Abstract
Industrial effluents containing dyes are the dominant pollutants, making the drinking water unfit. Among the dyes, methylene orange (MO) dye is mutagenic, carcinogenic and toxic to aquatic organisms. Therefore, its removal from water bodies through effective and economical approach is gaining increased attention in the last decades. Photocatalytic degradation has the ability to convert economically complex dye molecules into non-toxic and smaller species via redox reactions, by using photocatalysts. g-C3N4 is a metal-free n-type semiconductor, typical nonmetallic and non-toxici polymeric photocatalyst. It widely used in photocatalytic materials, due to its easy and simple synthesis, fascinating electronic band structure, high stability and abundant availability. As a photocatalyst, its major drawbacks are its limited efficiency in separating photo-excited electron–hole pairs, high separated charge recombination, low specific surface area, and low absorption coefficient. In this review, we report the recent modification strategies adopted for g-C3N4 for the efficient photodegradation of MO dye. The different modification approaches, such as nanocomposites and heterojunctions, as well as doping and defect introductions, are briefly discussed. The mechanism of the photodegradation of MO dye by g-C3N4 and future perspectives are discussed. This review paper will predict strategies for the fabrication of an efficient g-C3N4-based photocatalyst for the photodegradation of MO dye.
1. Introduction
Polluted wastewater containing industrial discharge is one of the main causes of the irreversible degradation of ecosystems [1]. Water pollution is considered a serious concern to the global community, since wastewaters from the textile, leather, food and chemical industries discharge hazardous dyes [2]. Organic dyes are the common coloring agents in textile, cosmetics, leather, paper, plastic, printing, rubber and pharmaceutical industries [3], and their use results in severe water pollution and create environmental and esthetic issues [4]. Most of these dyes are toxic, teratogenic, carcinogenic, xenobiotic, and non-biodegradable, owing to their complex structures and large size, and their accumulation create potential threats and risks to human and aquatic life [5,6]. Their continuous discharge in wastewaters into the natural aquatic environment causes non-aesthetic pollution and eutrophication, and can generate very toxic byproducts via oxidation, hydrolysis, or other chemical reactions occurring during water treatment [7]. Dyes affect the central nervous system, liver, kidney, skin, enzymatic system, chromosomes and reproductive systems of human [8]. Dye concentrations in textile wastewater are reported over a wide range of values. A study indicated that the level of dye in the textile effluent is 10–50 mg/L. The concentrations of reactive dyes in cotton factories are reported as 60 mg/L, as well as between 100 and 200 mg/L [9]. Cationic dyes are very toxic even at trace levels, e.g., the concentration of malachite green should not exceed 1.0 μg·L−1 in drinking water and 100 μg·L−1 in potable waters [10]. Similarly, the permissible levels of permitted food coloring Sunset yellow FCF dye are 100–200 mg/kg [11].
Methyl orange (MO) is an anionic azo dye, having high chemical stability [12], and it belongs to the sulphonated azo group [13]. The IUPAC name of MO is Sodium 4-[(4-dimethylamino) phenyldiazenyl] benzenesulfonate, having a molecular formula of C14H14N3NaO3S, a molar mass of 327.33 g/mole, and a density of 1.28 g/cm3 [14]. MO is partially soluble in normal water, highly soluble in hot water, and insoluble in ethanol. It is an orange–yellow powder and its melting point is greater than 300 °C [15]. MO can function as week acid, showing approximately 6.5 pH when dissolved in water, and it also works as a pH indicator (color changes from red to yellow in the range of 3.1–4.4) [16]. MO displays a red color in the acidic medium, and in basic media it shows an orange color [17]. The azo group, sulfur group and aromaticity of MO give it an intense coloration, high chemical stability, toxicity and low biodegradability [18]. The chemical structure and optimized structure of MO dye is shown in the Figure 1 [19]. MO dye has various industrial and laboratory applications [20].
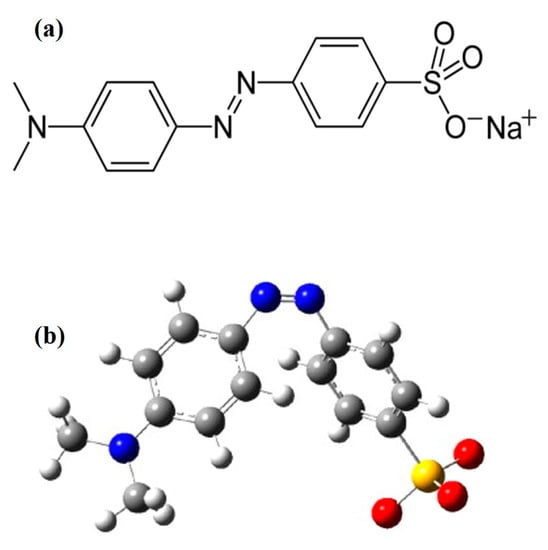
Figure 1.
(a) Chemical structure of Methyl orange; (b) the optimized MO molecular structure created using Gaussian 03 software package on the basis of the HF/6-31G method. Reprinted/adapted with permission from [19], 2023, Elsevier (License Number 5507170462000).
MO is extensively used in dyeing, leather, textile, pulp, paper and printing industries, and in research laboratories [21,22]. MO is used widely in the textile industry and is the major constituent of industrial waste discharge, polluting various water bodies [23]. The concentration of MO dye can reach up to 500 ppm in the textile effluent [24]. MO dye is mutagenic, carcinogenic and toxic to aquatic organisms [25,26]. MO is one of the most extensively used hazardous anionic azo dyes, is a highly recalcitrant and refractory xenobiotic, and causes a significant burden in the ecosystem [27]. Azo dyes show resistance in the natural environment, and their bio-degradation has serious hurdles [28]. MO is a more harmful dye, and cause serious water pollution when released into the environment. Its presence at maximum amounts in drinking water produces anemia, abdominal pain, headache, dizziness, mental confusion, excessive sweating and nausea [23]. Acute exposure to MO dye can cause shock, vomiting, cyanosis, increased heart rate, quadriplegia, tissue necrosis and jaundice in humans [29]. MO accidentally enters the body via ingestion, where intestinal microorganisms metabolize it into aromatic amines, which can cause intestinal cancer [30]. Thus, MO-containing wastewater should be decolorized and detoxified before being discharged into the environment [31].
Methyl orange is difficult to be decomposed using conventional methods at ambient conditions, as these methods can accumulate pollutants instead of decomposing them [32]. MO dye is nonbiodegradable, due to its complex aromatic structure and xenobiotic properties [33]. Several methods are described for MO removal, such as adsorption [34,35,36], biodegradation [37], phytoremediation [38] ozonation [39], coagulation/flocculation [40] and heterogenous photodegradation [41,42,43,44]. It has complex molecular structures, which make their removal from wastewater difficult when using conventional treatment methods [45]. Heterogeneous photodegradation has advantages over the other reported conventional techniques due to its process simplicity, complete pollutant mineralization, cost-effectiveness, no/fewer harmful byproduct production and its ability to be carried out at ambient temperature and pressure [46]. The heterogeneous photodegradation (PD) process usually requires a photocatalyst or semiconductor, light source, reactor system and the pollutant and oxygen [47].
Various photocatalysts are used for the PD of MO dye e.g., ZnO-rGO nanorods [48], CuO nanoparticles [29], Au/TiO2 nanoparticles [49], Ag–Ni and Al–Ni nanoparticles [50], silver nanoparticles/amidoxime-modified polyacrylonitrile nanofibers [51], g-C3N4 and B-doped g-C3N4 [52], SGCN/Fe3O4/PVIs/Pd [53], etc.
g-C3N4 is a metal free n-type semiconductor polymer, having several exceptional properties, such as unique structural, optical, electric and physiochemical properties, making g-C3N4-based materials an emerging class of materials that can be used in various photocatalytic applications [54]. The g-C3N4 can respond to the visible light (up to 460 nm), due to its suitable band gap of about 2.7 eV. Its conduction band potential (ECB) is about −1.1 V (vs. NHE), and, thus, its photogenerated electrons have a very strong reduction ability [55]. g-C3N4 is a typical, nonmetallic and non-toxic polymeric photocatalyst, which has attracted tremendous attention for environmental protection because of its easy synthesis, low cost, excellent photochemical stability, favorable band positions [56,57,58], etc. The use of g-C3N4, from an economical point of view, is considered as a viable choice in the photocatalysis field, because of its intrinsic properties and favorable internal qualities, such as its electrical conductive, photonic, distinctive 2D-stacked hierarchical features, high physical and chemical durability and non-toxicity [59]. Figure 2 illustrates the triazine (C3N3) and tri-striazine/heptazine (C6N7) rings, which are the basic structural tectonic units of g-C3N4 [60]. g-C3N4 is a layered structure, having a large specific surface area, electron-rich surface, and suitable band gap energy of 2.7 eV, which promotes its photocatalytic efficiency [61]. g-C3N4 composite-based materials are utilized widely in photocatalytic applications, owing to the low prices of their raw material, easy and simple synthesis, fascinating electronic band structure, high stability, as well as abundant availability [62,63]. g-C3N4 was synthesized, utilizing inexpensive nitrogen-rich substances (cyanamide, melamine, dicyandiamide, urea and thiourea) as precursors, via self-condensation by deamination, through a thermal reaction [64].
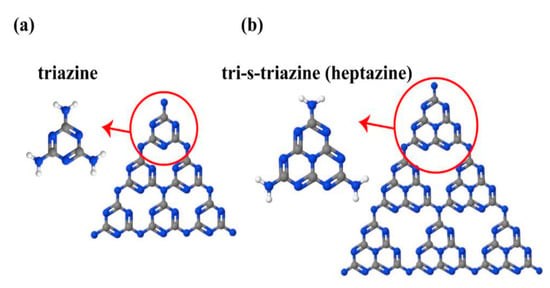
Figure 2.
(a) Triazine and (b) tri-striazine (heptazine) structures of g-C3N4 (blue, gray and white balls are nitrogen, carbon and hydrogen, respectively) [60].
Several reviews are reported in terms of the various aspects of g-C3N4-based photo-catalysts, such as their design strategies, properties, photocatalytic applications [65], and artificial photosynthesis, as well as their environmental remediation [66], modification with MXenes, the use of their derivatives for various photocatalytic applications [67], recent advances for photocatalytic CO2 reduction [68], their use in the disinfection of water and microbial control [69], quantum dot-modified g-C3N4-based photo-catalysts for different photo-catalytic applications [70], g-C3N4–metal oxide based nano-composites for photo-catalysis and sensing applications [60], the design and application of active sites in g-C3N4-based photo-catalysts [71], synthesis and structural developments in visible-light-active g-C3N4-based photo-catalysts [72], nonmetal modulation of morphology and composition of g-C3N4-based photo-catalysts [73], etc. There are no clear reviews reported specifically on recent modifications of g-C3N4 for dye photodegradation. However, a lot of work has been done on dye degradation using g-C3N4, hence, the present review is further specified to the recent modification in g-C3N4 for the efficient photodegradation of MO dye. This review will help those researchers who want to study the photodegradation of MO, by applying g-C3N4. The Scopus database indicates that limited literature is available on the photodegradation of MO dye using g-C3N4. Figure 3 shows the number of articles published per year on the photodegradation of MO dye by g-C3N4.
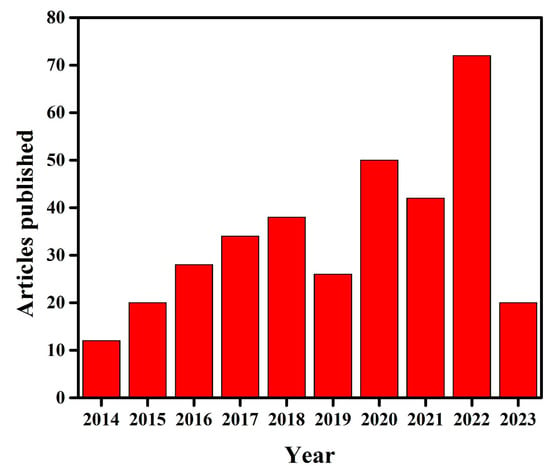
Figure 3.
Annual article frequency published extracted from the Scopus database on 13 March 2023 (Searched with the keyword ‘g-C3N4 for methyl orange degradation’).
In photodegradation, major drawbacks of g-C3N4 molecules are the limited efficiency of separating photo-excited electron–hole pairs, a narrow spectral absorption range, high-charge carrier recombination, insufficient sunlight absorption, low specific surface area, as well as a low absorption coefficient [74,75,76,77]. Several modifications have been attempted for improving the photo-catalytic activity of pure g-C3N4 for the efficient photodegradation of MO dye. Some of them are summarized below.
2. Composites and Heterojunctions
g-C3N4 composite materials were prepared with different materials for enhancing their photodegradation efficiency. Heterojunction systems were developed for improving the photo-catalytic activity of g-C3N4, enhancing its oxidation and reduction capabilities by utilizing the negative conduction band (CB) of one component, with the more positive valence band (VB) of the other component [78]. The ZnO/g-C3N4 composite (ZnO/g-C3N4-20 wt%) exhibited the highest photo-catalytic activity and good recyclability when compared to a pure ZnO catalyst and g-C3N4 catalyst [62]. The 5 wt% CuO/g-C3N4 nanolayer composites exhibited very efficient activity and removed 99.7% of the MO dye in 4 min, owing to their nano-size and porous nature [79]. MoS2/TiO2 heterostructure were decorated on g-C3N4 nanosheets to obtain a g-C3N4@MoS2/TiO2 nanocomposite photocatalyst, which shows a highly efficient removal of (98%) of MO dye in visible light and long-term stability when compared to neat g-C3N4 and MoS2/TiO2. The improved photo-catalytic activity of the g-C3N4@MoS2/TiO2 nanocomposite could be attributed to its strong response to visible light, the effective separation of photogenerated electron-hole pairs and the narrowed band gap. The catalyst caused a reduction of the C/C0 value of just 2.5%, from the first to the fifth cycle experiment [80]. Similarly, the AgBr/g-C3N4 composite system represents one with enhanced photo-catalytic activity when compared to mono-component systems under visible light irradiation. In this study, the composite AgBr:g-C3N4, having a mole ratio of 2:1, displayed efficient activity, and this ratio confirmed that the recombination of e−/h+ is the rate-limiting step in the coupled system. The re-used catalyst demonstrated good activities by being used in four successive runs without any significant loss in its activity [81]. A ternary ZnO/Fe3O4/g-C3N4 composite (magnetic recyclable) efficiently degraded MO dye. Among these composites, the ZnO/Fe3O4/g-C3N4-50% composite was found to be very effective, and degraded MO dye more effectively than pure ZnO and pure g-C3N4. There were three reasons discussed in terms of their enhanced photodegradation efficiency. Their UV-Vis spectra confirmed the strongest visible light response intensity of this composite. The electrochemical and PL results revealed that the rate of recombination of e−/h+ pair was the lowest. The electrochemical results showed that the photoelectron transfer rate was fastest in this composite than that observed in single components. The ZnO/Fe3O4/g-C3N4-50% composite exhibited a higher photocatalytic activity after five recycles [82]. Some g-C3N4 composites reported for the efficient PD of MO dye are AgBr:g-C3N4 [83], g-C3N4/TNTs heterojunctions [84], ternary g-C3N4/ZnO–W/M nanocomposites [85], g-C3N4-TiO2 nanocomposite [86], CdS/g-C3N4 hybrid nano-photocatalysts [87], MoO3–g-C3N4 composites [88], g-C3N4/ZnO composites [89], CoFe2O4/g-C3N4 nanocomposites [90], g-C3N4/Bi4O5I2 [91], ternary g-C3N4/Ag/γ-FeOOH photocatalysts [92], etc. Some g-C3N4-based composites/nanocomposites used for the efficient PD of MO dye are consolidated in Table 1.

Table 1.
g-C3N4 based composites/nanocomposites used for the PD of MO.
Apart from these, some metals/metallic compounds, such as Au [105], CoOx [106], Co3O4 [107], ZnO [108], SrTiO3 [109], Gd2O3 [110], and MnOx [111], were coupled to form g-C3N4 composite/heterojunctions for enhanced photodegradation of MO dyes.
Similarly, g-C3N4 quantum dot-based nanocomposites are also reported for the efficient photodegradation of MO dye. The g-C3N4 QDs/BiPO4 nanocrystal composite efficiently degraded about 92%, while g-C3N4 alone only degraded 75% in 180 min [112]. In another study, MoS2/Fe3O4/g-C3N4QDs nanocomposite degraded about 99.68% MO dye in 60 min under visible light [113]. Other g-C3N4 quantum dot-based nanocomposites reported for MO degradation are CaAl2O4:Eu2+,Nd3+ phosphor-coupled g-C3N4 quantum dot composites [114].
The photocatalytic enhancement, achieved by coupling g-C3N4 with other materials, can be easily understand from the mechanism of photodegradation of MO by BiOI/AgI/g-C3N4 composites. The photocatalyst exhibited a sandwich-type structure, with AgI sandwiched between g-C3N4 and BiOI. The CB levels of AgI, BiOI and g-C3N4 are about − 0.55 eV, 0.58 eV and − 1.12 eV (vs NHE), respectively, and the VB levels of AgI, BiOI and g-C3N4 are about 2.25 eV, 2.31 eV and 1.57 eV, respectively. Irradiating under visible light, AgI, BiOI and g-C3N4 become excited and generate e− and h+. The photogenerated e− present in the CB of g-C3N4 will freely transfer to the CB of AgI, and then transfer into the CB of BiOI. Similarly, h+ present in the VB of BiOI migrates to the VB of g-C3N4, using the pathway of AgI. Some photo-induced e− present in CB of g-C3N4 directly transfers to the CB of BiOI via contacted interfaces, however, a higher barrier height between the g-C3N4 and BiOI would hinder the process partially. Thus, the stepwise transfer of charge occurres, which results in the efficient transfer of e− and the enhanced separation of created charges. The e− reacts with O2 and generates •O2− that degrades MO dye efficiently, while the h+ can oxidize, directly, the MO dye molecules through the hole oxidation pathway. The VB edge potentials of BiOI, AgI, and g-C3N4 in the BiOI/AgI/g-C3N4 composites are insufficient for the formation of •OH radicals. The proposed possible MO degradation reaction mechanisms are represented schematically in the Figure 4 [115].
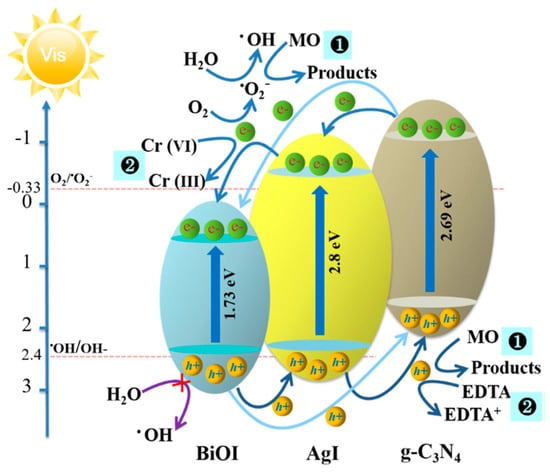
Figure 4.
The possible proposed photocatalytic mechanism for MO dye degradation (path 1), showing the BiOI/AgI/g−C3N4 composites’ irradiation under visible−light. Reprinted/adapted with permission from [115], 2023, Springer Nature (License Number 5507180161444).
3. Doping of g-C3N4
As g-C3N4 demonstrated poor catalytic efficiency due to low light adsorption and high recombination of the separated charges, which can be overcome by doping, in order to increase their conductivity. The LUMO and HOMO of the g-C3N4 are controllable. Owing to their tunable bandgap, the g-C3N4 photocatalytic efficiency is affected. Therefore, g-C3N4 can be modified by doping with elements. Doping is the intentional inserting of impurities into the certain semiconductor in order to tune its bandgap [116]. Sensitized doping can improve the catalytic activity of g-C3N4 photocatalysts [117]. For example, sulfur fluoride-doped g-C3N4 (F-SCN) shows enhanced MO degradation when compared to sulfur-doped g-C3N4 (SCN) and bulk g-C3N4 (BCN), as concluded from the Figure 5. Figure 5a shows that the F-SCN fluorescence intensity is the lowest, indicating a reduction in the created charges’ recombination rate to a certain extent, an increase in their numbers, and an improvement in its photocatalytic performance. The impedance spectrum (EIS) in the Figure 5b revealed that the radius of F-SCN is smaller than that of SCN and BCN, which revealed that F-SCN demonstrated the highest rate of electron transport. Similarly in the transient photocurrent response study, as represented in the Figure 5c, F-SCN displayed an enhanced photocurrent response, which suggests that the e−h+ photoexcitation in F-SCN materials requires a longer time, and their utilization time can also be extended as compared to SCN and BCN [118].
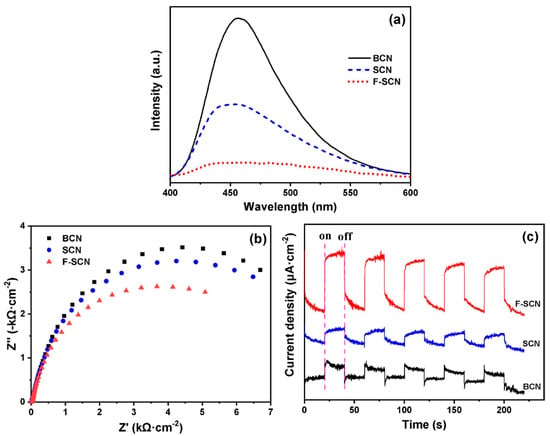
Figure 5.
(a) PL spectra, (b) EIS Nyquist spectra, and (c) transient photocurrent response of BCN, SCN, and F-SCN. Reprinted/adapted with permission from [118], 2023, Elsevier (License Number 5507180566714).
Doping of g-C3N4 with W improved its photocatalytic efficiency and degraded 99.6% within 60 min, using visible light [119]. Introducing boron into the structure of g-C3N4 will narrow its band gap, in order to absorb more visible light [52]. Boron-doped rGO/g-C3N4 nanocomposites (B-5%rGO/g-C3N4) approximately degraded 100% of the MO dyes within 240 min [120]. The P and S-codoped g-C3N4 enhanced the light absorption capability, surface area, and the charge separation efficiency, and showed higher catalytic activity. The pure g-C3N4, P-doped g-C3N4 and S-doped g-C3N4 degraded 15.26%, 22.67% and 24.86%, while the P and S codoped g-C3N4 (PSCN-50) degraded 73.25% of MO dye in 60 min under visible light irradiation. The PSCN-50 sample had no obvious activity even after five cycling runs, indicating high stability. [121]. BiVO4/pyridine-doped g-C3N4 shows enhanced performance when compared to the individual components, and photodegraded 97% of MO molecules within 150 min under visible light irradiation. The BiVO4/pyridine-doped g-C3N4 photocatalyst did not show an obvious reduction of activity, even after five cycling runs, implying the stability of photocatalysts [122]. A g-C3N4 molecule was reconstructed as a g-C3N4 nanoring by adding natural pollen, which results in abundant heteroatom (C)-doping, increase surface area and the formation of a porous hollow structure. The C-doped g-C3N4 demonstrated 2.8 times higher MO degradation activity than that of bulk g-C3N4, and the sample H–CN200–C degraded 95.5% dye after 120 min [123].
In some studies, both doping and coupling are applied to form doped g-C3N4 heterojunctions, in order to enhance MO dye degradation. For example, g-C3N4 was doped with Cl and then coupled with Bi2WO6 to obtain ClCN/Bi2WO6 heterojunctions. In this study, Bi2WO6 and ClCN degraded only 20% and 30%, while 10% ClCN/Bi2WO6 degraded 99% MO dye in 40 min. The enhanced photocatalytic efficiency was credited to the creation of S-scheme heterojunctions, which inhibited the separated charges’ recombination, but accelerated the recombination of the relatively useless holes and electrons [124]. Other such g-C3N4-based photocatalysts for MO dye degradation reported are B-doped g-C3N4/MoO3 [125], Fe-doped ZrO2 nanoparticle-supported g-C3N4 hybrids [126], CeO2/P-C3N4 composites [127], etc.
4. Crystal Phase Control and Defects Introduction
Modification of the bandgap for improved photocatalytic efficiency can also be achieved by variations in the crystal phase, as well as facet control [78]. The crystallinity of g-C3N4 has been altered by incorporating Ag with g-C3N4 via calcination for 8 h. The obtained Ag-decorated g-C3N4 achieved the degradation of MO, and degraded 98.7% of dye in 2 h. Calcination broadened the range of visible light responses, as well as conferred a high surface area, high Ag dispersibility, and low recombination rate of photogenerated e−/h+ recombination [128]. Porous graphitic carbon nitride (p-C3N4) has been prepared via a simple pyrolyzing treatment of g-C3N4, which introduces structural defects into the g-C3N4 via breaking some bonds, as shown in the Figure 6. The defects were found to be advantageous for the generation of e−/h+ charges and the prevention of the recombination of these charges. The p-C3N4 exhibited a narrow band gap for promoting visible light utilization as compared with g-C3N4. p-C3N4 degraded 90% of the MO dye in 90 min, which was sufficiently higher than the activity of g-C3N4 (only 19% after 90 min) under visible light [129]. Similarly, the results discussed in the doping section about the P and S-co-doped g-C3N4 indicate that P and S co-doping causes defects in the sample structure. The structure defects could trap photo-induced electrons, which can promote the e−/h+ separation, inhibiting the recombination of the photogenerated charges and prolonging their lifetime [121].
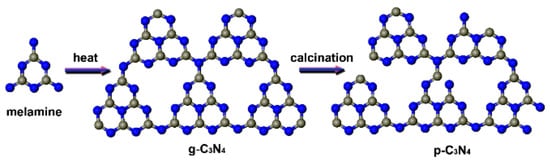
Figure 6.
Schematic diagram for the formation of p-C3N4 [129].
Similarly g-C3N4 molecules were also used for enhancing the photocatalytic activity of other materials, such as graphene aerogels [130], for the efficient photodegradation of MO dye.
5. Conclusions and Future Perspectives
The photodegradation of MO is the most effective method for its complete mineralization. g-C3N4 based materials are widely utilized in photo-catalytic materials owing to their low raw material prices, high physicochemical stability, easy and simple synthesis, earth-abundant nature and fascinating electronic band structure. The lower degradation efficiency of g-C3N4 can be enhanced via coupling with other materials to form composites and heterojunctions. Efficiency can also be enhanced through introducing structural defects, crystal phase control and doping with metals and non-metals. Preparation of composites and heterojunctions is the most followed approach, because the resulted materials exhibit improved photocatalytic efficiency, owing to their synergistic effects. Similarly, the structural defects were found to be beneficial for the e−/h+ generation and inhibition of the charge recombination.
There are a few dimensions that still need thorough investigations. Some of them are the following.
The majority of the g-C3N4 based photocatalysts are not economical and their preparation methods are quite complex. Therefore, exploring the unprecedented g-C3N4-based photocatalysts, which are economical, easy to prepare and have superior photocatalytic characteristics to cope with the industrial needs, is very important.
Each individual process of photodegradation of MO dye through g-C3N4 based photocatalysts needs to be visualized specifically through in situ characterization techniques. Through in situ characterizations, one can fabricate highly robust, cost-effective and perfectly photon energy-matched catalysts with commercial feasibility and effective photostability.
Monitoring of the photodegradation of dyes in aqueous solutions by recording the changes in UV-Vis absorption may not allow the accurate detection of the full degradation of organic pollutants. Therefore, it is suggested to use other monitoring techniques.
Mechanistic understanding of the PD of MO by g-C3N4-based photocatalysts has been examined, but researchers need to further investigate this with theoretical and computational and support. Through computational approaches, researchers are able to design g-C3N4-based photocatalysts for the efficient adsorption and photodegradation of MO dyes. Through this approach, researchers can also choose effective doping and coupling materials for g-C3N4, for the efficient adsorption and photodegradation of MO dyes. DFT calculations can suggest dye degradation mechanisms and are helpful for supporting the obtained experimental results.
Author Contributions
Conceptualization, A.A. (Abdulelah Aljuaid) and A.A.A.; methodology, M.A. (Mamdouh Allahyani); software, O.A.; validation, J.A.A.; formal analysis, M.S.; investigation, R.T.A.; resources, A.A. (Abdulaziz Alsharif); data curation, O.A.; writing—original draft preparation, I.K.; writing—review and editing, I.K.; visualization, M.A. (Mazen Almehmadi); supervision, I.K.; project administration, M.A. (Mazen Almehmadi); All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge Northwestern Polytechnical University China and Taif University Saudi Arabia for funding this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rajaram, T.; Das, A. Water pollution by industrial effluents in India: Discharge scenarios and case for participatory ecosystem specific local regulation. Futures 2008, 40, 56–69. [Google Scholar] [CrossRef]
- Bibi, S.; Ahmad, A.; Anjum, M.A.R.; Haleem, A.; Siddiq, M.; Shah, S.S.; Kahtani, A. Al Photocatalytic degradation of malachite green and methylene blue over reduced graphene oxide (rGO) based metal oxides (rGO-Fe3O4/TiO2) nanocomposite under UV-visible light irradiation. J. Environ. Chem. Eng. 2021, 9, 105580. [Google Scholar] [CrossRef]
- Prasad, A.R.; Joseph, A. Synthesis, characterization and investigation of methyl orange dye removal from aqueous solutions using waterborne poly vinyl pyrrolidone (PVP) stabilized poly aniline (PANI) core-shell nanoparticles. RSC Adv. 2017, 7, 20960–20968. [Google Scholar] [CrossRef]
- Khan, N.; Khan, I.; Zada, N.; Sadiq, M.; Saeed, K. Utilization of cross-linked chitosan for cobalt adsorption and its reutilization as a photocatalyst for the photodegradation of methyl violet dye in aqueous medium. Appl. Water Sci. 2022, 12, 107. [Google Scholar] [CrossRef]
- Khan, N.A.; Saeed, K.; Khan, I.; Gul, T.; Sadiq, M.; Uddin, A.; Zekker, I. Efficient photodegradation of orange II dye by nickel oxide nanoparticles and nanoclay supported nickel oxide nanocomposite. Appl. Water Sci. 2022, 12, 131. [Google Scholar] [CrossRef]
- Ahmad, S.; Almehmadi, M.; Janjuhah, H.T.; Kontakiotis, G.; Abdulaziz, O.; Saeed, K.; Ahmad, H.; Allahyani, M.; Aljuaid, A.; Alsaiari, A.A.; et al. The Effect of Mineral Ions Present in Tap Water on Photodegradation of Organic Pollutants: Future Perspectives. Water 2023, 15, 175. [Google Scholar] [CrossRef]
- Shah, L.A.; Malik, T.; Siddiq, M.; Haleem, A.; Sayed, M.; Naeem, A. TiO2 nanotubes doped poly(vinylidene fluoride) polymer membranes (PVDF/TNT) for efficient photocatalytic degradation of brilliant green dye. J. Environ. Chem. Eng. 2019, 7, 103291. [Google Scholar] [CrossRef]
- Haleem, A.; Shafiq, A.; Chen, S.Q.; Nazar, M. A Comprehensive Review on Adsorption, Photocatalytic and Chemical Degradation of Dyes and Nitro-Compounds over Different Kinds of Porous and Composite Materials. Molecules 2023, 28, 1081. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Al-Degs, Y.S.; Sweileh, J.A. Simultaneous determination of five commercial cationic dyes in stream waters using diatomite solid-phase extractant and multivariate calibration. Arab. J. Chem. 2012, 5, 219–224. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, A.; Tripathi, A.; Das, M. Sunset yellow FCF, a permitted food dye, alters functional responses of splenocytes at non-cytotoxic dose. Toxicol. Lett. 2013, 217, 197–204. [Google Scholar] [CrossRef]
- Liu, Y.; Song, L.; Du, L.; Gao, P.; Liang, N.; Wu, S.; Minami, T.; Zang, L.; Yu, C.; Xu, X. Preparation of polyaniline/emulsion microsphere composite for efficient adsorption of organic dyes. Polymers 2020, 12, 167. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Joshiba, G.J. Sustainable approach to decolourize methyl orange dye from aqueous solution using novel bacterial strain and its metabolites characterization. Clean Technol. Environ. Policy 2021, 23, 173–181. [Google Scholar] [CrossRef]
- Youssef, N.A.; Shaban, S.A.; Ibrahim, F.A.; Mahmoud, A.S. Degradation of methyl orange using Fenton catalytic reaction. Egypt. J. Pet. 2016, 25, 317–321. [Google Scholar] [CrossRef]
- Farhan Hanafi, M.; Sapawe, N. A review on the water problem associate with organic pollutants derived from phenol, methyl orange, and remazol brilliant blue dyes. Mater. Today Proc. 2020, 31, A141–A150. [Google Scholar] [CrossRef]
- Alghamdi, A.A.; Al-Odayni, A.-B.; Saeed, W.S.; Almutairi, M.S.; Alharthi, F.A.; Aouak, T.; Al-Kahtani, A. Adsorption of Azo Dye Methyl Orange from Aqueous Solutions Using Alkali-Activated Polypyrrole-Based Graphene Oxide. Molecules 2019, 24, 3685. [Google Scholar] [CrossRef] [PubMed]
- Rohilla, S.; Gupta, A.; Kumar, V.; Kumari, S.; Petru, M.; Amor, N.; Noman, M.T.; Dalal, J. Excellent UV-Light Triggered Photocatalytic Performance of ZnO.SiO2 Nanocomposite for Water Pollutant Compound Methyl Orange Dye. Nanomater 2021 2021, 11, 2548. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Obando, V.A.; García-Mora, A.-M.; Basante, J.S.; Hidalgo, A.; Galeano, L.-A. CWPO Degradation of Methyl Orange at Circumneutral pH: Multi-Response Statistical Optimization, Main Intermediates and by-Products. Front. Chem. 2019, 7, 772. [Google Scholar] [CrossRef]
- Huang, J.H.; Huang, K.L.; Liu, S.Q.; Wang, A.T.; Yan, C. Adsorption of Rhodamine B and methyl orange on a hypercrosslinked polymeric adsorbent in aqueous solution. Colloids Surfaces A Physicochem. Eng. Asp. 2008, 330, 55–61. [Google Scholar] [CrossRef]
- Masarbo, R.S.; Ismailsab, M.; Monisha, T.R.; Nayak, A.S.; Karegoudar, T.B. Enhanced decolorization of sulfonated azo dye methyl orange by single and mixed bacterial strains AK1, AK2 and VKY1. Bioremediat. J. 2018, 22, 136–146. [Google Scholar] [CrossRef]
- Modrogan, C.; Cǎprǎrescu, S.; Dǎncilǎ, A.M.; Orbuleț, O.D.; Grumezescu, A.M.; Purcar, V.; Radițoiu, V.; Fierascu, R.C. Modified Composite Based on Magnetite and Polyvinyl Alcohol: Synthesis, Characterization, and Degradation Studies of the Methyl Orange Dye from Synthetic Wastewater. Polymers 2021, 13, 3911. [Google Scholar] [CrossRef]
- Kishor, R.; Purchase, D.; Saratale, G.D.; Romanholo Ferreira, L.F.; Hussain, C.M.; Mulla, S.I.; Bharagava, R.N. Degradation mechanism and toxicity reduction of methyl orange dye by a newly isolated bacterium Pseudomonas aeruginosa MZ520730. J. Water Process. Eng. 2021, 43, 102300. [Google Scholar] [CrossRef]
- Dey, P.C.; Das, R. Enhanced photocatalytic degradation of methyl orange dye on interaction with synthesized ligand free CdS nanocrystals under visible light illumination. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2020, 231, 118122. [Google Scholar] [CrossRef] [PubMed]
- Trofimovaite, R.; Parlett, C.M.A.; Kumar, S.; Frattini, L.; Isaacs, M.A.; Wilson, K.; Olivi, L.; Coulson, B.; Debgupta, J.; Douthwaite, R.E.; et al. Single atom Cu(I) promoted mesoporous titanias for photocatalytic Methyl Orange depollution and H2 production. Appl. Catal. B Environ. 2018, 232, 501–511. [Google Scholar] [CrossRef]
- Qu, W.; He, D.; Huang, H.; Guo, Y.; Tang, Y.; Song, R.J. Characterization of amino-crosslinked hypromellose and its adsorption characteristics for methyl orange from water. J. Mater. Sci. 2020, 55, 7268–7282. [Google Scholar] [CrossRef]
- Ali, I.; Burakova, I.; Galunin, E.; Burakov, A.; Mkrtchyan, E.; Melezhik, A.; Kurnosov, D.; Tkachev, A.; Grachev, V. High-Speed and High-Capacity Removal of Methyl Orange and Malachite Green in Water Using Newly Developed Mesoporous Carbon: Kinetic and Isotherm Studies. ACS Omega 2019, 4, 19293–19306. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; El-Fakharany, E.M.; Abu-Serie, M.M.; Elkady, M.F.; Eltarahony, M. Methyl Orange Biodegradation by Immobilized Consortium Microspheres: Experimental Design Approach, Toxicity Study and Bioaugmentation Potential. Biology 2022, 11, 76. [Google Scholar] [CrossRef]
- Fan, J.; Guo, Y.; Wang, J.; Fan, M. Rapid decolorization of azo dye methyl orange in aqueous solution by nanoscale zerovalent iron particles. J. Hazard. Mater. 2009, 166, 904–910. [Google Scholar] [CrossRef]
- Khan, I.; Khan, I.; Usman, M.; Imran, M.; Saeed, K. Nanoclay-mediated photocatalytic activity enhancement of copper oxide nanoparticles for enhanced methyl orange photodegradation. J. Mater. Sci. Mater. Electron. 2020, 31, 8971–8985. [Google Scholar] [CrossRef]
- Maruthanayagam, A.; Mani, P.; Kaliappan, K.; Chinnappan, S. In vitro and In silico Studies on the Removal of Methyl Orange from Aqueous Solution Using Oedogonium subplagiostomum AP1. Water. Air. Soil Pollut. 2020, 231, 232. [Google Scholar] [CrossRef]
- Haque, M.M.; Haque, M.A.; Mosharaf, M.K.; Marcus, P.K. Decolorization, degradation and detoxification of carcinogenic sulfonated azo dye methyl orange by newly developed biofilm consortia. Saudi J. Biol. Sci. 2021, 28, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Aziz, A.R.A.; Mazari, S.A.; Baloch, A.G.; Nizamuddin, S. Photocatalytic degradation of methyl orange from wastewater using a newly developed Fe-Cu-Zn-ZSM-5 catalyst. Environ. Sci. Pollut. Res. 2020, 27, 26239–26248. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Gul, S.; Khan, M.I.; Khan, M.A.; Asiri, A.M.; Khan, S.B. Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange. Green Process. Synth. 2019, 8, 118–127. [Google Scholar] [CrossRef]
- Safavi-Mirmahalleh, S.A.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. Adsorption kinetics of methyl orange from water by pH-sensitive poly(2-(dimethylamino)ethyl methacrylate)/nanocrystalline cellulose hydrogels. Environ. Sci. Pollut. Res. 2020, 27, 28091–28103. [Google Scholar] [CrossRef]
- Wu, W.; Yao, T.; Xiang, Y.; Zou, H.; Zhou, Y. Efficient removal of methyl orange by a flower-like TiO2/MIL-101(Cr) composite nanomaterial. Dalt. Trans. 2020, 49, 5722–5729. [Google Scholar] [CrossRef] [PubMed]
- Haitham, K.; Razak, S.; Nawi, M.A. Kinetics and isotherm studies of methyl orange adsorption by a highly recyclable immobilized polyaniline on a glass plate. Arab. J. Chem. 2019, 12, 1595–1606. [Google Scholar] [CrossRef]
- Akansha, K.; Chakraborty, D.; Sachan, S.G. Decolorization and degradation of methyl orange by Bacillus stratosphericus SCA1007. Biocatal. Agric. Biotechnol. 2019, 18, 101044. [Google Scholar] [CrossRef]
- Al-Baldawi, I.A.; Abdullah, S.R.S.; Almansoory, A.F.; Ismail, N.; Hasan, H.A.; Anuar, N. Role of Salvinia molesta in biodecolorization of methyl orange dye from water. Sci. Rep. 2020, 10, 13980. [Google Scholar] [CrossRef]
- Nashmi, O.A.; Mohammed, A.A.; Abdulrazzaq, N.N. Investigation of Ozone Microbubbles for the Degradation of Methylene Orange Contaminated Wastewater. Iraqi J. Chem. Pet. Eng. 2020, 21, 1997–4884. [Google Scholar] [CrossRef]
- Othmani, B.; Gamelas, J.A.F.; Rasteiro, M.G.; Khadhraoui, M. Characterization of Two Cactus Formulation-Based Flocculants and Investigation on Their Flocculating Ability for Cationic and Anionic Dyes Removal. Polymers 2020, 12, 1964. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Hossaini, H.; Nasseri, S.; Azizi, N.; Shahmoradi, B.; Khosravi, T. Optimization of photocatalytic degradation of methyl orange using immobilized scoria-Ni/TiO2 nanoparticles. J. Nanostruct. Chem. 2020, 10, 143–159. [Google Scholar] [CrossRef]
- Shan, R.; Lu, L.; Gu, J.; Zhang, Y.; Yuan, H.; Chen, Y.; Luo, B. Photocatalytic degradation of methyl orange by Ag/TiO2/biochar composite catalysts in aqueous solutions. Mater. Sci. Semicond. Process. 2020, 114, 105088. [Google Scholar] [CrossRef]
- Li, M.; Guan, R.; Li, J.; Zhao, Z.; Zhang, J.; Qi, Y.; Zhai, H.; Wang, L. Photocatalytic Performance and Mechanism Research of Ag/HSTiO2on Degradation of Methyl Orange. ACS Omega 2020, 5, 21451–21457. [Google Scholar] [CrossRef]
- Chen, H.; Xue, C.; Cui, D.; Liu, M.; Chen, Y.; Li, Y.; Zhang, W. Co3O4–Ag photocatalysts for the efficient degradation of methyl orange. RSC Adv. 2020, 10, 15245–15251. [Google Scholar] [CrossRef]
- Oyarce, E.; Butter, B.; Santander, P.; Sánchez, J. Polyelectrolytes applied to remove methylene blue and methyl orange dyes from water via polymer-enhanced ultrafiltration. J. Environ. Chem. Eng. 2021, 9, 106297. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Ali, N.; Khan, I.; Zhang, B.; Sadiq, M. Heterogeneous photodegradation of industrial dyes: An insight to different mechanisms and rate affecting parameters. J. Environ. Chem. Eng. 2020, 8, 104364. [Google Scholar] [CrossRef]
- Mecha, A.C.; Chollom, M.N. Photocatalytic ozonation of wastewater: A review. Environ. Chem. Lett. 2020, 18, 1491–1507. [Google Scholar] [CrossRef]
- Ramos, P.G.; Luyo, C.; Sánchez, L.A.; Gomez, E.D.; Rodriguez, J.M. The Spinning Voltage Influence on the Growth of ZnO-rGO Nanorods for Photocatalytic Degradation of Methyl Orange Dye. Catalysts 2020, 10, 660. [Google Scholar] [CrossRef]
- Tsuji, M.; Matsuda, K.; Tanaka, M.; Kuboyama, S.; Uto, K.; Wada, N.; Kawazumi, H.; Tsuji, T.; Ago, H.; Hayashi, J. Enhanced Photocatalytic Degradation of Methyl Orange by Au/TiO 2 Nanoparticles under Neutral and Acidic Solutions. ChemistrySelect 2018, 3, 1432–1438. [Google Scholar] [CrossRef]
- Shaheen, K.; Suo, H.; Shah, Z.; Khush, L.; Arshad, T.; Khan, S.B.; Siddique, M.; Ma, L.; Liu, M.; Cui, J.; et al. Ag–Ni and Al–Ni nanoparticles for resistive response of humidity and photocatalytic degradation of Methyl Orange dye. Mater. Chem. Phys. 2020, 244, 122748. [Google Scholar] [CrossRef]
- Saeed, K.; Khan, I.; Shah, T.; Park, S.-Y. Synthesis, characterization and photocatalytic activity of silver nanoparticles/amidoxime-modified polyacrylonitrile nanofibers. Fibers Polym. 2015, 16, 1870–1875. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation of rhodamine B and methyl orange over boron-doped g-C 3N4 under visible light irradiation. Langmuir 2010, 26, 3894–3901. [Google Scholar] [CrossRef] [PubMed]
- Dorraj, M.; Sadjadi, S.; Heravi, M.M. Pd on poly(1-vinylimidazole) decorated magnetic S-doped grafitic carbon nitride: An efficient catalyst for catalytic reduction of organic dyes. Sci. Rep. 2020, 10, 13340. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Sun, Y.; Dong, F. Graphitic carbon nitride based nanocomposites: A review. Nanoscale 2015, 7, 15–37. [Google Scholar] [CrossRef]
- Lun, Y.; Liu, S.; Liang, Y.; Yan, G.; He, G.; Wang, Y.; He, Q. A nearly complete decomposition of MO, TC and OFX over a direct Z-scheme p-n heterojunction g-C3N4/La-Bi2O3 composite. J. Alloys Compd. 2023, 934, 167554. [Google Scholar] [CrossRef]
- Hou, L.; Wu, Z.; Jin, C.; Li, W.; Wei, Q.; Chen, Y.; Wang, T. Flower-like dual-defective z-scheme heterojunction g-C3N4/Znin2s4 high-efficiency photocatalytic hydrogen evolution and degradation of mixed pollutants. Nanomaterials 2021, 11, 2483. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Chen, Q.; Chao, C.; Sun, J.; Dong, S.; Sun, Y. Synthesis of rGO/g-C3N4 for methyl orange degradation in activating peroxydisulfate under simulated solar light irradiation. J. Alloys Compd. 2022, 907, 164500. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, J.; Jin, B.; Wu, Z.; Yang, S.; Zhang, S.; Tian, Y.; Fang, Y.; Hou, Y.; Zhou, X. Sustainable synthesis of low-cost nitrogen-doped-carbon coated Co3W3C@g-C3N4 composite photocatalyst for efficient hydrogen evolution. Chem. Eng. J. 2021, 426, 131208. [Google Scholar] [CrossRef]
- Hayat, A.; Shah Syed, J.A.; Al-Sehemi, A.G.; El-Nasser, K.S.; Taha, T.A.; Al-Ghamdi, A.A.; Amin, M.A.; Ajmal, Z.; Iqbal, W.; Palamanit, A.; et al. State of the art advancement in rational design of g-C3N4 photocatalyst for efficient solar fuel transformation, environmental decontamination and future perspectives. Int. J. Hydrog. Energy 2022, 47, 10837–10867. [Google Scholar] [CrossRef]
- Alaghmandfard, A.; Ghandi, K. A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)–Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing. Nanomaterials 2022, 12, 294. [Google Scholar] [CrossRef]
- Alharthi, F.A.; Alghamdi, A.A.; Alanazi, H.S.; Alsyahi, A.A.; Ahmad, N. Photocatalytic Degradation of the Light Sensitive Organic Dyes: Methylene Blue and Rose Bengal by Using Urea Derived g-C3N4/ZnO Nanocomposites. Catalysts 2020, 10, 1457. [Google Scholar] [CrossRef]
- Guan, R.; Li, J.; Zhang, J.; Zhao, Z.; Wang, D.; Zhai, H.; Sun, D. Photocatalytic Performance and Mechanistic Research of ZnO/g-C3N4 on Degradation of Methyl Orange. ACS Omega 2019, 4, 20742–20747. [Google Scholar] [CrossRef] [PubMed]
- Monga, D.; Basu, S. Enhanced photocatalytic degradation of industrial dye by g-C3N4/TiO2 nanocomposite: Role of shape of TiO2. Adv. Powder Technol. 2019, 30, 1089–1098. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, J.; Ding, Y.; Liu, B.; Zhao, L.; Zhang, S. Research progress on g–C3N4–based photocatalysts for organic pollutants degradation in wastewater: From exciton and carrier perspectives. Ceram. Int. 2021, 47, 31005–31030. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Hou, H.; Shao, G.; Yang, W. Recent advances in g-C3N4-based photocatalysts incorporated by MXenes and their derivatives. J. Mater. Chem. A 2021, 9, 13722–13745. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Z.; Yao, Y.; Li, Y.; Cheema, W.A.; Wang, D.; Zhu, S. Recent advancements in g-C3N4-based photocatalysts for photocatalytic CO2 reduction: A mini review. RSC Adv. 2020, 10, 29408–29418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Y.; Shuai, D.; Shen, Y.; Xiong, W.; Wang, L. Graphitic carbon nitride (g-C3N4)-based photocatalysts for water disinfection and microbial control: A review. Chemosphere 2019, 214, 462–479. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, X. A Review on Quantum Dots Modified g-C3N4-Based Photocatalysts with Improved Photocatalytic Activity. Catalysts 2020, 10, 142. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Zhang, H.; Fan, J.; Xiang, Q. Design and application of active sites in g-C3N4-based photocatalysts. J. Mater. Sci. Technol. 2020, 56, 69–88. [Google Scholar] [CrossRef]
- Zhang, S.; Gu, P.; Ma, R.; Luo, C.; Wen, T.; Zhao, G.; Cheng, W.; Wang, X. Recent developments in fabrication and structure regulation of visible-light-driven g-C3N4-based photocatalysts towards water purification: A critical review. Catal. Today 2019, 335, 65–77. [Google Scholar] [CrossRef]
- He, F.; Wang, Z.; Li, Y.; Peng, S.; Liu, B. The nonmetal modulation of composition and morphology of g-C3N4-based photocatalysts. Appl. Catal. B Environ. 2020, 269, 118828. [Google Scholar] [CrossRef]
- Sahoo, A.; Patra, S. A magnetically separable and recyclable g-C3N4/Fe3O4/porous ruthenium nanocatalyst for the photocatalytic degradation of water-soluble aromatic amines and azo dyes. RSC Adv. 2020, 10, 6043–6051. [Google Scholar] [CrossRef] [PubMed]
- Ismael, M. A review on graphitic carbon nitride (g-C3N4) based nanocomposites: Synthesis, categories, and their application in photocatalysis. J. Alloys Compd. 2020, 846, 156446. [Google Scholar] [CrossRef]
- Liang, H.; Zhu, C.; Wang, A.; Palanisamy, K.; Chen, F. Facile synthesis of NiAl2O4/g-C3N4 composite for efficient photocatalytic degradation of tetracycline. J. Environ. Sci. 2023, 127, 700–713. [Google Scholar] [CrossRef]
- Phoon, B.L.; Ong, C.C.; Lee, K.C.; Pan, G.T.; Leo, B.F.; Chong, S.; Pan, K.L. Co-Doped, Tri-Doped, and Rare-Earth-Doped g-C3N4 for Photocatalytic Applications: State-of-the-Art. Catalysts 2022, 12, 586. [Google Scholar] [CrossRef]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Recent progress in g-C3N4, TiO2 and ZnO based photocatalysts for dye degradation: Strategies to improve photocatalytic activity. Sci. Total Environ. 2021, 767, 144896. [Google Scholar] [CrossRef]
- Suresh, R.; Karthikeyan, N.S.; Gnanasekaran, L.; Rajendran, S.; Soto-Moscoso, M. Facile synthesis of CuO/g-C3N4nanolayer composites with superior catalytic reductive degradation behavior. Chemosphere 2023, 315, 137711. [Google Scholar] [CrossRef]
- Mahalakshmi, G.; Rajeswari, M.; Ponnarasi, P. Synthesis of few-layer g-C3N4nanosheets-coated MoS2/TiO2 heterojunction photocatalysts for photo-degradation of methyl orange (MO) and 4-nitrophenol (4-NP) pollutants. Inorg. Chem. Commun. 2020, 120, 108146. [Google Scholar] [CrossRef]
- Ghattavi, S.; Nezamzadeh-Ejhieh, A. A visible light driven AgBr/g-C3N4photocatalyst composite in methyl orange photodegradation: Focus on photoluminescence, mole ratio, synthesis method of g-C3N4 and scavengers. Compos. Part B Eng. 2020, 183, 107712. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, X.; Liu, X.; Yang, X.; Yang, Y. A Ternary Magnetic Recyclable ZnO/Fe3O4/g-C3N4 Composite Photocatalyst for Efficient Photodegradation of Monoazo Dye. Nanoscale Res. Lett. 2019, 14, 147. [Google Scholar] [CrossRef] [PubMed]
- Ghattavi, S.; Nezamzadeh-Ejhieh, A. GC-MASS detection of methyl orange degradation intermediates by AgBr/g-C3N4: Experimental design, bandgap study, and characterization of the catalyst. Int. J. Hydrogen Energy 2020, 45, 24636–24656. [Google Scholar] [CrossRef]
- Zeng, L.; He, Z.; Luo, Y.; Xu, J.; Chen, J.; Wu, L.; Huang, P.; Xu, S. A Simple g-C3N4/TNTs Heterojunction for Improving the Photoelectrocatalytic Degradation of Methyl Orange. J. Electrochem. Soc. 2021, 168, 116520. [Google Scholar] [CrossRef]
- Malik, M.; Len, T.; Luque, R.; Osman, S.M.; Paone, E.; Khan, M.I.; Wattoo, M.A.; Jamshaid, M.; Anum, A.; Rehman, A. ur Investigation on synthesis of ternary g-C3N4/ZnO–W/M nanocomposites integrated heterojunction II as efficient photocatalyst for environmental applications. Environ. Res. 2023, 217, 114621. [Google Scholar] [CrossRef]
- Kuldeep, A.R.; Dhabbe, R.S.; Garadkar, K.M. Development of g-C3N4-TiO2 visible active hybrid photocatalyst for the photodegradation of methyl orange. Res. Chem. Intermed. 2021, 47, 5155–5174. [Google Scholar] [CrossRef]
- Pourshirband, N.; Nezamzadeh-Ejhieh, A.; Mirsattari, S.N. The CdS/g-C3N4 nano-photocatalyst: Brief characterization and kinetic study of photodegradation and mineralization of methyl orange. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 248, 119110. [Google Scholar] [CrossRef]
- He, Y.; Zhang, L.; Wang, X.; Wu, Y.; Lin, H.; Zhao, L.; Weng, W.; Wan, H.; Fan, M. Enhanced photodegradation activity of methyl orange over Z-scheme type MoO3–g-C3N4 composite under visible light irradiation. RSC Adv. 2014, 4, 13610–13619. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, Y.; Wang, Y.; Yang, Y.; Wang, P.; Shi, L.; Feng, L.; Fang, S.; Liu, Q.; Ma, L.; et al. Synthesis and photocatalytic activity of g-C3N4/ZnO composite microspheres under visible light exposure. Ceram. Int. 2022, 48, 3293–3302. [Google Scholar] [CrossRef]
- Ismael, M.; Wark, M. Photocatalytic activity of CoFe2O4/g-C3N4 nanocomposite toward degradation of different organic pollutants and their inactivity toward hydrogen production: The role of the conduction band position. FlatChem 2022, 32, 100337. [Google Scholar] [CrossRef]
- Feng, Z.; Zeng, L.; Zhang, Q.; Ge, S.; Zhao, X.; Lin, H.; He, Y. In situ preparation of g-C3N4/Bi4O5I2 complex and its elevated photoactivity in Methyl Orange degradation under visible light. J. Environ. Sci. 2020, 87, 149–162. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Chen, Y.; Situ, Y.; Zhong, L.; Huang, H. Synthesis of ternary g-C3N4/Ag/γ-FeOOH photocatalyst: An integrated heterogeneous Fenton-like system for effectively degradation of azo dye methyl orange under visible light. Appl. Surf. Sci. 2017, 425, 862–872. [Google Scholar] [CrossRef]
- Shi, H.; Feng, D.; Li, H.; Yu, D.; Chen, X. Hydrophilic hydrogen-bonded organic frameworks/g-C3N4 all-organic Z-scheme heterojunction for efficient visible-light photocatalytic hydrogen production and dye degradation. J. Photochem. Photobiol. A Chem. 2023, 435, 114292. [Google Scholar] [CrossRef]
- Wang, H.; Lu, J.; Wang, F.; Wei, W.; Chang, Y.; Dong, S. Preparation, characterization and photocatalytic performance of g-C3N4/Bi2WO6 composites for methyl orange degradation. Ceram. Int. 2014, 40, 9077–9086. [Google Scholar] [CrossRef]
- Yassin, J.M.; Taddesse, A.M.; Sánchez-Sánchez, M. Sustainable synthesis of a new semiamorphous Ti-BDC MOF material and the photocatalytic performance of its ternary composites with Ag3PO4 and g-C3N4. Appl. Surf. Sci. 2022, 578, 151996. [Google Scholar] [CrossRef]
- Mukhair, H.M.; Abdullah, A.H.; Zainal, Z.; Lim, H.N. Pes-Ag3Po4/g-C3N4 mixed matrix film photocatalyst for degradation of methyl orange dye. Polymers 2021, 13, 1746. [Google Scholar] [CrossRef]
- Xian, T.; Yang, H.; Di, L.J.; Dai, J.F. Enhanced photocatalytic activity of BaTiO3@g-C3N4 for the degradation of methyl orange under simulated sunlight irradiation. J. Alloys Compd. 2015, 622, 1098–1104. [Google Scholar] [CrossRef]
- Hu, Q.; Ren, T.; Deng, D.; Xu, L.; Chen, J.; Xiao, Y.; He, P. Constructing carbon microspheres/MnFe2O4/g-C3N4 composite photocatalysts for enhanced photocatalytic activity under visible light irradiation. Inorg. Chem. Commun. 2021, 134, 108947. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, Y.; Du, Z.; He, J.; Zhong, J.; Zhao, L.; She, H.; Liu, G.; Su, B. Synthesis of Rod-Like g-C3N4/ZnS Composites with Superior Photocatalytic Activity for the Degradation of Methyl Orange. Eur. J. Inorg. Chem. 2015, 2015, 4108–4115. [Google Scholar] [CrossRef]
- Warshagha, M.; Muneer, M. Synthesis of ZnO Co-doped Ph-g-C3N4 for enhanced photocatalytic organic pollutants removal under visible light. Int. J. Environ. Anal. Chem. 2020, 102, 6339–6358. [Google Scholar] [CrossRef]
- Huang, Z.; Jia, S.; Wei, J.; Shao, Z. A visible light active, carbon–nitrogen–sulfur co-doped TiO2/g-C3N4 Z-scheme heterojunction as an effective photocatalyst to remove dye pollutants. RSC Adv. 2021, 11, 16747–16754. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Han, C.; Liu, J. Novel visible light-induced g-C3N4/Bi2WO6 composite photocatalysts for efficient degradation of methyl orange. Appl. Catal. B Environ. 2011, 108–109, 100–107. [Google Scholar] [CrossRef]
- Santha kumar, K.; Vellaichamy, B.; Paulmony, T. Visible light active metal-free photocatalysis: N-doped graphene covalently grafted with g-C3N4 for highly robust degradation of methyl orange. Solid State Sci. 2019, 94, 99–105. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, Y.; Yin, B.; Chen, J.; Guo, M.; Gao, X. Wood powder biochar in CdS-WPB-g-C3N4 heterojunction as an electron transfer medium for enhancing photocatalytic performance toward degradation methyl orange. J. Environ. Chem. Eng. 2023, 11, 109135. [Google Scholar] [CrossRef]
- Manjari Mishra, P.; Pattnaik, S.; Prabha Devi, A. Green synthesis of bio-based Au@g-C3N4 nanocomposite for photocatalytic degradation of methyl orange. Mater. Today Proc. 2021, 47, 1218–1223. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, M.; Wu, J.; Zheng, X.; Liao, S.; Ou, B.; Tian, L. In situ embedment of CoOx on g-C3N4 as Z scheme heterojunction for efficient photocatalytic degradation of methyl orange and phenol under visible light. J. Alloys Compd. 2022, 927, 167047. [Google Scholar] [CrossRef]
- Han, C.; Ge, L.; Chen, C.; Li, Y.; Xiao, X.; Zhang, Y.; Guo, L. Novel visible light induced Co3O4-g-C3N4 heterojunction photocatalysts for efficient degradation of methyl orange. Appl. Catal. B Environ. 2014, 147, 546–553. [Google Scholar] [CrossRef]
- Bhosale, A.; Gophane, A.; Kadam, J.; Sabale, S.; Sonawane, K.; Garadkar, K. Fabrication of visible-active ZnO-gC3N4 nanocomposites for photodegradation and cytotoxicity of methyl orange and antibacterial activity towards drug resistance pathogens. Opt. Mater. 2023, 136, 113392. [Google Scholar] [CrossRef]
- Sohrabian, M.; Mahdikhah, V.; Alimohammadi, E.; Sheibani, S. Improved photocatalytic performance of SrTiO3 through a Z-scheme polymeric-perovskite heterojunction with g-C3N4 and plasmonic resonance of Ag mediator. Appl. Surf. Sci. 2023, 618, 156682. [Google Scholar] [CrossRef]
- Rao, V.S.; Sharma, R.; Paul, D.R.; Almáši, M.; Sharma, A.; Kumar, S.; Nehra, S.P. Architecting the Z-scheme heterojunction of Gd2O3/g-C3N4 nanocomposites for enhanced visible-light-induced photoactivity towards organic pollutants degradation. Environ. Sci. Pollut. Res. 2023, 1–14. Online ahead of print. [Google Scholar] [CrossRef]
- Gong, W.; Wu, Q.; Ma, L.; Zhang, W.; Li, X.; Xu, A.; Zhao, S. MnOx/g-C3N4 nanocomposites mediated sulfite activation for enhanced organic pollutants degradation under visible light irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 659, 130812. [Google Scholar] [CrossRef]
- Li, Z.; Li, B.; Peng, S.; Li, D.; Yang, S.; Fang, Y. Novel visible light-induced g-C3N4 quantum dot/BiPO4 nanocrystal composite photocatalysts for efficient degradation of methyl orange. RSC Adv. 2014, 4, 35144–35148. [Google Scholar] [CrossRef]
- Zarei, M.; Mohammadzadeh, I.; Saidi, K.; Sheibani, H. g-C3N4 quantum dot decorated MoS2/Fe3O4 as a novel recoverable catalyst for photodegradation of organic pollutant under visible light. J. Mater. Sci. Mater. Electron. 2021, 32, 26213–26231. [Google Scholar] [CrossRef]
- Zhou, C.; Zhan, P.; Zhao, J.; Tang, X.; Liu, W.; Jin, M.; Wang, X. Long-lasting CaAl2O4:Eu2+,Nd3+ phosphor-coupled g-C3N4 QDs composites for the round-the-clock photocatalytic methyl orange degradation. Ceram. Int. 2020, 46, 27884–27891. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Zhang, K.; Lu, P.; Zhang, D. Facile fabrication of sandwich-like BiOI/AgI/g-C3N4 composites for efficient photocatalytic degradation of methyl orange and reduction of Cr(VI). J. Nanopart. Res. 2018, 20, 1–14. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Mishra, S.R. Photocatalytic performance of g-C3N4 based nanocomposites for effective degradation/removal of dyes from water and wastewater. Mater. Res. Bull. 2021, 143, 111417. [Google Scholar] [CrossRef]
- Bai, X.; Wang, X.; Lu, X.; Hou, S.; Sun, B.; Wang, C.; Jia, T.; Yang, S.; Bai, X. High crystallinity and conjugation promote the polarization degree in O-doped g-C3N4 for removing organic pollutants. CrystEngComm 2021, 23, 1366–1376. [Google Scholar] [CrossRef]
- Yang, X.; Luo, Z.; Wang, D.; Deng, C.; Zhao, Y.; Tang, F. Simple hydrothermal preparation of sulfur fluoride-doped g-C3N4 and its photocatalytic degradation of methyl orange. Mater. Sci. Eng. B 2023, 288, 116216. [Google Scholar] [CrossRef]
- Rong, X.; Qiu, F.; Rong, J.; Zhu, X.; Yan, J.; Yang, D. Enhanced visible light photocatalytic activity of W-doped porous g-C3N4 and effect of H2O2. Mater. Lett. 2016, 164, 127–131. [Google Scholar] [CrossRef]
- Li, S.; Zhu, T.; Dong, L.; Dong, M. Boosted visible light photodegradation activity of boron doped rGO/g-C3N4 nanocomposites: The role of C–O–C bonds. New J. Chem. 2018, 42, 17644–17651. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Zeng, G.; Chen, X.; Wu, Z.; Liang, J.; Zhang, J.; Wang, H.; Wang, H. Phosphorus- and Sulfur-Codoped g-C3N4: Facile Preparation, Mechanism Insight, and Application as Efficient Photocatalyst for Tetracycline and Methyl Orange Degradation under Visible Light Irradiation. ACS Sustain. Chem. Eng. 2017, 5, 5831–5841. [Google Scholar] [CrossRef]
- Meng, Q.; Lv, H.; Yuan, M.; Cheng, Z.; Chen, Z.; Wang, X. In Situ Hydrothermal Construction of Direct Solid-State Nano-Z-Scheme BiVO4/Pyridine-Doped g-C3N4 Photocatalyst with Efficient Visible-Light-Induced Photocatalytic Degradation of Phenol and Dyes. ACS Omega 2017, 2, 2728–2739. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Hong, Y.; Zeng, X.; Wei, J.; Wang, F.; Liu, M. Multilevel reconstruction of g-C3N4 nanorings via natural pollen for remarkable photocatalysis. Mater. Today Sustain. 2023, 21, 100267. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Qi, K.; Wang, Y.; Wen, F.; Wang, J. Superb photocatalytic activity of 2D/2D Cl doped g-C3N4 nanodisc/Bi2WO6 nanosheet heterojunction: Exploration of photoinduced carrier migration in S-scheme heterojunction. J. Alloys Compd. 2023, 933, 167789. [Google Scholar] [CrossRef]
- Chen, X.; Chen, C.; Zang, J. Construction of B-doped g-C3N4/MoO3 photocatalyst to promote light absorption and Z-scheme charge transfer. Diam. Relat. Mater. 2023, 132, 109606. [Google Scholar] [CrossRef]
- Reddy, C.V.; Kakarla, R.R.; Shim, J.; Aminabhavi, T.M. Synthesis of transition metal ions doped-ZrO2 nanoparticles supported g-C3N4 hybrids for solar light-induced photocatalytic removal of methyl orange and tetracycline pollutants. Chemosphere 2022, 308, 136414. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, X.; Ma, L.; Xu, X. Enhancing visible-light photocatalytic activity of g-C3N4 by doping phosphorus and coupling with CeO2 for the degradation of methyl orange under visible light irradiation. RSC Adv. 2015, 5, 68728–68735. [Google Scholar] [CrossRef]
- Liu, R.; Yang, W.; He, G.; Zheng, W.; Li, M.; Tao, W.; Tian, M. Ag-Modified g-C3N4 Prepared by a One-Step Calcination Method for Enhanced Catalytic Efficiency and Stability. ACS Omega 2020, 5, 19615–19624. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Wu, B.; Ning, Z.; Song, M.; Zhang, H.; Sun, X.; Wan, D.; Li, B. Porous g-C3N4 with defects for the efficient dye photodegradation under visible light. Water Sci. Technol. 2021, 84, 1354–1365. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Chao, C.; Yao, S.; Zhang, D.; Chen, Q. Enhanced visible-light activation of persulfate by g-C3N4 decorated graphene aerogel for methyl orange degradation. J. Alloys Compd. 2022, 926, 166904. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).