Controlling the Redox Catalytic Activity of a Cyclic Selenide Fused to 18-Crown-6 by the Conformational Transition Induced by Coordination to an Alkali Metal Ion
Abstract
1. Introduction
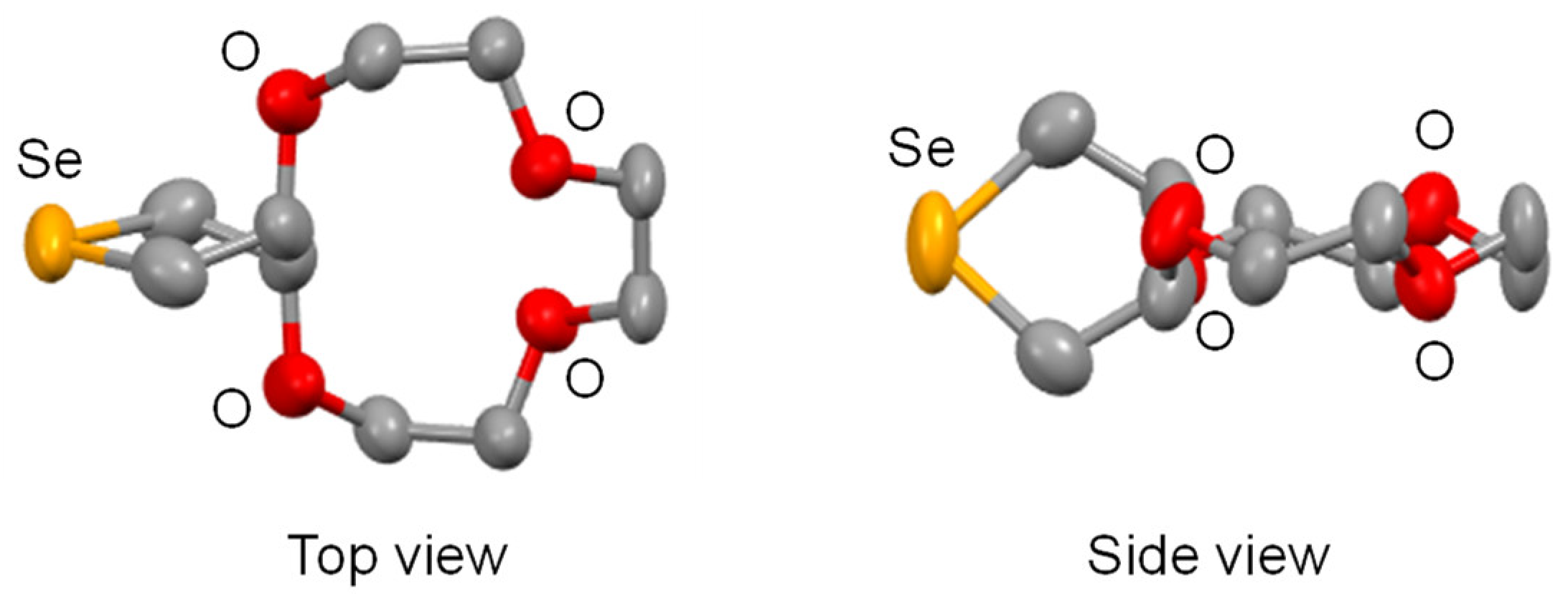
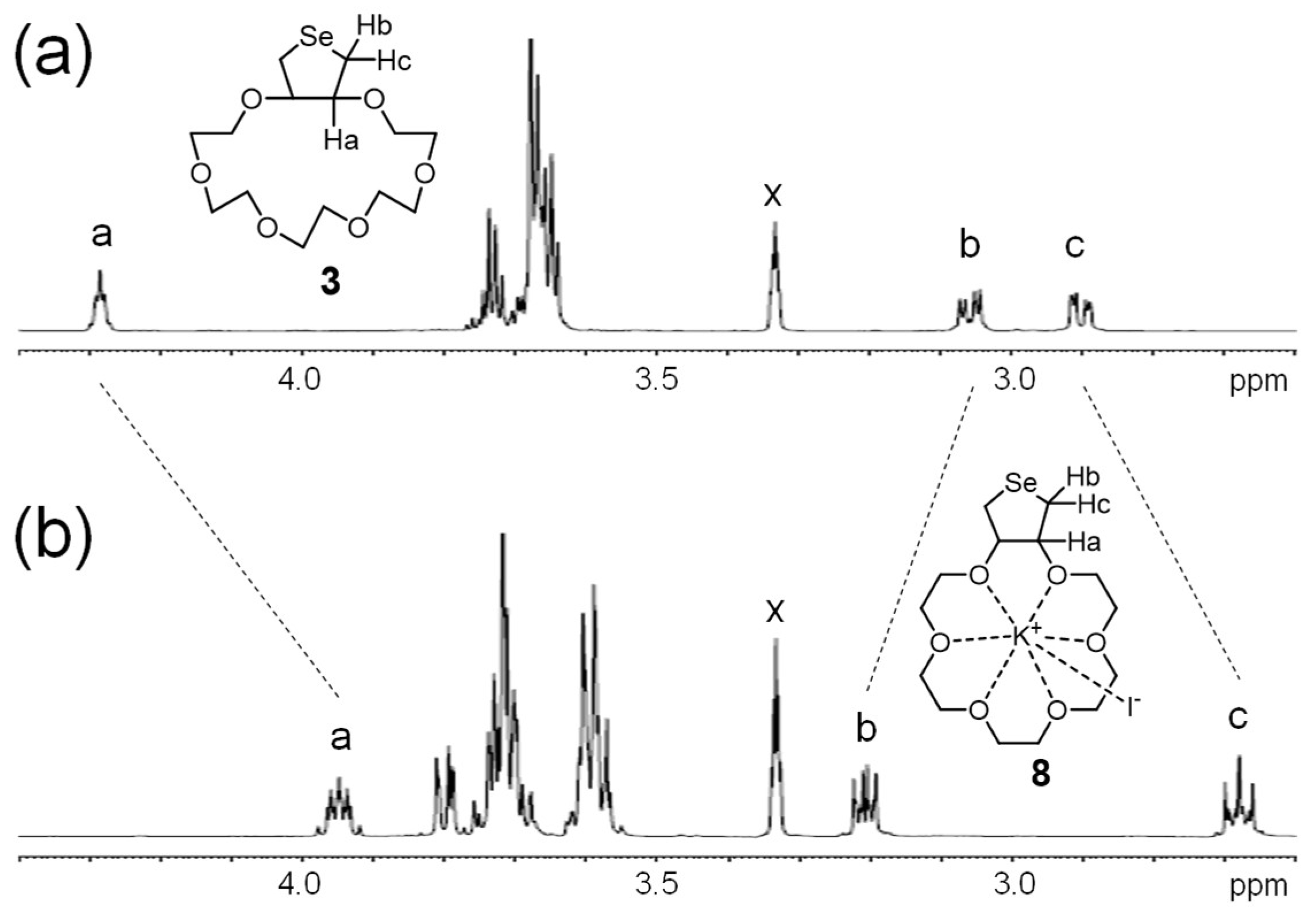
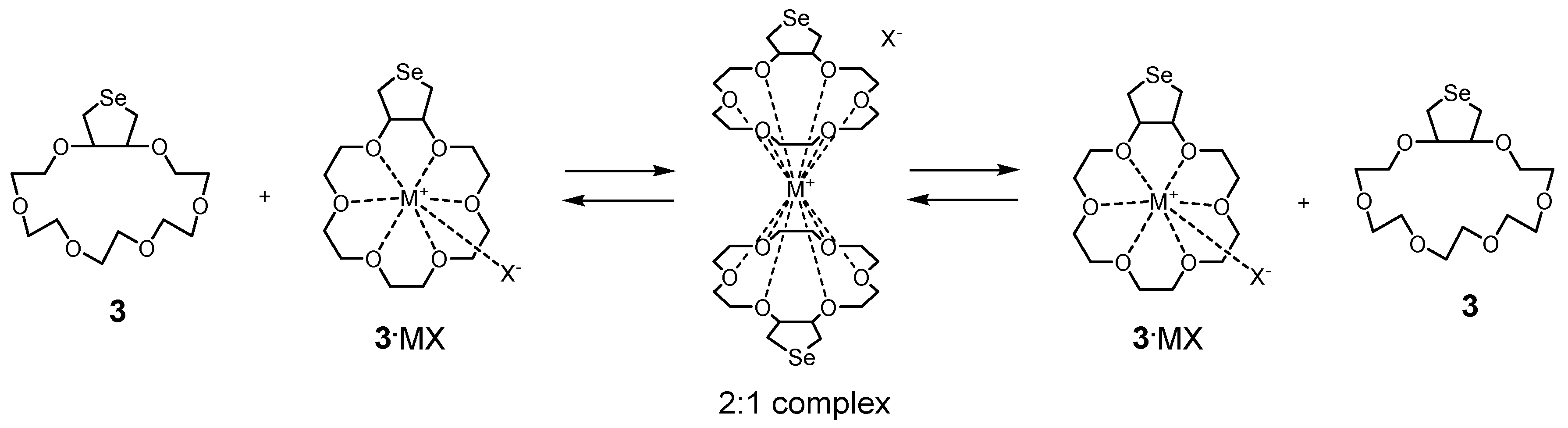
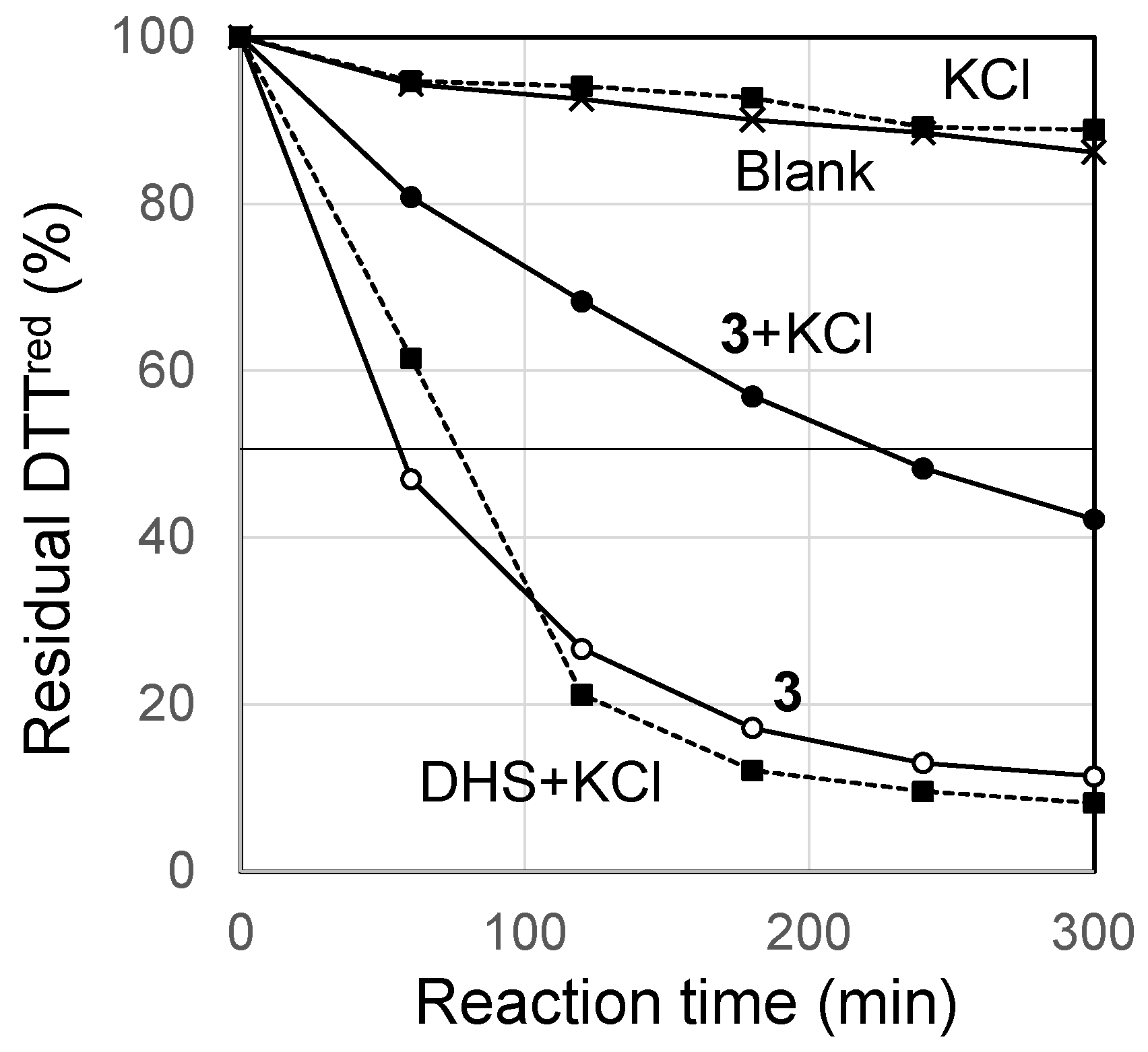
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Synthesis of 10a,13a-trans-Decahydroselenopheno[3,4-b][1,4,7,10]tetraoxacyclododecine (1)
3.3. Synthesis of 13a,16a-trans-Dodecahydroselenopheno[3,4-b][1,4,7,10,13]pentaoxacyclopentadecine (2)
3.4. Synthesis of 16a,19a-trans-Tetradecahydroselenopheno[3,4-b][1,4,7,10,13,16]hexaoxacyclooctadecine (3)
3.5. Synthesis of 19a,22a-Trans-hexadecahydroselenopheno[3,4-b][1,4,7,10,13,16,19]heptaoxacyclohenicosine (4)
3.6. Complex Preparation
3.7. X-ray Analysis
3.8. 1H NMR Titration Study in CD3OD
3.9. Redox Assay for DHS-crown-6 (3)
3.10. Theoretical Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panda, A. Chemistry of Selena Macrocycles. Coord. Chem. Rev. 2009, 253, 1056–1098. [Google Scholar] [CrossRef]
- Liu, Z.; Nalluri, S.K.M.; Fraser Stoddart, J. Surveying Macrocyclic Chemistry: From Flexible Crown Ethers to Rigid Cyclophanes. Chem. Soc. Rev. 2017, 46, 2459–2478. [Google Scholar] [CrossRef]
- Ren, Y.; Jamagne, R.; Tetlow, D.J.; Leigh, D.A. A Tape-Reading Molecular Ratchet. Nature 2022, 612, 78–82. [Google Scholar] [CrossRef]
- Pfeifer, L.; Crespi, S.; van der Meulen, P.; Kemmink, J.; Scheek, R.M.; Hilbers, M.F.; Buma, W.J.; Feringa, B.L. Controlling Forward and Backward Rotary Molecular Motion on Demand. Nat. Commun. 2022, 13, 2124. [Google Scholar] [CrossRef]
- Ariga, K. Molecular Machines and Microrobots: Nanoarchitectonics Developments and On-Water Performances. Micromachines 2022, 14, 25. [Google Scholar] [CrossRef]
- Li, J.; Yim, D.; Jang, W.D.; Yoon, J. Recent Progress in the Design and Applications of Fluorescence Probes Containing Crown Ethers. Chem. Soc. Rev. 2017, 46, 2437–2458. [Google Scholar] [CrossRef]
- Chowdhury, S.; Rooj, B.; Dutta, A.; Mandal, U. Review on Recent Advances in Metal Ions Sensing Using Different Fluorescent Probes. J. Fluoresc. 2018, 28, 999–1021. [Google Scholar] [CrossRef]
- Bruemmer, K.J.; Crossley, S.W.M.; Chang, C.J. Activity-Based Sensing: A Synthetic Methods Approach for Selective Molecular Imaging and Beyond. Angew. Chem. Int. Ed. 2020, 59, 13734–13762. [Google Scholar] [CrossRef] [PubMed]
- Krämer, J.; Kang, R.; Grimm, L.M.; De Cola, L.; Picchetti, P.; Biedermann, F. Molecular Probes, Chemosensors, and Nanosensors for Optical Detection of Biorelevant Molecules and Ions in Aqueous Media and Biofluids. Chem. Rev. 2022, 122, 3459–3636. [Google Scholar] [CrossRef] [PubMed]
- Barton, A.J.; Genge, A.R.J.; Hill, N.J.; Levason, W.; Orchard, S.D.; Patel, B.; Reid, G.; Ward, A.J. Recent Developments in Thio-, Seleno-, and Telluro-Ether Ligand Chemistry. Heteroat. Chem. 2002, 13, 550–560. [Google Scholar] [CrossRef]
- Laitinen, R.S.; Oilunkaniemi, R.; Weigand, W. Structure, Bonding, and Ligand Chemistry of Macrocyclic Seleno- and Telluroethers. In Chalcogen Chemistry: Fundamentals and Applications; Lippolis, V., Santi, C., Lenardao, E.J., Braga, A.L., Eds.; Royal Society of Chemistry: Croydon, UK, 2023; pp. 550–566. ISBN 978-1-83916-422-4. [Google Scholar]
- Tsuchiya, T.; Kurihara, H.; Sato, K.; Wakahara, T.; Akasaka, T.; Shimizu, T.; Kamigata, N.; Mizorogi, N.; Nagase, S. Supramolecular Complexes of La@C82 with Unsaturated Thiacrown Ethers. Chem. Commun. 2006, 14, 3585–3587. [Google Scholar] [CrossRef] [PubMed]
- Ferrier, M.G.; Kmak, K.N.; Kerlin, W.M.; Valdez, C.A.; Despotopulos, J.D. Transactinide Studies with Sulfur Macrocyclic Extractant Using Mercury. J. Radioanal. Nucl. Chem. 2020, 326, 215–222. [Google Scholar] [CrossRef]
- Sabah, K.J.; Sead, F.F.; Mohammed, H.J.; Abedul-Hussien, I.H. Novel Polymer Crafted Sugar Thiacrown Ether and Its Applications in Recovery of Metal Ions. Carbohydr. Res. 2020, 495, 108057. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.H.; Li, J.Z.; Yao, Z.X.; Cheng, X.; Yu, D.N.; Yan, X.W.; Wang, W.; Liu, K.G. 1,3,5-Trithian Mediated Formation of Two New Tetranuclear Silver-Alkynyl Clusters and Investigation of Their Optical Features. J. Clust. Sci. 2022, 33, 2363–2368. [Google Scholar] [CrossRef]
- Tomoda, S.; Iwaoka, M. Synthesis and Structures of Host Molecules Containing an Se-Se Bond. Intramolecular Hypervalent Nature of Selenium Atoms in the Crystal State. J. Chem. Soc. Chem. Commun. 1990, 231–233. [Google Scholar] [CrossRef]
- Raju, S.; Butcher, R.J.; Singh, H.B. Isolation and Structure of an 18-Membered Macrocycle Containing Two Diselenide Linkages and Its Precursor. Acta Crystallogr. C Struct. Chem. 2019, 75, 336–341. [Google Scholar] [CrossRef]
- Jayasree, E.G.; Sukumar, C. Integrating Redox-Response in Crown Ethers by Disulfide Incorporation: A Computational Approach. Struct. Chem. 2021, 32, 1833–1842. [Google Scholar] [CrossRef]
- Hirabayashi, K.; Nakashizuka, M.; Shimizu, T. Synthesis, Structure, and Properties of Unsaturated Thiacrown Ethers Possessing Sulfonium Groups. Chem. Asian J. 2022, 17, e202101329. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Li, B.; Shen, X.; Pan, T.; Cui, Z.; Wang, Y.; Ge, Y.; Qi, Z. Selenacrown Macrocycle in Aqueous Medium: Synthesis, Redox-Responsive Self-Assembly, and Enhanced Disulfide Formation Reaction. J. Org. Chem. 2021, 86, 1430–1436. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xu, Q.; Shen, X.; Pan, T.; Shang, J.; Ge, Y.; Qi, Z. Atom-Economic Macrocyclic Amphiphile Based on Guanidinium-Functionalized Selenacrown Ether Acting as Redox-Responsive Nanozyme. Chin. Chem. Lett. 2023, 34, 108015. [Google Scholar] [CrossRef]
- Rodewald, M.; Rautiainen, J.M.; Niksch, T.; Görls, H.; Oilunkaniemi, R.; Weigand, W.; Laitinen, R.S. Chalcogen-Bonding Interactions in Telluroether Heterocycles [Te(CH2)m]n (N = 1–4; M = 3–7). Chem. A Eur. J. 2020, 26, 13806–13818. [Google Scholar] [CrossRef] [PubMed]
- Iwaoka, M.; Kumakura, F. Applications of Water-Soluble Selenides and Selenoxides to Protein Chemistry. Phosphorus Sulfur Silicon Relat. Elem. 2008, 183, 1009–1017. [Google Scholar] [CrossRef]
- Kumakura, F.; Mishra, B.; Priyadarsini, K.I.; Iwaoka, M. A Water-Soluble Cyclic Selenide with Enhanced Glutathione Peroxidase-like Catalytic Activities. Eur. J. Org. Chem. 2010, 2010, 440–445. [Google Scholar] [CrossRef]
- Chakraborty, S.; Yadav, S.K.; Subramanian, M.; Priyadarsini, K.I.; Iwaoka, M.; Chattopadhyay, S. DL-Trans-3,4-Dihydroxy-1-Selenolane (DHSred) Accelerates Healing of Indomethacin-Induced Stomach Ulceration in Mice. Free Radic Res. 2012, 46, 1378–1386. [Google Scholar] [CrossRef]
- Chakraborty, S.; Yadav, S.K.; Subramanian, M.; Iwaoka, M.; Chattopadhyay, S. Dl-Trans-3,4-Dihydroxy-1-Selenolane (DHSred) Heals Indomethacin-Mediated Gastric Ulcer in Mice by Modulating Arginine Metabolism. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 3385–3392. [Google Scholar] [CrossRef]
- Iwaoka, M.; Katakura, A.; Mishima, J.; Ishihara, Y.; Kunwar, A.; Priyadarsini, K.I. Mimicking the Lipid Peroxidation Inhibitory Activity of Phospholipid Hydroperoxide Glutathione Peroxidase (GPx4) by Using Fatty Acid Conjugates of a Water-Soluble Selenolane. Molecules 2015, 20, 12364–12375. [Google Scholar] [CrossRef] [PubMed]
- Iwaoka, M.; Sano, N.; Lin, Y.Y.; Katakura, A.; Noguchi, M.; Takahashi, K.; Kumakura, F.; Arai, K.; Singh, B.G.; Kunwar, A.; et al. Fatty Acid Conjugates of Water-Soluble (±)-Trans-Selenolane-3,4-Diol: Effects of Alkyl Chain Length on the Antioxidant Capacity. ChemBioChem 2015, 16, 1226–1234. [Google Scholar] [CrossRef]
- Verma, P.; Kunwar, A.; Arai, K.; Iwaoka, M.; Priyadarsini, K.I. Mechanism of Radioprotection by Dihydroxy-1-Selenolane (DHS): Effect of Fatty Acid Conjugation and Role of Glutathione Peroxidase (GPx). Biochimie 2018, 144, 122–133. [Google Scholar] [CrossRef]
- Iwaoka, M.; Takahashi, T.; Tomoda, S. Syntheses and Structural Characterization of Water-Soluble Selenium Reagents for the Redox Control of Protein Disulfide Bonds. Heteroat. Chem. 2001, 12, 293–299. [Google Scholar] [CrossRef]
- Phadnis, P.P.; Wadawale, A.; Priyadarsini, K.I.; Jain, V.K.; Iwaoka, M. Synthesis, Characterization, and Structure of Trans-3,4-Dihydroxy-1-Selenolane {DHS(OH)2} Substituted Derivatives. Tetrahedron. Lett. 2015, 56, 2293–2296. [Google Scholar] [CrossRef]
- Iwaoka, M.; Hiyoshi, Y.; Arai, S.; Ito, T. Synthesis of 4-Selenothreofuranose Derivatives via Pummerer-Type Reactions of Trans-3,4-Dioxygenated Tetrahydroselenophenes Mediated by a Selenonium Intermediate. ACS Omega 2021, 6, 17621–17634. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Iwaoka, M. Oxidative Protein Folding Using Trans-3,4-Dihydroxyselenolane Oxide. In Methods in Molecular Biology; Humana Press Inc.: Torvalds, NJ, USA, 2019; Volume 1967, pp. 229–244. [Google Scholar]
- Bouzide, A.; Sauvé, G. Silver(I) Oxide Mediated Highly Selective Monotosylation of Symmetrical Diols. Application to the Synthesis of Polysubstituted Cyclic Ethers. J. Am. Chem. Soc 2002, 124, 16. [Google Scholar] [CrossRef]
- Minor, W.; Cymborowski, M.; Otwinowski, Z.; Chruszcz, M. HKL-3000: The Integration of Data Reduction and Structure Solution–from Diffraction Images to an Initial Model in Minutes. Acta Crystallogr. Sect. D Biol. Crystallogr. 2006, 62, 859–866. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A Found Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision B.01 2010; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Rastetter, W.H.; Phillion, D.P. Template-Driven Macrolide Closures. J. Org. Chem. 2002, 46, 3209–3214. [Google Scholar] [CrossRef]
- Kele, P.; Nagy, K.; Kotschy, A. The Development of Conformational-Dynamics-Based Sensors. Angew. Chem. Int. Ed. 2006, 45, 2565–2567. [Google Scholar] [CrossRef]
- Coppola, C.; Simeone, L.; Trotta, R.; De Napoli, L.; Randazzo, A.; Montesarchio, D. Synthesis and NMR Characterization of a Novel Crown-Ether Ring-Fused Uridine Analogue. Tetrahedron 2010, 66, 6769–6774. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwaoka, M.; Oba, H.; Ito, T. Controlling the Redox Catalytic Activity of a Cyclic Selenide Fused to 18-Crown-6 by the Conformational Transition Induced by Coordination to an Alkali Metal Ion. Molecules 2023, 28, 3607. https://doi.org/10.3390/molecules28083607
Iwaoka M, Oba H, Ito T. Controlling the Redox Catalytic Activity of a Cyclic Selenide Fused to 18-Crown-6 by the Conformational Transition Induced by Coordination to an Alkali Metal Ion. Molecules. 2023; 28(8):3607. https://doi.org/10.3390/molecules28083607
Chicago/Turabian StyleIwaoka, Michio, Hajime Oba, and Takeru Ito. 2023. "Controlling the Redox Catalytic Activity of a Cyclic Selenide Fused to 18-Crown-6 by the Conformational Transition Induced by Coordination to an Alkali Metal Ion" Molecules 28, no. 8: 3607. https://doi.org/10.3390/molecules28083607
APA StyleIwaoka, M., Oba, H., & Ito, T. (2023). Controlling the Redox Catalytic Activity of a Cyclic Selenide Fused to 18-Crown-6 by the Conformational Transition Induced by Coordination to an Alkali Metal Ion. Molecules, 28(8), 3607. https://doi.org/10.3390/molecules28083607






