticultural crops: A comprehensive review. 3 Biotech. 2017, 7, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Saddique, M.; Kamran, M.; Shahbaz, M. Differential responses of plants to biotic stress and the role of metabolites. In Plant Metabolites and Regulation under Environmental Stress; Academic Press: Cambridge, MA, USA, 2018; pp. 69–87. [Google Scholar]
- Meena, M.; Yadav, G.; Sonigra, P.; Nagda, A.; Mehta, T.; Swapnil, P.; Harish; Marwal, A. Role of elicitors to initiate the induction of systemic resistance in plants to biotic stress. Plant Stress 2022, 5, 100103. [Google Scholar] [CrossRef]
- Alkahtani, M.; Omer, S.A.; El-Naggar, M.A.; Abdelkareem, E.M.; Mahmoud, M.A. Pathogenesis-related protein and phytoalexin induction against cucumber powdery mildew by elicitors. Int. J. Plant Pathol. 2011, 2, 63–71. [Google Scholar] [CrossRef]
- Farahat, G. Potential Impacts of Copper Sulfate and Sodium Silicate Salts of Maize Late Wilt Disease and Synthase of Anti-defense Compounds. Environ. Biodivers. Soil Secur. 2019, 3, 269–282. [Google Scholar]
- Ortuño, A.; Díaz, L.; Alvarez, N.; Porras, I.; Garcia-Lidon, A.; Del Rio, J.A. Comparative study of flavonoid and scoparone accumulation in different Citrus species and their susceptibility to Penicillium digitatum. Food Chem. 2011, 125, 232–239. [Google Scholar] [CrossRef]
- François, G.A.; de Moraes Pontes, J.G.; Pereira, A.K.; Fill, T.P. Exploring the Citrus Sour Rot pathogen: Biochemical aspects, virulence factors, and strategies for disease management-a review. Fungal Biol. Rev. 2022, 41, 70–83. [Google Scholar] [CrossRef]
- Del Río, J.A.; Gómez, P.; Baidez, A.G.; Arcas, M.C.; Botía, J.M.; Ortuño, A. Changes in the levels of polymethoxyflavones and flavanones as part of the defense mechanism of Citrussinensis (cv. Valencia Late) fruits against Phytophthora citrophthora. J. Agric. Food Chem. 2004, 52, 1913–1917. [Google Scholar] [CrossRef]
- Ortuno, A.; Arcas, M.C.; Botia, J.M.; Fuster, M.D.; Del Río, J.A. Increasing resistance against Phytophthora citrophthora in tangelo Nova fruits by modulating polymethoxyflavones levels. J. Agric. Food Chem. 2002, 50, 2836–2839. [Google Scholar] [CrossRef]
- Boué, S.M.; Carter, C.H.; Ehrlich, K.C.; Cleveland, T.E. Induction of the soybean phytoalexins coumestrol and glyceollin by Aspergillus. J. Agric. Food Chem. 2000, 48, 2167–2172. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.Q.; Ren, L.C. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Tiku, A.R. Antimicrobial compounds (phytoanticipins and phytoalexins) and their role in plant defense. In Co-Evolution of Secondary Metabolites; Springer: Cham, Switzerland, 2020; pp. 845–868. [Google Scholar]
- Sharma, I.; Thakur, A.; Sharma, A.; Singh, N.; Kumar, R.; Sharma, A. Phytoalexins: Implications in Plant Defense and Human Health. In Plant Secondary Metabolites; Springer Nature Singapore: Singapore, 2022; pp. 329–353. [Google Scholar]
- Gnanamanickam, S.S.; Smith, D.A. Selective toxicity of isoflavonoid phytoalexins to gram-positive bacteria. Phytopathology 1980, 70, 894. [Google Scholar] [CrossRef]
- Lyon, F.M.; Wood, R.K.S. Production of phaseollin, coumestrol and related compounds in bean leaves inoculated with Pseudomonas spp. Physiol. Plant Pathol. 1975, 6, 117–124. [Google Scholar] [CrossRef]
- Li, P.; Ruan, Z.; Fei, Z.; Yan, J.; Tang, G. Integrated transcriptome and metabolome analysis revealed that flavonoid biosynthesis may dominate the resistance of Zanthoxylum bungeanum against stem canker. J. Agric. Food Chem. 2021, 69, 6360–6378. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Tanaka, H.; Tani, N.; Nagayama, M.; Yamaguchi, R. Different antibacterial actions of isoflavones isolated from Erythrina poeppigiana against methicillin-resistant Staphylococcus aureus. Lett. Appl. Microbiol. 2006, 43, 243–248. [Google Scholar] [CrossRef] [PubMed]
- de Barros, K.M.A.; Sardi, J.C.O.; Maria-Neto, S.; Macedo, A.J.; Ramalho, S.R. A new Kunitz trypsin inhibitor from Erythrina poeppigiana exhibits antimicrobial and antibiofilm properties against bacteria. Biomed. Pharmacother. 2021, 144, 112198. [Google Scholar] [CrossRef]
- Leney, A.C.; Heck, A.J.R. Native mass spectrometry: What is in the name? J. Am. Soc. Mass Spectr. 2016, 28, 5–13. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Medi. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Saewan, N.; Jimtaisong, A. Photoprotection of natural flavonoids. J. Appl. Pharm. Sci. 2013, 3, 129–141. [Google Scholar]
- Ferreyra, M.L.F.; Serra, P.; Casati, P. Recent advances on the roles of flavonoids as plant protective molecules after UV and high light exposure. Physiol. Plant. 2021, 173, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants and developmental regulators: Relative significance in plants and humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef]
- Ferdinando, M.D.; Brunetti, C.; Fini, A.; Brunetti, C.; Di Ferdinando, M. Flavonoids as antioxidants in plants under abiotic stresses. In Abiotic Stress Responses Plants; Springer: New York, NY, USA, 2012; pp. 159–179. [Google Scholar]
- Casati, P.; Walbot, V. Differential accumulation of maysin and rhamnosylisoorientin in leaves of high-altitude landraces of maize after UV-B exposure. Plant Cell Environ. 2005, 28, 788–799. [Google Scholar] [CrossRef]
- Casasa, M.I.; Falcone-Ferreyrac, M.L.; Nan, J.; Mejía-Guerra, M.K.; Rodríguez, E.; Wilson, T.; Engelmeier, J.; Casati, P.; Grotewold, E. Identification and characterization of maize salmon silks genes involved in 1 insecticidal maysin biosynthesis. Plant Cell 2016, 28, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- Righini, S.; Rodriguez, E.J.; Berosich, C.; Grotewold, E.; Casati, P.; Ferreyra, M.L.F. Apigenin produced by maize flavone synthase I and II protects plants against UV-B-induced damage. Plant Cell Environ. 2019, 42, 495–508. [Google Scholar] [CrossRef]
- Honda, C.; Moriya, S. Anthocyanin biosynthesis in apple fruit. Horticult. J. 2018, 87, 305–314. [Google Scholar] [CrossRef]
- Tohge, T.; de Souza, L.P.; Fernie, A.R. On the natural diversity of phenylacylated-flavonoid and their in planta function under conditions of stress. Phytochem. Rev. 2018, 17, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Benavente-Garcıa, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Galvez-Valdivesio, G.; Mullineaux, P. The role of reactive oxygen species in signalling from chloroplasts to the nucleus. Physiol. Plant. 2010, 138, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Pourcel, L.; Routaboul, J.M.; Cheynier, V.; Lepiniec, L.; Debeaujon, I. Debeaujon, Flavonoid oxidation in plants: From biochemical properties to physiological functions. Trends Plant Sci. 2006, 12, 29–36. [Google Scholar] [CrossRef]
- Laoué, J.; Fernandez, C.; Ormeño, E. Plant flavonoids in mediterranean species: A focus on flavonols as protective metabolites under climate stress. Plants 2022, 11, 172. [Google Scholar] [CrossRef]
- Li, W.; Tan, L.; Zou, Y.; Tan, X.Q.; Huang, J.C.; Chen, W.; Tang, Q. The effects of ultraviolet A/B treatments on anthocyanin accumulation and gene expression in dark-purple tea cultivar ‘Ziyan’(Camellia sinensis). Molecules 2020, 25, 354. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef] [PubMed]
- Chinnusamy, V.; Zhu, J.K.; Sunkar, R. Gene regulation during cold stress acclimation in plants. In Plant Stress Tolerance: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2010; pp. 39–55. [Google Scholar]
- Song, X.; Diao, J.; Ji, J.; Wang, G.; Guan, C.; Jin, C.; Wang, Y. Molecular cloning and identification of a flavanone 3-hydroxylase gene from Lycium chinense, and its overexpression enhances drought stress in tobacco. Plant Physiol. Biochem. 2016, 98, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, D.; Yang, Z.; Zeng, Q.; Luo, Y.; He, N. Flavones produced by mulberry flavone synthase type I constitute a defense line against the ultraviolet-B stress. Plants 2020, 9, 215. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, W.J.; Vu, T.T.; Jeong, C.Y.; Hong, S.W.; Lee, H. High accumulation of anthocyanins via the ectopic expression of AtDFR confers significant salt stress tolerance in Brassica napus L. Plant Cell Rep. 2017, 36, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Sun, D.; Wang, C.; Li, Y.; Guo, T. Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol. Biochem. 2014, 80, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Meraj, T.A.; Fu, J.; Raza, M.A.; Zhu, C.; Shen, Q.; Xu, D.; Wang, Q. Transcriptional factors regulate plant stress responses through mediating secondary metabolism. Genes 2020, 11, 346. [Google Scholar] [CrossRef]
- Li, H.; Yue, H.; Xie, J.; Bu, J.; Li, L.; Xin, X.; Zhao, Y.; Zhang, H.; Yang, L.; Wang, J.; et al. Transcriptomic profiling of the high-vigour maize (Zea mays L.) hybrid variety response to cold and drought stresses during seed germination. Sci. Rep. 2021, 11, 19345. [Google Scholar] [CrossRef]
- Song, Z.; Yang, Q.; Dong, B.; Li, N.; Wang, M.; Du, T.; Liu, N.; Niu, L.; Jin, H.; Meng, D. Melatonin enhances stress tolerance in pigeon pea by promoting flavonoid enrichment, particularly luteolin in response to salt stress. J. Exp. Bot. 2022, 73, 5992–6008. [Google Scholar] [CrossRef]
- Fang, H.; Qi, X.; Li, Y.; Yu, X.; Liu, X. De novo transcriptomic analysis of light-induced flavonoid pathway, transcription factors in the flower buds of Lonicera japonica. Trees 2020, 34, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Babitha, K.C.; Vemanna, R.S.; Nataraja, K.N.; Udayakumar, M. Overexpression of EcbHLH57 transcription factor from Eleusine coracana L. in tobacco confers tolerance to salt, oxidative and drought stress. PLoS ONE 2015, 10, e0137098. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Guo, S.; Zhao, Y.; Chen, D.; Chong, K.; Xu, Y. Overexpression of a homopeptide repeat-containing bHLH protein gene (OrbHLH001) from Dongxiang Wild Rice confers freezing and salt tolerance in transgenic Arabidopsis. Plant Cell Rep. 2010, 29, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Salehi-Lisar, S.Y.; Bakhshayeshan-Agdam, H. Drought stress in plants: Causes, consequences, and tolerance. In Drought Stress Tolerance in Plants; Springer: Cham, Switzerland, 2016; pp. 1–16. [Google Scholar]
- Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Aloui, A.; Dumas-Gaudot, E.; Daher, Z.; van Tuinen, D.; Aschi-Smit, S.; Morandi, D. Influence of arbuscular mycorrhizal colonisation on cadmium induced Medicago truncatula root isoflavonoid accumulation. Plant Physiol. Biochem. 2012, 60, 233–239. [Google Scholar] [CrossRef]
- Shin, D.H.; Choi, M.G.; Kang, C.S.; Par, C.S.; Choi, S.B.; Park, Y.I. Overexpressing the wheat dihydroflavonol 4-reductase gene TaDFR increases anthocyanin accumulation in an Arabidopsis dfr mutant. Genes Genom. 2016, 38, 333–340. [Google Scholar] [CrossRef]
- Li, C.; Liu, S.; Yao, X.; Wang, J.; Wang, T.; Zhang, Z.; Zhang, P.; Chen, K. PnF3H, a flavanone 3-hydroxylase from the Antarctic moss Pohlia nutans, confers tolerance to salt stress and ABA treatment in transgenic Arabidopsis. Plant Growth Regul. 2017, 83, 489–500. [Google Scholar] [CrossRef]
- Jeon, J.; Kim, J.K.; Wu, Q.; Park, S.U. Effects of cold stress on transcripts and metabolites in tartary buckwheat (Fagopyrum tataricum). Environ. Exp. Bot. 2018, 155, 488–496. [Google Scholar] [CrossRef]
- Song, Y.; Feng, J.; Liu, D.; Long, C. Different Phenylalanine Pathway Responses to Cold Stress Based on Metabolomics and Transcriptomics in Tartary Buckwheat Landraces. J. Agric. Food Chem. 2022, 70, 687–698. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Aslam, M.T.; Alhammad, B.A.; Hassan, M.U.; Maqbool, R.; Chattha, M.U.; Khan, I.; Gitari, H.I.; Uslu, O.S.; Roy, R. Salinity stress in wheat: Effects, mechanisms and management strategies. Phyton 2022, 91, 667. [Google Scholar] [CrossRef]
- Hu, J.; Fang, H.; Wang, J.; Yue, X.; Chen, X. Ultraviolet B-induced MdWRKY72 expression promotes anthocyanin synthesis in apple. Plant Sci. 2020, 292, 110377. [Google Scholar] [CrossRef]
- Hussain, S.S.; Kayani, M.A.; Amjad, M. Transcription factors as tools to engineer enhanced drought stress tolerance in plants. Biotechnol. Progr. 2011, 27, 297–306. [Google Scholar] [CrossRef]
- Wang, C.T.; Ru, J.N.; Liu, Y.W.; Yang, J.F.; Li, M.; Xu, Z.S.; Fu, J.D. The maize WRKY transcription factor ZmWRKY40 confers drought resistance in transgenic Arabidopsis. Int. J. Mol. Sci. 2018, 19, 2580. [Google Scholar] [CrossRef]
- Li, X.D.; Zhuang, K.Y.; Liu, Z.M.; Yang, D.Y.; Ma, N.N.; Meng, Q.W. Overexpression of a novel NAC-type tomato transcription factor, SlNAM1, enhances the chilling stress tolerance of transgenic tobacco. J. Plant Physiol. 2016, 204, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhu, H.; Chen, D.; Li, Z.; Peng, R.; Yao, Q. A grape bHLH transcription factor gene, VvbHLH1, increases the accumulation of flavonoids and enhances salt and drought tolerance in transgenic Arabidopsis thaliana. Plant Cell Tissue Organ Cul. 2016, 125, 387–398. [Google Scholar] [CrossRef]
- Jiao, X.; Teng, Y.; Zhan, Y.; Wu, J.; Lin, X. Soil heavy metal pollution and risk assessment in Shenyang industrial district, Northeast China. PLoS ONE 2015, 10, e0127736. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Chauhan, A.S.; Yusuf, M.A.; Sanyal, I.; Chauhan, P. Transcriptome sequencing of chickpea (Cicer arietinum L.) genotypes for identification of drought-responsive genes under drought stress condition. Plant Mol. Biol. Rep. 2019, 37, 186–203. [Google Scholar] [CrossRef]
- Lijuan, C.; Huiming, G.; Yi, L.; Hongmei, C. Chalcone synthase EaCHS1 from Eupatorium adenophorum functions in salt stress tolerance in tobacco. Plant Cell Rep. 2015, 34, 885–894. [Google Scholar] [CrossRef]
- Shi, H.; Chan, Z. The cysteine2/histidine2-type transcription factor ZINC FINGER of ARABIDOPSIS THALIANA 6-activated C-REPEAT-BINDING FACTOR pathway is essential for melatonin-mediated freezing stress resistance in Arabidopsis. J. Pineal Res. 2014, 57, 185–191. [Google Scholar] [CrossRef]
- Thakur, P.; Kumar, S.; Malik, J.A.; Berger, J.D.; Nayyar, H. Cold stress effects on reproductive development in grain crops: An overview. Environ. Exp. Bot. 2010, 67, 429–443. [Google Scholar] [CrossRef]
- Bhattacharya, A. Effect of low-temperature stress on germination, growth, and phenology of plants: A review. In Physiological Processes in Plants Under Low Temperature Stress; Springer: Singapore, 2022; pp. 1–106. [Google Scholar]
- Liu, X.; Zhou, Y.; Xiao, J.; Bao, F. Effects of chilling on the structure, function and development of chloroplasts. Front. Plant Sci. 2018, 9, 1715. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Bai, G.; Wang, S.; Yang, L.Y.; Yang, F.; Wang, Y.; Zhu, J.K.; Hua, J. Chloroplast RNA-binding protein RBD1 promotes chilling tolerance through 23S rRNA processing in Arabidopsis. PLoS Genet. 2016, 12, e1006027. [Google Scholar] [CrossRef] [PubMed]
- Gan, P.; Liu, F.; Li, R.; Wang, S.; Luo, J.; Pearce, R.S. Plant freezing and damage. Ann. Bot. 2001, 87, 417–424. [Google Scholar]
- Puyaubert, J.; Baudouin, E. New clues for a cold case: Nitric oxide response to low temperature. Plant Cell Environ. 2014, 37, 2623–2630. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Shi, Y.; Yang, S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019, 222, 1690–1704. [Google Scholar] [CrossRef]
- Mayr, S.; Ameglio, T. Freezing stress in tree xylem. Prog. Bot. 2016, 77, 381–414. [Google Scholar]
- Schulz, E.; Tohge, T.; Zuther, E.; Fernie, A.R.; Hincha, D.K. Flavonoids are determinants of freezing tolerance and cold acclimation in Arabidopsis thaliana. Sci. Rep. 2016, 6, 34027. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Hu, H.; Yang, J.; Cui, J.; Wei, G.; Xu, J. Transcriptome and metabolome changes in Chinese cedar during cold acclimation reveal the roles of flavonoids in needle discoloration and cold resistance. Tree Physiol. 2022, 9, 1858–1875. [Google Scholar] [CrossRef]
- Li, S.J.; Bai, Y.C.; Li, C.L.; Yao, H.P.; Chen, H.; Zhao, H.X.; Wu, Q. Anthocyanins accumulate in tartary buckwheat (Fagopyrum tataricum) sprout in response to cold stress. Acta Physiol. Plant. 2015, 37, 159. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.G. Osmotic adjustment and plant adaptation to environmental changes related to drought and salinity. Environ. Rev. 2010, 18, 309–319. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.; Tubeileh, A.; Akhtar, J.; Larbi, A.; Khan, M.A. Productivity enhancement of salt-affected environments through crop diversification. Land Degrad. Dev. 2008, 19, 429–453. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Safdar, H.; Amin, A.; Shafiq, Y.; Ali, A. A review: Impact of salinity on plant growth. Nat. Sci. 2019, 17, 34–40. [Google Scholar]
- Yan, J.; Wang, B.; Jiang, Y.; Cheng, L.; Wu, T. GmFNSII-controlled soybean flavone metabolism responds to abiotic stresses and regulates plant salt tolerance. Plant Cell Physiol. 2014, 55, 74–86. [Google Scholar] [CrossRef]
- Ahmad, H.; Maher, M.; Abdel-Salam, E.M.; Li, Y.; Yang, C.; Elsafty, N.; Ewas, M.; Nishawy, E.; Luo, J. Integrated de novo analysis of transcriptional and metabolic variations in salt-treated Solenostemma argel desert plants. Front. Plant Sci. 2021, 12, 744699. [Google Scholar] [CrossRef]
- Wang, X.; Dai, W.W.; Liu, C.; Yangchen, Y.C.; Gao, R.F.; Chen, Y.Y.; Yan, H. Evaluation of physiological coping strategies and quality substances in purple sweetpotato under different salinity levels. Genes 2022, 13, 1350. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; An, J.; Liu, Z.; Chen, J.; Yu, B. Salt stress induced soybean GmIFS1 expression and isoflavone accumulation and salt tolerance in transgenic soybean cotyledon hairy roots and tobacco. Plant Cell Tissue Organ Cul. 2017, 128, 469–477. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. [Google Scholar]
- Nabi, R.B.S.; Tayade, R.; Hussain, A.; Kulkarni, K.P.; Imran, Q.M.; Mun, B.G.; Yun, B.W. Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environ. Exp. Bot. 2019, 161, 120–133. [Google Scholar] [CrossRef]
- Chaudhry, S.; Sidhu, G.P.S. Climate change regulated abiotic stress mechanisms in plants: A comprehensive review. Plant Cell Rep. 2021, 41, 1–31. [Google Scholar] [CrossRef]
- Martignago, D.; Rico-Medina, A.; Blasco-Escámez, D.; Fontanet-Manzaneque, J.B.; Cao-Delgado, A.I. Drought resistance by engineering plant tissue-specific responses. Front. Plant Sci. 2020, 10, 1676. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Fahad, S.; Iqbal, M.; Alamzeb, M.; Ahmad, A.; Anwar, S.; Khan, A.A.; Arif, M.; Saeed, M.; Song, M. Special adaptive features of plant species in response to drought. In Salt and Drought Stress Tolerance in Plants; Springer: Cham, Switzerland, 2020; pp. 77–118. [Google Scholar]
- Amnan, M.A.M.; Aizat, W.M.; Khaidizar, F.D.; Tan, B.C. Drought stress induces morpho-physiological and proteome changes of Pandanus amaryllifolius. Plants 2022, 11, 221. [Google Scholar] [CrossRef]
- Salsinha, Y.C.F.; Indradewa, D.; Purwestri, Y.A.; Rachmawati, D. Leaf physiological and anatomical characters contribute to drought tolerance of Nusa Tenggara Timur local rice cultivars. J. Crops Sci. Biotechnol. 2021, 24, 337–348. [Google Scholar] [CrossRef]
- Lv, Z.; Zhang, C.; Shao, C.; Liu, B.; Shen, C. Research progress on the response of tea catechins to drought stress. J. Sci. Food Agric. 2021, 101, 5305–5313. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa-Gómez, J.; Martín-Hernández, C.S.; Heredia, J.B.; León-Félix, J.; Osuna-Enciso, T.; Muy-Rangel, M.D. Anthocyanin induction by drought stress in the calyx of roselle cultivars. Molecules 2020, 25, 1555. [Google Scholar] [CrossRef] [PubMed]
- Gholamreza, A.; Shokrpour, M.; Karami, L.; Salami, S.A. Prolonged water deficit stress and methyl jasmonate-mediated changes in metabolite profile, flavonoid concentrations and antioxidant activity in peppermint (Mentha piperita L.). Not. Bot. Horti Agrobo. 2019, 47, 70–80. [Google Scholar]
- Mechri, B.; Tekaya, M.; Hammami, M.; Chehab, H. Effects of drought stress on phenolic accumulation in greenhouse-grown olive trees (Olea europaea). Biochem. Syst. Ecol. 2020, 92, 104112. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Liu, G.; Tang, Y.; Lv, J. The spike plays important roles in the drought tolerance as compared to the flag leaf through the phenylpropanoid pathway in wheat. Plant Physiol. Biochem. 2020, 152, 100–111. [Google Scholar] [CrossRef]
- Kalaivanan, D.; Ganeshamurthy, A.N. Mechanisms of heavy metal toxicity in plants. In Abiotic Stress Physiology of Horticultural Crops; Springer: New Delhi, India, 2016; pp. 85–102. [Google Scholar]
- Ghori, N.H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Asati, A.; Pichhode, M.; Nikhil, K. Effect of heavy metals on plants: An overview. Int. J. Appl. Innov. Eng. Manag. 2016, 5, 56–66. [Google Scholar]
- Khan, M.; Aymen, U.; Mir, A.H.; Tiwari, A.; Prasad, S.M.; Singh, J.; Ramamurthy, P.C.; Singh, R.; Singh, S.; Parihar, P. Understanding Heavy Metal Stress in Plants Through Mineral Nutrients. In Heavy Metals in Plants Physiological to Molecular Approach; CRC Press: Boca Raton, FL, USA, 2022; pp. 281–309. [Google Scholar]
- Bhaduri, A.M.; Fulekar, M.H. Antioxidant enzyme responses of plants to heavy metal stress. Rev. Environ. Sci. Bio/Technol. 2012, 11, 55–69. [Google Scholar] [CrossRef]
- Mahmud, J.A.; Bhuyan, M.H.M.; Anee, T.I.; Nahar, K.; Fujita, M.; Hasanuzzaman, M. Reactive oxygen species metabolism and antioxidant defense in plants under metal/metalloid stress. In Plant Abiotic Stress Tolerance; Springer: Cham, Switzerland, 2019; pp. 221–257. [Google Scholar]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants-an overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef] [PubMed]
- Jamla, M.; Khare, T.; Joshi, S.; Patil, S.; Kumar, V. Omics approaches for understanding heavy metal responses and tolerance in plants. Curr. Plant Biol. 2021, 27, 100213. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Schaufelberger, M.; Li, X.; Ren, Z. Role of flavonol synthesized by nucleus FLS1 in Arabidopsis resistance to Pb stress. J. Agric. Food Chem. 2020, 68, 9646–9653. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.P.; Dong, X.J.; Ma, H.H. Molecular mechanism for cadmium-induced anthocyanin accumulation in Azolla imbricata. Chemospher 2012, 87, 319–325. [Google Scholar] [CrossRef]
- Barrows, G.; Sexton, S.; Zilberman, D. Agricultural biotechnology: The promise and prospects of genetically modified crops. J. Econ. Perspect. 2014, 28, 99–120. [Google Scholar] [CrossRef]
- Sedeek, K.E.M.; Mahas, A.; Mahfouz, M. Plant genome engineering for targeted improvement of crop traits. Front. Plant Sci. 2019, 10, 114. [Google Scholar] [CrossRef]
- Basu, S.K.; Dutta, M.; Goyal, A.; Bhowmik, P.K.; Kumar, J.; Nandy, S.; Scagliusi, S.M.; Prasad, R. Is genetically modified crop the answer for the next green revolution? GM Crops 2010, 1, 68–79. [Google Scholar] [CrossRef]
- Wani, S.H.; Sah, S.K.; Hossain, M.A.; Kumar, V.; Balachandran, S.M. Transgenic approaches for abiotic stress tolerance in crop plants. In Advances in Plant Breeding Strategies: Agronomic, Abiotic and Biotic Stress Traits; Springer: Cham, Switzerland, 2016; pp. 345–396. [Google Scholar]
- Zhang, X.H.; Zheng, X.T.; Sun, B.Y.; Peng, C.L.; Chow, W.S. Over-expression of the CHS gene enhances resistance of Arabidopsis leaves to high light. Environ. Exp. Bot. 2018, 154, 33–43. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Fan, F.; Yu, Q.; Zhang, P. A Moss 2-Oxoglutarate/Fe (II)-Dependent Dioxygenases (2-ODD) Gene of Flavonoids Biosynthesis Positively Regulates Plants Abiotic Stress Tolerance. Front. Plant Sci. 2022, 13, 850062. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.T.; Chen, Y.-H.L.; Zhang, X.H.; Cai, M.L.; Yu, Z.C.; Peng, C.L. ANS-deficient Arabidopsis is sensitive to high light due to impaired anthocyanin photoprotection. Funct. Plant Biol. 2019, 46, 756–765. [Google Scholar] [CrossRef]
- Li, X.-J.W.; Wang, Y.; Yan, F.; Li, J.W.; Zhao, Y.; Zhao, X.; Zhai, Y.; Wang, Q.Y. Overexpression of soybean R2R3-MYB transcription factor, GmMYB12B2, and tolerance to UV radiation and salt stress in transgenic Arabidopsis. Genet. Mol. Res. 2016, 15, gmr.15026573. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, D.; Chen, S.; He, J.; Wang, Z.; Yang, G.; Lu, Z. Identifying and expression analysis of WD40 transcription factors in walnut. Plant Genom. 2022, 15, e20229. [Google Scholar] [CrossRef]
- Wang, A.; Zhu, M.; Luo, Y.; Liu, Y.; Li, R.; Kou, M.; Wang, X.; Zhang, Y.; Meng, X.; Zheng, Y.; et al. A sweet potato cinnamate 4-hydroxylase gene, IbC4H, increases phenolics content and enhances drought tolerance in tobacco. Acta Physiol. Plant. 2017, 39, 276. [Google Scholar] [CrossRef]
- Ku, Y.S.; Ng, M.S.; Cheng, S.S.; Lo, A.W.Y.; Xiao, Z.; Shin, T.S.; Chung, G.; Lam, H.M. Understanding the composition, biosynthesis, accumulation and transport of flavonoids in crops for the promotion of crops as healthy sources of flavonoids for human consumption. Nutrients 2020, 12, 1717. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Zhang, S.; Deng, Y.S.; Wang, G.D.; Kong, F.Y. Overexpression of a tomato flavanone 3-hydroxylase-like protein gene improves chilling tolerance in tobacco. Plant Physiol. Biochem. 2015, 96, 388–400. [Google Scholar] [CrossRef]
- Ai, T.N.; Naing, A.H.; Yun, B.W.; Lim, S.; Kim, C.K. Overexpression of RsMYB1 enhances anthocyanin accumulation and heavy metal stress tolerance in transgenic petunia. Front. Plant Sci. 2018, 9, 1388. [Google Scholar] [CrossRef]
| Gene Name | Expression Status | Roles in Biosynthesis | Stress Resistance in Plants | References |
|---|---|---|---|---|
| Structural genes | ||||
| PAL | High expression under stress | Involved in the biosynthesis of flavonoids | Played positive roles in response to abiotic stress | [16,72,104,124] |
| C4H | High expression under stress | Involved in the biosynthesis of flavonoids | Played positive roles in response to abiotic stress | [105,216,217,218] |
| 4CL | High expression under stress | Involved in the biosynthesis of flavonoids | Played positive roles in response to abiotic stress | [106,216,217] |
| CHS | High expression under stress | Involved in the biosynthesis of flavonoids | Played positive roles in response to abiotic stress | [108,219,220] |
| CHI | High expression under stress | Involved in the biosynthesis of flavonoids | Played positive roles in response to abiotic stress | [109,221,222,223] |
| FNS | High expression under stress | Involved in the biosynthesis of flavones | Played positive roles in response to UV and salt stress | [224] |
| F3H | Constitutive of high expression in transgenic Arabidopsis and tobacco | Involved in the biosynthesis of flavan-3-ol (catechin and epicatechin) | Played positive roles in response to salt, drought and cold stress | [225,226,227] |
| F3’H | High expression under stress | Involved in the biosynthesis of flavonoids | Played positive roles in response to abiotic stress | [112,113,114,120,121] |
| F3’5’H | High expression under stress | Involved in the biosynthesis of flavonoids | Played positive roles in response to abiotic stress | [112,113,114,115,216,217] |
| IFS | High expression under stress | Involved in the biosynthesis of isoflavones | Played positive roles in response to salt osmotic stress | [228] |
| FLS | High expression under stress | Involved in the biosynthesis of flavonols (kaempferol, quercetin, and myricetin) | Played positive roles in response to UV stress, salinity stress, drought stress, cold stress and heavy metal stress | [114,133,215,229,230] |
| DFR | Constitutive of high expression in transgenic Arabidopsis | Involved in the biosynthesis of leucoanthocyanidin and anthocyanins | Played positive roles in response to salt, cold and UV stress | [114,231,232] |
| ANS | High expression under stress | Involved in the biosynthesis of anthocyanidin | Played positive roles in response to abiotic stress | [151,203,233,234,235] |
| UFGT | High expression under stress | Involved in synthesis of stable anthocyanins | Played positive roles in response to abiotic stress | [120,121,151,233,234,235] |
| Transcription factors | ||||
| MYB | Constitutive of high expression in transgenic Arabidopsis and Petunia | Regulated of anthocyanins and other flavonoids biosynthesis | Played positive roles in response to UV, salt and heavy metal stress | [236] |
| bHLH | Constitutive of high expression in transgenic Arabidopsis and tobacco | Regulated of flavonols, flavanols, anthocyanins, and flavanones biosynthesis | Played positive roles in response to drought, freezing and salt stress | [237,238,239] |
| WD40 | Constitutive of high expression in transgenic Arabidopsis | Regulated of flavonoids biosynthesis | Played positive roles in response to ABA and salt osmotic stress | [240] |
| bZIP | Constitutive of high expression in transgenic tobacco | Regulated of flavonoids biosynthesis | Played positive roles in response to High light, UV and salt stress | [147,216,217] |
| NAC | high expression; Constitutive of high expression in transgenic Arabidopsis | Regulated of anthocyanins and procyanidins biosynthesis | Played positive roles in response to High light and UV stress | [143,148,149] |
| MADS-box | High expression under stress | Regulated of flavonoids biosynthesis | Played positive roles in response to cold, salt, and drought stress | [223,233,234,235,241] |
| WRKY | High expression under stress; Constitutive of high expression in transgenic apple | Regulated of anthocyanins and other flavonoids biosynthesis | Played positive roles in response to cold, drought, and UV stress | [151,242,243] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, W.-B.; Li, Y.-H.; Shu, X.-C.; Pu, Y.-T.; Wang, X.-J.; Wang, T.; Wang, Z. The Classification, Molecular Structure and Biological Biosynthesis of Flavonoids, and Their Roles in Biotic and Abiotic Stresses. Molecules 2023, 28, 3599. https://doi.org/10.3390/molecules28083599
Zhuang W-B, Li Y-H, Shu X-C, Pu Y-T, Wang X-J, Wang T, Wang Z. The Classification, Molecular Structure and Biological Biosynthesis of Flavonoids, and Their Roles in Biotic and Abiotic Stresses. Molecules. 2023; 28(8):3599. https://doi.org/10.3390/molecules28083599
Chicago/Turabian StyleZhuang, Wei-Bing, Yu-Hang Li, Xiao-Chun Shu, Yu-Ting Pu, Xiao-Jing Wang, Tao Wang, and Zhong Wang. 2023. "The Classification, Molecular Structure and Biological Biosynthesis of Flavonoids, and Their Roles in Biotic and Abiotic Stresses" Molecules 28, no. 8: 3599. https://doi.org/10.3390/molecules28083599
APA StyleZhuang, W.-B., Li, Y.-H., Shu, X.-C., Pu, Y.-T., Wang, X.-J., Wang, T., & Wang, Z. (2023). The Classification, Molecular Structure and Biological Biosynthesis of Flavonoids, and Their Roles in Biotic and Abiotic Stresses. Molecules, 28(8), 3599. https://doi.org/10.3390/molecules28083599








